Abstract
Background
Diabetes mellitus (DM) presents impediment to wound healing. While ultraviolet B (UVB) exposure showed therapeutic potential in various skin conditions, its capacity to mediate diabetic wound healing remains unclear. To investigate the efficacy of UVB on wound healing and its underlying basis.
Materials and Methods
Male C57BL/6 mice were subjected to the high‐fat diet followed by streptozotocin administration to establish the diabetic model. Upon confirmation of diabetes, full‐thickness wounds were inflicted and the treatment group received UVB radiation at 50 mJ/cm2 for 5 min every alternate day for 2 weeks. Wound healing rate was then assessed, accompanied by evaluations of blood glucose, lipid profiles, CD31 expression, and concentrations of ghrelin and leptin. Concurrently, in vitro studies were executed to evaluate the protective role of ghrelin on human umbilical vein endothelial cells (HUVEC) under high glucose (HG) conditions.
Results
Post UVB exposure, there was a marked acceleration in wound healing in DM mice without alterations in hyperglycemia and lipid profiles. Compared to non‐UVB‐exposed mice, the UVB group showed enhanced angiogenesis manifested by a surge in CD31 expression. This trend appeared to be in harmony with the elevated ghrelin levels. In vitro experiments indicated that ghrelin significantly enhanced the migratory pace and angiogenic properties of HUVEC under HG‐induced stress, potentially mediated by an upregulation in vascular endothelial growth factor expression.
Conclusion
UVB exposure bolstered wound healing in diabetic mice, plausibly mediated through augmented angiogenesis induced by ghrelin secretion. Such findings underscore the vast potential of UVB‐induced ghrelin in therapeutic strategies targeting diabetic wound healing.
Keywords: angiogenesis, diabetes, ghrelin, ultraviolet B exposure, wound healing
1. INTRODUCTION
Diabetes mellitus (DM) represents a rapidly escalating global public health crisis, with an estimated prevalence exceeding 400 million individuals worldwide. 1 This metabolic disorder frequently culminates in a myriad of complications, including impaired wound healing. 2 A sustained hyperglycemic milieu can detrimentally affect microvasculature, leading to microcirculatory disorders which diminish the delivery of oxygen and essential nutrients to the wound site. 3 Furthermore, the immunological responses of patients are compromised, predisposing them to infections which, in turn, delay wound repair. 4 The chronic high glucose environment can also inflict neuropathic damage, resulting in sensory deficits; thus, minor injuries or pressure ulcers may go unnoticed. 5 Additionally, it disrupts the synthesis and deposition of skin collagen, augments oxidative stress exacerbating cellular and tissue damage, and leads to the formation of advanced glycation end products which impair both cellular function and matrix proteins. 6 These intricate, interplaying pathophysiological mechanisms account for the wound healing deficits and chronic non‐healing wounds observed in diabetic individuals. Beyond the primary treatments for diabetes itself, such as insulin administration and lifestyle modifications, there is a fervent research focus on therapeutic interventions targeting wound healing in diabetic patients. 7 Various methods, including pharmaceuticals, antibiotics, stem cell therapies, and physical therapies, have shown promising results in wound healing. 7
In the realm of physical therapy, ultraviolet B radiation (UVB) has been employed as a therapeutic modality in dermatology since the last century, with particular efficacy in treating conditions such as psoriasis, eczema, and other skin ailments. 8 UVB exposure is conventionally perceived to attenuate skin inflammation and modulate its immunological responses. Consequently, this modulates the skin's barrier function and fosters the normal growth and differentiation of cutaneous cells. 9 In the text of direct clinical application of UVB in wound healing, low‐dose UVB irradiation might be conducive in suppressing excessive inflammation, stimulating angiogenesis, and promoting collagen synthesis, which were vital facets of wound repair. 10 For example, a previous study based on magnetic resonance data demonstrated that UV radiation produces similar anti‐inflammatory effects to mid‐potency topical steroids. 11 Recent studies have indicated the new development on the mechanism that UVB‐induced expansion of PENK+ skin Treg cells plays a pivotal role in maintaining skin homeostasis and facilitating endogenous wound healing, validating the potential of UVB to promote skin healing. 12 Separately, there have been reports on UVB exerting differential impacts on the levels and functions of extracellular microRNAs according to radiation energy, potentially influencing diabetic progression, 13 but there is a dearth of studies directly investigating the impact of UVB on diabetic skin wound healing in experimental conditions, and its underlying mechanisms remain to be elucidated.
Recent studies have elucidated a compelling phenomenon wherein ghrelin's p53 transcriptional activation in cutaneous adipocytes is observed both in murine models and in human males under ultraviolet exposure, modulating individual feeding behaviors, food intake, and body weight. 14 This reveals a potential correlation between ultraviolet exposure and ghrelin. Ghrelin, predominantly synthesized by the stomach, is a peptide hormone intricately involved in appetite regulation, energy homeostasis, and gastrointestinal motility. 15 Beyond its canonical roles, which were previously identified as effective regulators for diabetes, ghrelin, in recent years, has demonstrated potential in augmenting wound healing. 16 This hormone, characterized as a natural antibacterial and anti‐inflammatory peptide, is ubiquitously present across various tissues, with an especially abundant presence in physical barriers like the skin, offering innate immunological protection and bolstering the defense against infections. 17 Furthermore, in rodents with combined radiological and burn injuries, ghrelin has been discerned to expedite wound recovery via the regulation of GHS‐R1a‐mediated MAPK‐NF‐κB/GR signaling pathways. 18
In light of these findings, we postulate that UVB‐induced ghrelin might potentiate the recovery of diabetic skin wounds. This hypothesis was subjected to rigorous evaluation by assessing the impact of UVB on wound recovery metrics, angiogenic markers, and relevant hormone fluctuations in a diabetic mouse model. Concurrently, in vitro evaluations were conducted on human umbilical vein endothelial cells, offering a comprehensive perspective of the positive influence of UVB‐induced ghrelin on diabetic wound healing and its underlying basis.
