Abstract
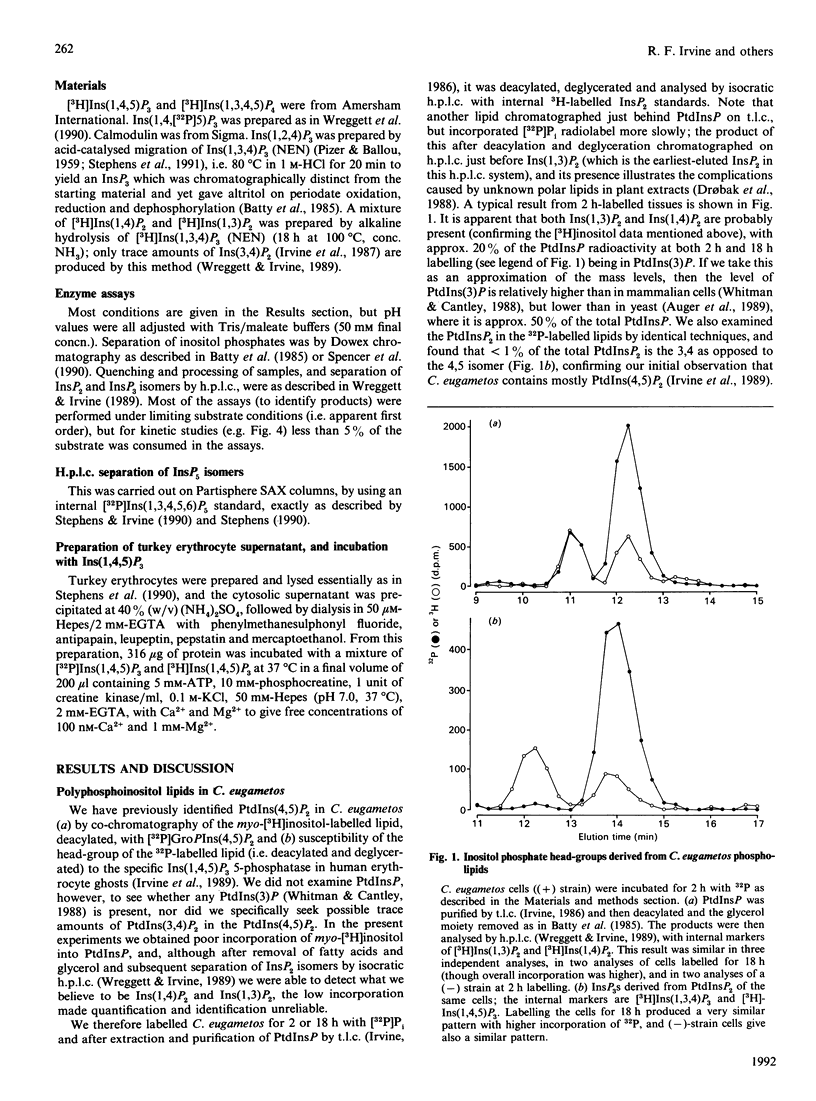
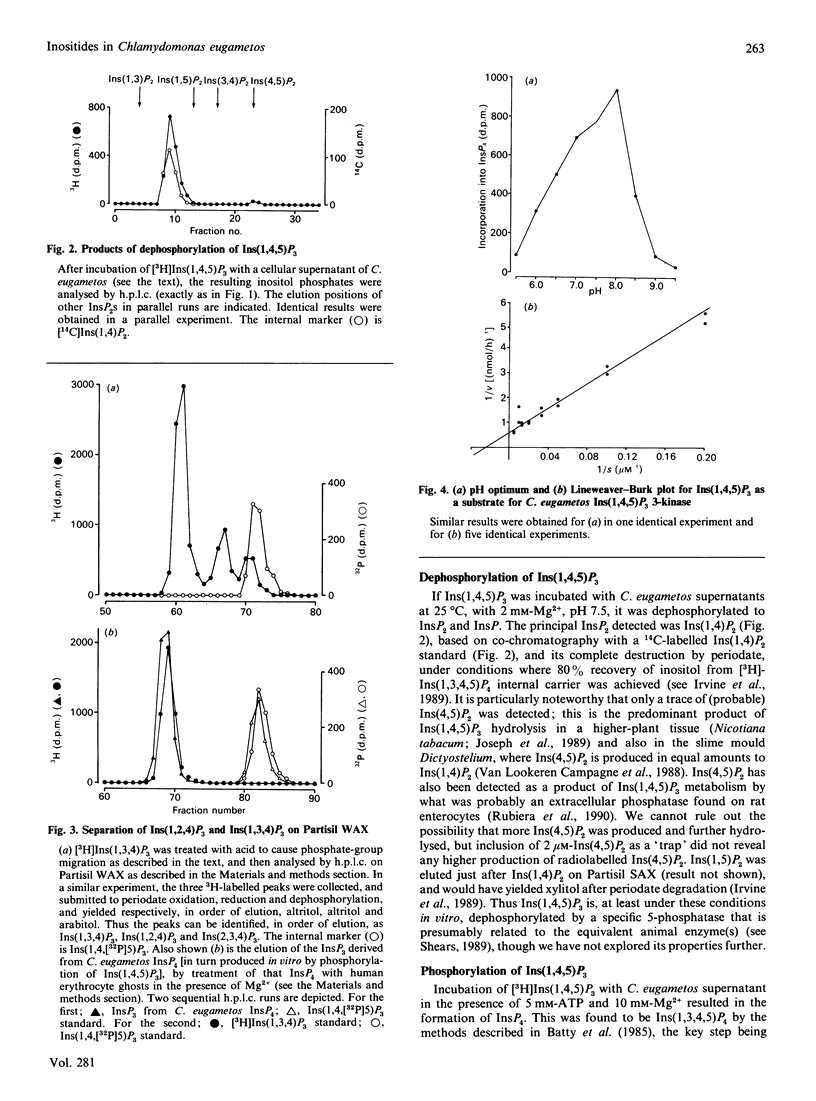
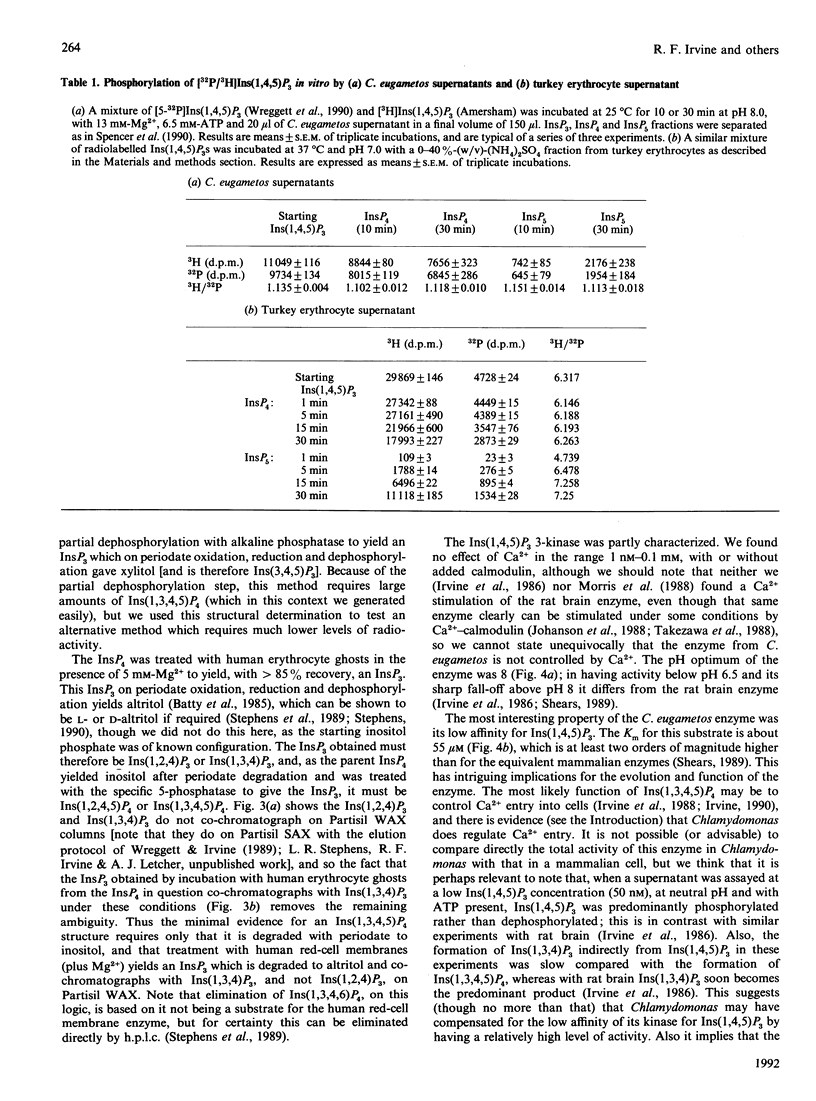
Swimming suspensions of Chlamydomonas eugametos were pelleted and homogenized, and the metabolism of inositol polyphosphates by cellular homogenates or supernatants was investigated. Ins(1,4,5)P3 was dephosphorylated under physiological conditions to yield a single InsP2, Ins(1,4]2. In the presence of ATP it was phosphorylated to give Ins(1,3,4,5)P3 as the only InsP4. The Ins(1,4,5)P3 3-kinase activity was predominantly soluble, was not detectably affected by calmodulin or Ca2+, and had a Km for Ins(1,4,5)P3 of 50 microM (two orders of magnitude higher than its mammalian counterpart). Ins(1,3,4,5)P4 was dephosphorylated by the cellular supernatants to Ins(1,3,4)P3 and Ins(1,4,5)P3, and could be phosphorylated to Ins(1,3,4,5,6)P4. No Ins(1,3,4)P3 6-kinase activity could be detected, and experiments with [3H]Ins(1,4,[32P]5)P3 revealed that Ins(1,3,4,5,6)P5 is formed from Ins(1,4,5)P3 with little loss of the 5-phosphate, i.e. the predominant route of synthesis is probably by a direct 6-phosphorylation of Ins(1,3,4,5)P4. Similar experiments with an (NH4)2SO4 fraction of turkey erythrocyte cytosol gave essentially the same result, i.e. direct phosphorylation of Ins(1,3,4,5)P4 in the 6 position is the predominant route of synthesis of InsP5 from that InsP4 in vitro. No InsP6 formation was detected in any of these experiments, but labelling of intact C. eugametos with [3H]inositol revealed that the cells do synthesize InsP6. The lipids of C. eugametos cells contain PtdIns, PtdIns(4)P and PtdIns(4,5)P2 [Irvine, Letcher, Lander, Drøbak, Dawson & Musgrave (1989) Plant Physiol. 