Abstract
It has been shown previously that the thioredoxin system (thioredoxin + thioredoxin reductase + NADPH) may replace dithiothreitol (DTT) as a cofactor for vitamin KO and K reductase in salt-washed detergent-solubilized bovine liver microsomes. Here we demonstrate that the system can be improved further by adding protein disulphide-isomerase (PDI) to the components mentioned above. Moreover, NADPH may be replaced by reduced RNAase as a hydrogen donor. In our in vitro system the various protein cofactors were required at concentrations 2-5 orders of magnitude lower than that of DDT, whereas the maximal reaction rate was about 3-fold higher. PDI stimulated the thioredoxin-driven reaction about 10-fold, with an apparent Km value of 8 microM. These data suggest that in the vitro system the formation of disulphide bonds is somehow linked to the vitamin K-dependent carboxylation of glutamate residues. In vivo, both disulphide formation and vitamin K-dependent carboxylation are post-translational modifications taking place at the luminal side of the endoplasmic reticulum of mammalian secretory cells. The possibility that the reactions are also coupled in vivo is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Engström N. E., Holmgren A., Larsson A., Söderhäll S. Isolation and characterization of calf liver thioredoxin. J Biol Chem. 1974 Jan 10;249(1):205–210. [PubMed] [Google Scholar]
- Freedman R. B., Bulleid N. J., Hawkins H. C., Paver J. L. Role of protein disulphide-isomerase in the expression of native proteins. Biochem Soc Symp. 1989;55:167–192. [PubMed] [Google Scholar]
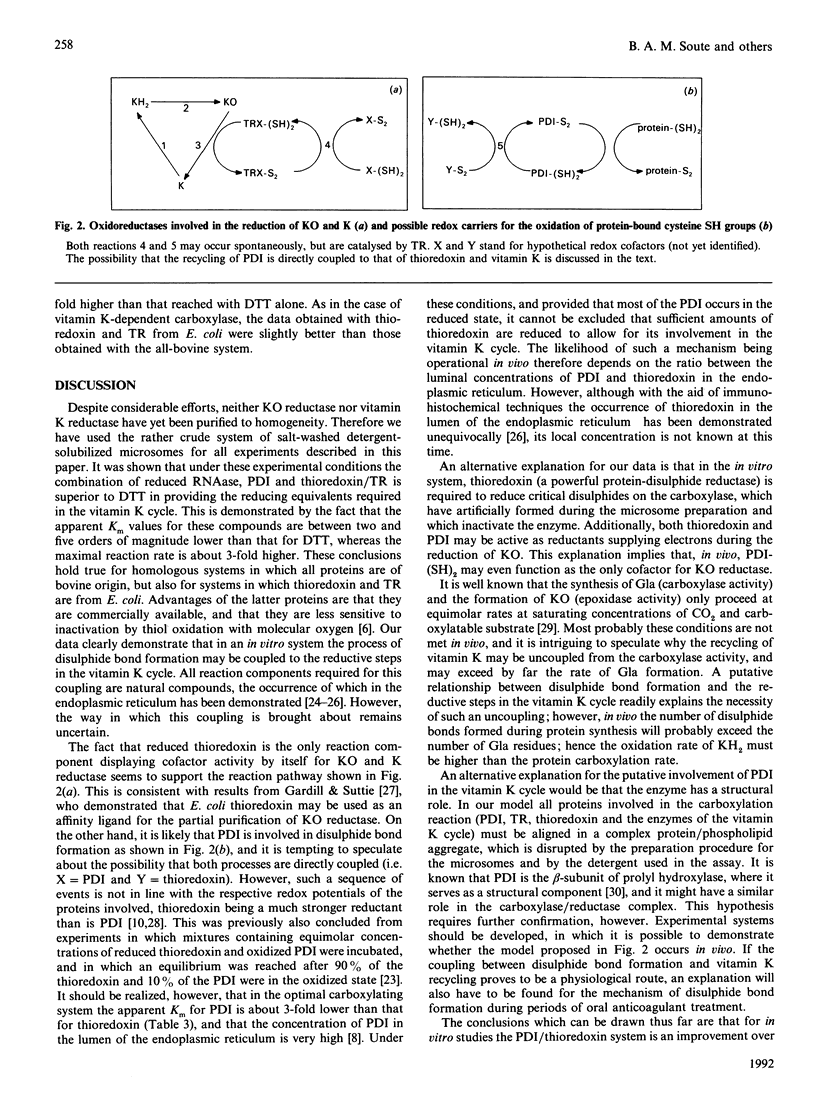
- Gardill S. L., Suttie J. W. Vitamin K epoxide and quinone reductase activities. Evidence for reduction by a common enzyme. Biochem Pharmacol. 1990 Sep 1;40(5):1055–1061. doi: 10.1016/0006-2952(90)90493-5. [DOI] [PubMed] [Google Scholar]
- Hawkins H. C., de Nardi M., Freedman R. B. Redox properties and cross-linking of the dithiol/disulphide active sites of mammalian protein disulphide-isomerase. Biochem J. 1991 Apr 15;275(Pt 2):341–348. doi: 10.1042/bj2750341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Bovine thioredoxin system. Purification of thioredoxin reductase from calf liver and thymus and studies of its function in disulfide reduction. J Biol Chem. 1977 Jul 10;252(13):4600–4606. [PubMed] [Google Scholar]
- Holmgren A., Luthman M. Tissue distrubution and subcellular localization of bovine thioredoxin determined by radioimmunoassay. Biochemistry. 1978 Sep 19;17(19):4071–4077. doi: 10.1021/bi00612a031. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989 Aug 25;264(24):13963–13966. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Hög J. O., von Bahr-Lindström H., Jörnvall H., Holmgren A. Cloning and expression of the glutaredoxin (grx) gene of Escherichia coli. Gene. 1986;43(1-2):13–21. doi: 10.1016/0378-1119(86)90003-x. [DOI] [PubMed] [Google Scholar]
- Johan L., van Haarlem M., Soute B. A., Vermeer C. Vitamin K-dependent carboxylase. Possible role for thioredoxin in the reduction of vitamin K metabolites in liver. FEBS Lett. 1987 Oct 5;222(2):353–357. doi: 10.1016/0014-5793(87)80401-5. [DOI] [PubMed] [Google Scholar]