2. MATERIALS AND METHODS
2.1. Ethics statement
All procedures involving animals were carried out in strict accordance with the guidelines provided by the International Council for Laboratory Animals. 19 Experimental protocols were pre‐approved by the Institutional Animal Care and Use Committee of Guangzhou Twelfth People's Hospital. Animals were housed under controlled environmental conditions (22°C ± 2°C, 60% ± 10% humidity, and 12 h light/dark cycle). All efforts were made to minimize animal suffering and they were euthanized by injecting pentobarbital sodium intravenously at the completion of the study.
2.2. Animal modeling and sample processing
A total of 18 male C57BL/6 mice were obtained from Guangdong Experimental Animal Center and randomly divided into three groups (n = 6 each) following a 1‐week acclimatization period: sham, DM, and DM + UVB. The DM and DM + UVB groups were subjected to a high‐fat diet, while the sham group received a normal diet. After 8 weeks of dietary intervention, the DM and DM + UVB groups were administered streptozotocin (STZ) injections for 5 consecutive days at a dose of 50 mg/kg/day, while the sham group received phosphate‐buffered saline (PBS) as control. For diabetic wound model establishment, all mice were anesthetized by the injection of pentobarbital sodium at a dose of 40 mg/kg intravenously prior to being shaved on their backs. Then, residual hair was removed using depilatory cream followed by sterile punch tools were employed to creating full‐thickness wounds with a diameter of 10 mm on the dorsum of the mice. Subsequently, mice in the DM + UVB group received UVB irradiation every other day for 2 consecutive weeks, with each session delivering 50 mJ/cm2 for 5 min, for therapeutic purposes.
At day 14 post‐treatment, blood was collected from the mice via orbital venous plexus puncture and transferred to serum separator tubes until clot formation. The tubes were centrifuged at 3000 rpm for 15 min to obtain serum, which were then stored at −80°C until further analysis. Afterwards, mice were euthanized by injecting 100 mg/kg of pentobarbital sodium intravenously and wound skin tissues were harvested using a scalpel and surgical scissors. A portion of the tissue was homogenized for subsequent molecular and protein assays, and the remaining tissue was immersed in 10% formalin for histochemical analysis.
2.3. Cell handling
The human umbilical vein endothelial cells (HUVECs; iCell‐h110) were purchased for iCell Research Institute (Bangkok, Thailand) and maintained in endothelial cell growth medium‐2 (EGM‐2) supplemented with the EGM‐2 BulletKit (Lonza Group Ltd, Basel, Switzerland) at 37°C with 5% CO₂. 20 For cellular experiment, HUVECs were subjected to three distinct treatments: (1) control; (2) high glucose (HG): d‐(+)‐glucose (Sigma‐Aldrich, MO, USA) was dissolved in the culture medium to achieve a final concentration of 33 mM; and (3) HG + ghrelin: HG‐treated cells were supplemented with 0.1 nM ghrelin (Abcam, Cambridge, UK). After finishing treatments, cells and supernatant were collected for further analysis.
2.4. Wound healing scratch assay
Following treatment, HUVECs were collected by centrifugation and cultured until they reached approximately 80% confluence in the six‐well plate. A consistent scratch was carefully introduced across the cell monolayer using a sterile pipette tip. Cells were then washed gently with PBS prior to fresh medium was added. The wound closure was monitored at 0, 6, and 12 h post‐scratching by capturing images using an Eclipse Ti2 Inverted Microscope (Nikon Corporation, Tokyo, Japan). The migration distance was quantified using ImageJ software (National Institutes of Health, NIH, MD, USA), considering the wound width's reduction over time.
2.5. Tube formation assay
Post‐treatment, the angiogenic potential of HUVECs was evaluated using a tube formation assay. 21 HUVECs were harvested and resuspended in a serum‐free medium. A total of 50 µL of Matrigel (Corning Inc., NY, USA) was placed into each well of a 96‐well plate for 1 h at 37°C. Subsequently, HUVECs were seeded at a density of 10 000 cells/well and incubated for 6–8 h at 37°C with 5% CO2. The formation of capillary‐like structures by cells was monitored and captured using an Eclipse Ti2 Inverted Microscope. The number of interconnected nodes was counted using ImageJ software and the angiogenic capacity was represented as the total branch points.
2.6. Biochemical and endocrine parameter detection
Biochemical and endocrine parameters were determined using commercially available enzyme‐linked immunosorbent assay (ELISA) kits. Serum glucose levels were determined using the Glucose Assay Kit (GAGO20, Sigma). The intensity of the pink color measured at 540 nm is proportional to the original glucose concentration. The concentrations of total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C), and high‐density lipoprotein cholesterol (HDL‐C) were assessed using corresponding assay kits (MyBioSource, CA, USA; MBS269999, MBS706909, MBS705286, respectively). The ghrelin concentration was assessed using the Mouse Ghrelin (GHRL) ELISA Kit (abx154080, Abbexa, Cambridge, UK) while the leptin level was determined with the Mouse Leptin ELISA Kit (PL696, Beyotime, Shanghai, China). The optical density of each well was detected within 5 min using a microplate reader (PR 3100 TSC, Bio‐Rad, Shanghai, China) set to 450 nm and compared with the standard curve to get the final concentration. All experiments were carried out according to the manufacturer's instructions.