64, 888-892]. Further examination of 32P-labelled lipids revealed that about 20% of the PtdInsP was the PtdIns(3)P isomer, and about 1% or less of the PtdInsP2 was the PtdIns(3,4)P2 isomer. The overall inositide metabolism of C. eugametos resembles that of a mammalian cell more closely than it does that of a plant cell or slime mould, and this suggests firstly that the known metabolism of inositol polyphosphates arose at an early time in eukaryotic evolution, and secondly that Chlamydomonas might prove a useful organism for genetic and comparative studies of inositide enzymology.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auger K. R., Carpenter C. L., Cantley L. C., Varticovski L. Phosphatidylinositol 3-kinase and its novel product, phosphatidylinositol 3-phosphate, are present in Saccharomyces cerevisiae. J Biol Chem. 1989 Dec 5;264(34):20181–20184. [PubMed] [Google Scholar]
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Blatt M. R., Thiel G., Trentham D. R. Reversible inactivation of K+ channels of Vicia stomatal guard cells following the photolysis of caged inositol 1,4,5-trisphosphate. Nature. 1990 Aug 23;346(6286):766–769. doi: 10.1038/346766a0. [DOI] [PubMed] [Google Scholar]
- Bloodgood R. A., Levin E. N. Transient increase in calcium efflux accompanies fertilization in Chlamydomonas. J Cell Biol. 1983 Aug;97(2):397–404. doi: 10.1083/jcb.97.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T. M., Bansal V. S., Bross T. E., Irvine R. F., Majerus P. W. The metabolism of tris- and tetraphosphates of inositol by 5-phosphomonoesterase and 3-kinase enzymes. J Biol Chem. 1987 Feb 15;262(5):2146–2149. [PubMed] [Google Scholar]
- Coté G. G., Depass A. L., Quarmby L. M., Tate B. F., Morse M. J., Satter R. L., Crain R. C. Separation and Characterization of Inositol Phospholipids from the Pulvini of Samanea saman. Plant Physiol. 1989 Aug;90(4):1422–1428. doi: 10.1104/pp.90.4.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drøbak B. K., Ferguson I. B., Dawson A. P., Irvine R. F. Inositol-containing lipids in suspension-cultured plant cells: an isotopic study. Plant Physiol. 1988 May;87(1):217–222. doi: 10.1104/pp.87.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S., Read N. D., Trewavas A. J. Elevation of cytoplasmic calcium by caged calcium or caged inositol triphosphate initiates stomatal closure. Nature. 1990 Aug 23;346(6286):769–771. doi: 10.1038/346769a0. [DOI] [PubMed] [Google Scholar]
