Abstract
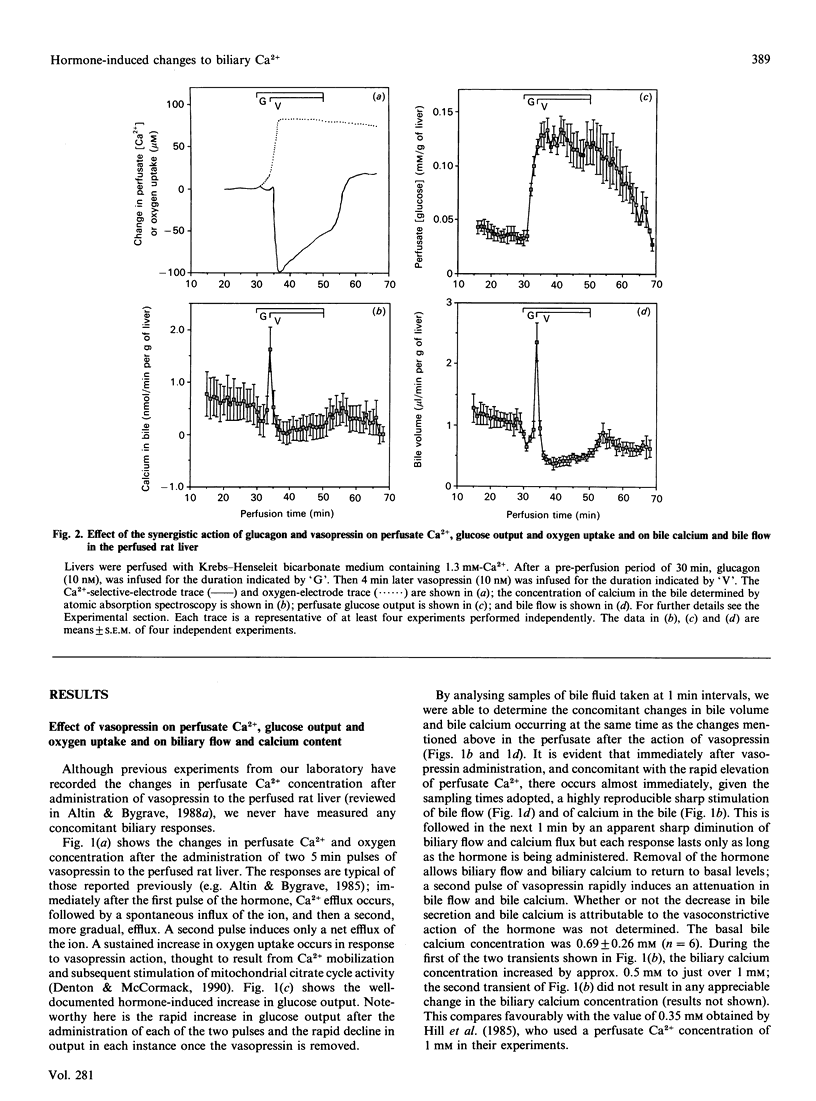
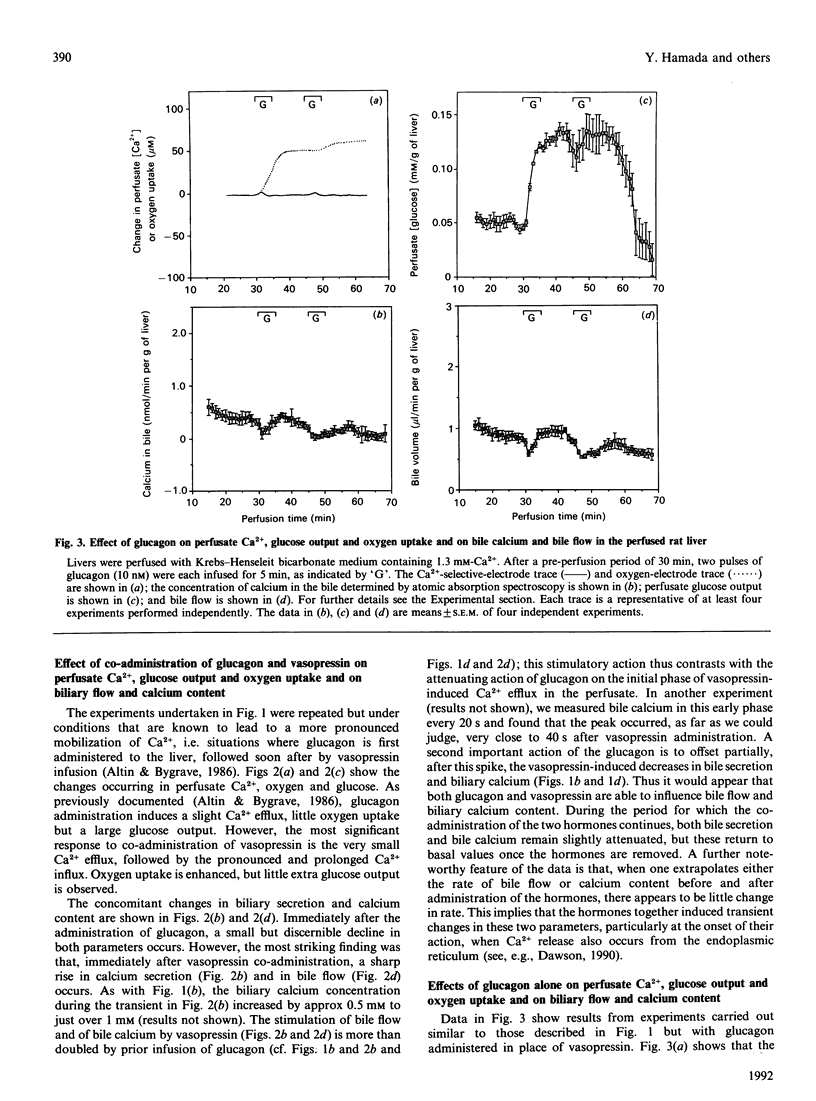
Changes in perfusate Ca2+ (measured with a Ca(2+)-selective electrode) and changes in bile calcium (measured by atomic absorption spectroscopy) were continuously and simultaneously monitored after infusion of (a) vasopressin, (b) glucagon and (c) both vasopressin and glucagon together to the perfused rat liver. Also monitored were perfusate glucose and oxygen concentrations and bile flow. Vasopressin induces a sharp, transient, pulse of increased bile flow and increased bile calcium within 1 min of infusion, concomitant with rapid changes in perfusate Ca2+ fluxes, glucose output and oxygen uptake. This is immediately followed by a decrease in both bile flow and bile calcium for as long as the hormone is administered. Changes induced by glucagon are a relatively slow onset of perfusate Ca2+ efflux and oxygen uptake, but rapid glucose output, and a small but significant and transient decrease in bile flow and bile calcium which, despite the continued infusion of the hormone, spontaneously and rapidly returns to normality. However, the greatest responses are observed after co-administration of both hormones. Coincident with the augmented perfusate Ca2+ fluxes (influx) seen in earlier work, there occurs within 1 min of vasopressin infusion a sharp increase in bile secretion and bile calcium greater in magnitude than that produced by vasopressin alone. Immediately thereafter bile secretion and bile calcium decline below basal values and remain there for as long as the hormones are administered. Glucagon and vasopressin therefore each have opposing effects on bile flow and bile calcium. However, the action of vasopressin is enhanced by the prior administration of glucagon. The data thus reveal features about the actions of glucagon and Ca(2+)-mobilizing hormones on bile flow and bile calcium not previously recorded and provide a novel framework around which the whole issue of hepato-biliary Ca2+ homoeostasis can be assessed in normal and diseased liver.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altin J. G., Bygrave F. L. Non-parenchymal cells as mediators of physiological responses in liver. Mol Cell Biochem. 1988 Sep;83(1):3–14. doi: 10.1007/BF00223193. [DOI] [PubMed] [Google Scholar]
