Abstract
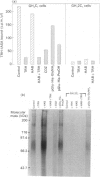
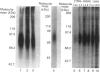
An analogue of thyrotropin-releasing hormone (TRH, pGlu-His-ProNH2), i.e. pGlu-His-ProNH-(CH2)6-(4-azidosalicylamide) (TRH-ASA), has been synthesized and, in a radioiodinated form (TRH-IASA), characterized and used as a photoaffinity reagent to label the TRH receptor on rat pituitary GH4C1 cells. TRH-IASA bound to GH4C1 cells with high affinity (Kd = 8 nM), comparable with that of TRH binding. The binding of TRH-IASA was competitive with binding of TRH, two TRH analogues and a TRH receptor antagonist, chlordiazepoxide. TRH-IASA did not bind to or label GH12C1 cells, which lack functional TRH receptors. Labelling of GH4C1 cells with TRH-IASA followed by SDS/PAGE and autoradiography of membrane proteins demonstrated labelling of a single polypeptide which ran as a diffuse band between 71 and 91 kDa, centred at 76 kDa. No change in this labelling pattern was observed as a function of the length of time (between 5 min and 2 h) that GH4C1 cells were incubated with 3 nM-TRH-IASA. Using either a very short (5 s) photolysis interval or low TRH-IASA concentrations, only the 76 kDa band was labelled. Minor bands appeared only after extended photolysis and use of high TRH-IASA concentrations. We conclude that the TRH receptor from rat pituitary GH4C1 cells is a single peptide with an apparent molecular mass of 76 kDa. Details of the chemical synthesis of TRH-ASA are given in Supplementary Publication SUP 50167 (5 pages), which has been deposited at the British Library Document Supply Centre, Boston Spa, Wetherby, West Yorkshire LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1992) 281, 5.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert P. R., Tashjian A. H., Jr Relationship of thyrotropin-releasing hormone-induced spike and plateau phases in cytosolic free Ca2+ concentrations to hormone secretion. Selective blockade using ionomycin and nifedipine. J Biol Chem. 1984 Dec 25;259(24):15350–15363. [PubMed] [Google Scholar]
- Albert P. R., Tashjian A. H., Jr Thyrotropin-releasing hormone-induced spike and plateau in cytosolic free Ca2+ concentrations in pituitary cells. Relation to prolactin release. J Biol Chem. 1984 May 10;259(9):5827–5832. [PubMed] [Google Scholar]
- Balk J. L., Johnston R. B., Pelton J. T. Comparative studies of thyrotropin releasing hormone diazomethyl ketone and chloromethyl ketone analogs. Biochem Biophys Res Commun. 1981 Jan 15;98(1):234–241. doi: 10.1016/0006-291x(81)91893-3. [DOI] [PubMed] [Google Scholar]
- Dannies P. S., Tasjian A. H., Jr Release and synthesis of prolactin by rat pituitary cell strains are regulated independently by thyrotropin-releasing hormone. Nature. 1976 Jun 24;261(5562):707–710. doi: 10.1038/261707a0. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Caron M. G., Lefkowitz R. J. A family of receptors coupled to guanine nucleotide regulatory proteins. Biochemistry. 1987 May 19;26(10):2657–2664. doi: 10.1021/bi00384a001. [DOI] [PubMed] [Google Scholar]
- Drummond A. H. Chlordiazepoxide is a competitive thyrotropin-releasing hormone receptor antagonist in GH3 pituitary tumour cells. Biochem Biophys Res Commun. 1985 Feb 28;127(1):63–70. doi: 10.1016/s0006-291x(85)80126-1. [DOI] [PubMed] [Google Scholar]
- Drummond A. H. Inositol lipid metabolism and signal transduction in clonal pituitary cells. J Exp Biol. 1986 Sep;124:337–358. doi: 10.1242/jeb.124.1.337. [DOI] [PubMed] [Google Scholar]
- Duong L. T., Hadac E. M., Miller L. J., Vlasuk G. P. Purification and characterization of the rat pancreatic cholecystokinin receptor. J Biol Chem. 1989 Oct 25;264(30):17990–17996. [PubMed] [Google Scholar]
