Abstract
Background
Preoperative evaluation of axillary lymph node status is crucial for the selection of both systemic and surgical treatment in early breast cancer. This study assessed the particular role of additional shear wave elastography (SWE) in axillary staging in patients undergoing initial breast cancer diagnostics.
Methods
One hundred patients undergoing axillary lymph node biopsy due to a sonographically suspicious axillary lymph node were prospectively evaluated with SWE using virtual touch tissue imaging quantification (VTIQ). Mean values of tissue stiffness for axillary tissue and lymph node tissue were measured prior to core-cut biopsy of the lymph node. All lymph nodes were clip-marked during the biopsy. Cut-off values to differentiate between malignant and benign lymph nodes were defined using Youden’s index.
Results
Lymph nodes with evidence of malignant tumor cells in the final pathological examination showed a significantly higher velocity as measured by SWE, with a mean velocity of 3.48 ± 1.58 m/s compared to 2.33 ± 0.62 m/s of benign lymph nodes (p < 0.0001). The statistically optimal cutoff to differentiate between malignant and benign lymph nodes was 2.66 m/s with a sensitivity of 69.8% and a specificity of 87.5%.
Conclusions
Lymph node metastases assessed with SWE showed significantly higher elasticity values compared to benign lymph nodes. Thus, SWE provides an additional useful and quantifiable parameter for the sonographic assessment of suspicious axillary lymph nodes in the context of pre-therapeutic axillary staging in order to differentiate between benign and metastatic processes and support the guidance of definitive biopsy work-up.
Critical relevance statement
Shear-wave elastography provides an additional useful and quantifiable parameter for the assessment of suspicious axillary lymph nodes in the context of pre-therapeutic axillary staging in order to differentiate between benign and metastatic processes and support guiding the definitive biopsy work-up.
Key Points
SWE is a quantifiable ultrasound parameter in breast cancer diagnosis.
SWE shows a significantly higher velocity in malignant lymph nodes.
SWE is useful in improving the sensitivity and specificity of axillary staging.
Graphical Abstract
Keywords: Breast cancer, Axillary staging, Ultrasound, Shear wave elastography
Introduction
B-mode ultrasound of the axillary region plays a crucial role in the pre-therapeutic diagnostics of patients with early breast cancer [1, 2]. The status of axillary lymph nodes (LN) is a significant prognostic factor for disease recurrence and overall survival, influencing the selection of therapy regimens, including both systemic and surgical approaches [3, 4]. To reduce the risk of surgical overtreatment, axillary B-mode ultrasound must effectively identify patients with an unsuspicious axillary LN status and thus do not benefit from extensive axillary surgery [5]. Patients with involvement of axillary LNs who are not receiving neoadjuvant systemic therapy are recommended to undergo axillary lymph node dissection (ALND), whereas patients with negative nodal status undergo sentinel lymph node biopsy (SLNB) [1]. SLNB is associated with less morbidity such as lymphedema, seroma, and arm mobility impairment and sensitivity [6, 7]. According to national and international guidelines, axillary B-mode ultrasound is widely used in clinical routine [1]. Despite this fact, its diagnostic accuracy is still low and a standardization of LN positivity criteria is not present yet [8, 9].
Accurate axillary staging is also becoming increasingly important in the context of axillary operative de-escalation. In patients with LN involvement, instead of performing a complete removal of all lymph nodes in the axilla (ALND), surgeons may selectively target specific lymph nodes for removal under certain conditions, based on preoperative imaging or intraoperative assessment. This approach is becoming increasingly relevant, particularly as the response rate to neoadjuvant systemic therapy increases. By selectively targeting specific lymph nodes, this approach reduces the extent of surgery while still effectively addressing nodal staging [10–12].
Sonomorphological criteria for metastatic axillary LNs are eccentric cortical thickening, loss of fatty hilum, rounder shape, pathological color Doppler images such as increased, peripheral, and disruptive vascularity, and irregular margins [13–18]. These criteria possess a low positive predictive value, as the ultrasound signs are nonspecific and can also be observed in inflammation or reactively changed LNs. Although conventional B-mode ultrasound is widely used in clinical practice a consensus score on LN assessment has not been identified yet. The sensitivity and specificity of axillary staging using conventional B-mode ultrasound vary significantly across different studies, ranging between approximately 45–95% for each, respectively [4, 19]. Therefore, confirmation of suspicious LN in ultrasound by fine needle aspiration or core-cut biopsy is indispensable. These methods have a specificity of up to 100% but lower sensitivity ranging from 25% to 94% [20, 21]. For this reason, up to 20% of patients require a secondary ALND after SLNB [4, 22, 23].