- Altin J. G., Bygrave F. L. Second messengers and the regulation of Ca2+ fluxes by Ca2+-mobilizing agonists in rat liver. Biol Rev Camb Philos Soc. 1988 Nov;63(4):551–611. doi: 10.1111/j.1469-185x.1988.tb00670.x. [DOI] [PubMed] [Google Scholar]
- Altin J. G., Bygrave F. L. Synergistic stimulation of Ca2+ uptake by glucagon and Ca2+-mobilizing hormones in the perfused rat liver. A role for mitochondria in long-term Ca2+ homoeostasis. Biochem J. 1986 Sep 15;238(3):653–661. doi: 10.1042/bj2380653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altin J. G., Bygrave F. L. The Ca2+-mobilizing actions of vasopressin and angiotensin differ from those of the alpha-adrenergic agonist phenylephrine in the perfused rat liver. Biochem J. 1985 Dec 15;232(3):911–917. doi: 10.1042/bj2320911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Dawson A. P. Regulation of intracellular Ca2+. Essays Biochem. 1990;25:1–37. [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ as a second messenger within mitochondria of the heart and other tissues. Annu Rev Physiol. 1990;52:451–466. doi: 10.1146/annurev.ph.52.030190.002315. [DOI] [PubMed] [Google Scholar]
- Exton J. H. The roles of calcium and phosphoinositides in the mechanisms of alpha 1-adrenergic and other agonists. Rev Physiol Biochem Pharmacol. 1988;111:117–224. doi: 10.1007/BFb0033873. [DOI] [PubMed] [Google Scholar]
- Hill C. E., Dawson A. P., Pryor J. S. Evidence for adrenergic control of transcellular calcium distribution in liver. Biochem J. 1985 Sep 15;230(3):733–737. doi: 10.1042/bj2300733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan K. S., Coleman R. The calcium ionophore A23187 increases the tight-junctional permeability in rat liver. Biochem J. 1988 Dec 15;256(3):1039–1041. doi: 10.1042/bj2561039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus-Friedmann N. Calcium sequestration in the liver. Cell Calcium. 1990 Nov-Dec;11(10):625–640. doi: 10.1016/0143-4160(90)90017-o. [DOI] [PubMed] [Google Scholar]
- Krell H., Jaeschke H., Pfaff E. Regulation of canalicular bile formation by alpha-adrenergic action and by external ATP in the isolated perfused rat liver. Biochem Biophys Res Commun. 1985 Aug 30;131(1):139–145. doi: 10.1016/0006-291x(85)91781-4. [DOI] [PubMed] [Google Scholar]
