Abstract
The biocatalytic degradation of poly(ethylene terephthalate) (PET) through enzymatic methods has garnered considerable attention due to its environmentally friendly and non-polluting nature, as well as its high specificity. While previous efforts in enhancing IsPETase performance have focused on amino acid substitutions in protein engineering, we introduced an amino acid insertion strategy in this work. By inserting a negatively charged acidic amino acid, Glu, at the right-angle bend of IsPETase, the binding capability between the enzyme’s active pocket and PET was improved. The resulted mutant IsPETase9394insE exhibited enhanced hydrolytic activity towards PET at various temperatures ranging from 30 to 45 ℃ compared with the wild-type IsPETase. Notably, a 10.04-fold increase was observed at 45 ℃. To further enhance PET hydrolysis, different carbohydrate-binding modules (CBMs) were incorporated at the C-terminus of IsPETase9394insE. Among these, the fusion of CBM from Verrucosispora sioxanthis exhibited the highest enhancement, resulting in a 1.82-fold increase in PET hydrolytic activity at 37 ℃ compared with the IsPETase9394insE. Finally, the engineered variant was successfully employed for the degradation of polyester filter cloth, demonstrating its promising hydrolytic capacity. In conclusion, this research presents an alternative enzyme engineering strategy for modifying PETases and enriches the pool of potential candidates for industrial PET degradation.
Keywords: Poly(ethylene terephthalate) (PET), Protein engineering, MD simulations, Carbohydrate-binding module
Introduction
Plastic pollution has emerged as a significant environmental issue in recent years, with its dangers and severity on the rise. Microplastics (Cox et al. 2019; Eshun and Pobee 2022), which are minuscule plastic particles often measuring less than 5 mm, have been discovered in virtually every corner of the planet, ranging from the deepest ocean trenches to remote polar regions (Citterich et al. 2023). Plastics can release harmful chemicals into the environment and water when exposed to sunlight and weathering. These chemicals can have harmful impacts on ecosystems and human health (Moyses et al. 2021). These microplastics can propagate through the food chain (Lin et al. 2023), ultimately accumulating within the human body, posing risks to human health (Li et al. 2023).
Nowadays, the enzymatic degradation of PET waste into recyclable oligomers or monomers represents the most ideal strategy for closed-loop recycling and disposal of PET waste, and researchers have been working on the discovery and modification of high-activity PET hydrolases. Since its discovery in 2016, IsPETase has undergone various modifications by researchers (Yoshida et al. 2016) as a model enzyme, including rational protein engineering and computational redesign, aimed at improving its PET hydrolytic activity. These efforts have led to the creation of remarkable mutants such as DuraPETase (Cui et al. 2021), HotPETase (Bell et al. 2022), FastPETase (Lu et al. 2022), and DepoPETase (Shi et al. 2023). At the same time, the LCCICCG constructed based on LCC by Tournier et al. also promoted the research progress of PET hydrolase (Tournier et al. 2020). However, it is worth noting that all the directed evolution of IsPETase thus far has focused on substituting amino acid residues, with no reports on the impact of amino acid insertions on IsPETase activity.
Carbohydrate-binding modules (CBMs) belong to a class of non-catalytic protein domains renowned for their exceptional binding affinity to cellulose and various other substances. CBMs can be categorized into three types: CBM type A, characterized by its high binding affinity to highly crystalline cellulose and chitin; CBM type B, which binds to internal glycan chains; and CBM type C, known for its binding to the termini of glycans (Roberts et al. 2021; Dai et al. 2021). These properties have been exploited to bind CBM-tagged recombinant proteins to different carbohydrates for various applications, especially for enzyme immobilization (Roberts et al. 2021).
Therefore, in this study, we attempted to insert an acidic amino acid into the active pocket of IsPETase. Experimental results have shown that inserting a negatively charged Glu between amino acid residues 93 and 94 in IsPETase effectively enhances its ability to degrade PET. MD simulations also indicate that the inserted amino acid increases the flexibility of IsPETase’s active pocket (Chen et al. 2021b). Finally, we tried to introduce carbohydrate modules that could improve the enzyme’s affinity for PET (Rennison et al. 2023).
Materials and methods
Chemicals and reagents
All analytical grade or higher purity chemicals were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Enzymes and kits used for plasmids construction and protein purification were purchased from Vazyme Biotech Co., Ltd (Nanjing, China). The PET film was purchased from Goodfellow (thickness 0.25 mm, code ES301450) and milled into PET powder (150–300 µm). The polyester filter cloth employed in this study was sourced from post-consumer waste collection.
Plasmid construction and mutagenesis
The genes encoding all the proteins utilized in this research were synthesized by General Biotech Co., Ltd (Anhui, China) and cloned into the pET22b(+). The amino acid sequences of these proteins are documented in Table 1. Plasmids containing variant were created using polymerase chain reaction (PCR)-based site-directed mutagenesis with the oligonucleotides provided in Table 2 (Yang et al. 2023). The validity of all plasmids was confirmed through sequencing at Tsingke Biotech Co., Ltd (Beijing, China).
Table 1.