Given this fact, additional tools are necessary to improve the diagnostic performance of axillary ultrasound. 2D shear wave elastography (SWE) measures tissue stiffness based on shear wave velocity and is an established complementary tool in diagnostics of the breast tissue since it has been integrated as a novel descriptor for malignancy in the BI-RADS 5th edition [24–26].
Increased tissue stiffness is a known predictor of malignancy. High stiffness is reported to be due to high levels of collagen, myofibroblasts, angiogenesis, inflammatory reaction, necrosis, and different tumor histologic biomarkers [27, 28].
It has been shown that SWE is also feasible in axillary LNs, but few studies have evaluated the diagnostic value of SWE in axillary staging [29–32]. In this single-center prospective diagnostic study, we assessed the potential of SWE to distinguish between benign and malignant axillary LNs in a clinical routine setting.
Materials and methods
Study design and enrollment
This is a single-center prospective diagnostic study. The study protocol was approved by the local ethics committee and additional written informed consent was obtained from each participating patient (S-396/2019). The study was conducted in a specialized diagnostic breast unit and included 100 consecutive patients with one or more suspicious axillary LNs and an indication for an axillary LN core-cut biopsy between June 2021 and December 2022. This cohort encompassed individuals attending the breast unit for various reasons, such as for routine surveillance following contralateral breast cancer treatment, those participating in routine breast cancer screening, as well as those seeking clarification of breast lesions. However, the study excluded patients diagnosed with malignancies other than breast cancer (e.g., lymphoma or sarcoma). Additional exclusion criteria comprised male sex, individuals younger than 18 years of age, inflammation of the breast, previous ipsilateral axillary surgery (i.e., history of SLNB or ALND), prior radiation therapy to the ipsilateral breast, or ongoing breast cancer treatment. All 100 LNs were evaluated using SWE prior to core needle biopsy. Each LN was clip-marked directly after the biopsy. SWE, as well as the biopsy, were performed by the same examiner. Two radiologists, as well as five gynecologists experienced in B-mode ultrasound, SWE, and biopsies, were eligible to include patients in this study.
Conventional B-mode ultrasound and selection of the LNs
Patients were positioned identically for imaging with the ipsilateral arm in an elevated position. B-mode ultrasound was performed using Siemens Acuson S2000 or S3000 with a 9 MHz probe (Siemens Healthineers). Criteria for suspicious LNs in ultrasound were the presence of cortical hypertrophy > 3 mm, eccentric or focal cortical hypertrophy, round shape with complete or partial effacement of the fatty hilus, or expressing pathological color Doppler images [8, 33]. If one or more of these criteria were applicable to one or more axillary LNs, a core-cut biopsy was performed. The selection of the respective LN was up to the examiner who performed a B-mode ultrasound. Generally, the LN judged to be the most suspicious was selected for biopsy, although the location, as well as the relation to nearby vessels, may also influence LN selection. The long-axis and short-axis diameters of each suspicious LN were measured and documented. Biopsy was performed using 14G HistoCore® Automatic Biopsy System by BIP Biomed (Tuerkenfeld, Germany). Two or three samples were taken from each LN.
SWE
SWE was performed using 2D-SWE systems Siemens Acuson S2000 or S3000 equipped with virtual touch tissue imaging quantification (VTIQ) software utilizing a 9 MHz probe (Siemens Healthineers). The VTIQ algorithm estimates the velocity of the induced shear waves which is correlated to tissue stiffness. SWE was always performed on the LN that was chosen for biopsy. The accuracy of the measurement was indicated by a quality map [34]. If the image was compromised due to compression or movement of the patient, the measurement had to be repeated.
SWE was performed with minimum compression induced by the transducer. Elasticity values were measured in meters per second (m/s), ranging from 0 to 10 m/s. Elasticity values from the regions of interest, namely the area with the highest velocity of the LN, as well as from the surrounding tissue were documented. The biopsy of the selected LN was performed in the same position. The previously selected LN was biopsied and marked with a clip. The biopsy of the LN was controlled with ultrasound. The clip was additionally documented by mammography in 96% of cases. In 80% of the cases, the clip could be seen on the mammogram; the other 20% could not be seen due to the clip’s localization.
Pathological reference
Pathological examinations and immunohistochemistry (IHC) of the core-cut biopsies were conducted by the division head of gynecopathology, who possesses over 25 years of experience in breast pathologies, in accordance with national guidelines. These assessments were performed in a blinded setting, ensuring unbiased evaluation (i.e., pathologists were not aware of SWE findings) [35].