- Hodgson M. E., Shears S. B. Rat liver contains a potent endogenous inhibitor of inositol 1,3,4,5-tetrakisphosphate 3-phosphatase. Biochem J. 1990 May 1;267(3):831–834. doi: 10.1042/bj2670831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyams J. S., Borisy G. G. Isolated flagellar apparatus of Chlamydomonas: characterization of forward swimming and alteration of waveform and reversal of motion by calcium ions in vitro. J Cell Sci. 1978 Oct;33:235–253. doi: 10.1242/jcs.33.1.235. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Drøbak B. K., Dawson A. P., Musgrave A. Phosphatidylinositol(4,5)bisphosphate and Phosphatidylinositol(4)phosphate in Plant Tissues. Plant Physiol. 1989 Mar;89(3):888–892. doi: 10.1104/pp.89.3.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Heslop J. P., Berridge M. J. Inositol(3,4)bisphosphate and inositol(1,3)bisphosphate in GH4 cells--evidence for complex breakdown of inositol(1,3,4)trisphosphate. Biochem Biophys Res Commun. 1987 Feb 27;143(1):353–359. doi: 10.1016/0006-291x(87)90672-3. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M., Pollock W. K., Smith P. M., Wreggett K. A. Inositol phosphates: proliferation, metabolism and function. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):281–298. doi: 10.1098/rstb.1988.0077. [DOI] [PubMed] [Google Scholar]
- Johanson R. A., Hansen C. A., Williamson J. R. Purification of D-myo-inositol 1,4,5-trisphosphate 3-kinase from rat brain. J Biol Chem. 1988 Jun 5;263(16):7465–7471. [PubMed] [Google Scholar]