To evaluate the secretion levels of Vascular Endothelial Growth Factor (VEGF) from treated HUVECs, cell culture supernatants were collected and centrifuged at 1500 rpm for 5 min to remove cell debris. Using the Mouse VEGF ELISA Kit (Pv957, Beyotime), the samples were processed as the manufacturer's protocol. Absorbance was then recorded at 450 nm.
2.7. Quantitative real‐time polymerase chain reaction (qPCR)
Total RNA from the wound skin tissues was isolated using the TRIzol Reagent (Invitrogen, CA, USA). 22 The concentration and purity of RNA were determined using the NanoDrop ND‐1000 Spectrophotometer (NanoDrop Technologies, DE, USA). Subsequently, 1 µg of total RNA from each sample was subjected to reverse transcription using the High‐Capacity cDNA Reverse Transcription Kit (Applied Biosystems, CA, USA) according to the manufacturer's instructions. For the qPCR analysis, the primer sequences for target genes were designed via Oligo 7 (Molecular Biology Insights, Inc., CO, USA) and listed as follows: CD31 (Forward: ACGCTGGTGCTCTATGCAAG; Reverse: TCAGTTGCTGCCCATTCATCA), ghrelin (Forward: CCAGAAAGCCCAGAGAAAGGAA; Reverse: GCCAACATCGAAGGGAGCAT), and leptin (Forward: GTGGCTTTGGTCCTATCTGTC; Reverse: CGTGTGTGAAATGTCATTGATCC). PCR reactions were set up using the SYBR Green PCR Master Mix (Applied Biosystems, CA, USA) and performed on the StepOnePlus Real‐Time PCR System (Applied Biosystems). The cycling conditions comprised an initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. The relative expression levels of the target genes were normalized to GAPDH (Forward: AGGTCGGTGTGAACGGATTTG; Reverse: TGTAGACCATGTAGTTGAGGTCA) and calculated using the 2−ΔΔCT method.
2.8. Western blot analysis
The proteins of wound skin tissues collected from mice or treated HUVECs were extracted using the RIPA Lysis Buffer (Thermo Fisher Scientific, MA, USA), supplemented with a protease and phosphatase inhibitor cocktail (Sigma‐Aldrich). The protein concentration was determined using the BCA Protein Assay Kit (Pierce Biotechnology, IL, USA). Equal amounts of proteins were then separated on a 10% SDS‐PAGE gel and transferred onto PVDF membranes (Millipore, MA, USA). After blocking for 1 h at room temperature, the membranes were incubated overnight at 4°C with the primary antibody against CD31 (AF6408), VEGF (AF1309). All primary antibodies were purchased from Beyotime (Shanghai, China) and diluted in 1: 1000 for use. The membranes were then washed three times and subsequently incubated with a horseradish peroxidase (HRP)‐conjugated secondary antibody (1:2000 dilution; AF0208, Beyotime) for 1 h at room temperature. After washing, protein bands were visualized using the ECL Western Blotting Detection System (Amersham Biosciences, Little Chalfont, UK) and images captured with the ChemiDoc Touch Imaging System (Bio‐Rad). GAPDH (AF1186, Beyotime) was often used as the loading control. The relative expression level of target proteins was calculated using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.9. Immunohistochemical (IHC) assay
Mouse wound skin tissues were excised and promptly fixed in 10% formalin (Sigma‐Aldrich) for 24 h for IHC assay. 23 Following fixation, tissues were embedded in paraffin blocks prior sections of 5 µm thickness were prepared. For antigen retrieval, the slides were immersed in citrate buffer (pH 6.0) and heated in a microwave for 10 min. Endogenous peroxidase activity was quenched by incubating sections in 3% hydrogen peroxide for 15 min. Following a rinse in PBS, 5% normal goat serum was used for blocking. Sections were then incubated overnight at 4°C with the CD31 Rabbit Polyclonal Antibody (1:100 dilution; AF6408, Beyotime) and the biotinylated secondary antibody (1:1000 dilution; AF0208, Beyotime) for 1 h at room temperature. Visualization was achieved using the Vectastain Elite ABC Kit (Vector Laboratories, CA, USA) followed by exposure to 3,3′‐diaminobenzidine (DAB; Sigma‐Aldrich). Finally, the sections were counterstained with hematoxylin, dehydrated in graded alcohols, cleared in xylene, and coverslipped with DPX mountant. All slides were examined under the DM4000B Microscope (Leica Microsystems, Wetzlar, Germany), and images were captured using the DFC295 Camera (Leica Microsystems).
2.10. Statistical analysis
Statistical evaluations and graphical representations were conducted using GraphPad Prism (Version 8.0; GraphPad Software Inc., CA, USA). Data were initially subjected to normality tests, followed by one‐way ANOVA for parametric data or Kruskal–Wallis test for non‐parametric data. Post hoc tests were utilized where applicable to discern specific group differences. For the assessment of the relationship between CD31 and the hormones ghrelin or leptin, Pearson's correlation analysis was conducted. Data were presented as means ± standard error of mean (SEM). A p‐value of less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Schematic representation of the diabetic wound model establishment and UVB treatment procedure
In this study, the diabetic model was established using 18 male C57BL/6 mice with 8‐week high‐fat diet followed by the administration of STZ for 5 consecutive days. Blood glucose levels were monitored 10 days post‐STZ injection, and mice with fasting blood glucose levels consistently exceeding 16.7 mM for 3 consecutive days were considered diabetic. After successful diabetes modeling for 2 weeks, a sterile punch tool was used to create full‐thickness wounds on the back. One day after that, mice in the DM + UVB group were received UVB irradiation every other day for 2 consecutive weeks before treatment effect observation (Figure 1).