Statistical analysis
This is an exploratory study. Statistical tests and resulting p-values can therefore only be interpreted descriptively. The study cohort was described by the measures of empirical distribution. Depending on the level of measurement, mean and standard deviation (SD), as well as absolute and relative frequencies were calculated. To compare the study cohort and sonomorphology of the LNs with respect to benign and malignant histopathology, an independent t-test and chi-square test were used. Receiver operating characteristic curves (ROC) were plotted to determine cutoff points yielding the maximal sum of sensitivity and specificity (Youden index). The area under the receiver operating characteristic curve (AUC) was additionally calculated. Statistical analysis was performed with R (version 4.1.0—© 2021, The R Foundation for Statistical Computing).
Results
Description of the study cohort
One hundred consecutive patients with suspicious axillary LNs in the ultrasound examination were enrolled. Five patients were excluded based on the exclusion criteria. In two cases, an axillary fibroadenoma was found, whereas in one case silicone deposits due to implant leakage after breast augmentation were detected. In one biopsy granulomatous inflammatory activity of the LN was detected due to a known sarcoidosis. One patient had previously undergone axillary surgery. These five patients/LNs were subsequently excluded from further analysis. Ninety-five patients were included in the further analysis. The mean age of the patients was 56 ± 16 years. Eighty-two patients received a biopsy of the ipsilateral breast due to a suspicious breast lesion and a biopsy of the axilla. Fifty-five biopsies were performed in the left axilla, and forty in the right axilla (Table 1).
Table 1.
Description of the study cohort
| Total, (n = 95) | Benign, (n = 32) | Malignant, (n = 63) | p-value | |
|---|---|---|---|---|
| Age (years ± SD) | 56 ± 15.59 | 54 ± 14.90 | 58 ± 15.94 | 0.12 |
| Simultaneous biopsy of the ipsilateral breast | 82 | 21 | 61 | < 0.001 |
| Side | ||||
| Left | 55 | 20 | 35 | |
| Right | 40 | 12 | 28 | 0.52 |
SD standard deviation
Pathology
Thirty-two (33.7%) biopsies contained healthy LN tissue and sixty-three (66.3%) LN metastases were detected in the remaining 95 patients. Sixty LN metastases were associated with ipsilateral breast cancer. One patient (1.1%) had an LN metastasis consistent with breast cancer but without evidence of a primary site in the breast (cancer of unknown primary, CUP). Two (2.1%) LN metastases were associated with ovarian cancer and head and neck cancer, respectively.
Of all patients, 79 had breast cancer of the ipsilateral breast where the LN biopsy was performed. The pathology including IHC of all patients with breast cancer (n = 80, including one patient with CUP) is presented in Table 2.
Table 2.
Pathology characteristics of patients with current breast cancer of the ipsilateral breast
| Total, (n = 80) | Benign, (n = 19) | Malignant, (n = 61) | |
|---|---|---|---|
| Histological subtype | |||
| NST | 73 | 18 | 55 |
| ILC | 4 | 1 | 3 |
| Other | 3a | 0 | 3 |
| Tumor biology | |||
| HR +/HER2 − | 55 | 8 | 47 |
| HR +/HER2 + | 9 | 4 | 5 |
| HR −/HER2 + | 6 | 3 | 3 |
| TNBC | 10 | 4 | 6 |
| Grading | |||
| 1 | 5 | 1 | 4 |
| 2 | 47 | 10 | 37 |
| 3 | 28 | 8 | 20 |
NST non-special type, ILC invasive lobular carcinoma, HR hormone receptor, TNBC triple-negative breast cancer
a Histological subtypes: two were apocrine and one a combination of NST and ILC
Conventional B-mode ultrasound
Axillary staging with conventional B-mode ultrasound was performed for all patients.
The mean number of sonographically suspicious LNs were 2.56 ± 1.96 among all patients. Considering the patients in which biopsy proved a benign LN, the mean number of initially sonographically suspicious LNs was 2.09 ± 1.15. In patients with LN metastasis, the mean number of suspicious LNs was 3.03 ± 2.54.
The mean size of the chosen LNs was 12.91 mm ± 4.71 mm × 7.28 mm ± 2.33 mm in benign LNs and 16.67 mm ± 8.10 mm × 10.28 mm ± 5.41 mm in malignant LNs. The mean depth of the chosen LNs was 13.25 mm ± 6.84 mm in benign LNs and 13.81 mm ± 4.32 mm in malignant LNs. There was no statistically significant difference in the depth of the chosen LNs (p = 0.62) whereas the size of the LNs was significantly larger in malignant LNs with p = 0.017 for the long axis diameter of the ln and p < 0.001 for the short axis diameter (Table 3).
Table 3.