- Gerard C. Purification of glycoproteins. Methods Enzymol. 1990;182:529–539. doi: 10.1016/0076-6879(90)82042-z. [DOI] [PubMed] [Google Scholar]
- Gershengorn M. C., Paul M. E. Evidence for tight coupling of receptor occupancy by thyrotropin-releasing hormone to phospholipase C-mediated phosphoinositide hydrolysis in rat pituitary cells: use of chlordiazepoxide as a competitive antagonist. Endocrinology. 1986 Aug;119(2):833–839. doi: 10.1210/endo-119-2-833. [DOI] [PubMed] [Google Scholar]
- Goebel R. J., Currie B. L., Bowers C. Y. Potential thyroliberin affinity labels. 1. Chloroacetyl-substituted phenylalanylpyrrolidines. J Med Chem. 1981 Apr;24(4):366–370. doi: 10.1021/jm00136a003. [DOI] [PubMed] [Google Scholar]
- Halpern J., Hinkle P. M. Direct visualization of receptors for thyrotropin-releasing hormone with a fluorescein-labeled analog. Proc Natl Acad Sci U S A. 1981 Jan;78(1):587–591. doi: 10.1073/pnas.78.1.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazum E. Photoaffinity labeling of peptide hormone receptors. Endocr Rev. 1983 Fall;4(4):352–362. doi: 10.1210/edrv-4-4-352. [DOI] [PubMed] [Google Scholar]
- Hinkle P. M., Kinsella P. A. Rapid temperature-dependent transformation of the thyrotropin-releasing hormone-receptor complex in rat pituitary tumor cells. J Biol Chem. 1982 May 25;257(10):5462–5470. [PubMed] [Google Scholar]
- Hinkle P. M., Kinsella P. A. Regulation of thyrotropin-releasing hormone binding by monovalent cations and guanyl nucleotides. J Biol Chem. 1984 Mar 25;259(6):3445–3449. [PubMed] [Google Scholar]
- Hinkle P. M. Pituitary TRH receptors. Ann N Y Acad Sci. 1989;553:176–187. doi: 10.1111/j.1749-6632.1989.tb46639.x. [DOI] [PubMed] [Google Scholar]
- Hinkle P. M., Tashjian A. H., Jr Receptors for thyrotropin-releasing hormone in prolactin producing rat pituitary cells in culture. J Biol Chem. 1973 Sep 10;248(17):6180–6186. [PubMed] [Google Scholar]
- Hinkle P. M., Woroch E. L., Tashjian A. H., Jr Receptor-binding affinities and biological activities of analogs of thyrotropin-releasing hormone in prolactin-producing pituitary cells in culture. J Biol Chem. 1974 May 25;249(10):3085–3090. [PubMed] [Google Scholar]
- Imai A., Gershengorn M. C. Evidence for tight coupling of thyrotropin-releasing hormone receptors to stimulated inositol trisphosphate formation in rat pituitary cells. J Biol Chem. 1985 Sep 5;260(19):10536–10540. [PubMed] [Google Scholar]
- Johnson W. A., Nathanson N. M., Horita A. Receptor binding and characterization of TRH receptors. Ann N Y Acad Sci. 1989;553:137–146. [PubMed] [Google Scholar]
- Labroo V. M., Kirk K. L., Cohen L. A., Delbeke D., Dannies P. S. Synthesis and biological activity of 5-fluoroimidazole-TRH. Biochem Biophys Res Commun. 1983 Jun 15;113(2):581–585. doi: 10.1016/0006-291x(83)91765-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavin T. N., Nambi P., Heald S. L., Jeffs P. W., Lefkowitz R. J., Caron M. G. 125I-labeled p-azidobenzylcarazolol, a photoaffinity label for the beta-adrenergic receptor. Characterization of the ligand and photoaffinity labeling of beta 1- and beta 2-adrenergic receptors. J Biol Chem. 1982 Oct 25;257(20):12332–12340. [PubMed] [Google Scholar]
- Martin T. F. Thyrotropin-releasing hormone rapidly activates the phosphodiester hydrolysis of polyphosphoinositides in GH3 pituitary cells. Evidence for the role of a polyphosphoinositide-specific phospholipase C in hormone action. J Biol Chem. 1983 Dec 25;258(24):14816–14822. [PubMed] [Google Scholar]
- Meyer J. P., Pelton J. T., Goeldner M., Hirth C. 2-Diazohistidinyl thyrotropin-releasing hormone (TRH): a photosensitive TRH analog. J Recept Res. 1991;11(1-4):371–377. doi: 10.3109/10799899109066415. [DOI] [PubMed] [Google Scholar]