Sequences of IsPETase and CBM1−5
| Gene name | Source | Sequence |
|---|---|---|
| IsPETase | Hydrolase from Ideonella sakaiensis (Uniprot number: A0A0K8P6T7) | QTNPYARGPNPTAASLEASAGPFTVRSFTVSRPSGYGAGTVYYPTNAGGTVGAIAIVPGYTARQSSIKWWGPRLASHGFVVITIDTNSTFDYPSSRSSQQMAALRQVASLNGDSSSPIYGKVDTARMGVMGHSMGGGASLRSAANNPSLKAAIPQAPWDSQTNFSSVTVPTLIFACENDSIAPVNSHALPIYDSMSRNAKQFLEINGGSHSCANSGNSNQALIGKKGVAWMKRFMDNDTRYSTFACENPNSTAVSDFRTANCS |
| CBM1 | Esterase from Verrucosispora sioxanthis (Uniprot number: A0A6M1LC17) | SGSANEIIGTQSGRCVDVPNASRNNGTRVQLYDCNKQTNQSWTYTTNKQLRVYDNMCLDAAGSGNGAAVQIYTCHSGTNQQWNVNSNGTITGVQSGRCLDVWSSNNGAQIQLYDCHGQPNQQFRLAALA |
| CBM2 | Endoglucanase from Bacillus subtilis (Uniprot number: P10475) | MASISVQYRAGDGSMNSNQIRPQLQIKNNGNTTVDLKDVTARYWYKAKNKGQNFDCDYAQIGCGNVTHKFVTLHKPKQGADTYLELGFKNGTLAPGASTGNIQLRLHNDDWSNYAQSGDYSFFKSNTFKTTKKITLYDQGKLIWGTEPN |
| CBM3 | Chitinase from a genome database of deep-sea bacteria (unpublished work) | VLRLRAIAPNPCASQTMIRFDLAGPAHTRVRVYSVAGRLVCTLLDAARGTGGHLVIWNRRDGADRPIPSGVYRCVVTAGPAAASRPLVVID |
| CBM4 | Chitinase from a genome database of deep-sea bacteria (unpublished work) | AAPSKPNIAWMPSQFNAPSSQTVTWNMWWGENGTDWTLYNNGSPVCSGSLTPNGQNAQSASCQIALAIGSNNLSVELCNRDGCQSDSKNIQVA |
| CBM5 | Chitinase from a genome database of deep-sea bacteria (unpublished work) | PAQPTIAWMPASASADDQTIRWDMWWGNNGNNWRLLHNGKKIHSGNLTPNGQQAQNGETVVNLQAGQHTFIVELCSNNLCTASESKTINIADT |
Table 2.
Oligonucleotide sequence of primers used for targeted mutagenesis
| Name | Sequence |
|---|---|
| 9394insE-F | 5ʹ-CGTCAAAGCTCCGAAATCAAATGGTGGGGTCCGCG-3ʹ |
| 9394insE-R | 5ʹ-CCATTTGATTTCGGAGCTTTGACGAGCAGTGTAACC-3ʹ |
Recombinant protein expression and purification in E. coli
The enzymes were expressed in E. coli BL21(DE3) for enzyme production. Recombinant strains were grown in 50 mL of LB medium at 37 ℃ and 200 rpm until the OD600 reached 0.6–0.8. Subsequently, 1 M isopropyl-β-thiogalactoside (IPTG) was added to the cultures, resulting in a final concentration of 1 mM, to induce protein expression for an additional 16 h at 20 ℃ with constant agitation at 200 rpm. Cells were then harvested through centrifugation (8000 rpm for 5 min), and the resulting cell pellet was resuspended in PBS (10 mM Phosphate buffer, pH 7.2–7.4). Sonication (240 W, 10 min) was used to disrupt the cells, and cell debris was removed by centrifugation (12,000 rpm for 30 min). Purification of all proteins was carried out using a Ni–NTA column. The Target protein were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and the total protein concentration of the enzyme solutions was determined using the BCA Protein Quantification Kit.
PET hydrolysis activity measurement
20 mg of PET powder (150–300 μm) was incubated with 500 nM of purified enzyme in a 10 mM phosphate buffer at pH 7.4, supplemented with 20% DMSO, at various temperatures. The enzyme reaction was terminated by the addition of an equal volume of methanol. The reaction was carried out in 2 mL Eppendorf tubes at 300 rpm.
As for the discarded polyester filter cloth, it was thoroughly cleaned with ultrapure water and allowed to dry. A strip of the cloth measuring approximately 15 mm in length and 5 mm in width, weighing around 20 mg, was then incubated with 500 nM of purified enzyme in a 10 mM phosphate buffer at pH 7.4, with the addition of 20% DMSO, at different temperatures. The enzyme reaction was stopped by adding an equal volume of methanol.
High-performance liquid chromatography (HPLC) analysis
The post-methanol-deactivated reaction solution was clarified by filtration through a 0.22-μm polyvinylidene fluoride (PVDF) syringe filter. The resulting supernatant was subjected to analysis via high-performance liquid chromatography (HPLC). Samples were examined using a C18 column (4.6 × 100 mm, 2.7 μm, Agilent, China). A mobile phase of 80% (V/V) 0.1% methanoic acid and 20% acetonitrile was employed, designated as mobile phase A and mobile phase B, respectively. The flow rate was set at 0.5 ml·min−1. The products were detected using a UV/Vis detector (SPD-20A) at a wavelength of 260 nm. The injected sample volume was 10 μL. The yield of hydrolytic products (BHET, MHET, and TPA) was determined by comparing retention times. The standards of BHET, MHET, and TPA were purchased from Sigma-Aldrich Co. (St.Louis, MO, USA). A standard curve was constructed by measuring peak areas of quantitative standard compounds, and concentrations were calculated from the peak areas of the analyzed samples using the standard curve (Brackmann et al. 2023).