FIGURE 1.
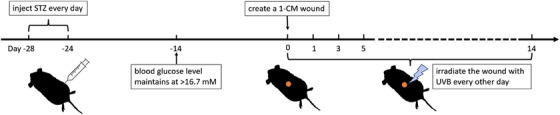
The diabetic wound model building and the treatment procedure with ultraviolet B (UVB). Male C57BL/6 mice were subjected to an 8‐week high‐fat diet, followed by a 5‐day consecutive administration of Streptozotocin. Ten days post‐injection, blood glucose levels were assessed. Post 2 weeks of successful diabetic modeling, full‐thickness wounds were created and mice in the diabetes mellitus with UVB treatment group were exposed to ultraviolet B irradiation on alternate days over a 2‐week course prior to observational assessments of treatment effects.
3.2. UVB exposure accelerates wound healing without anti‐hyperglycemic and anti‐hyperlipidemic effects in mice with diabetic wounds
To directly determine the effects of UVB exposure on wound healing in DM mice, wound healing progress was observed on days 0, 3, 7, and 14 (Figure 2A). Notably, starting from the day 3, discernible differences in wound closure rates among the groups became evident. Specifically, on days 3, 7, and 14 post‐injury, the DM group exhibited significantly lower wound closure levels compared to both the Sham group (p < 0.05) and the UVB treatment group (p < 0.001). On the day 14 post‐treatment, in serum of DM mice, it displayed significantly elevated blood glucose levels (Figure 2B), TC (Figure 2C), LDL‐C (Figure 2D), and decreased HDL‐C (Figure 2E) compared to the Sham control (p < 0.001). Intriguingly, UVB treatment, while promoting wound healing, did not exert statistically significant influence on these parameters (p > 0.05). These results demonstrate that UVB exposure accelerates the healing of diabetic wounds in mice, but UVB's beneficial impact on wound healing may operate independently of the anti‐hyperglycemic and anti‐hyperlipidemic effects.
FIGURE 2.
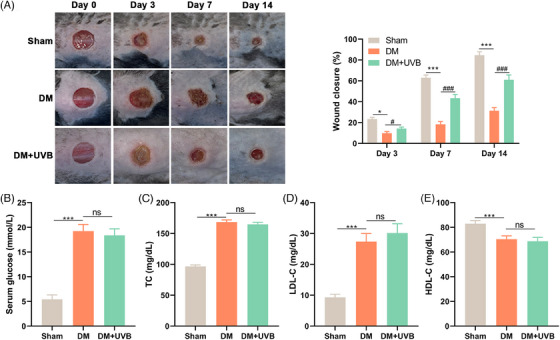
Ultraviolet B (UVB) exposure accelerates diabetic wound healing without anti‐hyperglycemic and anti‐hyperlipidemic effects. The wound healing progress in diabetes mellitus (DM) mice following UVB exposure was evaluated at intervals: days 0, 3, 7, and 14 (A). On day 14 post‐exposure, blood glucose levels (B), total cholesterol (C), low‐density lipoprotein cholesterol (D), and high‐density lipoprotein cholesterol (E) in serum of mice were determined by corresponding ELISA kits. Statistical analyses were conducted using [specific statistical method], with p‐values denoting significance. This collective data underscores that the therapeutic benefits of ultraviolet B exposure on diabetic wound repair might be distinct from its potential anti‐hyperglycemic and anti‐hyperlipidemic actions. Data were presented as mean ± SEM. ***p < 0.001; ###p < 0.001.
3.3. UVB exposure promotes angiogenesis and ghrelin secretion in mice with diabetic wounds
Wounded skin tissues and serum from the three mouse groups (sham, DM, DM + UVB) were harvested and analyzed. CD31 expression, indicative of angiogenesis, was examined using PCR (Figure 3A), Western blotting (Figure 3B), and IHC (Figure 3C), respectively. A consistent trend was observed across these tests. Specifically, a significant decrease in CD31 mRNA and protein levels in DM mice were illustrated compared to the sham group (p < 0.001). Notably, these diminished levels were significantly upregulated upon controlled UVB exposure (p < 0.001).
FIGURE 3.
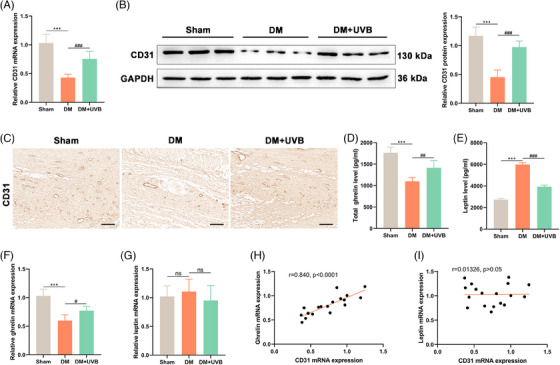
Effects of ultraviolet B (UVB) exposure on angiogenesis and hormone secretion in diabetic mice. On day 14 post‐treatments, CD31 expression in wounded skin tissues determined by PCR (A), Western blotting (B), and immunohistochemistry (C). Analysis of total ghrelin (D) and leptin levels (E) in serum were detected by ELISA. The mRNA of them (F and G) in skin tissues were measured by qPCR. Subsequently, the relationship between CD31 and ghrelin (H) or leptin (I) gene expressions were determined by Pearson's correlation analyses. Data were showed as mean ± SEM, with *** p < 0.001 and #p < 0.05, ##p < 0.01, ###p < 0.001.
