Abstract
Demyelination is a common pathological feature in a wide range of diseases, characterized by the loss of myelin sheath and myelin-supporting oligodendrocytes. These losses lead to impaired axonal function, increased vulnerability of axons to damage, and result in significant brain atrophy and neuro-axonal degeneration. Multiple pathomolecular processes contribute to neuroinflammation, oligodendrocyte cell death, and progressive neuronal dysfunction. In this study, we use the cuprizone mouse model of demyelination to investigate long-term non-invasive gamma entrainment using sensory stimulation as a potential therapeutic intervention for promoting myelination and reducing neuroinflammation in male mice. Here, we show that multisensory gamma stimulation mitigates demyelination, promotes oligodendrogenesis, preserves functional integrity and synaptic plasticity, attenuates oligodendrocyte ferroptosis-induced cell death, and reduces brain inflammation. Thus, the protective effects of multisensory gamma stimulation on myelin and anti-neuroinflammatory properties support its potential as a therapeutic approach for demyelinating disorders.
Subject terms: Myelin biology and repair, Oligodendrocyte, Microglia, Astrocyte
Demyelination leads to nerve damage and inflammation. Here, the authors show multisensory gamma stimulation’s potential to mitigate demyelination and neuroinflammation, suggesting that this might be a therapeutic strategy for demyelinating diseases.
Introduction
Myelin is a compact, multilayered structure produced by oligodendrocytes (OLs) that surrounds and insulates neurons, and is essential for proper central nervous system (CNS) functionality1. Direct insults to OLs and myelin can lead to demyelination, a phenomenon characterized by a progressive loss of myelin layers, which subsequently induces neuroaxonal degeneration2. Demyelination of the CNS may occur as a response to trauma, autoimmunity, or genetic factors being associated with several CNS diseases, such as multiple sclerosis (MS), neuromyelitis optica (NMO), acute disseminated encephalomyelitis (ADEM), among others3–5. The course of these devastating neurological conditions comprises severe neurodegenerative processes, including neuroinflammation, profound myelin damage (high degree of demyelination), and brain atrophy6–8.
It is recognized that demyelinating diseases are multifactorial, with associations to genetic susceptibility, lifestyle, and environmental factors, such as potential risk due to viral infections, which trigger distinct pathogenic mechanisms6,9,10. In particular, central myelin degeneration promoted by pathological interactions involving both peripheral and central cell-mediated immune attacks induces the accumulation of dysfunctional myelin and neurological deficits1,11. Faced with the insults to the CNS microenvironment, microglia, the resident macrophages of the brain, rapidly become reactive and together with other brain immune sentinel cells (e.g. astrocytes), contribute to neuroinflammation and neurodegeneration12–14. Furthermore, it is noteworthy that the pro-inflammatory environment, metabolic stress, and the accumulation of toxic products of biological origin lead to injury and loss of OLs15.
Thus, discovering treatments that promote remyelination and myelin repair is imperative. Current hypotheses posit that post-natal modulation of myelination may depend on the activity of mature neurons16. Ensheathment of neurons by OLs is a highly coordinated process, requiring a specific signal that appears to be modulated in a neuronal activity-dependent manner17. Previously published studies have shown that non-invasive gamma entrainment using sensory stimulation (GENUS) at 40 Hz preserved neuronal and synaptic density, induced glial cell response changes, and prevented brain atrophy in Alzheimer’s disease (AD)18–20. Importantly, despite recent research indicating distinct findings regarding the 40 Hz effect, particularly concerning amyloid load in AD21,22, multiple studies in both mouse models and humans have consistently affirmed the beneficial effects of GENUS on disease pathology18,19,23–30.
Major insights into the demyelination and remyelination processes that occur in the CNS have been made through the use of mouse models, such as with the cuprizone (CPZ) mouse model. CPZ intoxication mimics several degenerative events in the brain, inducing demyelination, loss of OLs, axonal and synaptic dysfunction, glial cell activation, and oxidative stress, recapitulating cellular and molecular degenerative features of demyelinating diseases31–34.
In this context, we hypothesized that long-term non-invasive GENUS could prevent demyelination and reduce neuroinflammation in demyelinating conditions. Thus, we perform a therapeutic approach using the CPZ-induced demyelination model and test the effectiveness of GENUS. Here, we show that GENUS significantly decreases demyelination induced by CPZ, preserves the functional integrity of white matter tracts, and enhances synaptic plasticity. Furthermore, GENUS stimulates oligodendrogenesis in the corpus callosum (CC) and decreases OL cell death by reducing ferroptosis. In addition, long-term GENUS shows a notable anti-inflammatory effect in the brain by decreasing microgliosis and astrogliosis, along with a decrease in potent proinflammatory molecules, such as high mobility group box 1 (HMGB1), complement component 1q (C1q), and complement C3 (C3). Taken together, these data suggest that GENUS may be a potential therapeutic for myelin degeneration disorders.
Results
GENUS attenuated cuprizone-induced demyelination
To study whether long-term auditory plus visual GENUS influences the demyelination process induced by CPZ, we assessed its effects on the CC. In the CC of CPZ-treated mice, the peak of demyelination occurs at 4-5 weeks31,35. Here, 12-week-old male wild-type mice (C57BL/6 J) were treated with a 0.3% CPZ diet for 3 weeks. At that point, mice were exposed to GENUS (40 Hz) or control stimulation (constant light and sound with matched lux intensity and decibels) 1 h/d for 3 weeks, maintaining the CPZ diet until week 6 (Supplementary Fig. 1A). To assess the baseline levels, a normal diet (ND) cohort of 12-week-old male C57BL/6 J was subjected to the same experimental paradigm (Supplementary Fig. 1B). To investigate whether GENUS can entrain gamma oscillations in both the primary somatosensory (SSp) and prefrontal frontal (PFC) cortices of CPZ mice, we conducted recordings of local field potentials (LFP) during combined auditory and visual stimulation at 40 Hz (Fig. 1A). Our findings revealed a clear elevation in LFP power specifically at the 40 Hz frequency band in response to multisensory stimulation (Fig. 1B, C). LFP analysis showed a significant interaction effect in the PFC, indicating varied gamma power response between 3 and 6 weeks. In contrast, no significant interaction was found in the SSp, suggesting a stable gamma power response. This indicates potential variability in the specific vulnerability of the PFC compared to the SSp in CPZ-induced demyelination, as evidenced by the levels of MBP in this region, which are not as dramatically reduced as those in the PFC (Supplementary Fig. 1C–H). Immunohistochemical (IHC) analysis of myelination using the myelin-related markers Fluoromyelin™ and myelin oligodendrocyte glycoprotein (MOG), demonstrated a reduction in myelin content in the CPZ cohort compared to the normal diet (ND) cohort at week 6, which is in agreement with the findings reported in the literature on the cytotoxic effect of CPZ35. In the CC, we observed that GENUS significantly reduces CPZ-induced demyelination at the 6-week time point (Fig. 1D–F and Supplementary Fig. 1I, J). Morphometric analysis of the CC revealed that GENUS effectively protects against CPZ cytotoxicity. Remarkably, both the area and thickness of CC were preserved in the CPZ + 40 Hz group (Fig. 1G, H). To understand the dynamics of myelin changes during CPZ intoxication, we introduced a cohort of animals that experienced both interventions simultaneously for 3 weeks (CPZ plus either 40 Hz or control constant light and sound) (Supplementary Fig. 7A). Our findings indicate that after 3 weeks, GENUS shows a trend in protecting against CPZ-induced demyelination, as evidenced by increased levels of both Fluoromyelin and MOG in the CPZ + 40 Hz group compared to the CPZ control group (Supplementary Fig. 7B–F). We found an increment in MBP expression between CPZ + 40 Hz compared with CPZ control mice in different brain areas (cortex, CC, and striatum), supporting a protective role of GENUS against CPZ-induced demyelination throughout multiple brain regions and CC tracts. Additionally, we identified an elevated expression of structural proteins such as ankyrin G (ANKG) and neurofilament heavy chain (NFH) in these regions (Fig. 1I and Supplementary Fig. 2A-H). These findings were further corroborated by transmission electron microscopy (TEM). TEM images obtained at both high and low magnifications revealed a substantial increase in myelinated axons, accompanied by a reduction in the G-ratio and axon diameter in the CPZ + 40 Hz group (Fig. 1J–M and Supplementary Fig. 2I). Axon diameter distribution exhibited a distinctive pattern, and larger G-ratio axons were found in CPZ control group (Fig. 1N, O and Supplementary Fig. 2J–L).
Fig. 1. GENUS reduces cuprizone-induced demyelination.
A LFP schematic approach. B LFP spectrograms from the PFC and SSp at two different time points. C Tile scan of mouse brain slices of one mouse to confirm probe implantation using MBP (green) and GFAP (magenta) antibodies (mouse brain atlas by Matt Gaidica87, scale bar: 1000 μm). D–H IHC of Fluoromyelin (green) and MOG antibody (red) of the CC (n = 8 mice per group, each dot corresponds to a technical replicate, tile scan, scale bar—100 μm). E Normalized fluorescence intensity of Fluoromyelin® in the CC area (mean ± SD). F (1,28) = 11,56; p = 0.0020. F Normalized fluorescence intensity of MOG in the CC area (mean ± SD). F (1,28) = 1.359; p = 0.2535. G Morphometric analysis of CC area (mean ± SD). F (1,28) = 8.330; p = 0.0074. H Morphometric analysis of CC thickness (mean ± SD). F (1,28) = 8.660; p = 0.0065. I IHC of MBP antibody (green) in different brain areas (Ctx cortex, AC anterior commissure, CC corpus callosum and STR striatum; representative image, one mouse per group). J–M Representative TEM images of the CC of (n = 4 mice per group; 14 technical replicates for high magnification images and one technical replicate for low magnification images, 200 axons per mouse). K G-ratio quantification (mean ± SD). F (597,2398) = 0.8396; p = 0.9959. L Myelin thickness quantification (mean ± SD). F (597,2398) = 0.8617; p = 0.9879. M Axon diameter quantification (mean ± SD). F (597,2398) = 0.9513; p = 0.7747. N Scatter plot displays the linear regression of g-ratios of individual myelinated axons per their axon diameter for each group. O Bar graph displays the percentage of myelinated axons to their axon diameter, for each group. P–S Experimental detail of stimulated and recording electrodes implanted in the CC to detect CAPs (n = 3 mice per group, two technical replicates). Q CAPs detected across the experimental groups showed N1 (myelinated axons) and N2 (non-myelinated axons) peaks. R Quantification of N1 CAP amplitude. F (60,2079) = 9.366; p < 0.0001. S Quantification of N2 CAP amplitude. F(60,2079) = 1.119; p = 0.2498. All p-values were calculated using two-way ANOVA followed by Bonferroni’s multiple comparisons test (Supplementary Data S14). Schematic panel A and P were created with BioRender. Source data are provided as a Source Data file. **** p ≤ 0.0001, ** p ≤ 0.01, *p < 0.005. LFP local field potentials, CPZ cuprizone, ND normal diet, SSp somatosensory cortex, PFC prefrontal cortex, CAPs compound action potentials.
To explore the functional implications of the demyelination process and the potential neuroprotective effect of GENUS, we performed callosal electrophysiological measurements of compound action potentials (CAPs) in 400 μm-coronal brain sections (Fig. 1P). The evoked waveform pattern exhibited two distinct negative peaks, with the first peak, N1, corresponding to the rapid depolarization of myelinated axons, and the second peak, N2, associated with the depolarization of non-myelinated axons36. By utilizing electrodes positioned in the CC of ex vivo acute sections, we recorded a prominent deficiency in the negative peak N1 and observed a weak response signal from non-myelinated axons in the CPZ control group, indicating a primary injury in the myelinating axons (Fig. 1Q). The statistical analysis of CAPs revealed a significant improvement in the axonal function of the CPZ + 40 Hz group compared to the CPZ control group in terms of both N1 and N2 peaks (Fig. 1R, S and Supplementary Fig. 2M, N). These observations provide compelling evidence that supports the beneficial effect of GENUS on preserving the structural and functional integrity of myelinated axons even under CPZ-induced cytotoxicity.
Brain proteomics revealed distinct pathological signatures rescued by GENUS
In an effort to understand how GENUS may rescue the CPZ phenotype, we conducted quantitative proteomics of brain tissue using tandem mass spectrometry (TMT) (Fig. 2A and Supplementary Fig. 3A, B). The computational analysis through a convectional pipeline identified 7242 proteins (Supplementary Data S1–3). A total of 473 significantly differentially expressed proteins (DEPs) were identified across the 4 groups (Supplementary Fig. 3C, D and Supplementary Data S4). In order to understand the overall pathway-specific biology of these DEPs, we explored the enriched networks. We observed a high involvement of metabolic pathways, an expected result due to the effect of the CPZ diet. Interestingly, neurodegeneration and disease pathways, such as AD and Parkinson’s disease, glutamatergic synapse, energy metabolism, or amino acids metabolism pathways were also prominently identified (Fig. 2B and Supplementary Fig. 3E, F). To detect whether DEPs could be related to the GENUS effect, we restricted the analysis to the DEPs that showed an altered pattern between CPZ groups in the variance analysis. After applying this criterion, 142 DEPs were filtered, and 3 panels associated with the GENUS effect were identified based on their expression pattern, with these proteins being linked to more specific pathways (Fig. 2C, Supplementary Fig. 3G, Supplementary Data S5).