Characteristics of LNs in B-mode ultrasound and velocities measured by SWE (in m/s)
| Total, (n = 95) | Benign, (n = 32) | Malignant, (n = 63) | p-value | |
|---|---|---|---|---|
| Number of suspicious LNsa ± SDb | 2.78 ± 2.31 | 2.09 ± 1.15 | 3.10 ± 2.64 | 0.041 |
| Mean depth of the LN ± SD | 13.52 ± 5.39 | 13.25 ± 6.84 | 13.81 ± 4.32 | 0.62 |
| Diameter of the LN in mm | ||||
| Long axis (mean ± SD) | 15.5 ± 7.24 | 12.91 ± 4.71 | 16.67 ± 8.10 | 0.017 |
| Short axis (mean ± SD) | 9.34 ± 4.76 | 7.28 ± 2.33 | 10.28 ± 5.41 | < 0.001 |
| LNa tissue | ||||
| Mean ± SDb | 3.10 ± 1.44 | 2.33 ± 0.62 | 3.48 ± 1.58 | < 0.001 |
| Max | 10.00 | 4.97 | 10.00 | – |
| Min | 1.00 | 1.52 | 1.00 | – |
| Surrounding tissue | ||||
| Mean ± SD | 1.67 ± 0.37 | 1.58 ± 0.32 | 1.73 ± 0.39 | 0.06 |
| Max | 3.25 | 2.36 | 3.25 | – |
| Min | 0.98 | 1.04 | 0.98 | – |
a Lymph node
b Standard deviation
SWE
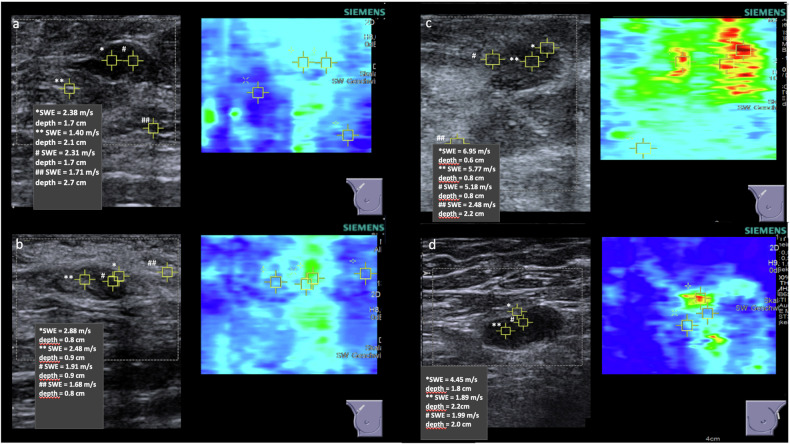
SWE was performed in all 95 LNs. The region of interest (ROI) was defined as the area with the highest velocity within the respective LN. An example measurement is shown in Fig. 1. Additionally, SWE was measured in the surrounding tissue.
Fig. 1.
Example measurement of SWE in benign (a) and malignant (b–d) axillary LNs
Velocities of LN tissue and surrounding tissue showed a mean shear wave velocity of 3.10 ± 1.44 m/s and 1.67 ± 0.37 m/s, respectively. The mean velocity of LN tissue in benign LNs was 2.33 ± 0.62 m/s, while the mean velocity in metastatic LNs was 3.48 ± 1.58 m/s. The velocities of the benign and malignant LNs demonstrate a statistically significant difference (p = 0.00015). The mean velocities measured in the surrounding tissue showed no significant difference in both groups. The velocities measured by SWE are presented in Table 3 and Fig. 2.
Fig. 2.
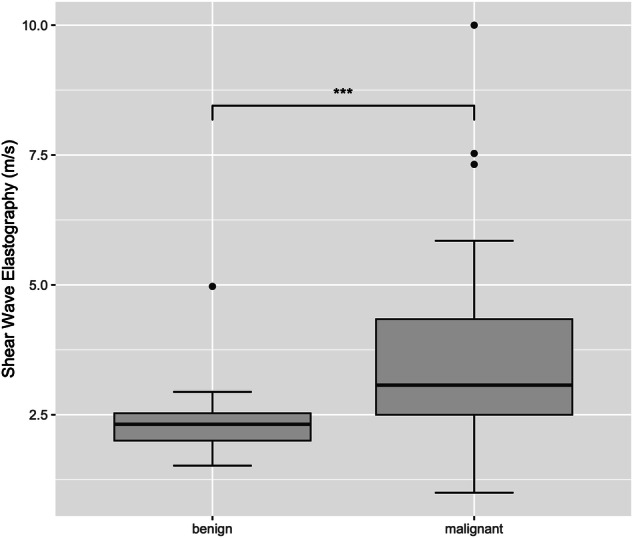
Velocities measured by SWE in benign and malignant LN tissue. *** p < 0.001
ROC analysis
AUC for the mean velocity was measured in LN tissue to discriminate between benign and malignant LNs and found to be 0.79 (Fig. 3). The statistically optimal cutoff with the highest Youden Index (0.58) was 2.66 m/s measured in LN tissue. This threshold yielded a sensitivity of 69.8% and a specificity of 87.5%. The corresponding false negative rate stood at 20%, while the false positive rate was 4%.