Additionally, a pronounced reduction in total ghrelin levels in the serum (Figure 3D) and its mRNA in the skin tissues (Figure 3F) of DM mice compared to the sham controls (p < 0.001) was detected, but UVB treatment markedly elevated both measures (p < 0.05). Conversely, the leptin concentration, in the serum of DM mice (Figure 3E) was significantly elevated compared to the sham (p < 0.001), and this was effectively attenuated by UVB exposure (p < 0.001). In skin tissues, however, no significant difference in leptin mRNA levels was found across three groups (p > 0.05; Figure 3G). To further understand the association between gene expressions of CD31 and ghrelin (Figure 3H) or leptin (Figure 3I), the Pearson's correlation analyses were performed. A significant positive correlation was observed between CD31 and ghrelin (r = 0.840, p < 0.0001), suggesting a potential synergistic role in wound healing. However, no significant correlation was detected between CD31 and leptin mRNA levels (r = 0.01326, p > 0.05). These findings suggest that UVB exposure potentially promotes angiogenesis in diabetic wounds, and the alteration in ghrelin in response to UVB treatment might play crucial roles in wound healing in DM mice.
3.4. Ghrelin elicits cell migratory and angiogenic activities of human umbilical vein endothelial cells injured by high glucose
We further assessed the influence of ghrelin on HUVECs subjected to HG conditions in vitro. In the wound healing assay (Figure 4A), the HG group demonstrated a significantly diminished migration distance at 6 h post‐wounding in comparison to the control group (p < 0.001). Intriguingly, upon ghrelin administration, the HG + ghrelin cohort exhibited a pronounced enhancement in migration distance at 6 h relative to the HG group (p < 0.001). This trend persisted at the 12‐h mark post‐wounding across the experimental groups. In the tube formation assay (Figure 4B), there was a marked decrement in the total number of branch points in the HG group compared to the control. However, the introduction of ghrelin ameliorated this decline, as evidenced by a significant augmentation in branch points in the HG + ghrelin group relative to the HG‐treated cells (p < 0.05). To further substantiate ghrelin's role in promoting angiogenesis, we quantified VEGF protein expression and secretion levels. There was a significant downturn in VEGF relative protein expression in the HG group compared to the control (Figure 4C, p < 0.001), which was notably elevated by the treatment with ghrelin (p < 0.001). Consistently, results from the ELISA assay (Figure 4D) mirrored this trend, signifying that Ghrelin treatment markedly mitigates the reduction in VEGF secretion levels in HUVEC subjected to HG‐induced injury (p < 0.01). As such, elevated glucose concentrations appear to detrimentally influence HUVEC migration and angiogenesis via lowering VEGF expression. Notably, ghrelin confers a protective effect against these glucose‐mediated adversities, underscoring its potential therapeutic implications for post‐injury healing in diabetes contexts.
FIGURE 4.
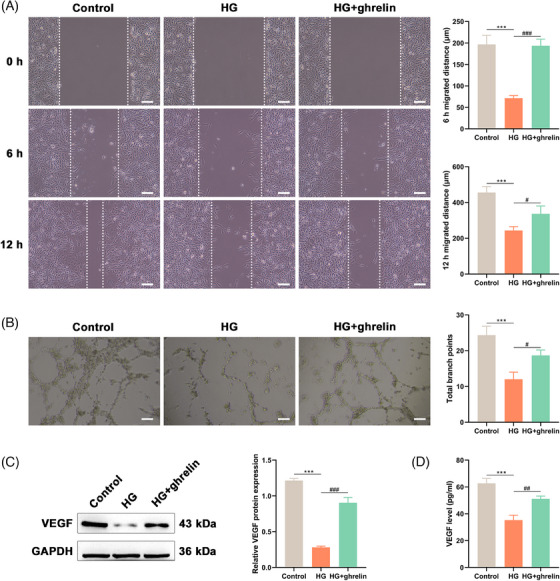
Ghrelin protects human umbilical vein endothelial cells (HUVECs) against high glucose‐induced injury on cell migratory and angiogenic activities. Cells were added with 33 mM glucose with or without ghrelin treatment. After treatment, wound healing assay (A) showcasing the migration distance of HUVECs at 6 and 12 h post‐wounding. Tube formation assay (B) were conducted to highlight the number of branch points of treated cells. The protein expression (C) and secreted level (D) of vascular endothelial growth factor were determined by the western blot analysis and ELISA, respectively. Data were presented as mean ± SEM. *** p < 0.001; #p < 0.05, ##p < 0.01, and ###p < 0.001.
4. DISCUSSION
DM can precipitate a myriad of organ complications, such as impediments in wound healing, augmented susceptibility to infections, and consequent complications 2 . Physical therapy emerges as a non‐pharmacological intervention, enhancing the pace and efficacy of wound recovery and UVB radiation among which has been extensively utilized in clinical settings to promote dermal health via modulation of immune responses and attenuation of inflammation. 24 Our research delineates the pivotal role of ghrelin, induced under UVB exposure, in ameliorating diabetic wound healing. Notably, this enhancement is mediated through the promotion of angiogenesis, without manifest alterations in glucose or lipid profiles.