Fig. 2. GENUS rescued distinct CPZ-induced pathological signatures.
A TMT proteomics workflow. B Overall pathway-specific biology analysis of DEPs (a total of 473). C Heatmap of DEPs after filtering the proteins of interest. D–H From the heatmap, 3 panels were associated with the GENUS effect (n = 4 CPZ + 40 Hz; n = 3 CPZ control; n = 4 ND + 40 Hz; n = 4 ND control, one technical replicate). D Panel I shows the marked overexpression of DEPs in the CPZ control group, but also in the ND control group. F (72,275) = 0.4664; p > 0.9999. E Sankey diagram of significantly enriched biological pathways (top 5 GO terms). F Panel II shows the marked underexpression of DEPs in the CPZ control group, as well as, in the ND control group. F (12,55) = 0.1093; p > 0.9999. G Sankey diagram of significantly enriched biological pathways (top 5 GO terms). H Panel III shows the marked underexpression of DEPs in the CPZ control group. F (24,99) = 0.1095; p > 0.9999. I Sankey diagram of significantly enriched biological pathways (top 5 GO terms). J–M IHC of HMGB1 (red), OLIG2 (green), and GFAP (magenta) of the CC of 12-week WT mice under CPZ and ND (n = 6 CPZ + 40 Hz; n = 6 CPZ control; n = 6 ND + 40 Hz; n = 6 ND control, scale bar—5 μm). K Normalized mean intensity of HMGB1 in the CC area (mean ± SD). F (1,18) = 62.38; p < 0.0001. L Normalized colocalization of HMGB1 GFAP+ cells in the CC area (mean ± SD). F(1,19) = 7.167; p = 0.0149. M Normalized colocalization of HMGB1 OLIG2+ cells in the CC area (mean ± SD). F (1,19) = 15.11; p = 0.0010. N–Q IHC of SYN1 (green), SHANK3 (red), and MAP2 (magenta) of the anterior cingulate cortical area (ACA) (n = 6 mice per group, scale bar—5 μm). O Normalized mean intensity of SYN in the ACA area (mean ± SD). F (1,20) = 6.710; p = 0.0175. P Normalized mean intensity of SHANK3 in the ACA area (mean ± SD). F(1,20) = 5.525; p = 0.0291. Q Normalized mean intensity of MAP2 in the ACA area (mean ± SD). F (1,20) = 7.737; p = 0.0115. All P-values were calculated using two-way ANOVA followed by Bonferroni’s multiple comparisons test (Supplementary Data S14). Schematic panel A was created with BioRender. Source data are provided as a Source Data file. CPZ cuprizone, ND normal diet.
In Panel 1, DEPs are overexpressed in both CPZ and ND control groups and gene ontology (GO) enrichment analysis implicated these DEPs in intracellular ion homeostasis and transport, such as calcium-regulated proteins (e.g., sarcoplasmic/endoplasmic reticulum calcium ATPase 3—SERCA3, inositol 1,4,5-Trisphosphate Receptor Type 1—ITPR1, voltage-dependent anion-selective channel—VDAC), glucose metabolism (e.g., enolase 1—ENO1, aldolase C—ALDOC, aldehyde dehydrogenase 1 family member A1—ALDH1A1), monoatomic anion epigenetic regulation of gene expression (e.g., high mobility group box 1—HMGB1) (Fig. 2D, E). Dysregulation of intracellular calcium through increased IP3 signaling and mitochondrial uptake (e.g., VDAC2 and VDAC3) has been strongly associated with reactive oxygen species (ROS) production and cell death, both of which are hallmarks of neurodegeneration. Potentially, this calcium dyshomeostasis induces mitochondrial dysfunction and bioenergetic deficits, ultimately contributing to axonal degeneration37. Conversely, we observed the upregulation of ALDOC, ALDH1A1, and excitatory amino acid transporter 1 (SLC1A3, also known as EAAT1), which are markers of a reactive astrocyte phenotype38,39. Consequently, this phenomenon could explain the detection of the nuclear protein HMGB1, a prototypical damage-associated molecular pattern (DAMP) molecule with a proinflammatory function released by both ferroptotic cells and astrocytes, which triggers different immune responses40. IHC analysis further demonstrated the overexpression of HMGB1 and its colocalization within ferroptotic OLs (previously described in the CPZ model41) and astrocytes in the CC, indicating that both cell types contribute to the elevated levels of HMGB1 (Fig. 2J–M). Notably, GENUS exhibited potent anti-inflammatory and anti-ferroptotic properties by specifically targeting HMGB1 and reducing its levels. Intriguingly, we observed an unexpected increase in parvalbumin (PVALB) levels in both the CPZ and ND control groups. Considering its role in inhibitory neurons, we investigated the reduction of PVALB by GENUS. Through in-depth IHC analysis, we discovered that PVALB did not exhibit any significant differences in expression across groups in the cortex (Supplementary Fig. 3H–O). However, it was markedly expressed in the ependymal layer of the third ventricle in the control groups. Previous literature has shown that ependymal cells can express PVALB in reactive states, such as in response to brain injury or aging42,43. In this context, GENUS diminishes PVALB-expressing ependymal cells—a factor associated with pathological states or aging. This reduction in PVALB is relevant because it is associated with preserving barrier properties and integrity in ependymal cells.
In Panel 2, despite the absence of significant interaction and main effects in the two-way ANOVA test, DEPs show underexpression in both CPZ and ND control groups during pairwise multiple comparisons tests. This observation suggests the involvement of proteins linked to cell morphogenesis and cytoskeleton organization, exemplified by microtubule-associated protein 2 (MAP2). The differential expression of MAP2 is further confirmed through IHC. In Panel 3, DEPs are only underexpressed in the CPZ control group, indicating synaptic deficits (e.g., SH3 and multiple ankyrin repeat domains 3—SHANK3, synapsin 1—SYN1) (Fig. 2F–I). The IHC analysis of MAP2, SHANK3, and SYN1 in the anterior cingulate area (ACA) corroborated proteomics findings (Fig. 2N–Q). Extensive evidence supports that neuronal activity plays a key role in myelination, OL lineage dynamics, and axonal structure44. Optogenetic stimulation of deep-layer neurons of the premotor cortex showed that neuronal firing elicits OPC differentiation and proliferation, and increased myelin sheath and thickness17. In this sense, the synchronization of neuronal firing activity via GENUS and enhancement of synaptic plasticity may underlie how the CPZ + 40 Hz group exhibited less demyelination and increased oligodendrogenesis45. Additionally, we sought to assess what effect GENUS may be having on stress pathways. By analyzing the corticotrophin-releasing hormone (CRH) levels detected in this experiment, we provide evidence that GENUS does not induce stress in animals (Supplementary Fig. 3P).
Neuroinflammation induced by cuprizone is reduced by GENUS
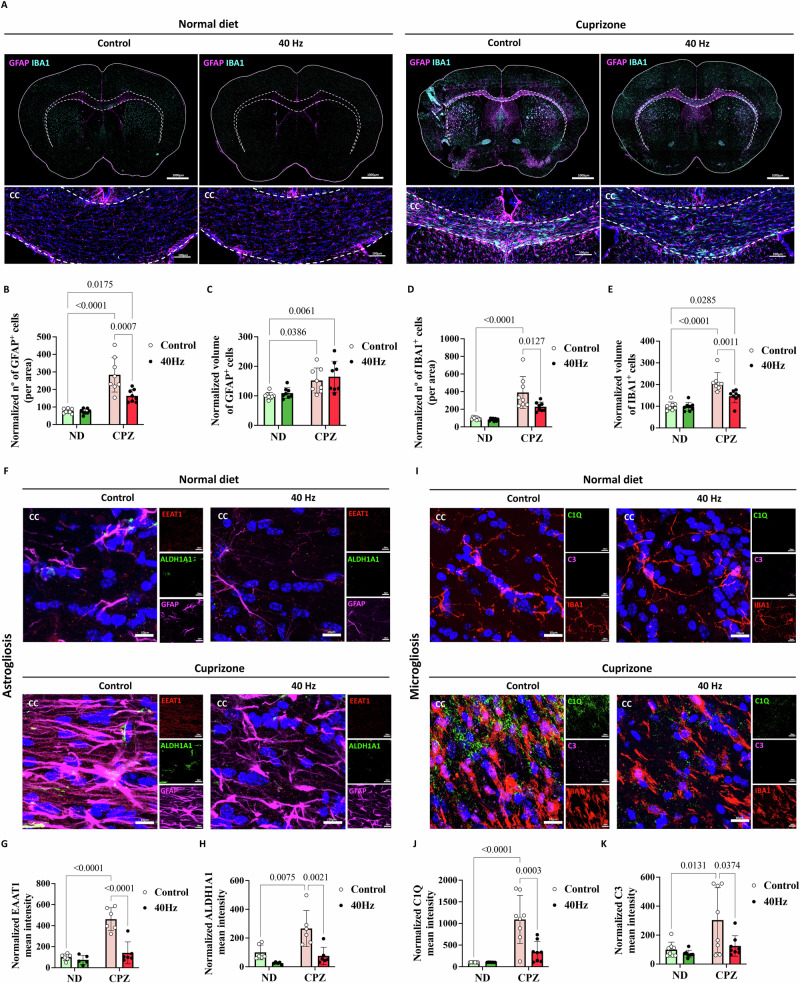
The CPZ mouse model has proven invaluable in advancing the understanding of demyelination and remyelination events in the CNS and in characterizing brain inflammation indicators, such as microgliosis and astrogliosis35. During the acute CPZ-induced demyelination process, microglia demonstrate significant proliferation and high activation levels at approximately weeks 2–3, playing a crucial role in clearing myelin debris. As the demyelination process reaches completion, microgliosis decreases, but chronically activated microglia persist in demyelinated areas31. Additionally, astrocytes begin to show a significant increase at week 3, with astrogliosis reaching its peak during maximal demyelination occurring around weeks 5–646. Astrogliosis plays a pivotal role in recruiting microglia to demyelinated areas, thereby facilitating microglia-mediated phagocytosis and contributing to the clearance of myelin debris and remyelination14. Previously published studies have shown that GENUS induced changes in glial cell response, reducing neuroinflammation and neurodegeneration in AD18,47. Here, we measured the expression of the glial fibrillary acidic protein (GFAP) and ionized calcium-binding adaptor molecule 1 (IBA1) in the CC, which are markers of astrocytes and microglia, respectively. Tile scans of the CC were acquired and GFAP and IBA1+ cells were quantified. We observed that long-term GENUS reduced the number of both glial cell types, in addition to microglial cell volume, demonstrating that GENUS modulates microgliosis and astrogliosis in the CC, thereby reducing neuroinflammation (Fig. 3A–E and Supplementary Fig. 4A). In response to CNS insults, astrocytes become reactive and hypertrophic promoting a proinflammatory environment48. Our proteomics data showed a massive involvement of astrocytes in the CPZ-induced demyelination model. To study the active response of astrocytes to CPZ and the GENUS effect, here we measured the levels of EAAT1 and ALDH1A1 colocalization within GFAP+ cells. We observed that CPZ control group presented high levels of both markers within GFAP+ cells, corroborating the disproportionate response of reactive astrocytes in the CC area (Fig. 3F–H and Supplementary Fig. 4B–F). Previous literature has also implicated complement-cascade-related products to be involved in neurodegeneration. Different mouse models of neurodegeneration, such as the CK-p25, Tau P301S have demonstrated elevated C1q levels19,49, and previous data have shown that GENUS reduced C1q signal in both CK-p25 and P301S mice by GENUS19. Due to the pathogenic role of C1q and C3 in OL death, axons damage, and myelin loss49, we analyzed the C1q and C3 expression in the CC of CPZ mice. Both complement markers were decreased in the CPZ + 40 Hz group. Colocalization of C1q with the IBA1 marker did not show differences across CPZ groups, however, colocalization of C3 deposits within microglia showed a significant interaction in the CPZ control group (Fig. 3I–K and Supplementary Fig. 4G–I). These results together provide additional evidence for the anti-neuroinflammatory and reduced immune cell effects of GENUS.
Fig. 3. Neuroinflammation induced by cuprizone is reduced by GENUS.
