Abstract
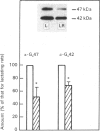
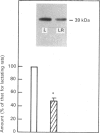
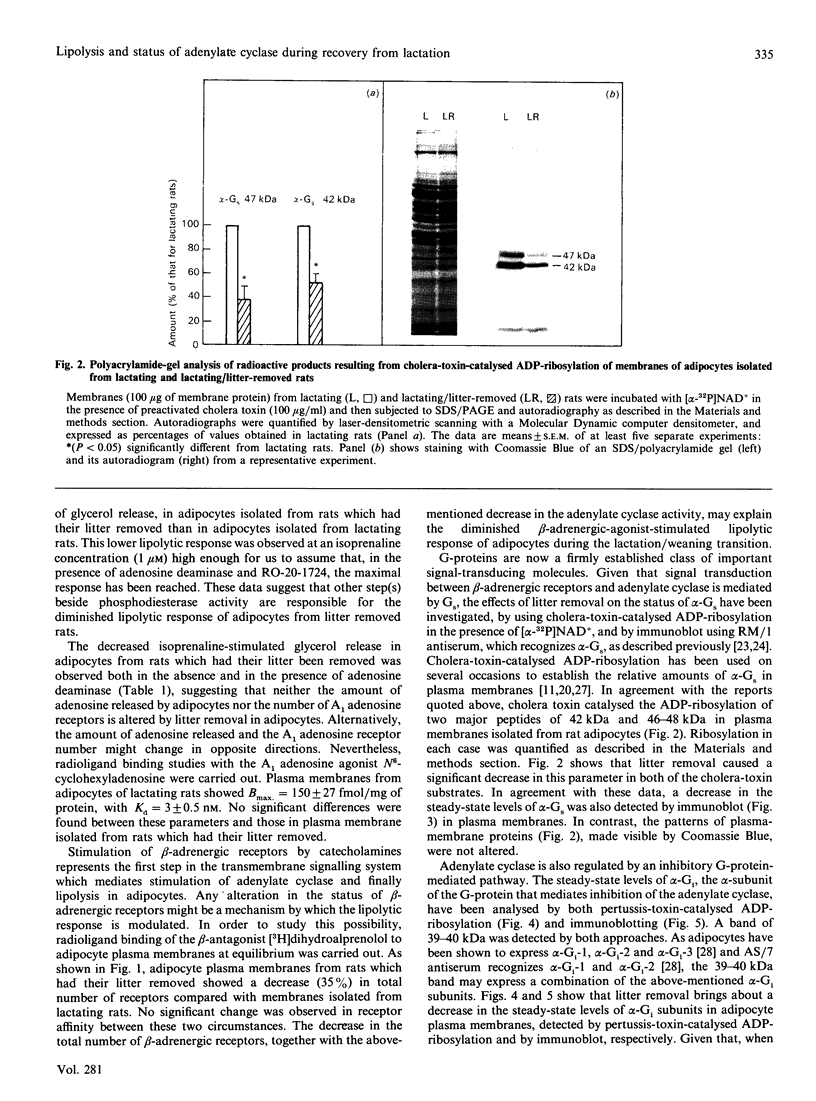
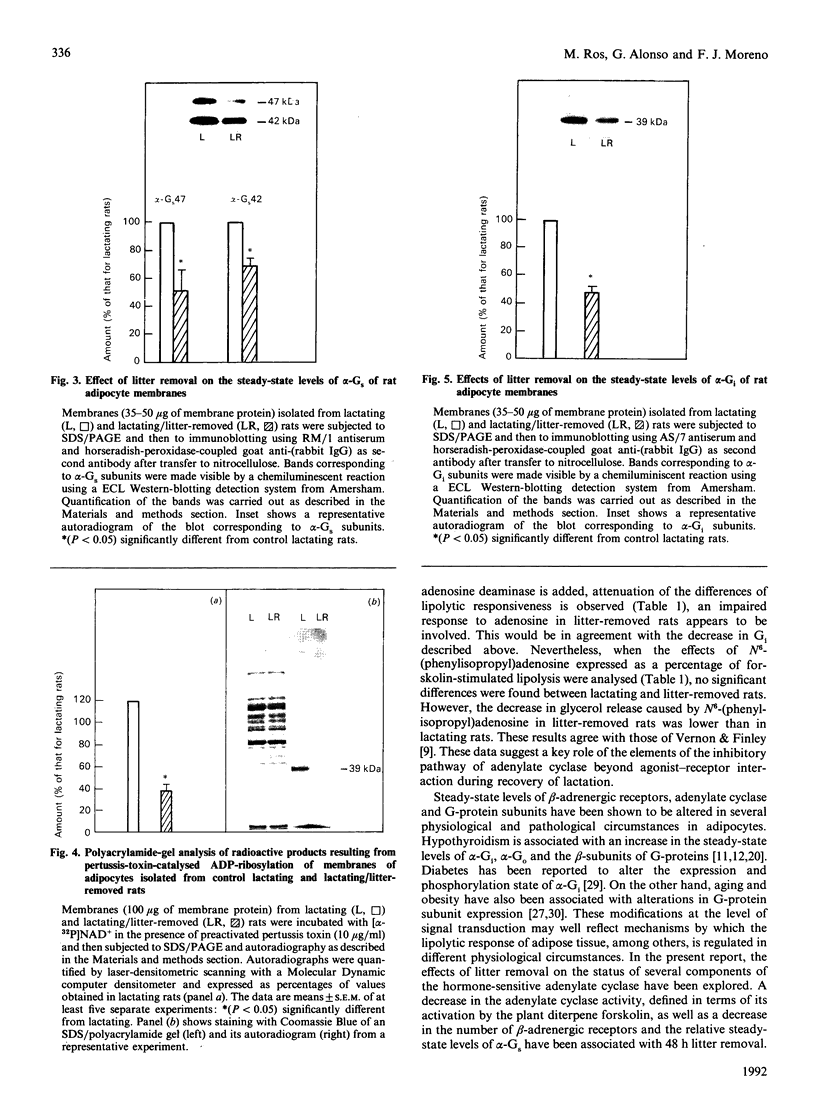
The effects of litter removal on the status of different components of the hormone-sensitive adenylate cyclase system were analysed in plasma membranes of rat adipocytes. These effects were correlated with the decreased lipolytic response of adipose tissue. No changes in total number of A1 adenosine receptors or their affinity were detected in response to litter removal. In contrast, beta-adrenergic receptors showed a decrease (35%) in total number of receptors, without any significant change in their affinity. The status of alpha-GS and alpha-Gi, the alpha-subunits of G proteins which mediate stimulation and inhibition respectively of adenylate cyclase, were probed by cholera- and pertussis-toxin-catalysed ADP-ribosylation respectively and by immunoblot. Associated with litter removal, decreases of 63% and 62% in the incorporation of [alpha 32P]ADP-ribose catalysed by cholera toxin and pertussis toxin into alpha-Gs and alpha-Gi respectively were detected. Immunoblotting using RM/1 (anti-alpha-Gs) and AS/7 (anti-alpha-Gi) antisera also showed decreases in the levels of alpha-Gs (52%) and alpha-Gi (55%) in adipocyte membranes from litter-removed rats compared with lactating rats. Alterations in the status of hormone-sensitive adenylate cyclase components, such as those described herein, may be biochemical mechanism(s) by which adipose tissue shows a decreased lipolytic response during recovery from lactation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agius L., Robinson A. M., Girard J. R., Williamson D. H. Alterations in the rate of lipogenesis in vivo in maternal liver and adipose tissue on premature weaning of lactating rats: a possible regulatory role of prolactin. Biochem J. 1979 Jun 15;180(3):689–692. doi: 10.1042/bj1800689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitchison R. E., Clegg R. A., Vernon R. G. Lipolysis in rat adipocytes during pregnancy and lactation. The response to noradrenaline. Biochem J. 1982 Jan 15;202(1):243–247. doi: 10.1042/bj2020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. F., Daly J. W., Snyder S. H. Adenosine receptor binding: structure-activity analysis generates extremely potent xanthine antagonists. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2077–2080. doi: 10.1073/pnas.80.7.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushfield M., Griffiths S. L., Murphy G. J., Pyne N. J., Knowler J. T., Milligan G., Parker P. J., Mollner S., Houslay M. D. Diabetes-induced alterations in the expression, functioning and phosphorylation state of the inhibitory guanine nucleotide regulatory protein Gi-2 in hepatocytes. Biochem J. 1990 Oct 15;271(2):365–372. doi: 10.1042/bj2710365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bégin-Heick N. Quantification of the alpha and beta subunits of the transducing elements (Gs and Gi) of adenylate cyclase in adipocyte membranes from lean and obese (ob/ob) mice. Biochem J. 1990 May 15;268(1):83–89. doi: 10.1042/bj2680083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubero A., Ros M., Lobato M. F., Carcía-Ruiz J. P., Moreno F. J. Coordination of glucose metabolism and NADPH formation in the adipose tissue and mammary gland during the lactation-weaning transition. Enzyme. 1983;30(1):38–47. doi: 10.1159/000469543. [DOI] [PubMed] [Google Scholar]
- Flint D. J., Sinnett-Smith P. A., Clegg R. A., Vernon R. G. Role of insulin receptors in the changing metabolism of adipose tissue during pregnancy and lactation in the rat. Biochem J. 1979 Aug 15;182(2):421–427. doi: 10.1042/bj1820421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawler D., Milligan G., Spiegel A. M., Unson C. G., Houslay M. D. Abolition of the expression of inhibitory guanine nucleotide regulatory protein Gi activity in diabetes. Nature. 1987 May 21;327(6119):229–232. doi: 10.1038/327229a0. [DOI] [PubMed] [Google Scholar]
- Goldsmith P., Gierschik P., Milligan G., Unson C. G., Vinitsky R., Malech H. L., Spiegel A. M. Antibodies directed against synthetic peptides distinguish between GTP-binding proteins in neutrophil and brain. J Biol Chem. 1987 Oct 25;262(30):14683–14688. [PubMed] [Google Scholar]
- Goldsmith P., Rossiter K., Carter A., Simonds W., Unson C. G., Vinitsky R., Spiegel A. M. Identification of the GTP-binding protein encoded by Gi3 complementary DNA. J Biol Chem. 1988 May 15;263(14):6476–6479. [PubMed] [Google Scholar]