Recently, the European Union launched an initiative to use skin models instead of animal testing. For example, Li et al. proposed a framework for dynamically calculating and visualizing UVB doses on the skin. 25 However, since DM is a systemic disease, using only skin models will not be conducive to our understanding of the mechanism of action of UVB in diabetic wound healing. Thus, we established the murine model of DM via a high‐fat diet and STZ injection, which manifested quintessential diabetic attributes, as corroborated by prior studies. 26 These included hyperglycemia, elevated total TC levels, heightened LDL levels, and diminished HDL levels. Subsequent to wound induction, the model presented conspicuous delays in wound healing. However, subjecting these mice to a fortnightly regimen of daily UVB exposure at a dose of 50 mJ/cm2 for a duration of 5 min each proved beneficial in augmenting diabetic wound healing, as evidenced by a marked disparity in wound closure rates between UVB‐exposed and non‐exposed groups. UVB, a fraction of the solar radiation spectrum, has a wavelength ranging from 280 to 320 nanometers. Notwithstanding UVB's plethora of benefits, injudicious or excessive exposure can inflict dermatological damage and escalate the risk of skin carcinomas. 27 Considering the findings wherein narrow‐band UVB irradiation administered every other day, accumulating a dosage of 2000mJ/cm2, has been demonstrated to modulate the expression of inflammatory factors and alleviate psoriatic plaques in a mouse model of psoriasis, 28 and the observation that daily exposure of 10 min for 5 days a week, over a span of 3 weeks, with each session delivering approximately 308 mJ/cm2 of UVB irradiation, could effectively enhance the condition of mice with vitamin D synthesis disorders, 29 each specific model and disease context may warrant its distinct therapeutic irradiation dosage to ensure safety and efficacy. As UVB utilization mandates judicious discretion on dosage and time of use, the efficacious dosage of UVB without observable conspicuous adverse effects found in the present study could provide a reference for other basic experiments focused on treating diabetic wounds in murine models.
Elevated glucose levels have been demonstrated to impede wound healing via several mechanisms, encompassing HG‐induce tissue damage, persistent inflammation, attenuation of leukocyte functionality leading to heightened susceptibility to infections, and alterations in the structural and functional integrity of connective tissues. 30 , 31 , 32 , 33 Concurrently, dyslipidemia, commonly associated with diabetic patients, not only induces inflammation and weakens the immune response akin to hyperglycemia but may also promote atherosclerosis and plaque formation. 34 This results in ischemic conditions for the wound, characterized by inadequate blood and oxygen supply, thereby adversely affecting the rate of wound healing. 34 It is indisputable that regulating the hyperglycemic and hyperlipidemic states in diabetic individuals can ameliorate complications related to wound healing. Interestingly, while UVB radiation did enhance the wound healing rate in diabetic mice, we did not observe any significant anti‐hyperglycemic or anti‐hyperlipidemic effects of UVB. This observation raises a pivotal question: Does UVB possess alternative mechanisms mediating wound healing that operate independently of the primary symptoms associated with DM?
Ghrelin and leptin are pivotal endocrine hormones intricately linked to appetite regulation, body weight, lipid storage, and metabolic processes. Ghrelin functions to stimulate appetite, reduce energy expenditure, and promote fat accumulation. In contrast, elevated leptin secretion diminishes appetite and amplifies energy expenditure. 35 Consistent with previous research, 36 DM mice demonstrated notably decreased ghrelin levels and elevated leptin concentrations, a trend that was reversed upon exposure to UVB. This observation provides an insight, suggesting an alternative mechanism by which UVB exposure facilitates wound healing, beyond its glucose and lipid‐lowering effects. Analogously, earlier investigations have recognized ghrelin as a multifunctional peptide, involved in diverse physiological processes, including wound healing. 16 Concurrently, our data, corroborated through multiple methodologies, accentuates the promotional effect of UVB exposure on CD31 mRNA and protein expression, consistent with prior studies. 37 , 38 Angiogenesis, the formation of new blood vessels, is fundamental to wound healing, serving as the conduit for essential oxygen and nutrients to rejuvenating tissues and CD31 is frequently utilized as an angiogenic marker, indicating neovascularization. 39 Beyond this, CD31 plays an instrumental role in intercellular adhesion, signal transduction, and augmenting cellular permeability—factors paramount to endothelial cell interactions and new vessel formation. 40 The observed significant positive correlation between CD31 and ghrelin in this study fortifies the hypothesis that an increase in ghrelin in response to UVB exposure potentially aids wound healing in DM mice via promoting angiogenesis. This also aligns with preceding findings where UVB exposure was confirmed to bolster angiogenesis. 37 Intriguingly, while serum leptin concentrations were markedly diminished following UVB exposure, its mRNA levels in the skin tissues across the groups remained relatively unchanged and bore no evident correlation with CD31. Given prior studies attesting to leptin's role in angiogenesis, 35 further investigations are warranted to elucidate whether leptin acts via signaling factors independent of CD31, if Leptin is subject to negative feedback from ghrelin, and the potential influence of UVB exposure dosages on Leptin expression.
Subsequent in vitro analyses corroborated that ghrelin treatment can directly ameliorate the diminished cell migration and impaired angiogenic capabilities of HUVEC under HG conditions. This mitigation can be, at least in part, attributed to the upregulated expression of Vascular Endothelial Growth Factor (VEGF) induced by ghrelin, which is a pivotal signaling protein associated with vascular formation and maturation. 41 It holds the potential to stimulate endothelial cell proliferation and enhance vascular permeability, thus allowing plasma and proteins to permeate into tissues, facilitating the formation of new blood vessels. 42 A previous study has elucidated the capacity of ghrelin to foster cardiac protection and repair through the VEGF‐related blood vessel formation. 43 Our findings, similarly, represent the inaugural evidence that ghrelin can promote angiogenesis and damage repair in HUVEC under HG conditions via the secretion of VEGF. However, our findings are primarily based on a murine model, which may not fully replicate the human physiological and pathophysiological conditions. Therefore, translational potential in humans remains to be verified. And, the long‐term consequences, both positive and adverse, of UVB exposure and increased ghrelin levels in diabetic individuals require further investigation before putting it into application.