A–E IHC of GFAP (magenta) and IBA1 (cyan) of the CC of 12-week WT mice under CPZ and ND after 6 weeks of diet intervention and 3 weeks of 1 h/day of GENUS or control (n = 8 mice per group, one technical replicate, tile scan, scale bar—50 μm). B Normalized number of GFAP+ cells in the CC area (mean ± SD). F(1,28) = 9.763; p = 0.0041. C Normalized volume of GFAP cells in the CC area (mean ± SD). F(1,28) = 0.0194; p = 0.8902. D Normalized number of IBA1+ cells in the CC area (mean ± SD). F(1,28) = 4.866; p = 0.0358. E Normalized volume of IBA1 cells in the CC area (mean ± SD). F(1,28) = 8.653; p = 0.0065. F–H IHC of EAAT1 (red), ALDH1A1 (green), and GFAP (magenta) of the CC of 12-weeks WT mice under CPZ and ND after 6 weeks of diet intervention and 3 control weeks of 1 h/day of GENUS or control (n = 6 mice per group, one technical replicate, scale bar—10 μm). G Normalized mean intensity of EAAT1 in the CC area (mean ± SD). F(1,19) = 18.63; p = 0.0004. H Normalized mean intensity of ALDH1A1 in the CC area (mean ± SD). F(1,19) = 3.327; p = 0.0839. I–K IHC of IBA1 (red), C1Q (green), and C3 (magenta) of the CC of 12-weeks WT mice under CPZ and ND after 6 weeks of diet intervention and 3 weeks of 1 h/day of GENUS or control (n = 8 mice per group, two technical replicates, scale bar—10 μm). J Normalized mean intensity of C1Q in the CC area (mean ± SD). F(1,27) = 11.47; p = 0.0022. K Normalized mean intensity of C3 in the CC area (mean ± SD). F(1,28) = 3.071; p = 0.0907. All p-values were calculated using two-way ANOVA followed by Bonferroni’s multiple comparisons test (Supplementary Data S14). Source data are provided as a Source Data file.
Single-nucleus RNA sequencing analysis decodes the transcriptional profile diversity within the CC
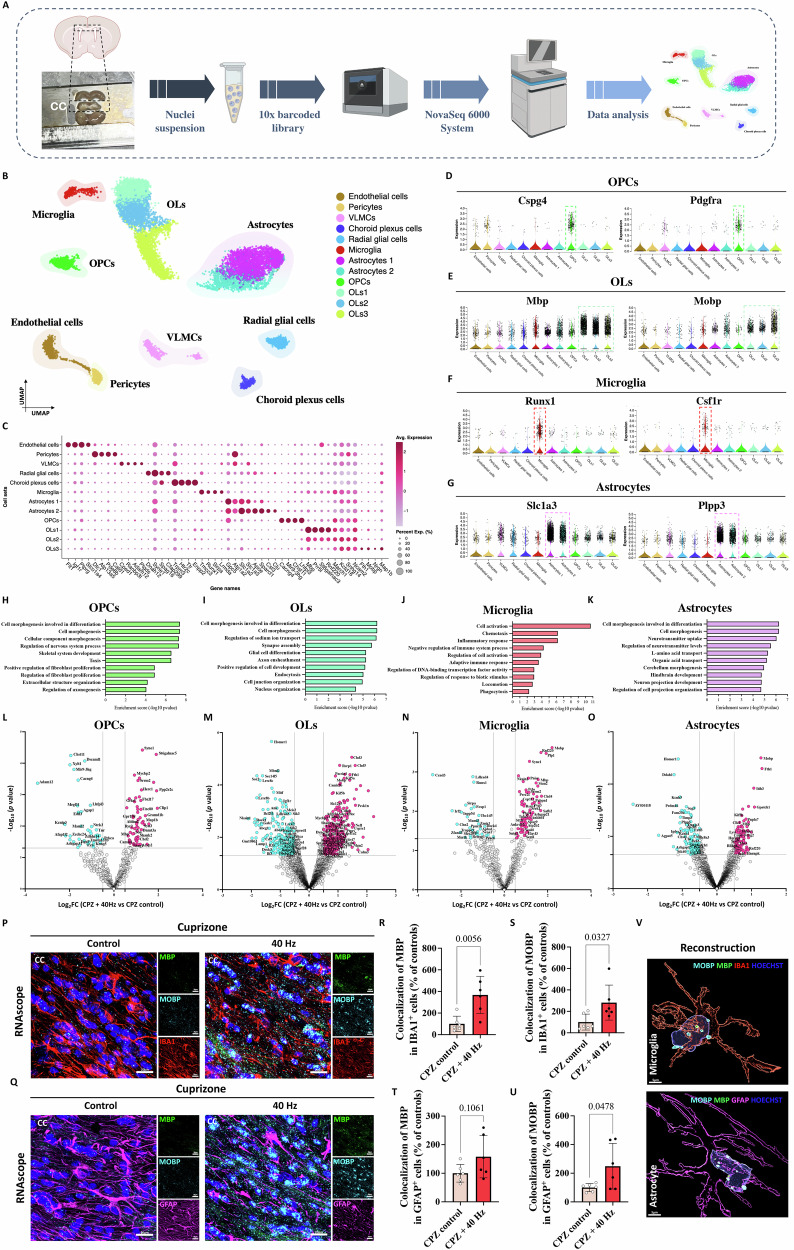
To capture the cellular transcriptional profile of the CC, we specifically microdissected and flash-froze the CC from sixteen mice in both the CPZ and ND groups. Single-nucleus RNAseq was performed using 10x Genomics Chromium to profile the cellular diversity of the CC (Fig. 4A). After conducting quality control (QC) and data clearance procedures (Supplementary Data S6-7), we successfully retained a total of 11,333 high-quality nuclei with an average of 15,457 genes. This analysis, combined with UMAP visualization, revealed the existence of nine main clusters: OPCs, OLs, astrocytes, microglia, radial glial cells, choroid plexus cells, vascular leptomeningeal cells (VLMCs), endothelial cells, and pericytes (Fig. 4B). To understand the biological significance of the primary clusters and discern potential subclusters, we conducted a differential expression analysis of a set of upregulated genes (Fig. 4C). Within the OL population, distinct subtypes were identified: OPCs expressing Pdgfrα, Vcan, and Cspg4; OLs1 exhibiting expression of Mbp, Prr5l, and Rnf220; OLs2, while also expressing Mbp, displaying enrichment for Atp1a2 and Nkain2; and OLs3, which demonstrated enrichment for Fth1, Mobp, and Nrg. Furthermore, we anticipated capturing other cell types due to the close proximity of the CC with the lateral ventricle and longitudinal fissure. These include choroid plexus cells (Ttr and Enpp2); endothelial cells (Ftl1, Slco1a4, and Mecom); pericytes (Atp13a5 and Cald1); astrocytes type 1 (Slc1a3, Gpc5, and Slc1a2); astrocytes type 2 (Apoe, Clu, and Cst3); microglia (Runx1, Csf1r, and Cx3cr1); VLMCs (Cped1, Bnc2, and Ranbp3l); and radial glial cells (Dnah12 and Cfap44) (Fig. 4B–G and Supplementary Data S8). GO analysis of the top 10 terms associated with the upregulated genes in the clusters revealed that cell morphogenesis, proliferation, and regulation of the CNS are prominent terms for OPCs. Conversely, for OLs, the analysis indicated involvement in glial cell differentiation and axon ensheathment. In the context of microglia, an observable pattern of activation, chemotaxis, and a role in phagocytosis was observed. Finally, astrocytes revealed a pronounced significance in cell differentiation, morphogenesis, and neurotransmitter regulation (Fig. 4H–K, Supplementary Fig. 5H–L and Supplementary Data S9–S12).
Fig. 4. Single-nucleus RNA sequencing analysis of the CC.
A Overview of the experimental design. Single-nucleus RNA sequencing (snRNA-seq) was performed in 12-week WT mice under CPZ and ND after 6 weeks of diet intervention and 3 weeks of 1 h/day of GENUS or control (n = 4 mice per group, one technical replicate). B UMAP plot of sn-RNAseq data. C Bubble plot showing expression levels of marker genes for each cell type. D Violin plot of upregulated genes in OPCs (Cspg4 and Pdgfra). E Violin plot of upregulated genes in OLs (Mbp and Mobp). F Violin plot of upregulated genes in microglia (Runx1 and Csf1r). G Violin plot of upregulated genes in astrocytes (Slc1a3 and Plpp3). H–K Bar plots of significantly enriched biological pathways of top-50 upregulated genes in OPCs, OLs, microglia and astrocytes, respectively. p-Values are calculated based on the accumulative hypergeometric distribution. L–O Volcano plot showing the top DEGs within OPCs, OLs, microglia and astrocytes, respectively. p-Values are calculated based on the Wilcoxon rank-sum test. P–U RNAscope using Mbp and Mobp probes combined with IHC for IBA1 staining of the CC (n = 6 CPZ + 40 Hz and n = 6 CPZ control, one technical replicate, scale bar—20 μm). Q RNAscope using Mbp and Mobp probes combined with IHC for GFAP staining of the CC (n = 6 CPZ + 40 Hz and n = 6 CPZ control, one technical replicate, scale bar—20 μm). R Normalized colocalization of Mbp transcripts in IBA1+ cells in the CC area. The graph displays normalized values using an unpaired two-tailed Student’s t-test (mean ± SD). S Normalized colocalization of Mobp transcripts in IBA1+ cells in the CC area. The graph displays normalized using an unpaired two-tailed Student’s t-test (mean ± SD). T Normalized colocalization of Mbp transcripts in GFAP+ cells in the CC area. The graph displays normalized values using an unpaired two-tailed Student’s t-test (mean ± SD). U Normalized colocalization of Mobp transcripts in GFAP+ cells in the CC area. The graph displays normalized values using an unpaired two-tailed Student’s t-test (mean ± SD). V 3D reconstruction of the RNAscope images. Schematic panel A was created with BioRender. Source data are provided as a Source Data file. CC corpus callosum, OPCs oligodendrocyte progenitor cell, OLs oligodendrocytes.
Subsequently, we performed an examination of the cellular composition distribution within each cluster across groups (both CPZ and ND) (Supplementary Fig. 5A, B and Supplementary Data S13). We observed statistically significant differences, particularly in the context of Astrocytes 1, OLs1, and OLs3. The observed variations could be attributed to disparities in sample sizes, resulting from the exclusion of a sample in the CPZ + 40 Hz group due to technical issues. This exclusion might have led to a lower number of cells being detected in the CPZ + 40 Hz group. Following this, a comparative analysis of DEGs among the CPZ groups and ND groups was carried out, aiming to uncover the modifications occurring following GENUS stimulation (Fig. 4L–O and Supplementary Fig. 5H–K). Focusing on OPCs, we observed that GENUS increased the expression of genes associated with the regulation of cell projection, cellular morphogenesis, and synaptic assembly (e.g., Mbp, Map1b, Pacsin1, Clip1, Syne1, Ntng1, Zmynd8 and Gabra2) (Fig. 4L). Regarding OLs (including OLs1, OLs2 and OLs3), GENUS led to increased expression of genes related to neuron projection extension, axogenesis and myelination, modulation of chemical synaptic signaling, and cell growth (e.g., Mobp, Kif5a and Kif5b, Camk2b and Camk2a, Slc12a5, Plec, Cdh4, Syt7, Shank1 and Shank2, Nefl, Kcnc3 and Eno2) (Fig. 4M).
In the context of the CPZ diet, the clearance of injured myelin and OLs relies on the phagocytic capabilities of immune glial cells. Accumulated myelin debris has been identified as a hindrance to white matter repair, with direct inhibition of remyelination50. Interestingly, within the microglial cluster, we observed a remarkable upregulation of myelin-specific genes specific (Plp1, Mobp, Rnf220, and Mbp) (Fig. 4N, Q), which had previously been suggested to result from the phagocytic process and subsequent enrichment of OL-derived mRNA molecules in microglial nuclear and perinuclear spaces51,52. This result suggests that GENUS enhances microglial phagocytosis of myelin debris, a crucial and essential step for successful remyelination, and furthermore, this effect was even observed in the healthy ND + 40 Hz group (Supplementary Figs. 5J and 4L, M). This finding is further substantiated by the GO analysis, which highlights phagocytosis as a prominent GO term associated with microglia. Intriguingly, a similar observation was found in the astrocytes cluster, which also expressed typical OL-transcripts, such as Mobp, Mbp, and Rfn220 (Fig. 4O). Due to the harsh microenvironment during CPZ-induced demyelination, astrocyte behavior could be compromised. Astrocytes have phagocytic machinery to compensate for microglial dysfunction. Although debris clearance is beneficial, the phagocytic activity of astrocytes has also been associated with inadequate remyelination and myelin damage in multiple sclerosis53. However, our previous results indicate that the CPZ + 40 Hz group exhibits greater myelination and reduced astrogliosis compared to the CPZ control group. We hypothesize that GENUS could promote cellular plasticity—a functional compensatory mechanism that might attempt to counterbalance demyelination by contributing to repair processes or stabilizing the damaged tissue. In light of our previous findings demonstrating the structural preservation of myelin and significant reductions in microgliosis and astrogliosis in the CPZ + 40 Hz group, the presence of myelin debris within microglia and astrocytes may potentially serve as a mechanism aimed at promoting remyelination. To validate this hypothesis, we performed an RNAscope assay using Mobp and Mbp probes, co-stained with IHC employing GFAP and IBA1 antibodies. The results are consistent with the data from the sn-RNAseq experiment, indicating an increase in myelin transcripts within microglia and astrocytes (Fig. 4P–V and Supplementary Fig. 5O-T).