Fig. 3.
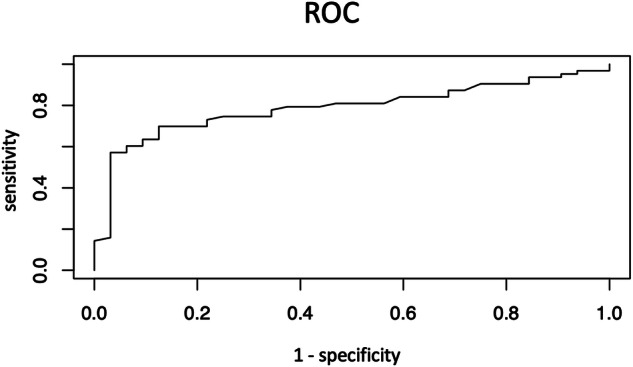
ROC for the velocities measured in LN tissue
Discussion
Ninety-five sonographically suspicious axillary LNs were examined using SWE in a clinically routine setting. The mean velocity measured in LN tissue was 3.10 ± 1.44 m/s for the whole cohort. These values are higher than in sonographically unsuspicious LN, which were collected in a previous study (1.90 ± 0.34 m/s in LN cortex and 2.02 ± 0.37 m/s in LN hilus) [29]. A distinction between the cortex and hilus area of an LN, as performed in the previous study, was not possible in this cohort, as a reliable distinction between these areas was not always possible due to the ultrasound changes in the LNs. In this cohort, LNs with evidence of malignant cells in histopathology had significantly higher mean velocities than benign LNs.
These data are in line with previous literature examining SWE in axillary LNs. Several studies have indicated that velocities measured by SWE correlate with malignancy in axillary LNs. A French study group examined in 2012 eighty-one sentinel LNs using SWE, with seventy LNs being benign and eleven showing metastasis. In their analysis, benign LN showed a significantly lower velocity with 11.32 kPa (converted 1.94 m/s) while LN metastasis showed a higher velocity of 17.47 kPa (converted 2.41 m/s) [36]. Conversion between kPa and m/s was done by using the simplified formula for stiffness in kPa = 3 × (velocity in m/s)2 [37, 38]. However, the comparability of these findings to the present study may be limited due to the small number of cases in the metastatic LN group. Bae et al examined SWE on sixty-three LN ex vivo during breast cancer surgery. They demonstrated that LNs later identified as metastatic had a higher mean SWE than benign ones. The mean velocities were 47.7 kPa (converted 3.88 m/s) in metastatic LN and 17.7 kPa (converted 2.43 m/s) in benign ones [39]. The values reported by Bae et al were higher compared to those in the current study; however, direct comparison isn’t feasible as the examination was conducted ex vivo. Several other studies evaluated the use of SWE as part of preoperative axillary staging. In a study by Ng et al qualitative SWE with color patterns had a higher discriminatory power compared with quantitative SWE or B-mode ultrasound [30]. In another analysis conducted by Luo et al, they examined one hundred twenty-one axillary LNs and proposed a cutoff value of 26.90 kPa (equivalent to 3.0 m/s when converted) to distinguish between benign and malignant LNs. It is important to note that this study included patients who underwent both SLNB and ALND. However, there was no guarantee that the LNs measured using SWE corresponded directly to the LNs subsequently operated on. Their findings revealed that the cutoff value of 26.90 kPa (3.0 m/s) achieved a sensitivity of 86.7% and a specificity of 96.7%, both of which were higher than the values reported in the current study [31]. Pulappadi et al additionally demonstrated in a cohort of 48 patients that a velocity measured in the LN cortex higher than 14.9 kPa (converted 2.23 m/s) is associated with malignancy with a sensitivity of 73.7% and a specificity of 81.8% [32]. A further study by Seo et al with fifty-three patients reported a cut-off value of 23.8 kPa (converted 2.82 m/s) to be associated with metastatic LN with a sensitivity of 76.5% and a specificity of 100% [40].
What these discussed studies have in common is the inherent challenge in definitely allocating between LN measured by SWE and those examined pathologically. Pulappadi et al correlated the SWE velocities to the results of the biopsy only, while in the study by Tourasse et al marking on the patients’ skin, as well as the long and short axis diameter of the LN were considered for the paring process [32, 36]. Seo et al used only skin markings for the pairing process [40].