Molecular dynamics (MD) simulations
Molecular dynamics (MD) simulations were conducted using IsPETase (PDB: 5XJH) and IsPETase9394insE (predicted by Alphafold 2) (Jumper et al. 2021; Barrio-Hernandez et al. 2023). Variants were generated with PYMOL. The OPLS-AA/L all-atom force field was applied. The enzymes were individually placed at the center of cubic simulation boxes, ensuring that all box edges were at least 2 Å away from any point on the protein surface. The protein was solvated with water, using the simple point charge (SPC) explicit solvent model. To neutralize the system, Na+ or Cl− ions were randomly introduced into the simulation box, replacing water molecules. The system was then energy-minimized using the steepest descent method. Subsequently, with position restraints on the heavy atoms, NVT equilibrations were conducted at 45 ℃ (318.15 K) for 100 ps (50,000 steps), followed by NPT ensemble equilibrations at 1 bar for 100 ps (50,000 steps). MD simulations were then performed for 100 ns (10,000,000 steps). The analysis included the assessment of root mean square deviation (RMSD) to gauge simulation accuracy and protein structural stability, radius of gyration (Rg) to measure protein conformation compactness, and root mean square fluctuation (RMSF) to examine the per amino acid fluctuation (Chen et al. 2021b).
Molecular docking calculations
Molecular docking of the tetrahedral intermediate, 2-HE(MHET)4, to PET hydrolase structures was performed using a combination of flexible and covalent docking approaches employing AutoDock Vina. The 2-HE(MHET)4 molecule was prepared using ChemDraw 19.0 (Maheswari and Salamun 2023).
For the generation of pdbqt files for both rigid and flexible receptors, specific flexible residues were selected. In the case of IsPETase, the flexible residues included Tyr87, Trp159, Ser160, Met161, Trp185, Ile208, His237, Ser238, and Asn241. For IsPETase9394insE, the selected flexible residues were Tyr87, Glu94, Trp160, Ser161, Met162, Trp186, Ile209, His238, Ser239, and Asn242. The bonds in the side chains of these selected residues were allowed to rotate (Joo et al. 2018).
Scanning electron microscope (SEM) analysis of polyester filter cloth
Polyester filter cloths were affixed onto a 3.2-mm SEM stub with the aid of carbon tape. Subsequently, the samples underwent sputter coating with an 8-nm layer of platinum/palladium, employing a Cressington 208HR Sputter Coater. The deposition of this metallic layer was accomplished through plasma generated within the chamber, utilizing argon as the process gas. The SEM imaging was conducted under vacuum conditions utilizing a Hitachi Regulus 8100 SEM, with an electron beam intensity set at 5 kV.
Results and discussion
Inserting additional acidic amino acids improves the PET hydrolytic capacity of IsPETase
Enzyme activity pockets are vital structural elements that have a pivotal role in facilitating enzyme catalytic functions (Pfaff et al. 2022; Aboelnga and Kalyaanamoorthy 2022; Prabmark et al. 2022). These pockets represent specific regions within the enzyme’s three-dimensional structure that interact with substrates (Guo et al. 2022; Wei et al. 2022), enabling and accelerating chemical reactions. The presence and properties of these enzyme activity pockets profoundly impact the enzyme’s activity, and they are closely related to substrate binding (Chen et al. 2022; Fang et al. 2023). The amino acid composition of enzyme activity pockets ensures effective substrate binding, which is crucial for initiating and facilitating the hydrolysis reaction (Erickson et al. 2022).
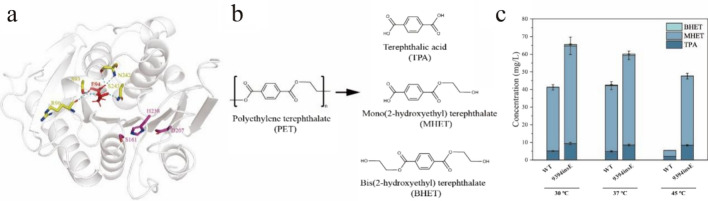
Since PET is a high-molecular-weight polymer, the size of the active pocket is closely related to PET hydrolysis capabilities (Pfaff et al. 2022). Therefore, researchers also aim to modify the activity pocket size of PET-hydrolyzing enzymes to reduce the difficulty of PET interacting with the catalytic triad, thereby enhancing enzyme activity (Nakamura et al. 2021; Chen et al. 2021a; Hong et al. 2023). In our efforts to boost the activity of IsPETase, we also turned our attention to the IsPETase activity pocket (Tournier et al. 2020; Han et al. 2017; Qu et al. 2020). According to literature, IsPETase assumes a right-angle conformation with the small molecule substrate 2-HE(MHET)4 (Joo et al. 2018). We introduced a negatively charged acidic amino acid, Glu (IsPETase9394insE), at the right-angle bend to improve the binding capability between IsPETase’s activity pocket and PET, with the expectation of enhancing enzyme activity.
We used Alphafold 2 to predict the protein structure of IsPETase9394insE (Jumper et al. 2021), and we observed that E94 can form hydrogen bonds with R90, S93, N242, and S243 (Fig. 1a). This suggests that the inserted Glu might be able to stabilize the enzyme’s active pocket. Subsequently, we measured the degradation abilities of IsPETase and IsPETase9394insE on PET powder at different temperatures. We used high-performance liquid chromatography (HPLC) to measure the concentrations of PET degradation products TPA, MHET, and BHET (Fig. 1b). Under various temperature conditions, IsPETase9394insE produced higher levels of TPA and MHET when degrading PET powder compared to IsPETase. Compared to IsPETase, IsPETase9394insE demonstrated a significant enhancement in hydrolytic activity at different temperatures. Specifically, the PET hydrolytic activity increased by 1.59-fold, 1.42-fold, and 10.04-fold at 30 ℃, 37 ℃ and 45 ℃ (Fig. 1c), respectively. What is particularly noteworthy is that at 45 ℃, IsPETase loses 86.38% of its activity compared to its optimal temperature of 37 ℃, while IsPETase9394insE only experiences a 3.74% reduction compared to its optimal temperature conditions. These suggest that the insertion of a negatively charged Glu has a stabilizing effect on the structure of IsPETase at elevated temperatures.