5. CONCLUSION
In conclusion, this study elucidated the instrumental role of UVB exposure in enhancing diabetic wound healing, underpinned primarily by the induction of ghrelin. While no significant alterations in glucose or lipid profiles were observed post‐UVB treatment, a pronounced enhancement in wound healing was evident. This was seemingly contributed to the promoting angiogenesis effect induced by ghrelin, underscored by elevated CD31 expression and VEGF secretion. These results presented herein not only offer valuable insights into the multifaceted mechanisms by which UVB radiation promotes wound healing in DM mice but also lay the groundwork for future investigations aimed at optimizing therapeutic UVB applications for diabetic wound management.
CONFLICT OF INTEREST STATEMENT
The authors of this work have nothing to disclose.
ACKNOWLEDGMENT
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
Fu QR, Peng S, Zhu CQ, Chen LS, Sun Y, Li WM. Ghrelin induced by ultraviolet B exposure promotes the restoration of diabetic cutaneous wound healing. Skin Res Technol. 2024;30:e13919. 10.1111/srt.13919
Qi‐Rui Fu and Sha Peng contributed equally to this work and should be considered as equal first coauthors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Lu M, Pilla S, Oh S. Diabetes mellitus: dietary management. Encyclopedia of Human Nutrition (Volume 1–4). 4th ed. Elsevier; 2023:234‐251. [Google Scholar]
- 2. Burgess JL, Wyant WA, Abdo Abujamra B, Kirsner RS, Jozic I. Diabetic wound‐healing science. Medicina. 2021;57(10):1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharma S, Schaper N, Rayman G. Microangiopathy: is it relevant to wound healing in diabetic foot disease? Diabetes/Metab Res Rev. 2020;36:e3244. [DOI] [PubMed] [Google Scholar]
- 4. Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, Saparov A. Immunology of acute and chronic wound healing. Biomolecules. 2021;11(5):700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fan L, Xiao C, Guan P, et al. Extracellular matrix‐based conductive interpenetrating network hydrogels with enhanced neurovascular regeneration properties for diabetic wounds repair. Adv Healthcare Mater. 2022;11(1):2101556. [DOI] [PubMed] [Google Scholar]
- 6. Wan L, Bai X, Zhou Q, et al. The advanced glycation end‐products (AGEs)/ROS/NLRP3 inflammasome axis contributes to delayed diabetic corneal wound healing and nerve regeneration. Int J Biol Sci. 2022;18(2):809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel S, Srivastava S, Singh MR, Singh D. Mechanistic insight into diabetic wounds: pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother. 2019;112:108615. [DOI] [PubMed] [Google Scholar]
- 8. Krutmann J, Morita A. Mechanisms of Ultraviolet (UV) B and UVA Phototherapy. Elsevier; 1999:70‐72. [DOI] [PubMed] [Google Scholar]
- 9. Byrne SN, Beaugie C, O'Sullivan C, Leighton S, Halliday GM. The immune‐modulating cytokine and endogenous Alarmin interleukin‐33 is upregulated in skin exposed to inflammatory UVB radiation. Am J Pathol. 2011;179(1):211‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varricchi G, Granata F, Loffredo S, Genovese A, Marone G. Angiogenesis and lymphangiogenesis in inflammatory skin disorders. J Am Acad Dermatol. 2015;73(1):144‐153. [DOI] [PubMed] [Google Scholar]
- 11. Zemtsov A. Measurement of suberythema UVA radiation effects on skin by 31P magnetic resonance spectroscopy. Photodermatol Photoimmunol Photomed. 1997;13(1‐2):24‐26. doi: 10.1111/j.1600-0781.1997.tb00104.x [DOI] [PubMed] [Google Scholar]
- 12. Shime H, Odanaka M, Tsuiji M, et al. Proenkephalin+ regulatory T cells expanded by ultraviolet B exposure maintain skin homeostasis with a healing function. Proc Natl Acad Sci. 2020;117(34):20696‐20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang J, Pothana K, Chen S, et al. Ultraviolet B irradiation alters the level and miR contents of exosomes released by keratinocytes in diabetic condition. Photochem Photobiol. 2022;98(5):1122‐1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parikh S, Parikh R, Michael K, et al. Food‐seeking behavior is triggered by skin ultraviolet exposure in males. Nat Metab. 2022;4(7):883‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiao Z‐T, Luo Q. Molecular mechanisms and health benefits of ghrelin: a narrative review. Nutrients. 2022;14(19):4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akalu Y, Molla MD, Dessie G, Ayelign B. Physiological effect of ghrelin on body systems. Int J Endocrinol.1‐26, 2020;2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang W‐L, Ma G, Zhou M, et al. Combined administration of human ghrelin and human growth hormone attenuates organ injury and improves survival in aged septic rats. Mol Med. 2016;22(1):124‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu C, Huang J, Li H, et al. Ghrelin accelerates wound healing through GHS‐R1a‐mediated MAPK‐NF‐κB/GR signaling pathways in combined radiation and burn injury in rats. Sci Rep. 2016;6(1):27499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vergara P, Pekow C. Laboratory Animal Science and Service Organizations. Handbook of Laboratory Animal Science. CRC Press; 2021:923‐936. [Google Scholar]