In general, based on the currently available evidence, the threshold velocity warranting a biopsy remains uncertain, given the limited and inconclusive data, ranging between 2.23 m/s and 3.0 m/s, and sensitivity and specificity ranging between 73.7% and 86.7% and 81.8% and 100%, respectively. In this study, a maximum velocity of 2.66 m/s measured in the LN tissue is proposed to adequately differentiate between malignant and benign axillary LN. Within this cut-off velocity, a sensitivity of 69.8% and a specificity of 87.5% can be achieved.
There are several limitations to consider. First, it must be noted that the selection of LNs is solely based on ultrasound criteria. It is further known that non-suspicious LNs may still contain malignant cells as seen in positive SLNBs [41]. It also must be noted that the results of this study only apply to female patients since male patients were excluded from the study cohort. Due to the limited sample size, an analysis of cancer subtypes has not been performed. A final limitation includes the presence of subjective observer bias, which is a common limitation in general ultrasound techniques.
In conclusion, additional SWE provides a quantifiable parameter for axillary staging in breast cancer patients. A recommended cutoff velocity of 2.66 m/s aids in distinguishing between benign and metastatic processes. However, further studies are essential to assess SWE’s efficacy in reducing biopsies in benign axillary LNs and decreasing positive SLNBs due to insufficient preoperative diagnostics.
Acknowledgements
The article was edited by Chi Ho.
Abbreviations
- ALND
Axillary lymph node dissection
- AUC
Area under the receiver operating characteristic curve
- IHC
Immunohistochemistry
- LN
Lymph node
- ROC
Receiver operating curve
- SD
Standard deviation
- SLNB
Sentinel lymph node biopsy
- SWE
Shear wave elastography
- VTIQ
Virtual touch tissue imaging quantification
Authors contributions
R.T. and F.R. analyzed and interpreted the patient data and were major contributors to writing the manuscript. M.F. analyzed the data and supervised the statistics. S.F., C.G., A.H., J.N., A.P., B.S., A.S., M.W., and J.H. contributed to patient recruitment. M.G. conceptualized and supervised the study. All authors read and approved the final manuscript.
Funding
The authors state that this work has not received any funding. Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate/for publication
The study protocol was approved by the ethics committee of Heidelberg University medical faculty and additional written informed consent including consent for publication was obtained from each participating patient (S-396/2019).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Riku Togawa and Fabian Riedel contributed equally to this work.
References
- 1.AGO (2021) AGO guidelines (S3-Leitlinie, 2021). Available via https://healthcare-quality.jrc.ec.europa.eu/ecibc/european-breast-cancer-guidelines. Accessed 04 Dec 2021
- 2.ECIBC (2021) European guidelines on breast cancer screening and diagnosis. Available via https://healthcare-quality.jrc.ec.europa.eu/ecibc/european-breast-cancer-guidelines. Accessed 04 Dec 2021
- 3.Riedel F, Hoffmann AS, Moderow M et al (2020) Time trends of neoadjuvant chemotherapy for early breast cancer. Int J Cancer. 10.1002/ijc.33122 [DOI] [PubMed]
- 4.Jamaris S, Jamaluddin J, Islam T et al (2021) Is pre-operative axillary ultrasound alone sufficient to determine need for axillary dissection in early breast cancer patients? Medicine (Baltimore) 100:e25412. 10.1097/md.0000000000025412 10.1097/md.0000000000025412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson JL, Wright GP (2022) Contemporary approaches to the axilla in breast cancer. Am J Surg. 10.1016/j.amjsurg.2022.11.036 [DOI] [PubMed]
- 6.Verbelen H, Gebruers N, Eeckhout FM et al (2014) Shoulder and arm morbidity in sentinel node-negative breast cancer patients: a systematic review. Breast Cancer Res Treat 144:21–31. 10.1007/s10549-014-2846-5 10.1007/s10549-014-2846-5 [DOI] [PubMed] [Google Scholar]
- 7.Kuijer A, Dominici LS, Rosenberg SM et al (2021) Arm morbidity after local therapy for young breast cancer patients. Ann Surg Oncol 28:6071–6082. 10.1245/s10434-021-09947-3 10.1245/s10434-021-09947-3 [DOI] [PubMed] [Google Scholar]
- 8.Riedel F, Schaefgen B, Sinn H-P et al (2021) Diagnostic accuracy of axillary staging by ultrasound in early breast cancer patients. Eur J Radiol. 135:109468 10.1016/j.ejrad.2020.109468 [DOI] [PubMed]