Fig. 1.
a Schematic diagram of inserting acidic amino acids to form hydrogen bonds with surrounding amino acids. The inserted amino acids are represented as red sticks. The catalytic triad is depicted as magenta sticks. The amino acids forming hydrogen bonds with the inserted amino acids are illustrated as yellow sticks. b Chemical structural formulas of PET and its degradation products TPA, MHET, BHET. c Comparison of TPA, MHET and BHET released by IsPETase, and IsPETase9394insE at different temperatures (30–45 ℃). WT represented IsPETase, 9394insE represented IsPETase9394insE
MD simulations reveal the effect of amino acid insertion on the structure of IsPETase
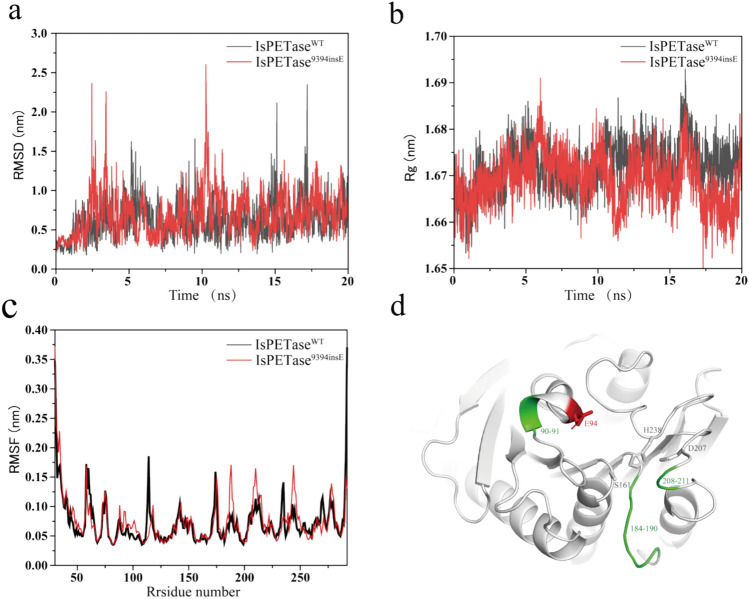
MD simulation trajectories offered valuable insights into the protein’s changes in conformation over the course of the simulations (Fig. 2). Figure 2a, b also demonstrates that IsPETase9394insE sustains Cα-RMSD and radius of gyration values similar to those of IsPETase throughout the simulations. For a more in-depth analysis of conformational stability, we computed the root mean square fluctuation (RMSF) of Cα atoms per residue during the MD simulations (Chen et al. 2021b).
Fig. 2.
Comparison of MD simulations of IsPETaseWT and IsPETase9394insE. a Root mean square deviation (RMSD) of the Cα atoms, and b the time course of radius of gyration (Rg), and c the root mean square fluctuation (RMSF) of the Cα atoms per residue during the MD simulations. Black line represented IsPETaseWT, and red line represented IsPETase9394insE d RMSF results for MD simulation showing flexibly enhanced amino acid residues are shown in green, inserted amino acids are shown in red stick, and the active amino acids is shown in white stick
As shown in Fig. 2c, it became evident that the average RMSF values of IsPETase were higher than those of IsPETase9394insE in specific regions, namely residues 90–91, 184–190, and 208–211. This indicates that amino acid residues in these regions display greater flexibility. Furthermore, Fig. 2d highlights that these particular regions are strategically located within the active pocket, underscoring the substantial influence of inserting a negatively charged Glu on the structure of IsPETase.
Molecular docking reveals the acute angle substrate binding conformation of IsPETase9394insE
To gain further insights into the impact of insertion amino acids on the protein structure, we conducted covalent docking calculations to speculate on the substrate binding mode of IsPETase and IsPETase9394insE. In this study, we employed 2-hydroxyethyl-(monohydroxyethyl terephthalate)4, referred to as 2-HE(MHET)4, which mimics PET, a four-MHET molecule. Our findings were in line with the report by Sang Yup Lee and his colleagues. IsPETase exhibited a right-angle binding conformation with 2-HE(MHET)4 (Fig. 3a, c) (Joo et al. 2018). However, IsPETase9394insE introduced a negatively charged Glu residue in the right-angle binding region of IsPETase with 2-HE(MHET)4, which led to increased electrostatic repulsion with the negatively charged PET in that region and caused a displacement in the spatial arrangement of surrounding amino acids. Consequently, IsPETase9394insE exhibited an acute angle binding conformation with 2-HE(MHET)4 (Fig. 3b, d).
Fig. 3.
Comparison of the structures of IsPETase and IsPETase9394insE. The IsPETase (a) and IsPETase9394insE (b) structure are presented as a cartoon diagram with residues involved in binding of 2-HE(MHET)4. The 2-HE(MHET)4 docking model is shown as a vermilion-colored stick. The catalytic triad shown as rose sticks. c and d The schematic structures of IsPETase and IsPETase9394insE with amino acid residues. The 2-HE(MHET)4 model is shown as a rose-colored stick. The existence of amino acids is shown in rose, the inserted amino acids are shown in red
In AutoDock Vina, the binding free energy serves as an indicator to assess the interactions between ligands and receptors. A lower binding free energy implies stronger interactions between the ligand and receptor, signifying a tighter binding. Hence, we also scrutinized the binding free energy for two different binding conformations. The results revealed that IsPETase exhibited a binding free energy of – 4.573 kcal/mol, while IsPETaseins displayed a binding free energy of – 5.128 kcal/mol. This suggests a stronger interaction between IsPETase9394insE and 2-HE(MHET)4, providing an explanation for the enhanced activity of IsPETase9394insE.