GENUS stimulates oligodendrogenesis and reduces OL loss
In the CPZ model, oligodendroglia cell death occurs shortly after the administration of CPZ, which translates into a profound demyelination process evident in multiple brain structures including in the CC and neocortex54. Here, we tested the effectiveness of GENUS in preventing OL cell death. Expression of OL subtype markers was done using adenomatous polyposis coli clone CC1 (APCCC1) (mature OLs) and platelet-derived growth factor receptor α (PDGFRα) (immature OLs). In the CPZ + 40 Hz condition, both APCCC1+ and PDGFRα+ cells were significantly increased compared with the CPZ control group (Fig. 5A–C and Supplementary Fig. 6A). To better understand whether the protective effects of GENUS were affecting either the proliferation of new cells or preventing cell death, we assessed this by using the proliferation marker Ki67 and performed the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay to quantify apoptotic cells (Supplementary Fig. 6B, C). We observed that expression of the oligodendroglial lineage marker, oligodendrocyte transcription factor 2 (OLIG2) co-labeled with Ki67 increased substantially in the CPZ + 40HZ group (Fig. 5D–F), and conversely, TUNEL-positive cells were decreased in this group (Fig. 5G). Furthermore, the quantification of both OLIG2+ and SOX10+ cells at the earlier 3-week time point demonstrated how CPZ affects OL densities, confirming that GENUS enhances the rate of cell survival (Supplementary Fig. 7G–K). Additionally, we quantified HSP70, a protective chaperone protein known for enhancing cell survival and inhibiting apoptosis55,56. In the CC, HSP70 showed significantly higher expression in the 3-week CPZ + 40 Hz group compared to the 3-week CPZ control, 6-week CPZ, and ND cohorts in the extracellular milieu (Supplementary Fig. 7I, M). Colocalization of HSP70 with SOX10+ cells increased in the 3-week cohort, predominantly in the CPZ + 40 Hz group. Given HSP70’s potent antiapoptotic properties that impede multiple steps of programmed cell-death signaling, its increased expression in week 3 provides further evidence that GENUS prevents cell death by boosting cytoprotectant proteins like HSP70.
Fig. 5. GENUS stimulates oligodendrogenesis and reduces OL cell death.
A–C IHC of APCCC1 (red) and PDGFRα (green) of the CC of 12-week WT mice under CPZ and ND after 6 weeks of diet intervention and 3 weeks of 1 h/day of GENUS or control (n = 7 CPZ + 40 Hz; n = 8 CPZ control; n = 8 ND + 40 Hz; n = 8 ND control, one technical replicate, scale bar—20 μm). Dashed lines indicate the boundaries of the CC. B Normalized number of PDGFR+ cells in the CC area (mean ± SD). F(1,27) = 12.53; p = 0.0015. C Normalized number of APCCC1+ cells in the CC area (mean ± SD). F(1,27) = 7.964; p = 0.0088. D–F IHC of Ki67 (green) and OLIG2 (red) of the CC of 12-week WT mice under CPZ and ND after 6 weeks of diet intervention and 3 weeks of 1 h/day of GENUS or control (n = 8 mice per group, one technical replicate, scale bar—20 μm). E Normalized number of OLIG2+ cells in the CC area (mean ± SD). F(1,26) = 7.894; p = 0.0093. F Normalized number of Ki67+OLIG2+ cells in the CC area (mean ± SD). F(1,27) = 3.981; p = 0.0562. G Representative image of the TUNEL colorimetric assay in CC comparing the four conditions, CPZ + 40 Hz, CPZ control, ND + 40 Hz, ND control (scale bar—100 μm). TUNEL-positive cells (apoptotic cells) acquired a dark brown color. H–J IHC of GPX4 (green) and OLIG2 (red) of the CC of 12-week WT mice under CPZ and ND after 6 weeks of diet intervention and 3 weeks of 1 h/day of GENUS or control (n = 7 CPZ + 40 Hz; n = 7 CPZ control; n = 8 ND + 40 Hz; n = 8 ND control, one technical replicate, scale bar—20 μm). I Normalized mean intensity of GPX4 in the CC (mean ± SD). F(1,26) = 1.149; p = 0.2936. J Normalized number of GPX4+ cells colocalized with OLIG2 in the CC area (mean ± SD). F(1,26) = 2.515; p = 0.1248. All p-values were calculated using two-way ANOVA followed by Bonferroni’s multiple comparisons test (Supplementary Data S14). Source data are provided as a Source Data file.
To validate the potential impact of GENUS on myelination and oligodendrogenesis, we employed an advanced aging model using 27-month-old mice. The well-established association between aging and a decline in both myelination and oligodendrogenesis makes this model particularly relevant for investigating long-term GENUS’s effect in enhancing regeneration efficiency during aging (Supplementary Fig. S8A–C). Structural alterations in myelin sheaths over time can lead to their loss, compromising interregional connectivity in the brain and contributing to cognitive decline56. Recent studies also suggest that the loss of myelin integrity may be involved in neuronal amyloid-β deposition, a hallmark of AD pathology57. In our study with aged mice, we confirmed that GENUS promotes myelination, as evidenced by increased MBP content in the anterior cingulate cortical area (ACA) and CC. Additionally, markers for OLs (OLIG2 and APCCC1) showed higher levels in the CC after GENUS compared to the aged-control group (Supplementary Fig. S8D–K). GENUS also stimulated oligodendrocyte proliferation in aged mice, leading to a significant increase in the number of OLIG2+Ki67+ cells compared to the control group (Supplementary Fig. S8L, M). Consistent findings emerged in the cisplatin chemobrain mouse model within the context of chemobrain pathology, demonstrating the protective effects of GENUS on the mouse brain against cisplatin-induced demyelination57.
Relatedly, ferroptosis is an iron-dependent lipid peroxidation cell death caused by an accumulation of ROS. Abnormal iron metabolism and deposition have been implicated in the pathophysiology of demyelinating diseases58,59. CPZ-induced toxic demyelination generates an accumulation of myelin debris. These lipidic debris may undergo peroxidation promoting the deposition of highly neurotoxic species60. Given the critical role of ROS in the CPZ mouse model, this makes it a suitable target to properly study ROS accumulation and ferroptosis. Therefore, to try to understand whether GENUS could exert a neuroprotective effect decreasing ROS, lipid peroxidation, and iron accumulation, we analyzed the expression of glutathione peroxidase 4 (GPX4), which is the master regulator of ferroptosis by reduction of cytotoxic lipid peroxides61. We found that GPX4 was significantly increased in CPZ + 40 Hz group, but not in the CPZ control group, suggesting a beneficial effect of GENUS in decreasing ferroptosis (Fig. 5H–J and Supplementary Fig. 6D, E). We measured the levels of oxidative stress markers, such as oxidized phospholipids (E06) and C11 Bodipy™ 581/591. We observed a high deposition of both C11 Bodipy™ and E06 markers in the CC of the CPZ control group compared with the CPZ + 40 Hz group (Supplementary Fig. 6F–H). As lipid peroxidation products have been identified in peripheral samples (e.g., cerebrospinal fluid)62, suggesting their possible use as fluid biomarkers, we measured the levels of malondialdehyde (MDA) in plasma. High levels of MDA were detected in the plasma of the CPZ control group (Supplementary Fig. 6I). Additionally, we used Prussian blue staining for the detection of ferric iron (Fe3+) accumulation in brain tissue. Iron dyshomeostasis accompanies several neurodegenerative diseases and has been associated with ROS and lipid peroxidation63. Deposits of Fe3+ were detected only in the CPZ control group (Supplementary Fig. 6J). These results confirm that GENUS not only reduces ferroptosis-mediated loss of OLs but also promotes OPC proliferation and differentiation.
Discussion
Demyelinating diseases are a group of heterogeneous neurological conditions that share a common pathological feature: loss of myelin. Previous research has shown that myelination is a highly regulated process that is influenced by neuronal electrical activity64. Increased neuronal firing has been demonstrated to promote myelination, while dark-reared mice exhibited optic nerve hypomyelination17. In this study, we used long-term non-invasive GENUS to assess its efficacy in rescuing pathological hallmarks of the CPZ mouse model which replicates several underlying pathomolecular processes associated with demyelinating conditions, such as the dramatic processes of demyelination, OL cell death, and neuroinflammation. Our findings consistently demonstrated the benefit of GENUS in reducing demyelination and preserving axonal integrity, including myelin thickness and axon diameter. Importantly, these structural improvements were strongly correlated with the electrophysiological response of the white matter tracts. The CC, a crucial commissural structure housing white matter tracts, has been the subject of extensive research regarding its electrophysiological and pharmacological implications for axonal function65. This study highlights the significant enhancement of axonal function within the CC of the CPZ + 40 Hz group following long-term GENUS. In contrast, the CPZ control group exhibited evident axonal physiological dysfunction, which correlated with substantial degeneration in myelin and axons.
In an effort to understand the mechanism behind how GENUS rescued the strong CPZ phenotype, we conducted brain proteomics. Previous literature has shown that synaptic dysregulation, such as that seen in SHANK3 deficiency, can cause significant disruption of white matter integrity66. In our study, we observed a remarkable positive effect of GENUS on synaptic plasticity, cellular homeostasis, and morphogenesis. Specifically, we observed that in the CPZ + 40 Hz group, levels of SHANK3, SYN1, and MAP2 were comparable to those of the healthy ND condition. Our data demonstrate the powerful protection of synaptic integrity by GENUS, which has also been previously demonstrated in other animal models19,28,67. The observed increase in myelination within the CPZ + 40 Hz group can be attributed, at least in part, to the improvement in synaptic plasticity and transmission efficiency.
In the CPZ model, neuroglial proliferation is an extremely pronounced process68. Both microglia and astrocytes become activated, and several inflammatory markers, including complement factors, are upregulated14. Potent astrogliosis has been detected in the CC and gray matter structures (e.g., cortex, hippocampus, or basal ganglia) in response to CPZ treatment31. In our brain proteome dataset, we observed prominent overexpression of astrocytic markers such as EAAT1, ALDH1A1, ALDOC, and ENO1 in the CPZ control group. The activation of reactive astrocytes results in the release of pro-inflammatory cytokines, chemokines, and other molecules that contribute to neuroinflammation69. We investigated the expression of HMGB1 and detected significantly elevated levels of this DAMP molecule colocalized within both astrocytes and OLs in the CC of the CPZ control group and was reduced in the CPZ + 40 Hz group. This discovery provides insights into astrogliosis and supports the role of ferroptosis as a significant mechanism of OL cell death during demyelination.
Microglia, another key player in the neuroinflammatory process, exhibit transient activation that coincides with the peak of myelin debris in the CPZ model, in contrast to the persistent activation observed in astrocytes31. As the first-line resident cells in the defense against myelin debris accumulation, activated microglia release C1q, which stimulates a neurotoxic phenotype in astrocytes and leads to the death of both neurons and OL cells70. Our data found that GENUS reduced microgliosis in the CC of the CPZ + 40 Hz group, thereby also reducing complement factor expression.
Simultaneously, the results of the sn-RNA seq experiment revealed the phagocytic profile of microglia after GENUS. Notably, the identification of OL-derived transcripts within the analysis of microglial DEGs in CPZ clusters underscores the crucial importance of efficiently clearing myelin debris to facilitate effective remyelination processes. It is well known that any delays in this debris clearance process significantly impairs the remyelination process31. Equally notable, astrocytes showed potential plasticity to contribute to maintaining some aspects of the myelin sheath’s protective and supportive functions.
We subsequently proceeded to investigate whether GENUS could prevent the CPZ-induced loss of OLs and stimulate oligodendrogenesis. Existing evidence suggests that neuronal activity, via optogenetic stimulation, can promote OPC maturation and myelin remodeling16,71. Our findings here indicated that non-invasive GENUS could exert a similar effect, as there was a significant increase in OPCs in the CC of the CPZ + 40 Hz group, along with a higher number of mature OLs. These results suggest that the activity-dependent synchronized gamma oscillations at 40 Hz play a crucial role in boosting myelination. Given the characteristic accumulation of ROS and lipid peroxides in the CPZ model, we speculated on the involvement of ferroptosis, a non-apoptotic form of cell death that involves lipid peroxidation and iron-overloading41,72. We focused on GPX4, a critical enzyme that catalyzes the detoxification of lipid peroxides in mammalian phospholipid membranes73. We found that GPX4 was increased in the CPZ + 40 Hz group, and that toxic lipids such as oxidized phospholipids were decreased, which could explain the high survival rate of mature OLs in the CPZ + 40 Hz group, even at the peak of CPZ cytotoxicity.