- 9.Harnett A, Smallwood J, Titshall V, Champion A (2009) Diagnosis and treatment of early breast cancer, including locally advanced disease-summary of NICE guidance. BMJ 338:b438. 10.1136/bmj.b438 10.1136/bmj.b438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kontos M, Kanavidis P, Kühn T et al (2024) Targeted axillary dissection: worldwide variations in clinical practice. Breast Cancer Res Treat 204:389–396. 10.1007/s10549-023-07204-7 10.1007/s10549-023-07204-7 [DOI] [PubMed] [Google Scholar]
- 11.Friedrich M, Kühn T, Janni W et al (2021) AGO recommendations for the surgical therapy of the axilla after neoadjuvant chemotherapy: 2021 update. Geburtshilfe Frauenheilkd 81:1112–1120. 10.1055/a-1499-8431 10.1055/a-1499-8431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann S, Kühn T, Hauptmann M et al (2022) Axillary staging after neoadjuvant chemotherapy for initially node-positive breast carcinoma in Germany: initial data from the AXSANA study. Geburtshilfe Frauenheilkd 82:932–940. 10.1055/a-1889-7883 10.1055/a-1889-7883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshimura G, Sakurai T, Oura S et al (1999) Evaluation of axillary lymph node status in breast cancer with MRI. Breast Cancer 6:249–258. 10.1007/bf02967179 10.1007/bf02967179 [DOI] [PubMed] [Google Scholar]
- 14.Farrell TP, Adams NC, Stenson M et al (2015) The Z0011 trial: Is this the end of axillary ultrasound in the pre-operative assessment of breast cancer patients? Eur Radiol 25:2682–2687. 10.1007/s00330-015-3683-6 10.1007/s00330-015-3683-6 [DOI] [PubMed] [Google Scholar]
- 15.Cho N, Moon WK, Han W et al (2009) Preoperative sonographic classification of axillary lymph nodes in patients with breast cancer: node-to-node correlation with surgical histology and sentinel node biopsy results. AJR Am J Roentgenol 193:1731–1737. 10.2214/ajr.09.3122 10.2214/ajr.09.3122 [DOI] [PubMed] [Google Scholar]
- 16.Yang WT, Chang J, Metreweli C (2000) Patients with breast cancer: differences in color Doppler flow and gray-scale US features of benign and malignant axillary lymph nodes. Radiology 215:568–573. 10.1148/radiology.215.2.r00ap20568 10.1148/radiology.215.2.r00ap20568 [DOI] [PubMed] [Google Scholar]
- 17.Na DG, Lim HK, Byun HS et al (1997) Differential diagnosis of cervical lymphadenopathy: usefulness of color Doppler sonography. AJR Am J Roentgenol 168:1311–1316. 10.2214/ajr.168.5.9129432 10.2214/ajr.168.5.9129432 [DOI] [PubMed] [Google Scholar]
- 18.Zhou J, Zhang B, Dong Y et al (2020) Value on the diagnosis of axillary lymph node metastasis in breast cancer by color Doppler ultrasound combined with computed tomography. J BUON 25:1784–1791 [PubMed] [Google Scholar]
- 19.Hotton J, Salleron J, Henrot P et al (2020) Pre-operative axillary ultrasound with fine-needle aspiration cytology performance and predictive factors of false negatives in axillary lymph node involvement in early breast cancer. Breast Cancer Res Treat 183:639–647. 10.1007/s10549-020-05830-z 10.1007/s10549-020-05830-z [DOI] [PubMed] [Google Scholar]
- 20.Marino MA, Avendano D, Zapata P et al (2020) Lymph node imaging in patients with primary breast cancer: concurrent diagnostic tools. Oncologist 25:e231–e242. 10.1634/theoncologist.2019-0427 10.1634/theoncologist.2019-0427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humphrey KL, Saksena MA, Freer PE et al (2014) To do or not to do: axillary nodal evaluation after ACOSOG Z0011 trial. Radiographics 34:1807–1816. 10.1148/rg.347130141 10.1148/rg.347130141 [DOI] [PubMed] [Google Scholar]
- 22.Rautiainen S, Masarwah A, Sudah M et al (2013) Axillary lymph node biopsy in newly diagnosed invasive breast cancer: comparative accuracy of fine-needle aspiration biopsy versus core-needle biopsy. Radiology 269:54–60. 10.1148/radiol.13122637 10.1148/radiol.13122637 [DOI] [PubMed] [Google Scholar]
- 23.Nakamura R, Yamamoto N, Miyaki T et al (2018) Impact of sentinel lymph node biopsy by ultrasound-guided core needle biopsy for patients with suspicious node positive breast cancer. Breast Cancer 25:86–93. 10.1007/s12282-017-0795-7 10.1007/s12282-017-0795-7 [DOI] [PubMed] [Google Scholar]
- 24.Golatta M, Pfob A, Büsch C et al (2022) The potential of combined shear wave and strain elastography to reduce unnecessary biopsies in breast cancer diagnostics—an international, multicentre trial. Eur J Cancer 161:1–9. 10.1016/j.ejca.2021.11.005 10.1016/j.ejca.2021.11.005 [DOI] [PubMed] [Google Scholar]