CBM fusion to further enhance the PET hydrolytic capability
The hydrophobic nature of PET material surfaces presents a challenge for enzyme binding in aqueous solutions (Rennison et al. 2023; Knott et al. 2020; Hwang et al. 2022). To enhance the enzyme’s affinity for PET, we explored the possibility of appending an additional carbohydrate-binding module (CBM) to the C-terminus of IsPETase9394insE.
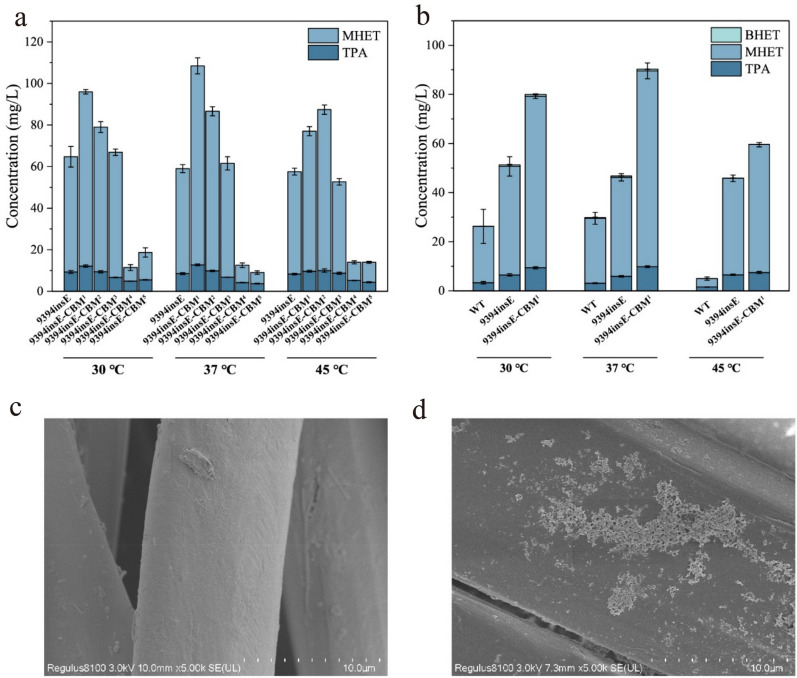
We compared the ability of five different CBMs to enhance the degradation of PET powder by IsPETase9394insE. These CBMs were attached to the C-terminus of IsPETase9394insE and were named IsPETase9394insE-CBM1−5 (Roberts et al. 2021; Chen et al. 2020). They included the CBM in esterase from Verrucosispora sioxanthis (Uniprot number: A0A6M1LC17) (Jiang et al. 2024), and the CBM in endoglucanase from Bacillus subtilis (Uniprot number: P10475) (Hwang et al. 2022), which was reported to be effective in enhancing PET hydrolase activity. In addition, three CBMs from chitinases in the hydrolase family 18 (GH18) identified from a genome database of deep-sea bacteria in an unpublished work are included. The successful expression and purification of IsPETase9394insE-CBM1−5 were confirmed through SDS-PAGE (Fig. 4). HPLC results showed that, under the conditions of 37 ℃, the CBM from Verrucosispora sioxanthis exhibited the highest enhancement of IsPETase9394insE activity, with a 1.82-fold increase. Interestingly, under higher temperature conditions (45 ℃), the CBM from Bacillus subtilis endoglucanase showed the most significant enhancement, increasing the MHET production by 1.51-fold. This suggests that this CBM may stabilize the overall enzyme structure, leading to improved enzyme activity (Fig. 5a).
Fig. 4.
a SDS-PAGE analysis of IsPETase and IsPETase9394insE. Expected proteins (contain his-tag) were marked in red box. b SDS-APGE analysis of IsPETaseCBM1, IsPETaseCBM2, IsPETaseCBM3, IsPETaseCBM4, and IsPETaseCBM5. Expected proteins (contain his-tag) were marked in red box. S represented supernatant after cell fragmentation; P represented precipitation after cell fragmentation; T represented purified target protein
Fig. 5.
a A comparison of TPA, MHET, and BHET released during the degradation of PET powder at different temperatures (30–45 ℃) by IsPETase9394insE, and IsPETase9394insE-CBM1−5. As BHET was detected in trace amounts, the calculation of degradation products for PET focused solely on the content of TPA and MHET. 9394insE represented IsPETase9394insE, 9394insE-CBM1−5 represented IsPETase9394insE-CBM1−5. b A comparison of TPA, MHET, and BHET released during the degradation of polyester by IsPETase, IsPETase9394insE, and IsPETase9394insE-CBM1 at different temperatures (30–45 ℃). WT represented IsPETase, 9394insE represented IsPETase9394insE. c Surface analysis of the polyester filter cloth using scanning electron microscopy (SEM) represents the non-enzyme system fabric surface. d depicts the polyester filter cloth surface after treatment with IsPETase9394insE-CBM1
Compared to IsPETase, IsPETase9394insE-CBM1 has shown a significant improvement in its ability to degrade PET, and thus, we further anticipated that the constructed IsPETase9394insE-CBM1 could contribute to addressing the challenges of recycling PET waste in the environment. Given that polyester filter cloth is one of the most important textile materials, its degradation has garnered increasing attention (Palacios-Mateo et al. 2021). Conventional methods such as chemical incineration and physical landfilling for handling discarded textiles not only harm the environment but also squander natural resources. Therefore, we also examined the ability of IsPETase9394insE-CBM1 to degrade waste polyester filter fabric at different temperatures. IsPETase9394insE-CBM1 displayed a similar trend in degrading polyester fabric as it did with PET powder, with optimal activity observed at 37 ℃ (Fig. 5a). In contrast to IsPETase9394insE, IsPETase9394insE-CBM1 exhibited a pronounced boost in activity at various temperatures. Its activity increased by 1.47-fold at 30 ℃, 1.82-fold at 37 ℃, and 1.33-fold at 45 ℃. This clearly demonstrated that the fusion of CBM enhanced the polyester filter cloth degradation capability of IsPETase9394insE to varying degrees. What is even more remarkable was that at 45 ℃, IsPETase9394insE-CBM1 displayed an 11.42-fold increase in polyester filter cloth degradation activity compared to IsPETase (Fig. 5b). This underscores the successful incorporation of a negatively charged Glu and the fusion of CBM in our approach. Figure 5c and d illustrates the surface changes of the polyester filter cloth before and after the enzymatic reaction, showing a transition from a relatively smooth texture to a corroded appearance, which further validated the good PET hydrolytic capacity of IsPETase9394insE-CBM1.