- Llopis J., Kass G. E., Duddy S. K., Farell G. C., Gahm A., Orrenius S. Mobilization of the hormone-sensitive calcium pool increases hepatocyte tight junctional permeability in the perfused rat liver. FEBS Lett. 1991 Mar 11;280(1):84–86. doi: 10.1016/0014-5793(91)80209-l. [DOI] [PubMed] [Google Scholar]
- Lowe P. J., Miyai K., Steinbach J. H., Hardison W. G. Hormonal regulation of hepatocyte tight junctional permeability. Am J Physiol. 1988 Oct;255(4 Pt 1):G454–G461. doi: 10.1152/ajpgi.1988.255.4.G454. [DOI] [PubMed] [Google Scholar]
- Mallat A., Pavoine C., Dufour M., Lotersztajn S., Bataille D., Pecker F. A glucagon fragment is responsible for the inhibition of the liver Ca2+ pump by glucagon. Nature. 1987 Feb 12;325(6105):620–622. doi: 10.1038/325620a0. [DOI] [PubMed] [Google Scholar]
- Mauger J. P., Claret M. Calcium channels in hepatocytes. J Hepatol. 1988 Oct;7(2):278–282. doi: 10.1016/s0168-8278(88)80492-6. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. Calcium ion fluxes induced by the action of alpha-adrenergic agonists in perfused rat liver. Biochem J. 1982 Dec 15;208(3):619–630. doi: 10.1042/bj2080619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. Studies on alpha-adrenergic-induced respiration and glycogenolysis in perfused rat liver. J Biol Chem. 1982 Feb 25;257(4):1906–1912. [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. The role of calcium ions in the mechanism of action of alpha-adrenergic agonists in rat liver. Biochem J. 1984 Oct 1;223(1):1–13. doi: 10.1042/bj2230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Imase M. Hormonal regulation of biliary calcium excretion in rats: inhibition of calcitonin action by alpha 1-adrenergic stimulation. Horm Metab Res. 1988 Apr;20(4):221–224. doi: 10.1055/s-2007-1010798. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Sugii K. Movement of subcellular calcium in bile pool of hepatocyte in rats: effect of thyroparathyroidectomy. Endocrinol Jpn. 1980 Oct;27(5):613–618. doi: 10.1507/endocrj1954.27.613. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Yamamoto T. Dietary and thyroparathyroidal regulation of calcium excretion into the bile of rats. Chem Pharm Bull (Tokyo) 1981 Apr;29(4):1172–1175. doi: 10.1248/cpb.29.1172. [DOI] [PubMed] [Google Scholar]