- Gonzalez-Calero G., Martín M., Cubero A., Andrés A. Bovine brain coated vesicles contain adenosine A1 receptors. Presence of adenylate cyclase coupled to the receptor. J Neurochem. 1990 Jul;55(1):106–113. doi: 10.1111/j.1471-4159.1990.tb08827.x. [DOI] [PubMed] [Google Scholar]
- Green A., Johnson J. L. Evidence for altered expression of the GTP-dependent regulatory proteins, Gs and Gi, in adipocytes from aged rats. Biochem J. 1989 Mar 1;258(2):607–610. doi: 10.1042/bj2580607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malbon C. C., Li S., Fain J. N. Hormonal activation of glycogen phosphorylase in hepatocytes from hypothyroid rats. J Biol Chem. 1978 Dec 25;253(24):8820–8825. [PubMed] [Google Scholar]
- Malbon C. C., Rapiejko P. J., Mangano T. J. Fat cell adenylate cyclase system. Enhanced inhibition by adenosine and GTP in the hypothyroid rat. J Biol Chem. 1985 Feb 25;260(4):2558–2564. [PubMed] [Google Scholar]
- McKeel D. W., Jarett L. Preparation and characterization of a plasma membrane fraction from isolated fat cells. J Cell Biol. 1970 Feb;44(2):417–432. doi: 10.1083/jcb.44.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G., Spiegel A. M., Unson C. G., Saggerson E. D. Chemically induced hypothyroidism produces elevated amounts of the alpha subunit of the inhibitory guanine nucleotide binding protein (Gi) and the beta subunit common to all G-proteins. Biochem J. 1987 Oct 1;247(1):223–227. doi: 10.1042/bj2470223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno F. J., Mills I., García-Sáinz J. A., Fain J. N. Effects of pertussis toxin treatment on the metabolism of rat adipocytes. J Biol Chem. 1983 Sep 25;258(18):10938–10943. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rapiejko P. J., Malbon C. C. Short-term hyperthyroidism modulates adenosine receptors and catalytic activity of adenylate cyclase in adipocytes. Biochem J. 1987 Feb 1;241(3):765–771. doi: 10.1042/bj2410765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. M., Girard J. R., Williamson D. H. Evidence for a role of insulin in the regulation of lipogenesis in lactating rat mammary gland. Measurements of lipogenesis in vivo and plasma hormone concentrations in response to starvation and refeeding. Biochem J. 1978 Oct 15;176(1):343–346. doi: 10.1042/bj1760343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros M., Lobato M. F., García-Ruíz J. P., Moreno F. J. Integration of lipid metabolism in the mammary gland and adipose tissue by prolactin during lactation. Mol Cell Biochem. 