- 20. Chen Z, Htay A, Santos WD, et al. In vitro angiogenesis by human umbilical vein endothelial cells (HUVEC) induced by three‐dimensional co‐culture with glioblastoma cells. J Neuro‐oncol. 2009;92:121‐128. [DOI] [PubMed] [Google Scholar]
- 21. Gentile MT, Pastorino O, Bifulco M, Colucci‐D'Amato L. HUVEC tube‐formation assay to evaluate the impact of natural products on angiogenesis. JoVE (J Vis Exp). 2019;(148):e58591. [DOI] [PubMed] [Google Scholar]
- 22. Turabelidze A, Guo S, DiPietro LA. Importance of housekeeping gene selection for accurate reverse transcription‐quantitative polymerase chain reaction in a wound healing model. Wound Repair Regen. 2010;18(5):460‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shukla A, Choudhury S, Chaudhary G, et al. Chitosan and gelatin biopolymer supplemented with mesenchymal stem cells (Velgraft®) enhanced wound healing in goats (Capra hircus): involvement of VEGF, TGF and CD31. J Tissue Viabil. 2021;30(1):59‐66. [DOI] [PubMed] [Google Scholar]
- 24. Myers E, Kheradmand S, Miller R. An update on narrowband ultraviolet B therapy for the treatment of skin diseases. Cureus. 2021;13(11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Z, Kim MA, Kim E, Jung YC, Kim JJ, Shin HS. Dynamic visualization of ultraviolet dose on skin with sunscreen applied using minimum erythema dose. Skin Res Technol. 2022;28(4):614‐622. doi: 10.1111/srt.13176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu Y, Zhu C, Yang H, Deng J, Fan D. Protective effect of ginsenoside Rg5 against kidney injury via inhibition of NLRP3 inflammasome activation and the MAPK signaling pathway in high‐fat diet/streptozotocin‐induced diabetic mice. Pharmacol Res. 2020;155:104746. [DOI] [PubMed] [Google Scholar]
- 27. Bahamondes Lorca VA, McCulloch MK, Ávalos‐Ovando Ó, Govorov AO, Rahman F, Wu S. Characterization of UVB and UVA‐340 lamps and determination of their effects on ER stress and DNA damage. Photochem Photobiol. 2022;98(5):1140‐1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ye J, Huang H, Luo G, et al. NB‐UVB irradiation attenuates inflammatory response in psoriasis. Dermatol Ther. 2020;33(4):e13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin M‐Y, Lim LM, Tsai S‐P, et al. Low dose ultraviolet B irradiation at 308 nm with light‐emitting diode device effectively increases serum levels of 25 (OH) D. Sci Rep. 2021;11(1):2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deng L, Du C, Song P, et al. The role of oxidative stress and antioxidants in diabetic wound healing. Oxid Med Cell Long. 1‐11, 2021;2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dong R, Guo B. Smart wound dressings for wound healing. Nano Today. 2021;41:101290. [Google Scholar]
- 32. Simpson DM, Ross R. The neutrophilic leukocyte in wound repair: a study with antineutrophil serum. J Clin Invest. 1972;51(8):2009‐2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Argyropoulos AJ, Robichaud P, Balimunkwe RM, et al. Alterations of dermal connective tissue collagen in diabetes: molecular basis of aged‐appearing skin. PloS One. 2016;11(4):e0153806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deora N, Venkatraman K. Aloe vera in diabetic dyslipidemia: improving blood glucose and lipoprotein levels in pre‐clinical and clinical studies. J Ayurv Integr Med. 2022;13(4):100675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kroemer G, Zitvogel L. Ghrelin and leptin regulating wound healing. Trends Immunol. 777‐779, 2022. [DOI] [PubMed] [Google Scholar]
- 36. Hekim MG, Kelestemur MM, Bulmus FG, et al. Asprosin, a novel glucogenic adipokine: a potential therapeutic implication in diabetes mellitus. Arch Physiol Biochem. 2021:1‐7. [DOI] [PubMed] [Google Scholar]
- 37. Kim KM, Im A‐R, Lee JY, et al. Hesperidin inhibits UVB‐induced VEGF production and angiogenesis via the inhibition of PI3K/Akt pathway in HR‐1 hairless mice. Biol Pharm Bull. 2021;44(10):1492‐1498. [DOI] [PubMed] [Google Scholar]
- 38. Lu K, Bhat M, Peters S, et al. Dopamine prevents ultraviolet B–induced development and progression of premalignant cutaneous lesions through its D2 receptors. Cancer Prevent Res. 2021;14(7):687‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu J, Yan Z, Yang F, et al. Exosomes derived from human umbilical cord mesenchymal stem cells accelerate cutaneous wound healing by enhancing angiogenesis through delivering angiopoietin‐2. Stem Cell Rev Rep. 2021;17:305‐317. [DOI] [PubMed] [Google Scholar]
- 40. Naito H, Iba T, Takakura N. Mechanisms of new blood‐vessel formation and proliferative heterogeneity of endothelial cells. Int Immunol. 2020;32(5):295‐305. [DOI] [PubMed] [Google Scholar]
- 41. Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006;12(17):5018‐5022. [DOI] [PubMed] [Google Scholar]
- 42. Weis SM, Cheresh DA. Pathophysiological consequences of VEGF‐induced vascular permeability. Nature. 2005;437(7058):497‐504. [DOI] [PubMed] [Google Scholar]
- 43. Carmeliet P, Collen D. Molecular basis of angiogenesis: role of VEGF and VE‐cadherin. Ann N Y Acad Sci. 2000;902(1):249‐264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