- Lambert N., Freedman R. B. The latency of rat liver microsomal protein disulphide-isomerase. Biochem J. 1985 Jun 15;228(3):635–645. doi: 10.1042/bj2280635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson A. E., Friedman P. A., Suttie J. W. Vitamin K-dependent carboxylase. Stoichiometry of carboxylation and vitamin K 2,3-epoxide formation. J Biol Chem. 1981 Nov 10;256(21):11032–11035. [PubMed] [Google Scholar]
- Lundström J., Holmgren A. Protein disulfide-isomerase is a substrate for thioredoxin reductase and has thioredoxin-like activity. J Biol Chem. 1990 Jun 5;265(16):9114–9120. [PubMed] [Google Scholar]
- Luthman M., Holmgren A. Glutaredoxin from calf thymus. Purification to homogeneity. J Biol Chem. 1982 Jun 25;257(12):6686–6690. [PubMed] [Google Scholar]
- Luthman M., Holmgren A. Rat liver thioredoxin and thioredoxin reductase: purification and characterization. Biochemistry. 1982 Dec 21;21(26):6628–6633. doi: 10.1021/bi00269a003. [DOI] [PubMed] [Google Scholar]
- Parkkonen T., Kivirikko K. I., Pihlajaniemi T. Molecular cloning of a multifunctional chicken protein acting as the prolyl 4-hydroxylase beta-subunit, protein disulphide-isomerase and a cellular thyroid-hormone-binding protein. Comparison of cDNA-deduced amino acid sequences with those in other species. Biochem J. 1988 Dec 15;256(3):1005–1011. doi: 10.1042/bj2561005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozell B., Hansson H. A., Luthman M., Holmgren A. Immunohistochemical localization of thioredoxin and thioredoxin reductase in adult rats. Eur J Cell Biol. 1985 Jul;38(1):79–86. [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Silverman R. B., Nandi D. L. Reduced thioredoxin: a possible physiological cofactor for vitamin K epoxide reductase. Further support for an active site disulfide. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1248–1254. doi: 10.1016/s0006-291x(88)81274-9. [DOI] [PubMed] [Google Scholar]
- Soute B. A., Ulrich M. M., Vermeer C. Vitamin K-dependent carboxylase: increased efficiency of the carboxylation reaction. Thromb Haemost. 1987 Feb 3;57(1):77–81. [PubMed] [Google Scholar]
- Suttie J. W. Vitamin K-dependent carboxylase. Annu Rev Biochem. 1985;54:459–477. doi: 10.1146/annurev.bi.54.070185.002331. [DOI] [PubMed] [Google Scholar]
- Ulrich M. M., Soute B. A., de Boer-van den Berg M. A., Vermeer C. Isoenzymes of vitamin-K-dependent carboxylase. Biochim Biophys Acta. 1985 Jul 18;830(1):105–108. doi: 10.1016/0167-4838(85)90138-4. [DOI] [PubMed] [Google Scholar]
- Vermeer C. Gamma-carboxyglutamate-containing proteins and the vitamin K-dependent carboxylase. Biochem J. 1990 Mar 15;266(3):625–636. doi: 10.1042/bj2660625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin R., Martin L. F. Warfarin poisoning and vitamin K antagonism in rat and human liver. Design of a system in vitro that mimics the situation in vivo. Biochem J. 1987 Jan 15;241(2):389–396. doi: 10.1042/bj2410389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer-van den Berg M. A., Thijssen H. H., Vermeer C. The in vivo effects of acenocoumarol, phenprocoumon and warfarin on vitamin K epoxide reductase and vitamin K-dependent carboxylase in various tissues of the rat. Biochim Biophys Acta. 1986 Oct 29;884(1):150–157. doi: 10.1016/0304-4165(86)90238-2. [DOI] [PubMed] [Google Scholar]