Conclusion
In summary, we introduced additional acidic amino acids into the active pocket of wild-type IsPETase and the resulted variant IsPETase9394insE exhibited enhanced PET degradation capacity at different temperatures, with the most significant increase in enzyme activity observed at 45 ℃. Molecular dynamics simulations further indicated that the insertion of amino acids improved the flexibility of amino acid residues within the enzyme’s active pocket, providing valuable insights for the future modification of PET hydrolytic enzymes. In addition, by incorporating an extra carbohydrate-binding module (CBM), we enhanced the binding affinity between IsPETase and PET substrates. We also validated the degradation capability of IsPETase9394insE-CBM1 towards polyester, showing a 11.42-fold increase in degradation efficiency compared to IsPETase. These findings suggest the potential application of the IsPETase variant obtained in this research for the recycling of industrial PET waste.
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2021YFC2102700) and the National Natural Science Foundation of China (U2106228, 32101887).
Author contributions
The research was designed by LJ and WL. WL and CL wrote the manuscript. The experiments were performed by CL and QQZ. Manuscript was critically revised by LJ, WL, and QYZ. All the authors read and approved the final manuscript.
Funding
National Key Research and Development Program of China, 2021YFC2102700, Ling Jiang, National Natural Science Foundation of China, U2106228, Ling Jiang, 32101887, Wei Liu.
Data availability
All data generated or analyzed during this study are included in this article.
Declarations
Conflict of interest
The authors declare they have no conflict of interest in the publication.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Wei Liu, Email: liuwei6775@njtech.edu.cn.
Ling Jiang, Email: jiangling@njtech.edu.cn.
References
- Aboelnga MM, Kalyaanamoorthy S (2022) QM/MM investigation to identify the hallmarks of superior PET biodegradation activity of PETase over cutinase. ACS Sustain Chem Eng 10(48):15857–15868. 10.1021/acssuschemeng.2c04913 10.1021/acssuschemeng.2c04913 [DOI] [Google Scholar]
- Barrio-Hernandez I, Yeo J, Jänes J, Mirdita M, Gilchrist CLM, Wein T, Varadi M, Velankar S, Beltrao P, Steinegger M (2023) Clustering predicted structures at the scale of the known protein universe. Nature 622(7983):637–645. 10.1038/s41586-023-06510-w 10.1038/s41586-023-06510-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EL, Smithson R, Kilbride S, Foster J, Hardy FJ, Ramachandran S, Tedstone AA, Haigh SJ, Garforth AA, Day PJR, Levy C, Shaver MP, Green AP (2022) Directed evolution of an efficient and thermostable PET depolymerase. Nat Catal 5(8):673–681. 10.1038/s41929-022-00821-3 10.1038/s41929-022-00821-3 [DOI] [Google Scholar]
- Brackmann R, de Oliveira Veloso C, de Castro AM, Langone MAP (2023) Enzymatic post-consumer poly(ethylene terephthalate) (PET) depolymerization using commercial enzymes. 3 Biotech 13(5):135. 10.1007/s13205-023-03555-6 10.1007/s13205-023-03555-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SJ, Lam MQ, Thevarajoo S, Abd Manan F, Yahya A, Chong CS (2020) Genome analysis of cellulose and hemicellulose degrading Micromonospora sp. CP22. 3 Biotech 10(4):160. 10.1007/s13205-020-2148-z 10.1007/s13205-020-2148-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-C, Han X, Li X, Jiang P, Niu D, Ma L, Liu W, Li S, Qu Y, Hu H, Min J, Yang Y, Zhang L, Zeng W, Huang J-W, Dai L, Guo R-T (2021a) General features to enhance enzymatic activity of poly(ethylene terephthalate) hydrolysis. Nat Catal 4(5):425–430. 10.1038/s41929-021-00616-y 10.1038/s41929-021-00616-y [DOI] [Google Scholar]
- Chen K, Hu Y, Dong X, Sun Y (2021b) Molecular insights into the enhanced performance of EKylated PETase toward PET degradation. Acs Catal 11(12):7358–7370. 10.1021/acscatal.1c01062 10.1021/acscatal.1c01062 [DOI] [Google Scholar]
- Chen XQ, Guo ZY, Wang L, Yan ZF, Jin CX, Huang QS, Kong DM, Rao DM, Wu J (2022) Directional-path modification strategy enhances PET hydrolase catalysis of plastic degradation. J Hazard Mater 433:128816. 10.1016/j.jhazmat.2022.128816 10.1016/j.jhazmat.2022.128816 [DOI] [PubMed] [Google Scholar]
- Citterich F, Lo Giudice A, Azzaro M (2023) A plastic world: a review of microplastic pollution in the freshwaters of the Earth’s poles. Sci Total Environ 869:161847. 10.1016/j.scitotenv.2023.161847 10.1016/j.scitotenv.2023.161847 [DOI] [PubMed] [Google Scholar]
- Cox KD, Covernton GA, Davies HL, Dower JF, Juanes F, Dudas SE (2019) Human consumption of microplastics. Environ Sci Technol 53(12):7068–7074. 10.1021/acs.est.9b01517 10.1021/acs.est.9b01517 [DOI] [PubMed] [Google Scholar]