- 25.Golatta M, Pfob A, Büsch C et al (2021) The potential of shear wave elastography to reduce unnecessary biopsies in breast cancer diagnosis: an international, diagnostic, multicenter trial. Ultraschall Med. 10.1055/a-1543-615610.1055/a-1543-6156 [DOI] [PubMed]
- 26.Spak DA, Plaxco JS, Santiago L et al (2017) BI-RADS(®) fifth edition: a summary of changes. Diagn Interv Imaging 98:179–190. 10.1016/j.diii.2017.01.001 10.1016/j.diii.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 27.Yoo J, Seo BK, Park EK et al (2020) Tumor stiffness measured by shear wave elastography correlates with tumor hypoxia as well as histologic biomarkers in breast cancer. Cancer Imaging 20:85. 10.1186/s40644-020-00362-7 10.1186/s40644-020-00362-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plekhanov AA, Sirotkina MA, Sovetsky AA et al (2020) Histological validation of in vivo assessment of cancer tissue inhomogeneity and automated morphological segmentation enabled by optical coherence elastography. Sci Rep 10:11781. 10.1038/s41598-020-68631-w 10.1038/s41598-020-68631-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Togawa R, Binder LL, Feisst M et al (2022) Shear wave elastography as a supplemental tool in the assessment of unsuspicious axillary lymph nodes in patients undergoing breast ultrasound examination. Br J Radiol 95:20220372 10.1259/bjr.20220372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng WL, Omar N, Ab Mumin N et al (2021) Diagnostic accuracy of shear wave elastography as an adjunct tool in detecting axillary lymph nodes metastasis. Acad Radiol. 10.1016/j.acra.2021.03.01810.1016/j.acra.2021.03.018 [DOI] [PubMed]
- 31.Luo S, Yao G, Hong Z et al (2019) Qualitative classification of shear wave elastography for differential diagnosis between benign and metastatic axillary lymph nodes in breast cancer. Front Oncol 9:533. 10.3389/fonc.2019.00533 10.3389/fonc.2019.00533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pulappadi VP, Paul S, Hari S et al (2022) Role of shear wave elastography as an adjunct to axillary ultrasonography in predicting nodal metastasis in breast cancer patients with suspicious nodes. Br J Radiol 95:20220055. 10.1259/bjr.20220055 10.1259/bjr.20220055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng X, Yao Z, Huang Y et al (2020) Deep learning radiomics can predict axillary lymph node status in early-stage breast cancer. Nat Commun 11:1236. 10.1038/s41467-020-15027-z 10.1038/s41467-020-15027-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barr RG, Zhang Z (2015) Shear-wave elastography of the breast: value of a quality measure and comparison with strain elastography. Radiology 275:45–53. 10.1148/radiol.14132404 10.1148/radiol.14132404 [DOI] [PubMed] [Google Scholar]
- 35.Oncology guideline programme (German Cancer Society, German Cancer Aid, AWMF): S3 guideline Early detection, diagnosis, therapy and follow-up of breast cancer breast cancer, version 4.4, 2021, AWMF register number: 032-045OL, http://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom/ (retrieved on: 6th may 2023)
- 36.Tourasse C, Dénier JF, Awada A et al (2012) Elastography in the assessment of sentinel lymph nodes prior to dissection. Eur J Radiol 81:3154–3159. 10.1016/j.ejrad.2012.04.031 10.1016/j.ejrad.2012.04.031 [DOI] [PubMed] [Google Scholar]
- 37.Youk JH, Son EJ, Park AY, Kim JA (2014) Shear-wave elastography for breast masses: local shear wave speed (m/sec) versus young modulus (kPa). Ultrasonography 33:34–39. 10.14366/usg.13005 10.14366/usg.13005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sigrist RMS, Liau J, Kaffas AE et al (2017) Ultrasound elastography: review of techniques and clinical applications. Theranostics 7:1303–1329. 10.7150/thno.18650 10.7150/thno.18650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bae SJ, Park JT, Park AY et al (2018) Ex vivo shear-wave elastography of axillary lymph nodes to predict nodal metastasis in patients with primary breast cancer. J Breast Cancer 21:190–196. 10.4048/jbc.2018.21.2.190 10.4048/jbc.2018.21.2.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seo M, Sohn YM (2018) Differentiation of benign and metastatic axillary lymph nodes in breast cancer: additive value of shear wave elastography to B-mode ultrasound. Clin Imaging 50:258–263. 10.1016/j.clinimag.2018.04.013 10.1016/j.clinimag.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 41.Veronesi P, Corso G (2019) Standard and controversies in sentinel node in breast cancer patients. Breast 48:S53–S56. 10.1016/s0960-9776(19)31124-5 10.1016/s0960-9776(19)31124-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.