Overall, long-term non-invasive GENUS demonstrates multiple benefits in mitigating the detrimental effects of demyelination, preserving axonal integrity, and improving the electrophysiological function of white matter tracks within the CC. The observed increase in myelination associated with neuronal firing at 40 Hz suggests a direct influence on the activity of OLs or the provision of critical signals and metabolic support necessary for OL maturation and efficient myelin production. Additionally, the enhanced synaptic plasticity induced by 40 Hz may provide a mechanistic basis for the observed improvements in myelin-related processes, contributing to the overall restoration of neural circuitry and function. Furthermore, by dampening neuroinflammation and increasing myelin debris clearance, GENUS may create a more favorable environment for oligodendrogenesis and myelin remodeling. In summary, GENUS demonstrated protective effects on myelin and anti-neuroinflammatory activity, promoting a harmonious environment for myelin repair and overall functional recovery.
Methods
Mice and experimental model
Male C57BL/6 J mice (12 weeks old), were obtained from the Jackson Laboratory (https://www.jax.org/) while aged male C57BL/6 J mice (25 months old) were sourced from the National Institute of Health (NIH) (https://www.nia.nih.gov/research/dab/aged-rodent-colonies). The mice were housed in groups of four based on a standard cycle of 12-h light/12-h dark, with weekly cage maintenance. All experimental procedures were conducted following the approval of the Committee for Animal Care of the Division of Comparative Medicine at the Massachusetts Institute of Technology and the Institutional Animal Care and Use Committee (protocol n° 0621-033-24). Demyelination was induced by administering CPZ-containing pellets to the mice for a duration of 6 weeks. During the experiment, all animals had access to chow and water without restrictions. Experiments were conducted in animals at 12 weeks of age.
Experimental design
Two cohorts of mice were used for this study. Each cohort consisted of male C57BL/6 J mice at 12 weeks of age. The mice were assigned to different dietary interventions, involving either a diet consisting of 0.3% CPZ-containing pellets or ND for a duration of 6 weeks. In the first cohort, after 3 weeks of CPZ feeding, the therapeutic group was subjected to GENUS for 3 weeks, while the control group received CPZ and constant light and sound with matched lux intensity and decibels maintaining the diet intervention until week 6. In the second cohort, mice were fed with ND and were also exposed to GENUS stimulation or received constant light and sound, following the same timeline as the CPZ cohort (Supplementary Fig. 1A, B). A third cohort of mice was employed to evaluate the dynamics of myelin content and OL densities. These mice underwent a 3-week regimen of CPZ and GENUS or constant light and sound simultaneously and were subsequently sacrificed (Supplementary Fig. 7A).
The advanced-aged mice experiment involved two groups of approximately 25–27-month-old male C57BL/6 J mice. One group received GENUS for 4 weeks, while the control group received constant light and sound for the same duration.
Simultaneous 40 Hz visual and auditory flicker stimulation
Mice were habituated daily in a quiet area for 30 min prior to being placed in control or 40 Hz stimulation which consisted of the mice being placed individually in a dark chamber illuminated by a light-emitting diode (LED) bulb (12.5 ms light on/off, 60 W, 40 Hz) and exposed to an auditory tone train of 40 Hz, which speakers (AYL, AC-48073), for 1 h during 3 weeks in a quiet room insulated with sound-proof foam (McMaster-Carr, 5692T49)18,74. Control groups (CPZ and ND) were subjected to the same protocol, that is, in a quiet and insulated room, but were instead exposed to a continuous “constant” light (LED bulb) and white noise for 1 h during 24 days (both conditions were matched in lux intensity and decibels)74.
Electrode probes implantation
LFP probes were custom-built with soldering perfluoroalkoxy-coated tungsten wire electrodes (50 μm bare diameter, 101.6 μm coated diameter; A–M Systems, #795500) to connectors (Pinnacle Technology). For probe implantation, animals (4 mice) were anesthetized with isoflurane, and their heads were restrained in a stereotaxic apparatus. A midline sagittal incision was made, and a dental drill was employed to expose the skull (hole diameter: 0.5–1.0 mm). Probes were meticulously positioned on the SSp and PFC cortices (stereotaxic coordinates relative to bregma: SSp: AP = −0.50, ML = 2.50, DV = −1.30; PFC: AP = 1.5, ML = 0.5, DV = −2.3) in CPZ mice during the third week under the CPZ diet. Reference and ground screw electrodes were implanted above the cerebellum. Subsequently, the implant was sealed with dental cement. Mice were administered buprenorphine (1 mg/kg) and meloxicam (5 mg/kg for 3 days) post-surgery and allowed a 7-day recovery period before recordings.
Electrophysiology data acquisition
Freely moving mice subjected to the 6-week protocol were connected to the tethered electrophysiology recording device at weeks 4 and 6. Recording sessions were structured to include 1 minute of baseline recordings, followed by 5 min with GENUS (comprising auditory and visual 40 Hz stimulation), and concluded with an additional 1 min of baseline recordings. Raw data was acquired using Synapse (Tucker-Davis Technologies), and LFP signals were resampled at a sampling rate of 2 kHz and bandpass filtering between 1 and 300 Hz. Subsequently, the brain was collected, and IHC was conducted to confirm the anatomical location of the recordings.
Immunohistochemistry
Mice were transcardially perfused with phosphate-buffered saline (PBS) and the brains were post-fixed with 4% paraformaldehyde in PBS overnight at 4 °C. Brains were sectioned in a Leica CM1520 cryostat at a thickness of 20 μm. Sections were permeabilized and blocked in 5% donkey serum with 0.3% Triton in PBS for 2 h at room temperature (RT). Next, sections were incubated with the first antibody in 5% donkey serum with 0.3% Triton in PBS, overnight at 4 °C. The primary antibodies used were: anti-MOG (1:500, AB32760, Abcam), anti-MBP (1:300, MCA409S, BioRad), anti-APCCC1 (1:50, OP80, Sigma-Aldrich), anti-PDGFRα (1:50, AF1062, R&D Systems), anti-OLIG2 (1:200, MABN50, Sigma-Aldrich), anti-Ki67 (1:500, 14569882, Thermo Fisher), anti-IBA1 (1:500, 234004, SYSY), anti-GFAP (1:500, 130300, Thermo Fisher), anti-GPX4 (1:200, ab25066, Abcam), anti-HMGB1 (1:200, ab18256, Abcam), anti-MAP2 (1:500, 822501, BioLegend), anti-SYN1 (1:500, D12G5, Cell Signaling Technology), anti-SHANK3 (1:500, 162304, Synaptic Systems), anti-EAAT1 (1:50, ab181036, Abcam), anti-ALDH1A1 (1:200, AF5869, R&D Systems), anti-C1Q (1:500, ab182451, Abcam), anti-C3 (1:500, 11862, Abcam), anti-RUNX1 (1:100, sc-365644, Santa Cruz Biotechnology), anti-MOBP (1:50, Thermo Fisher), and anti-PVALB (1:300, ab11427, Abcam), anti-ANKG (1:500, 386005, SySy); anti-NFH (1:1000, ab4680, Abcam), anti-HSP70 (1:100, PA5-77828, Thermo Fisher) and anti-SOX10 (1:100, 703439, Thermo Fisher). For staining of E06, CD16/CD32 Fc blocking antibody (1:500, 553141, BD) and 0.1% fish gelatin blocking agent were added to the blocking solution. Sections were washed with PBS, and stained with Alexa Fluor 488, 594, or 647 conjugated secondary antibodies in PBS, for 2 h at RT. The secondary antibodies used were: anti-rabbit IgG Alexa 488 conjugated (1:500, A-21206, Thermo Fisher), anti-rat IgG Alexa 488 conjugated (1:500, A-21208, Thermo Fisher), anti-mouse IgG Alexa 488 conjugated (1:500, A-28175, Thermo Fisher), anti-rabbit IgG Alexa 594 conjugated (1:500, A-11037, Thermo Fisher), anti-mouse IgG Alexa 594 conjugated (1:500, A-11032, Thermo Fisher), anti-guinea pig IgG Alexa 594 conjugated (1:500, A-11076, Thermo Fisher), anti-chicken IgY Alexa 647 conjugated (1:500, A-21449, Thermo Fisher), anti-rat IgG Alexa 647 conjugated (1:500, A-21247, Thermo Fisher) and anti-mouse IgG Alexa 647 conjugated (1:500, A-28181, Thermo Fisher). Finally, sections were washed with PBS and incubated with Hoechst 33342 (1:10,000, H3570, Thermo Fisher) for 10 min at RT. C11 Bodipy (1:300, D3871, ThermoFisher) was incubated simultaneously with Hoechst when required, both for 20 min. For FluoroMyelin™ Green Fluorescent Myelin Stain, sections were incubated for 20 min at RT (1:300, F34651, Thermo Fisher), and were mounted with fluoromount-G (Electron Microscopic Sciences). Images were acquired using Zeiss LSM 710, 880, and 900 confocal microscopes with 5×, 40×, and 63× objectives. CC images were acquired from the medial portion of the CC at bregma coordinates 1.09–0.49 mm.
Transmission electron microscopy
Mice were transcardially perfused with formaldehyde/glutaraldehyde 2.5% in 0.1 M sodium cacodylate buffer, 7.4 pH (Electron Microscopy Sciences). Brains were sectioned using a coronal brain matrix (1 mm) and immediately immersed in the fresh fixative solution containing formaldehyde/glutaraldehyde 2.5% in 0.1 M sodium cacodylate at RT. Sections of the corpus callosum were washed in cacodylate buffer and post-fixed with 1%osmiumtetroxide/1.5% potassiumferrocyanide (in H2O) for 1 h at RT. Then, sections were washed with malelate buffer pH 5.15 and immersed in 1% uranyl acetate (in H2O) for 1 h at RT. Sections were washed with H2O and dehydrated in increasing concentrations of ethanol (70% EtOH for 15 min, 90% EtOH for 15 min, and 100% EtOH for 15 min). Next, sections were incubated in popyleneoxide for 1 h and embedded in epon mixed 1:1 with propyleneoxide, overnight at 4 °C. Lastly, samples were placed in an embedding mold filled with freshly mixed epon and polymerized in a 60 °C oven for approximately 48 h. Thin sections (60–90 nm) were placed on copper slot grids and stained with 2% uranyl acetate and 1% lead citrate. Images were acquired with the Tecnai G2 Spirit BioTWIN Transmission Electron Microscope in the electron microscopy core of Harvard Medical School.
Processing imaging data
Maximum intensity projections of z-stacks and tile scans of images were obtained using Zen Black or Zen Blue software (ZEISS Microscopy Software). To quantify and reconstruct three-dimensional representations of images, were used. In cases where images were acquired at low magnification or via the tile scan function, the CC area was defined by drawing a region of interest (ROI) for normalization purposes (e.g., myelin content, number of cells in the CC area). Conversely, for high-magnification images (e.g., 40× or 63×), the entire field of the image represented the medial CC, and in such cases, no area normalization was applied. TEM images were analyzed using AxonDeepSeg (https://axondeepseg.readthedocs.io/en/latest/) with a resolution of 0.001 micrometers per pixel. The analysis included measurements of axon diameter, myelin thickness, and g-ratio, with 200 axons per animal being analyzed. Schematic and some figures features were generated using Biorender (https://www.biorender.com/), released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International licence.
Slice preparation for electrophysiological recordings
Mice were anesthetized by intraperitoneal injection of sodium pentobarbital (200 mg/kg) and were transcardially perfused with ice-cold cutting solution containing (in mM): 2.5 KCl, 1.25 NaH2PO4·H2O, 20 HEPES, 2 Thiourea, 5 Na-ascorbate, 92 NMDG, 30 NaHCO3, 25 d-Glucose, 0.5 CaCl2·2H2O and 10 MgSO4·7H2O. The brain was quickly removed and placed in an oxygenated ice-cold cutting solution. A total of 400-μm-thick coronal sections containing corpus callosum (CC) were made using a Leica VT1000 vibratome (Leica Biosystems Inc.). The brain slices were immediately transferred into an incubation chamber containing oxygenated cutting solution at 34 °C for 20 min, then transferred to a holding chamber with oxygenated artificial cerebrospinal fluid (ACSF) at room temperature (24 °C). Slices were allowed to recover for 1 hour prior to recording. ACSF solution contains (in mM): 125 NaCl, 2.5 KCl, 1.2 NaH2PO4·H2O, 1.2 MgCl2·6H2O, 2.4 CaCl2·2H2O, 26 NaHCO3, 11 d-Glucose. Single slices were transferred into a recording chamber continually superperfused with oxygenated ACSF (30−32 °C) at a flow rate of ∼2 mL/min for recording. Slice structures were visualized using infrared differential interference contrast (IR-DIC) imaging on an Olympus BX-50WI microscope.