- Cui Y, Chen Y, Liu X, Dong S, Ye T, Qiao Y, Mitra R, Han J, Li C, Han X, Liu W, Chen Q, Wei W, Wang X, Du W, Tang S, Xiang H, Liu H, Liang Y, Houk KN, Wu B (2021) Computational redesign of a PETase for plastic biodegradation under ambient condition by the GRAPE strategy. Acs Catal 11(3):1340–1350. 10.1021/acscatal.0c05126 10.1021/acscatal.0c05126 [DOI] [Google Scholar]
- Dai L, Qu Y, Huang J-W, Hu Y, Hu H, Li S, Chen C-C, Guo R-T (2021) Enhancing PET hydrolytic enzyme activity by fusion of the cellulose–binding domain of cellobiohydrolase I from Trichodermareesei. J Biotechnol 334:47–50. 10.1016/j.jbiotec.2021.05.006 10.1016/j.jbiotec.2021.05.006 [DOI] [PubMed] [Google Scholar]
- Erickson E, Gado JE, Avilán L, Bratti F, Brizendine RK, Cox PA, Gill R, Graham R, Kim D-J, König G, Michener WE, Poudel S, Ramirez KJ, Shakespeare TJ, Zahn M, Boyd ES, Payne CM, DuBois JL, Pickford AR, Beckham GT, McGeehan JE (2022) Sourcing thermotolerant poly(ethylene terephthalate) hydrolase scaffolds from natural diversity. Nat Commun 13(1):7850. 10.1038/s41467-022-35237-x 10.1038/s41467-022-35237-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshun F, Pobee ANA (2022) Effects of frying on microplastics load in fish and implications on health. Food Front 3(4):543–549. 10.1002/fft2.164 10.1002/fft2.164 [DOI] [Google Scholar]
- Fang Y, Chao K, He J, Wang Z, Chen Z (2023) High-efficiency depolymerization/degradation of polyethylene terephthalate plastic by a whole-cell biocatalyst. 3 Biotech 13(5):138. 10.1007/s13205-023-03557-4 10.1007/s13205-023-03557-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Vanga SR, Lopez-Lorenzo X, Saenz-Mendez P, Ericsson SR, Fang Y, Ye X, Schriever K, Bäckström E, Biundo A, Zubarev RA, Furó I, Hakkarainen M, Syrén P-O (2022) Conformational selection in biocatalytic plastic degradation by PETase. Acs Catal 12(6):3397–3409. 10.1021/acscatal.1c05548 10.1021/acscatal.1c05548 [DOI] [Google Scholar]
- Han X, Liu W, Huang J-W, Ma J, Zheng Y, Ko T-P, Xu L, Cheng Y-S, Chen C-C, Guo R-T (2017) Structural insight into catalytic mechanism of PET hydrolase. Nat Commun. 10.1038/s41467-017-02255-z 10.1038/s41467-017-02255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Ki D, Seo H, Park J, Jang J, Kim K-J (2023) Discovery and rational engineering of PET hydrolase with both mesophilic and thermophilic PET hydrolase properties. Nat Commun. 10.1038/s41467-023-40233-w 10.1038/s41467-023-40233-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DH, Lee ME, Cho BH, Oh JW, You SK, Ko YJ, Hyeon JE, Han SO (2022) Enhanced biodegradation of waste poly(ethylene terephthalate) using a reinforced plastic degrading enzyme complex. Sci Total Environ 842:156890. 10.1016/j.scitotenv.2022.156890 10.1016/j.scitotenv.2022.156890 [DOI] [PubMed] [Google Scholar]
- Jiang L, Li C, Liu W, Zhu LY, Wang CY (2024) A screening method and application of CBM for improving affinity between PET and enzyme. Patent, CN202311506262.0, 2024-02-0.
- Joo S, Cho IJ, Seo H, Son HF, Sagong HY, Shin TJ, Choi SY, Lee SY, Kim KJ (2018) Structural insight into molecular mechanism of poly(ethylene terephthalate) degradation. Nat Commun 9(1):382. 10.1038/s41467-018-02881-1 10.1038/s41467-018-02881-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D (2021) Highly accurate protein structure prediction with AlphaFold. Nature 596(7873):583–589. 10.1038/s41586-021-03819-2 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott BC, Erickson E, Allen MD, Gado JE, Graham R, Kearns FL, Pardo I, Topuzlu E, Anderson JJ, Austin HP, Dominick G, Johnson CW, Rorrer NA, Szostkiewicz CJ, Copie V, Payne CM, Woodcock HL, Donohoe BS, Beckham GT, McGeehan JE (2020) Characterization and engineering of a two-enzyme system for plastics depolymerization. Proc Natl Acad Sci U S A 117(41):25476–25485. 10.1073/pnas.2006753117 10.1073/pnas.2006753117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Sheng Y, Cui H, Wang M, Wu L, Song Y, Yang R, Li X, Huang H (2023) Discovery and mechanism-guided engineering of BHET hydrolases for improved PET recycling and upcycling. Nat Commun 14(1):4169. 10.1038/s41467-023-39929-w 10.1038/s41467-023-39929-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Xie H, Zhang Y, Tian X, Cui L, Shi N, Wang L, Zhao J, An L, Wang J, Li B, Li Y-F (2023) The toxicity of nano polyethylene terephthalate to mice: intestinal obstruction, growth retardant, gut microbiota dysbiosis and lipid metabolism disorders. Food Chem Toxicol. 10.1016/j.fct.2022.113585 10.1016/j.fct.2022.113585 [DOI] [PubMed] [Google Scholar]