- Moseley J. M., Findlay D. M., Gorman J. J., Martin T. J. Photoaffinity labeling of the calcitonin receptor. Pharmacol Ther. 1987;34(1):51–58. doi: 10.1016/0163-7258(87)90091-x. [DOI] [PubMed] [Google Scholar]
- Murdoch G. H., Franco R., Evans R. M., Rosenfeld M. G. Polypeptide hormone regulation of gene expression. Thyrotropin-releasing hormone rapidly stimulates both transcription of the prolactin gene and the phosphorylation of a specific nuclear protein. J Biol Chem. 1983 Dec 25;258(24):15329–15335. [PubMed] [Google Scholar]
- Phillips W. J., Hinkle P. M. Solubilization and characterization of pituitary thyrotropin-releasing hormone receptors. Mol Pharmacol. 1989 Apr;35(4):533–540. [PubMed] [Google Scholar]
- Shorr R. G., Heald S. L., Jeffs P. W., Lavin T. N., Strohsacker M. W., Lefkowitz R. J., Caron M. G. The beta-adrenergic receptor: rapid purification and covalent labeling by photoaffinity crosslinking. Proc Natl Acad Sci U S A. 1982 May;79(9):2778–2782. doi: 10.1073/pnas.79.9.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simasko S., Horita A. Chlordiazepoxide displaces thyrotropin-releasing hormone (TRH) binding. Eur J Pharmacol. 1984 Mar 2;98(3-4):419–423. doi: 10.1016/0014-2999(84)90291-7. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sojar H. T., Bahl O. P. Chemical deglycosylation of glycoproteins. Methods Enzymol. 1987;138:341–350. doi: 10.1016/0076-6879(87)38029-2. [DOI] [PubMed] [Google Scholar]
- Straub R. E., Frech G. C., Joho R. H., Gershengorn M. C. Expression cloning of a cDNA encoding the mouse pituitary thyrotropin-releasing hormone receptor. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9514–9518. doi: 10.1073/pnas.87.24.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szirtes T., Kisfaludy L., Pálosi E., Szporny L. Synthesis of thyrotropin-releasing hormone analogues. 1. Complete dissociation of central nervous system effects from thyrotropin-releasing activity. J Med Chem. 1984 Jun;27(6):741–745. doi: 10.1021/jm00372a006. [DOI] [PubMed] [Google Scholar]
- Szirtes T., Kisfaludy L., Pálosi E., Szporny L. Synthesis of thyrotropin-releasing hormone analogues. 2. Tripeptides structurally greatly differing from TRH with high central nervous system activity. J Med Chem. 1986 Sep;29(9):1654–1658. doi: 10.1021/jm00159a015. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr Clonal strains of hormone-producing pituitary cells. Methods Enzymol. 1979;58:527–535. doi: 10.1016/s0076-6879(79)58167-1. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr Hormonal regulation of cytosolic free calcium and its functional consequences: the GH-cell model. Environ Health Perspect. 1990 Mar;84:27–30. doi: 10.1289/ehp.908427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Yasumura Y., Levine L., Sato G. H., Parker M. L. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968 Feb;82(2):342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- Taylor C. W. The role of G proteins in transmembrane signalling. Biochem J. 1990 Nov 15;272(1):1–13. doi: 10.1042/bj2720001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winicov I., Cory R. N., Gershengorn M. C. Sphingosine interacts directly with the receptor complex to inhibit thyrotropin-releasing hormone binding. Endocrinology. 1990 Mar;126(3):1668–1672. doi: 10.1210/endo-126-3-1668. [DOI] [PubMed] [Google Scholar]
- Wright M., Høgset A., Alestrøm P., Gautvik K. M. A 64 kDa protein is a candidate for a thyrotropin-releasing hormone receptor in prolactin-producing rat pituitary tumor cells (GH4C1 cells). Biochem Biophys Res Commun. 1988 Dec 30;157(3):875–882. doi: 10.1016/s0006-291x(88)80956-2. [DOI] [PubMed] [Google Scholar]