Compound action potentials measurements
Electrophysiological recordings were recorded using an Axon Multiclamp 700B patch-clamp amplifier (Molecular Devices) and Clampex software (version 11.2, Molecular Devices). Signals were filtered at 1 kHz using the amplifier’s four-pole, low-pass Bessel filter, digitized with an Axon Digidata 1550B interface (Molecular Devices). A concentric bipolar stimulating microelectrode electrode (FHC) was lowered into the CC at approximately ~1 mm lateral to midline. The recording electrode filled with 3 M NaCl (a resistance of 1–3 MΩ) is to be placed into the contralateral CC at ~2 mm away from the stimulating electrode. Adjustments were made in the depths of both stimulating and recording electrodes to optimize the signal amplitude. For analyses of the Compound action potential amplitude, standardized input–output functions were generated for each slice by varying the intensity of stimulus pulses (200 ms duration). Stimulus intensity was adjusted manually using a SIU91A stimulator (Cygnus Technology). Data were stored on a personal computer and analyzed with Clampfit (version 11.2, Molecular Devices).
Preparation of mouse brain tissue for proteomics
Isobaric labeling through tandem mass tag (TMT) was employed for the comprehensive analysis of the brain proteome75,76. Flash-frozen brains were homogenized in 5 mL of urea lysis buffer (9 M urea, 20 mM N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES) pH 8.0, 1 mM sodium orthovanadate, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate). The lysates were sonicated at 15 W output for three bursts of 30 s with 1 min rest on ice in between bursts. Then the samples were centrifuged at 20,000×g for 15 min and the protein concentration was assayed in the supernatants using a Pierce 660 nm assay. One hundred micrograms of protein were digested from each sample. Disulfides were reduced with 4 mM dithiothreitol (DTT) at RT for 1 h and then alkylated with 10 mM indole-3-acetic acid (IAA) for 15 min in the dark. One microgram of LysC was added for 2 h at 37 °C, then samples were diluted 4× with 20 mM HEPES pH 8. Samples were digested overnight at room temp with 2 µg MS-grade trypsin. After digestion, the samples were acidified with 1% trifluoroacetic acid (TFA) and were allowed to sit at 4 °C for 15 min until lipids precipitated. This precipitate was removed by centrifugation at 10,000×g for 10 min. The peptides were then purified by solid phase extraction on 50 mg C18 Sep Paks (Waters™). The Sep Pak cartridges were first wet with 1 mL acetonitrile (ACN) and equilibrated with 2 mL of 0.1% TFA in H20. The peptides were loaded on the cartridges and then, washed with 2 mL of 0.1% TFA in H20. Finally, peptides were eluted with 50% ACN/0.1% TFA and dried in a speedvac.
Tandem mass tag labeling and solid-phase extraction
For sample labeling, TMT16-plex reagents were used76. In brief, the dry peptides were resuspended in 200 mM HEPES pH 8.0 at 1 µg/uL. Twenty micrograms of peptide were mixed with 100 µg of TMTpro reagent in 10 µL of ACN and allowed to react for 1 h at RT. The labeling reaction was quenched with 0.3% hydroxylamine for 15 min, then all channels were pooled into one plex. The plex was acidified with TFA to pH 2, dried to remove ACN, then desalted on a 50 mg C18 Sep Pak as previously described. The desalted peptide eluate was dried in a speedvac prior to fractionation.
Basic reverse-phase fractionation
Basic reverse-phase fractionation (bRP) was carried out on an HPLC equipped with an Agilent Zorbax 300 2.1 mm × 150 mm column packed with 5 µm C18 beads. Buffer A was 10 mM ammonium bicarbonate/5% ACN, and buffer B was 10 mM ammonium bicarbonate/90% ACN. Then, 150 µg of the pooled peptide was loaded on the column, and then 96 fractions were collected starting 5 min into the gradient. The gradient began at 0% B, then increased to 5% B at 2 min and ramped to 40% B at 60 min. At 65 min, the gradient jumped to 100% B, which was held for 5 min before returning to initial conditions. The 96 fractions were concatenated non-sequentially down to 12 fractions and stagetipped prior to LCMS analysis.
Liquid chromatography–tandem mass spectrometry
One-tenth of each peptide fraction was injected on-column for LCMS analysis. The LC used a 100 µm × 50 cm column packed with 1.8 µm C18 beads. Flowing at 275 nL/min, the analytical gradient ramped from 5 to 41% ACN in 151 min with a total run time of 171 min. Peptides were electrosprayed into a Thermo Fusion Lumos Orbitrap mass spectrometer for detection. Mass spec analysis used a MultiNotch SPS-MS3 approach as described previously75. MS1 scans were collected with Orbitrap (OT) resolving power at 120 K, scanning from 350–1400 m/z. Charge states from 2–5 were allowed and dynamic exclusion was set to 120 s. The maximum cycle time for data-dependent acquisition was set at 3 s. Precursors were isolated with a 0.7 m/z window prior to collision-induced dissociation (CID) fragmentation at 30% normalized collision energy (NCE) and MS2 detection in the ion trap. The trap scan rate was set to “Rapid” and the automatic gain control (AGC) target was 200%. MS3 scans also used a 0.7 m/z isolation window followed by higher-energy C-trap dissociation (HCD) fragmentation at 55% NCE. Fragments were detected in the OT at 30 K resolving power using a maximum injection time of 54 ms and an AGC target of 500%.
Data analysis
MS raw files were searched against the Swissprot mouse database downloaded 25 May 2022 using the Comet search algorithm77 implemented in GFY Core software from Harvard. Peptides were required to have at least one tryptic end (cleavage at K or R but not KP or RP) with up to five missed cleavages allowed. The precursor ion tolerance was 20 ppm and the fragment mass tolerance was 1.005 Da for the ion trap MS2 spectra and 0.02 Da for OT collected MS3 spectra. Carbamidomethylcysteine and TMT on Lys and the peptide N-termini were static modifications, while oxidized Met was a variable modification. Both peptide and protein identifications were controlled at a 1% FDR cutoff using the target-decoy search approach. Protein quantification was calculated from TMT reporter ion signal:noise (S/N) ratios summed across all peptides in each protein. Corrections were applied for isotopic impurities in the TMT reagents as indicated by the manufacturer. Only peptides that had summed S/N ≥ 160 across all 16 channels and had ≥50% isolation specificity in both the MS2 and MS3 spectra were considered for quantitation. Log-median normalization was applied across all channels to reflect equal sample inputs.
Proteome differential expression analysis
DEPs in the CPZ and ND cohorts were identified using a two-way ANOVA correcting for multiple comparisons by an FDR cutoff of 1% using the two-stage step-up method from Benjamini, Krieger, and Yekuteli method (Supplementary Data S4). Although different comparisons across groups were performed, in order to avoid the DEPs associated with the massive effect of the CPZ diet (e.g., metabolic proteins), and be able to identify DEPs correlated with the GENUS effect, we focused on the DEPs across CPZ + 40 Hz versus CPZ control in the comparative analysis. A list of all proteins identified is provided in Supplementary Data S2 and 3.
Gene ontology analysis of brain proteome
The network analysis of DEPs (473 proteins) consisting of 473 proteins was conducted using ShinyGo v0.77 (http://bioinformatics.sdstate.edu/go/) with an FDR cutoff of 0.01. To gain further insights into the functional implications of the DEPs, functional enrichment analysis was performed on a subset of 142 proteins using ToppGene (https://toppgene.cchmc.org/), Metascape (https://metascape.org/) and Revigo (http://revigo.irb.hr/). The overlap and relationships among the DEPs were visualized using a Venn diagram generated with InteractiVenn (http://www.interactivenn.net/). Furthermore, the top five significantly enriched Gene Ontology (GO) terms from the ranked list of DEPs were analyzed. Sankey diagrams were employed to visualize the DEPs and their correlation with GO terms, utilizing SRplot (https://www.bioinformatics.com.cn/en). These analyses collectively provide a comprehensive understanding of the functional characteristics and interplay of the DEPs in the context of the studied conditions
Corpus callosum dissection for single-nucleus RNA-seq
Brain samples were collected from a total of 16 male C57BL/6 J mice (4 mice per group). After perfusion with 1% PBS, the brains were promptly placed in a coronal stainless steel brain matrix that had been pre-chilled on ice. Clean disposable blades were positioned at 2 mm intervals, and the CC was dissected using a scalpel directly from the tissue sections adhered to the blades. Both the dissected CC and the remaining brain tissue were immediately transferred to tubes and placed on dry ice.
Preparation of single-nucleus suspensions
The tubes containing frozen tissue (CC) were transferred from the −80 °C freezer to ice, along with the necessary solutions for isolating the nuclei. The isolation of CC nuclei was performed using a commercial kit (Minute™ Single Nucleus Isolation Kit for Neuronal Tissues/Cells, BN-020, Invent Biotechnologies, Inc.). The cold buffer A was added to the tissue, which was manually homogenized using a motorized pestle mixer. Subsequently, cold buffer A was added to achieve a final volume of 700 μl, and the tissue was homogenized again. The tubes were then incubated on ice for 5 min, followed by transferring the homogenate to a column. The tubes with the column were incubated at −20 °C for 10 min with the cap open. Afterward, the tubes were capped and centrifuged at 13,000×g for 30 s. The column was discarded, and the pellet was gently resuspended. Next, the tubes were centrifuged at 600×g for 5 min, the supernatant was discarded, and the pellet was resuspended in 1% bovine serum albumin (BSA). Cold buffer B (1.5 ml) was carefully added to the tubes, overlaying the previous BSA suspension. Finally, the tubes were centrifuged at 1000×g for 10 min. The supernatant was discarded, and the pellet containing the nuclei was resuspended in 100 μl of 1% BSA in PBS containing 0.2 U μl − 1 RNase inhibitors. Trypan blue was used to visually inspect and count the number of nuclei.
cDNA library preparation and sequencing
cDNA libraries were prepared using the Chromium Next GEM Single Cell 3′ Kit v3.1 (10× Genomics), according to manufacture protocol. The isolated nuclei were subjected to reverse transcription and barcoding using the provided reagents and enzymes. The resulting cDNA was then amplified, and sequencing adapters were integrated. To assess the quality of the cDNA libraries, 1 μl of each sample (dilution factor 1:10) was run on the Agilent 2100 Bioanalyzer System. The prepared cDNA libraries were subsequently sequenced on the NovaSeq 6000 system, generating 2 × 150 bp paired-end reads.
Single-nucleus RNA-seq data processing
The sn-RNA seq FASTQ files were analyzed using Cell Ranger v3.0.1 from 10× Genomics. The data alignment was performed against the mm10/GRCm38 reference genome. Subsequently, the sn-RNA seq dataset underwent processing, analysis, and visualization through the Cellenics® platform, which is accessible at GitHub repository (https://github.com/hms-dbmi-cellenics) and the community instance (https://scp.biomage.net/) hosted by Biomage (https://biomage.net/). Count matrices were pre-filtered by removing all barcodes with more than 20000 UMIs and using the Cellranger Empty Drops method78 with FDR threshold of 0.1. Due to technical problems in the preparation of the samples that were revealed as irremediable in the analysis, sample number 3 had to be excluded. Barcodes exhibiting fewer than 100 or more than 5000 features were also excluded. Empty Drops failed to properly identify empty droplets for samples 7, 15 and 16. After manual visualization of diagnostic plots a threshold of 800, 450 and 550 UMIs was selected for each sample respectively, and only barcodes surpassing their respective thresholds were retained for further analysis. For the last step of pre-filtering, clustering was calculated for each sample using Seurat’s implementation of the Louvain method. The pre-filtered count matrices were then uploaded to Cellenics®. Following this step, barcodes were then filtered in a series of two sequentially applied steps. First, dead and dying cells were removed by filtering out barcodes with a percentage of mitochondrial reads above 10%. Secondly, droplets outside the upper and lower boundaries of the prediction interval were filtered out. Lastly, the probability of droplets containing more than one cell was calculated using the scDblFinder R package (v. 1.11.3)79. Barcodes classified as doublets by the algorithm were filtered out. For each filtering step, sample-specific thresholds are reported in Supplementary Data S6. After filtering, each sample contained between 1255 and 4556 high-quality barcodes, totaling 37,001 cells, which were input to the integration pipeline. The initial stage of integration involved log-normalization of the data. Subsequently, the top 2000 genes with the highest variability were chosen using the variance stabilizing transformation (VST) method. Principal-component analysis (PCA) was performed, and the top 30 principal components, explaining 85.23% of the total variance, were used for batch correction with the Harmony R package80. Finally, clustering was performed using Seurat’s implementation of the Louvain method and a total of 18 clusters were identified. To facilitate result visualization, a uniform manifold approximation and projection (UMAP) embedding was computed. This was achieved using Seurat’s encapsulation of the Uniform Manifold Approximation and Projection (UMAP) package81. For the purpose of identifying marker genes specific to each cluster, cells within each cluster were subjected to comparison against all other cells. This comparative analysis was executed utilizing the presto package’s implementation of the Wilcoxon rank-sum test81.