- Lu H, Diaz DJ, Czarnecki NJ, Zhu C, Kim W, Shroff R, Acosta DJ, Alexander BR, Cole HO, Zhang Y, Lynd NA, Ellington AD, Alper HS (2022) Machine learning-aided engineering of hydrolases for PET depolymerization. Nature 604(7907):662–667. 10.1038/s41586-022-04599-z 10.1038/s41586-022-04599-z [DOI] [PubMed] [Google Scholar]
- Maheswari A, Salamun DE (2023) In silico molecular docking of cyclooxygenase (COX-2) ADME-toxicity and in vitro evaluation of antioxidant and anti-inflammatory activities of marine macro algae. 3 Biotech 13(11):359. 10.1007/s13205-023-03770-1 10.1007/s13205-023-03770-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyses DN, Teixeira DA, Waldow VA, Freire DMG, Castro AM (2021) Fungal and enzymatic bio-depolymerization of waste post-consumer poly(ethylene terephthalate) (PET) bottles using Penicillium species. 3 Biotech 11(10):435. 10.1007/s13205-021-02988-1 10.1007/s13205-021-02988-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Kobayashi N, Koga N, Iino R (2021) Positive charge introduction on the surface of thermostabilized PET hydrolase facilitates PET binding and degradation. Acs Catal 11(14):8550–8564. 10.1021/acscatal.1c01204 10.1021/acscatal.1c01204 [DOI] [Google Scholar]
- Palacios-Mateo C, van der Meer Y, Seide G (2021) Analysis of the polyester clothing value chain to identify key intervention points for sustainability. Environ Sci Eur 33(1):2. 10.1186/s12302-020-00447-x 10.1186/s12302-020-00447-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff L, Gao J, Li Z, Jackering A, Weber G, Mican J, Chen Y, Dong W, Han X, Feiler CG, Ao YF, Badenhorst CPS, Bednar D, Palm GJ, Lammers M, Damborsky J, Strodel B, Liu W, Bornscheuer UT, Wei R (2022) Multiple substrate binding mode-guided engineering of a thermophilic PET hydrolase. Acs Catal 12(15):9790–9800. 10.1021/acscatal.2c02275 10.1021/acscatal.2c02275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabmark K, Boonyapakron K, Bunterngsook B, Arunrattanamook N, Uengwetwanit T, Chitnumsub P, Champreda V (2022) Enhancement of catalytic activity and alkaline stability of cellobiohydrolase by structure-based protein engineering. 3 Biotech 12(10):269. 10.1007/s13205-022-03339-4 10.1007/s13205-022-03339-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu G, Li A, Acevedo-Rocha CG, Sun Z, Reetz MT (2020) The crucial role of methodology development in directed evolution of selective enzymes. Angew Chem Int Ed Engl 59(32):13204–13231. 10.1002/anie.201901491 10.1002/anie.201901491 [DOI] [PubMed] [Google Scholar]
- Rennison AP, Westh P, Møller MS (2023) Protein-plastic interactions: the driving forces behind the high affinity of a carbohydrate-binding module for polyethylene terephthalate. Sci Total Environ. 10.1016/j.scitotenv.2023.161948 10.1016/j.scitotenv.2023.161948 [DOI] [PubMed] [Google Scholar]
- Roberts AD, Payne KAP, Cosgrove S, Tilakaratna V, Penafiel I, Finnigan W, Turner NJ, Scrutton NS (2021) Enzyme immobilisation on wood-derived cellulose scaffolds via carbohydrate-binding module fusion constructs. Green Chem 23(13):4716–4732. 10.1039/d1gc01008e 10.1039/d1gc01008e [DOI] [Google Scholar]
- Shi L, Liu P, Tan Z, Zhao W, Gao J, Gu Q, Ma H, Liu H, Zhu L (2023) Complete depolymerization of PET wastes by an evolved PET hydrolase from directed evolution. Angew Chem Int Ed. 10.1002/anie.202218390 10.1002/anie.202218390 [DOI] [PubMed] [Google Scholar]
- Tournier V, Topham CM, Gilles A, David B, Folgoas C, Moya-Leclair E, Kamionka E, Desrousseaux ML, Texier H, Gavalda S, Cot M, Guemard E, Dalibey M, Nomme J, Cioci G, Barbe S, Chateau M, Andre I, Duquesne S, Marty A (2020) An engineered PET depolymerase to break down and recycle plastic bottles. Nature 580(7802):216–219. 10.1038/s41586-020-2149-4 10.1038/s41586-020-2149-4 [DOI] [PubMed] [Google Scholar]
- Wei R, von Haugwitz G, Pfaff L, Mican J, Badenhorst CPS, Liu W, Weber G, Austin HP, Bednar D, Damborsky J, Bornscheuer UT (2022) Mechanism-based design of efficient PET hydrolases. Acs Catal 12(6):3382–3396. 10.1021/acscatal.1c05856 10.1021/acscatal.1c05856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Yang Z, Liu J, Liu Z, Liu Y, Zhu L, Zhu Z, Jiang L (2023) Deciphering the contribution of PerR to oxidative stress defense system in Clostridiumtyrobutyricum. Food Frontiers 4(1):343–357. 10.1002/fft2.205 10.1002/fft2.205 [DOI] [Google Scholar]
- Yoshida S, Hiraga K, Takehana T, Taniguchi I, Yamaji H, Maeda Y, Toyohara K, Miyamoto K, Kimura Y, Oda K (2016) Response to comment on “A bacterium that degrades and assimilates poly(ethylene terephthalate).” Science 353(6301):759. 10.1126/science.aaf8625 10.1126/science.aaf8625 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.