1990 Mar 27;93(2):185–194. doi: 10.1007/BF00226191. [DOI] [PubMed] [Google Scholar]
- Ros M., Northup J. K., Malbon C. C. Adipocyte G-proteins and adenylate cyclase. Effects of adrenalectomy. Biochem J. 1989 Feb 1;257(3):737–744. doi: 10.1042/bj2570737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros M., Northup J. K., Malbon C. C. Steady-state levels of G-proteins and beta-adrenergic receptors in rat fat cells. Permissive effects of thyroid hormones. J Biol Chem. 1988 Mar 25;263(9):4362–4368. [PubMed] [Google Scholar]
- Simonds W. F., Goldsmith P. K., Codina J., Unson C. G., Spiegel A. M. Gi2 mediates alpha 2-adrenergic inhibition of adenylyl cyclase in platelet membranes: in situ identification with G alpha C-terminal antibodies. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7809–7813. doi: 10.1073/pnas.86.20.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds W. F., Goldsmith P. K., Woodard C. J., Unson C. G., Spiegel A. M. Receptor and effector interactions of Gs. Functional studies with antibodies to the alpha s carboxyl-terminal decapeptide. FEBS Lett. 1989 Jun 5;249(2):189–194. doi: 10.1016/0014-5793(89)80622-2. [DOI] [PubMed] [Google Scholar]
- Sinnett-Smith P. A., Vernon R. G., Mayer R. J. Lipogenic enzymes in rat maternal adipose tissue in the perinatal period. Biochem J. 1980 Mar 15;186(3):937–944. doi: 10.1042/bj1860937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon R. G., Finley E. Lipolysis in rat adipocytes during recovery from lactation. Response to noradrenaline and adenosine. Biochem J. 1986 Feb 15;234(1):229–231. doi: 10.1042/bj2340229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon R. G., Flint D. J. Control of fatty acid synthesis in lactation. Proc Nutr Soc. 1983 Jun;42(2):315–331. doi: 10.1079/pns19830035. [DOI] [PubMed] [Google Scholar]
- Williamson D. H. Integration of metabolism in tissues of the lactating rat. FEBS Lett. 1980 Aug 25;117 (Suppl):K93–105. doi: 10.1016/0014-5793(80)80574-6. [DOI] [PubMed] [Google Scholar]