Cell type clustering and annotation
Clusters that were identified as non-neuronal cell types were isolated from the complete experiment. This isolation process involved extracting manually annotated barcodes and then creating a subset of the Seurat object. To distinguish non-neuronal cell types, clusters with fewer than 50% of cells expressing the marker gene Rbfox3 were retained, while the remaining clusters were excluded from further analysis. The subset samples were subsequently inputted into Cellenics®. A more stringent mitochondrial content threshold of 5% was applied to the data, but all the remaining filters of the pipeline were disabled because the data had already been filtered. The data was subjected to the same integration pipeline as the full experiment, and the top 23 principal components, explaining 78.7% of the total variance, were used for batch correction (Supplementary Data S7). Mitochondrial genes were removed from the analysis before integration. Overall, the resulting filtered matrix consisted of 11,333 high-quality nuclei with an average of 15,457 genes. A total of 13 clusters were identified after performing clustering with the Seurat implementation of the Louvain method and used for differential expression analysis. Next, annotation was performed in two steps82. Automatic annotation was conducted using the ScType platform83 followed by a manual inspection and specific annotation using established cell-type markers widely recognized84–86. These markers encompassed Pdgfrα, Vcan, and Cspg4 (for OPCs), Plp1, Mbp, Mobp, and Prr5l (for OLs), Ttr and Enpp2 (for choroid plexus), Ftl1, Slco1a4, and Mecom (for endothelial cells), Atp13a5 and Cald1 (for pericytes), Slc1a3, Plpp3, Clu, and Gpc5 (for astrocytes), Runx1, Csf1r and Cx3cr1 (for microglia), Cped1, Bnc2, and Ranbp3l (for VLMCs), and Dnah12 and Cfap44 (for radial glial cells). Subclusters within these cell types were delineated based on the distinct expression patterns of marker genes. For instance, within Astrocytes, the first subcluster (Astrocytes 1) was characterized by the presence of Gpc5, Atp1a2, Slc1a2, and Slc1a3 as markers, while the second subcluster (Astrocytes 2) exhibited Apoe, Clu, and Sparcl expression. Concerning Oligodendrocytes (OLs), three subclusters were discerned. Ols1 displayed expression of Mbp, Prr5l, and Rnf220, Ols2 also expressed Mbp but showed enrichment for Atp1a2 and Nkain2, and OLs3 exhibited enrichment for Fth1, Mobp, and Nrg (Supplementary Data S8).
Differential expression analysis across groups
We employed volcano plots to effectively visualize the outcomes of our differential expression analysis conducted across multiple experimental groups. The analysis method utilized was the pseudobulk limma-voom workflow as elaborated in the comprehensive resource accessible at http://bioconductor.org/books/3.14/OSCA.multisample/multi-sample-comparisons.html. For the purpose of identifying significant differential expression, we considered genes with an absolute log fold change (FC) exceeding 0.5, and concurrently, a false discovery rate (FDR) lower than 0.05. This threshold provided us with the confidence to confidently attribute differential expression to specific genes within the unique context of our experiments.
Gene ontology analysis of sn-RNAseq data
The top-upregulated genes resulting from the analysis, ordered by adjusted p value and fold change, were employed for the enrichment GO analysis, following the methodology previously outlined in the proteomics section. To summarize, the top genes were subjected to analysis using the Metascape platform (www.metascape.org/), and any redundant GO terms were eliminated through the utilization of the Revigo platform (www.revigo.irb.hr/) (Supplementary Data S9–12). GO terms significantly enriched at FDR < 0.05 were considered.
mRNA in situ hybridization of brain sections
The RNAscope™ Multiplex Fluorescent V2 Assay (323100, ACD) was conducted on 20-μm free-floating sections for the detection of MOBP (431721-C2, ACD) and MBP (451491, ACD) transcripts. The assay was performed according to the manufacturer’s instructions. In brief, sections were mounted on Superfrost Plus slides, baked, and fixed using 4% PFA for 15 min at room temperature (RT). Following dehydration with serial dilutions of ethanol, hydrogen peroxide was added to the sections for 10 minutes at RT. Subsequently, antigen retrieval was carried out as per the protocol, and RNA protease III was applied to slides, which were then placed in a humidified tray and incubated for 30 min at 40 °C in the HybEZ oven. Probes for MOBP and MBP were prepared and added to the slides, incubating for 2 h at 40 °C. After washing, slides were stored in a 5× saline sodium citrate solution overnight. Next, RNAscope Amp 1 and Amp 2 reagents were added to amplify the signal from hybridized probes. Sections were then stained with Opal 520 and Opal 650 fluorophores. Following the wash, the IHC protocol, as described previously, was carried out using anti-GFAP and anti-IBA1 antibodies (see “IHC” section). Slides were mounted, and images were acquired at 40× magnification using the 900 confocal microscope. To quantify and reconstruct three-dimensional images, Imaris software (v.9.5, Bitplane) was used.
TUNEL colorimetric assay
To assess cell death in the brain floating sections, we performed the TUNEL assay using the TUNEL colorimetric assay kit (C10625, Thermo Fisher). Free-floating sections were mounted on gelatin-coated slides and air-dried overnight. Proteinase K was added to the sections and incubated for 10 min at RT. Then, the brain sections were first permeabilized to facilitate the access of the terminal deoxynucleotidyl transferase (TdT) enzyme to the DNA fragments. Subsequently, the sections were incubated with a TUNEL reaction mixture containing the TdT enzyme and 2-deoxyuridine 5-triphosphate (dUTP). To visualize the TUNEL-positive cells, which acquire a dark-brown color, we utilized a Zeiss LSM 900 confocal microscope.
Statistics
The GraphPad Prism 9 software (GraphPad Software Inc., San Diego, USA) was used to perform the statistical analysis. A two-way ANOVA was conducted to assess significant differences between CPZ and ND groups, followed by the Bonferroni post hoc test (Supplementary Data S14). For comparisons between the two groups within the CPZ condition and aging conditions, Student’ t-tests were conducted. Statistically significant results were assumed considering a p ≤ 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
We would like to acknowledge the following individuals and organizations for their support: Fundacion Bancaria la Caixa, The JPB Foundation, Carol and Gene Ludwig Family Foundation, Lester A. Gimpelson, Eduardo Eurnekian, The Dolby Family, Kathy and Miguel Octavio, the Marc Haas Foundation, Ben Lenail and Laurie Yoler, and NIH RO1 grants AG069232, AT011460 and R01NS122742 to L.-H.T.
Author contributions
D.R.-A., L.B., T.K., and L.-H.T. designed the study. D.R.-A, T.K., and F.A. performed gamma entrainment using sensory stimulus (GENUS). D.R.-A., L.B., and D.S.P. performed and analyzed imaging experiments. L.L. performed the electrophysiological analysis of evoked CAPs. D.R.-A., A.J.N, J.M.R, V.Y., and M.P.S. performed quantitative proteomics. D.R.-A, M.M, F.G., and M.R.I. performed RNA sequencing experiments and O.G. analyzed RNA sequencing data. C.Y. performed LFP recordings. BS, AD and RMR conducted the RNAscope assay. D.R.-A., L.B., and L.-H.T. wrote the paper.
Peer review
Peer review information
Nature Communications thanks Alban Gaultier, Markus Kipp and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
Sequence data have been deposited at the NIH GEO database under accession code GSE240815. The raw mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD044572. Source data are provided as a Source Data file. Source data are provided with this paper.
Code availability
The code used in this study is available on the GitHub repository.
Competing interests
L.-H.T. is a scientific founder and serves on the scientific advisory board of Cognito Therapeutics. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-51003-7.
References
- 1.Aggarwal, S., Yurlova, L. & Simons, M. Central nervous system myelin: structure, synthesis and assembly. Trends Cell Biol.21, 585–593 (2011). 10.1016/j.tcb.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 2.Franklin, R. J. M. & Ffrench-Constant, C. Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci.9, 839–855 (2008). 10.1038/nrn2480 [DOI] [PubMed] [Google Scholar]
- 3.Love, S. Demyelinating diseases. J. Clin. Pathol.59, 1151–1159 (2006). 10.1136/jcp.2005.031195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graves, J. et al. Protective environmental factors for neuromyelitis optica. Neurology83, 1923–1929 (2014). 10.1212/WNL.0000000000001001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menge, T. et al. Acute disseminated encephalomyelitis: an update. Arch. Neurol.62, 1673–1680 (2005). 10.1001/archneur.62.11.1673 [DOI] [PubMed] [Google Scholar]
- 6.Storch, M. & Lassmann, H. Pathology and pathogenesis of demyelinating diseases. Curr. Opin. Neurobiol.10, 186–192 (1997). 10.1097/00019052-199706000-00004 [DOI] [PubMed] [Google Scholar]
- 7.Fisher, E., Lee, J. C., Nakamura, K. & Rudick, R. A. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann. Neurol.64, 255–265 (2008). 10.1002/ana.21436 [DOI] [PubMed] [Google Scholar]
- 8.Blanc, F. et al. White matter atrophy and cognitive dysfunctions in neuromyelitis optica. PLoS ONE7, 33878 (2012). 10.1371/journal.pone.0033878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjornevik, K. et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science375, 296–301 (2022). 10.1126/science.abj8222 [DOI] [PubMed] [Google Scholar]
- 10.Olsson, T., Barcellos, L. F. & Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2017 13113, 25–36 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Centonze, D. et al. The link between inflammation, synaptic transmission and neurodegeneration in multiple sclerosis. Cell Death Differ.17, 1083–1091 (2010). 10.1038/cdd.2009.179 [DOI] [PubMed] [Google Scholar]
- 12.Voet, S., Prinz, M. & van Loo, G. Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol. Med.25, 112–123 (2019). 10.1016/j.molmed.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 13.Remington, L. T., Babcock, A. A., Zehntner, S. P. & Owens, T. Microglial recruitment, activation, and proliferation in response to primary demyelination. Am. J. Pathol.170, 1713–1724 (2007). 10.2353/ajpath.2007.060783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sen, M. K., Mahns, D. A., Coorssen, J. R. & Shortland, P. J. The roles of microglia and astrocytes in phagocytosis and myelination: Insights from the cuprizone model of multiple sclerosis. Glia70, 1215–1250 (2022). 10.1002/glia.24148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molina-Gonzalez, I., Miron, V. E. & Antel, J. P. Chronic oligodendrocyte injury in central nervous system pathologies. Commun. Biol.5, 1–11 (2022). 10.1038/s42003-022-04248-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson, E. M. et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science344, 1252304 (2014). 10.1126/science.1252304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradl, M. & Lassmann, H. Oligodendrocytes: Biology and pathology. Acta Neuropathol.119, 37–53 (2010). 10.1007/s00401-009-0601-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martorell, A. J. et al. Multi-sensory gamma stimulation ameliorates Alzheimer’s-associated pathology and improves cognition. Cell177, 256–271.e22 (2019). 10.1016/j.cell.2019.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adaikkan, C. et al. Gamma entrainment binds higher-order brain regions and offers neuroprotection. Neuron102, 929–943.e8 (2019). 10.1016/j.neuron.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan, D. et al. Gamma frequency sensory stimulation prevents brain atrophy, improves sleep and memory in probable mild Alzheimer’s patients. Alzheimer’s. Dement.17, e054218 (2021). 10.1002/alz.054218 [DOI] [Google Scholar]
- 21.Soula, M. et al. Forty-hertz light stimulation does not entrain native gamma oscillations in Alzheimer’s disease model mice. Nat. Neurosci.26, 570–578 (2023). 10.1038/s41593-023-01270-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang, Y. L. & Lai, T. W. Chronic visual stimulation with LED light flickering at 24, 40, or 80 Hz failed to reduce amyloid b load in the 5XFAD Alzheimer’s disease mouse model. eNeuro10, 1–7 (2023). 10.1523/ENEURO.0189-23.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanco-Duque, C., Chan, D., Kahn, M. C., Murdock, M. H. & Tsai, L. H. Audiovisual gamma stimulation for the treatment of neurodegeneration. J. Intern. Med.295, 146–170 (2023). 10.1111/joim.13755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahn, M. et al. Gamma sensory stimulation and effects on the brain. Preprint at bioRxiv10.1101/2023.10.30.564197 (2023).