- Joseph S. K., Esch T., Bonner W. D., Jr Hydrolysis of inositol phosphates by plant cell extracts. Biochem J. 1989 Dec 15;264(3):851–856. doi: 10.1042/bj2640851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya R., Witman G. B. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J Cell Biol. 1984 Jan;98(1):97–107. doi: 10.1083/jcb.98.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. J., Murray K. J., England P. J., Downes C. P., Michell R. H. Partial purification and some properties of rat brain inositol 1,4,5-trisphosphate 3-kinase. Biochem J. 1988 Apr 1;251(1):157–163. doi: 10.1042/bj2510157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubiera C., Lazo P. S., Shears S. B. Polarized subcellular distribution of the 1-, 4- and 5-phosphatase activities that metabolize inositol 1,4,5-trisphosphate in intestinal epithelial cells. Biochem J. 1990 Jul 15;269(2):353–358. doi: 10.1042/bj2690353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuring F., Brederoo J., Musgrave A., van den Ende H. Increase in calcium triggers mating structure activation in Chlamydomonas eugametos. FEMS Microbiol Lett. 1990 Sep 15;59(3):237–240. doi: 10.1016/0378-1097(90)90226-g. [DOI] [PubMed] [Google Scholar]
- Shears S. B. Metabolism of the inositol phosphates produced upon receptor activation. Biochem J. 1989 Jun 1;260(2):313–324. doi: 10.1042/bj2600313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Berrie C. P., Irvine R. F. Agonist-stimulated inositol phosphate metabolism in avian erythrocytes. Biochem J. 1990 Jul 1;269(1):65–72. doi: 10.1042/bj2690065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Downes C. P. Product-precursor relationships amongst inositol polyphosphates. Incorporation of [32P]Pi into myo-inositol 1,3,4,6-tetrakisphosphate, myo-inositol 1,3,4,5-tetrakisphosphate, myo-inositol 3,4,5,6-tetrakisphosphate and myo-inositol 1,3,4,5,6-pentakisphosphate in intact avian erythrocytes. Biochem J. 1990 Jan 15;265(2):435–452. doi: 10.1042/bj2650435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Hawkins P. T., Barker C. J., Downes C. P. Synthesis of myo-inositol 1,3,4,5,6-pentakisphosphate from inositol phosphates generated by receptor activation. Biochem J. 1988 Aug 1;253(3):721–733. doi: 10.1042/bj2530721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Hughes K. T., Irvine R. F. Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature. 1991 May 2;351(6321):33–39. doi: 10.1038/351033a0. [DOI] [PubMed] [Google Scholar]