- 25.Iaccarino, H. et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature540, 230–235 (2016). 10.1038/nature20587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen, Q. et al. Gamma frequency light flicker regulates amyloid precursor protein trafficking for reducing β-amyloid load in Alzheimer’s disease model. Aging Cell21, e13573 (2022). 10.1111/acel.13573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park, S. S. et al. Physical exercise during exposure to 40-Hz light flicker improves cognitive functions in the 3xTg mouse model of Alzheimer’s disease. Alzheimer’s. Res. Ther.12, 62 (2020). 10.1186/s13195-020-00631-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao, Y. et al. Non-invasive 40-Hz light flicker ameliorates Alzheimer’s-associated rhythm disorder via regulating central circadian clock in mice. Front. Physiol.11, 294 (2020). 10.3389/fphys.2020.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng, L. et al. Rhythmic light flicker rescues hippocampal low gamma and protects ischemic neurons by enhancing presynaptic plasticity. Nat. Commun.11, 1–16 (2020). 10.1038/s41467-020-16826-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murdock, M. H. et al. Multisensory gamma stimulation promotes glymphatic clearance of amyloid. Nature627, 149–156 (2024). 10.1038/s41586-024-07132-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gudi, V., Gingele, S., Skripuletz, T. & Stangel, M. Glial response during cuprizone-induced de- and remyelination in the CNS: lessons learned. Front. Cell. Neurosci.8, 1–24 (2014). 10.3389/fncel.2014.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiremath, M. M. et al. Microglial/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice. J. Neuroimmunol.92, 38–49 (1998). 10.1016/S0165-5728(98)00168-4 [DOI] [PubMed] [Google Scholar]
- 33.Dubey, M. et al. Myelination synchronizes cortical oscillations by consolidating parvalbumin-mediated phasic inhibition. Elife11, 1–24 (2022). 10.7554/eLife.73827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varga, E. et al. Cuprizone administration alters the iron metabolism in the mouse model of multiple sclerosis. Cell. Mol. Neurobiol.38, 1081–1097 (2018). 10.1007/s10571-018-0578-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guglielmetti, C. et al. Hyperpolarized 13C MR metabolic imaging can detect neuroinflammation in vivo in a multiple sclerosis murine model. Proc. Natl Acad. Sci. USA114, E6982–E6991 (2017). 10.1073/pnas.1613345114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reeves, T. M., Phillips, L. L. & Povlishock, J. T. Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp. Neurol.196, 126–137 (2005). 10.1016/j.expneurol.2005.07.014 [DOI] [PubMed] [Google Scholar]
- 37.Tai, Y.-H. et al. Targeting the TCA cycle can ameliorate widespread axonal energy deficiency in neuroinflammatory lesions. Nat. Metab.5, 1364–1381 (2023). 10.1038/s42255-023-00838-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedrazzi, M. et al. Selective proinflammatory activation of astrocytes by high-mobility group Box 1 protein signaling. J. Immunol.179, 8525–8532 (2007). 10.4049/jimmunol.179.12.8525 [DOI] [PubMed] [Google Scholar]
- 39.Escartin, C. et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci.24, 312–325 (2021). 10.1038/s41593-020-00783-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang, D., Chen, X., Kang, R. & Kroemer, G. Ferroptosis: molecular mechanisms and health implications. Cell Res.31, 107–125 (2021). 10.1038/s41422-020-00441-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jhelum, P. et al. Ferroptosis mediates cuprizone-induced loss of oligodendrocytes and demyelination. J. Neurosci.40, 9327–9341 (2020). 10.1523/JNEUROSCI.1749-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Filice, F., Celio, M. R., Babalian, A., Blum, W. & Szabolcsi, V. Parvalbumin-expressing ependymal cells in rostral lateral ventricle wall adhesions contribute to aging-related ventricle stenosis in mice. J. Comp. Neurol.525, 3266–3285 (2017). 10.1002/cne.24276 [DOI] [PubMed] [Google Scholar]
- 43.Szabolcsi, V. & Celio, M. R. De novo expression of parvalbumin in ependymal cells in response to brain injury promotes ependymal remodeling and wound repair. Glia63, 567–594 (2015). 10.1002/glia.22768 [DOI] [PubMed] [Google Scholar]
- 44.Almeida, R. G. & Lyons, D. A. On myelinated axon plasticity and neuronal circuit formation and function. J. Neurosci.37, 10023–10034 (2017). 10.1523/JNEUROSCI.3185-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Rourke, M., Gasperini, R. & Young, K. M. Adult myelination: wrapping up neuronal plasticity. Neural Regen. Res.9, 1261–1264 (2014). 10.4103/1673-5374.137571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zirngibl, M., Assinck, P., Sizov, A., Caprariello, A. V. & Plemel, J. R. Oligodendrocyte death and myelin loss in the cuprizone model: an updated overview of the intrinsic and extrinsic causes of cuprizone demyelination. Mol. Neurodegener.17, 34 (2022). 10.1186/s13024-022-00538-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adaikkan, C. & Tsai, L. H. Gamma entrainment: impact on neurocircuits, glia, and therapeutic opportunities. Trends Neurosci.43, 24–41 (2020). 10.1016/j.tins.2019.11.001 [DOI] [PubMed] [Google Scholar]
- 48.Pekny, M. & Pekna, M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim. Biophys. Acta. 1862, 483–491 (2016). 10.1016/j.bbadis.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 49.Gao, Z. et al. C1q inhibits differentiation of oligodendrocyte progenitor cells via Wnt/β-catenin signaling activation in a cuprizone-induced mouse model of multiple sclerosis. Exp. Neurol.348, 113947 (2022). 10.1016/j.expneurol.2021.113947 [DOI] [PubMed] [Google Scholar]
- 50.Cao, Q. et al. Astrocytic CXCL5 hinders microglial phagocytosis of myelin debris and aggravates white matter injury in chronic cerebral ischemia. J. Neuroinflamm.20, 1–18 (2023). 10.1186/s12974-023-02780-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schirmer, L. et al. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature573, 75–82 (2019). 10.1038/s41586-019-1404-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borggrewe, M. et al. Regionally diverse astrocyte subtypes and their heterogeneous response to EAE. Glia69, 1140–1154 (2021). 10.1002/glia.23954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ponath, G. et al. Myelin phagocytosis by astrocytes after myelin damage promotes lesion pathology. Brain140, 399–413 (2017). 10.1093/brain/aww298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhan, J. et al. The Cuprizone Model: Dos and Do Nots. Cells9, 843 (2020). 10.3390/cells9040843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pavlik, A., Aneja, I. S., Lexa, J. & Al-Zoabi, B. A. Identification of cerebral neurons and glial cell types inducing heat shock protein Hsp70 following heat stress in the rat. Brain Res.973, 179–189 (2003). 10.1016/S0006-8993(03)02476-4 [DOI] [PubMed] [Google Scholar]
- 56.Mansilla, M. J., Montalban, X. & Espejo, C. Heat shock protein 70: roles in multiple sclerosis. Mol. Med.18, 1018–1028 (2012). 10.2119/molmed.2012.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim, T. et al. Gamma entrainment using audiovisual stimuli alleviates chemobrain pathology and cognitive impairment induced by chemotherapy in mice. Sci. Transl. Med.16, 4601 (2024). 10.1126/scitranslmed.adf4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rayatpour, A., Foolad, F., Heibatollahi, M., Khajeh, K. & Javan, M. Ferroptosis inhibition by deferiprone, attenuates myelin damage and promotes neuroprotection in demyelinated optic nerve. Sci. Rep.12, 1–16 (2022). 10.1038/s41598-022-24152-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haider, L. et al. Multiple sclerosis deep grey matter: The relation between demyelination, neurodegeneration, inflammation and iron. J. Neurol. Neurosurg. Psychiatry85, 1386–1395 (2014). 10.1136/jnnp-2014-307712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dong, Y. et al. Oxidized phosphatidylcholines found in multiple sclerosis lesions mediate neurodegeneration and are neutralized by microglia. Nat. Neurosci.24, 489–503 (2021). 10.1038/s41593-021-00801-z [DOI] [PubMed] [Google Scholar]
- 61.Ursini, F. & Maiorino, M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic. Biol. Med.152, 175–185 (2020). 10.1016/j.freeradbiomed.2020.02.027 [DOI] [PubMed] [Google Scholar]
- 62.Qin, J., Goswami, R., Balabanov, R. & Dawson, G. Oxidized phosphatidylcholine is a marker for neuroinflammation in multiple sclerosis brain. J. Neurosci. Res.85, 977–984 (2007). 10.1002/jnr.21206 [DOI] [PubMed] [Google Scholar]
- 63.Yan et al. Ferroptosis: mechanisms and links with diseases. Signal Transduct. Target. Ther.6, 1–16 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Demerens, C. et al. Induction of myelination in the central nervous system by electrical activity. Proc. Natl Acad. Sci. USA93, 9887–9892 (1996). 10.1073/pnas.93.18.9887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li, L., Velumian, A. A., Samoilova, M. & Fehlings, M. G. A novel approach for studying the physiology and pathophysiology of myelinated and non-myelinated axons in the CNS white matter. PLoS ONE11, e0165637 (2016). 10.1371/journal.pone.0165637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Malara, M. et al. SHANK3 deficiency leads to myelin defects in the central and peripheral nervous system. Cell. Mol. Life Sci.79, 371 (2022). 10.1007/s00018-022-04400-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manippa, V. et al. An update on the use of gamma (multi)sensory stimulation for Alzheimer’s disease treatment. Front. Aging Neurosci.14, 1095081 (2022). 10.3389/fnagi.2022.1095081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vega-Riquer, J. M., Mendez-Victoriano, G., Morales-Luckie, R. A. & Gonzalez-Perez, O. Five decades of cuprizone, an updated model to replicate demyelinating diseases. Curr. Neuropharmacol.17, 129–141 (2019). 10.2174/1570159X15666170717120343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Linnerbauer, M., Wheeler, M. A. & Quintana, F. J. Astrocyte crosstalk in CNS inflammation. Neuron108, 608–622 (2020). 10.1016/j.neuron.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Borst, K., Dumas, A. A. & Prinz, M. Microglia: immune and non-immune functions. Immunity54, 2194–2208 (2021). 10.1016/j.immuni.2021.09.014 [DOI] [PubMed] [Google Scholar]
- 71.Ortiz, F. C. et al. Neuronal activity in vivo enhances functional myelin repair. JCI Insight4, e123434 (2019). 10.1172/jci.insight.123434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conrad, M. et al. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev.32, 602–619 (2018). 10.1101/gad.314674.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ding, J. et al. Relationship between the plasma levels of neurodegenerative proteins and motor subtypes of Parkinson’s disease. J. Neural Transm.124, 353–360 (2017). 10.1007/s00702-016-1650-2 [DOI] [PubMed] [Google Scholar]
- 74.Singer, A. C. et al. Noninvasive 40-Hz light flicker to recruit microglia and reduce amyloid beta load. Nat. Protoc.13, 1850–1868 (2018). 10.1038/s41596-018-0021-x [DOI] [PubMed] [Google Scholar]
- 75.McAlister, G. C. et al. MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal. Chem.86, 7150–7158 (2014). 10.1021/ac502040v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li, J. et al. TMTpro reagents: a set of isobaric labeling mass tags enables simultaneous proteome-wide measurements across 16 samples. Nat. Methods17, 399–404 (2020). 10.1038/s41592-020-0781-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eng, J. K., Jahan, T. A. & Hoopmann, M. R. Comet: an open-source MS/MS sequence database search tool. Proteomics13, 22–24 (2013). 10.1002/pmic.201200439 [DOI] [PubMed] [Google Scholar]
- 78.Kaminow, B., Yunusov, D. & Dobin, A. STARsolo: accurate, fast and versatile mapping/quantification of single-cell and single-nucleus RNA-seq data. Preprint at bioRxiv10.1101/2021.05.05.442755 (2021).
- 79.Germain, P. L., Lun, A., Macnair, W. & Robinson, M. D. Doublet identification in single-cell sequencing data using scDblFinder. F1000Research10, 979 (2021). 10.12688/f1000research.73600.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods16, 1289–1296 (2019). 10.1038/s41592-019-0619-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sainburg, T., McInnes, L. & Gentner, T. Q. Parametric umap embeddings for representation and semisupervised learning. Neural Comput.33, 2881–2907 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heumos, L. et al. Best practices for single-cell analysis across modalities. Nat. Rev. Genet.24, 550–572 (2023). 10.1038/s41576-023-00586-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ianevski, A., Giri, A. K. & Aittokallio, T. Fully-automated and ultra-fast cell-type identification using specific marker combinations from single-cell transcriptomic data. Nat. Commun.13, 1246 (2022). 10.1038/s41467-022-28803-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Macnair, W. et al. Single nuclei RNAseq stratifies multiple sclerosis patients into three distinct white matter glia responses. Preprint at bioRxiv10.1101/2022.04.06.487263 (2022).
- 85.Marques, S. et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science352, 1326–1329 (2016). 10.1126/science.aaf6463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pandey, S. et al. Disease-associated oligodendrocyte responses across neurodegenerative diseases. Cell Rep.40, 111189 (2022). 10.1016/j.celrep.2022.111189 [DOI] [PubMed] [Google Scholar]
- 87.George, P., Franklin., K. B. J. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates: Hard Cover Edition. (Elservier, 2001).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Sequence data have been deposited at the NIH GEO database under accession code GSE240815. The raw mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD044572. Source data are provided as a Source Data file. Source data are provided with this paper.
The code used in this study is available on the GitHub repository.