- Stephens L. R., Irvine R. F. Stepwise phosphorylation of myo-inositol leading to myo-inositol hexakisphosphate in Dictyostelium. Nature. 1990 Aug 9;346(6284):580–583. doi: 10.1038/346580a0. [DOI] [PubMed] [Google Scholar]
- Stephens L., Hawkins P. T., Carter N., Chahwala S. B., Morris A. J., Whetton A. D., Downes P. C. L-myo-inositol 1,4,5,6-tetrakisphosphate is present in both mammalian and avian cells. Biochem J. 1988 Jan 1;249(1):271–282. doi: 10.1042/bj2490271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takazawa K., Passareiro H., Dumont J. E., Erneux C. Ca2+/calmodulin-sensitive inositol 1,4,5-trisphosphate 3-kinase in rat and bovine brain tissues. Biochem Biophys Res Commun. 1988 Jun 16;153(2):632–641. doi: 10.1016/s0006-291x(88)81142-2. [DOI] [PubMed] [Google Scholar]
- Van Lookeren Campagne M. M., Erneux C., Van Eijk R., Van Haastert P. J. Two dephosphorylation pathways of inositol 1,4,5-trisphosphate in homogenates of the cellular slime mould Dictyostelium discoideum. Biochem J. 1988 Sep 1;254(2):343–350. doi: 10.1042/bj2540343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M., Cantley L. Phosphoinositide metabolism and the control of cell proliferation. Biochim Biophys Acta. 1989 Feb;948(3):327–344. doi: 10.1016/0304-419x(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Wreggett K. A., Irvine R. F. Automated isocratic high-performance liquid chromatography of inositol phosphate isomers. Biochem J. 1989 Sep 15;262(3):997–1000. doi: 10.1042/bj2620997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreggett K. A., Lander D. J., Irvine R. F. Two-stage analysis of radiolabeled inositol phosphate isomers. Methods Enzymol. 1990;191:707–718. doi: 10.1016/0076-6879(90)91043-6. [DOI] [PubMed] [Google Scholar]


