Abstract
OBJECTIVE:
This study aimed to identify clinical measures that have been used to evaluate function, health related quality of life (HRQoL), and/or satisfaction in children who use lower limb prostheses (LLP). The data reported on psychometric properties for children who use LLP were collected for each measure.
METHODS:
First, PubMed, CINAHL, and Web of Science databases were searched using broad search terms to identify standardized outcome measures of function, HRQoL, and/or satisfaction with treatment used in pediatric LLP research published in 2001 or after. For each of the eligible measures found, a second search was performed to identify psychometric properties (e.g., validity, reliability) assessed with children who use LLP.
RESULTS:
Forty-four standardized outcome measures were identified from 41 pediatric LLP research articles. Five measures (i.e., Gait Outcomes Assessment for Lower Limb Differences, Functional Mobility Assessment, Child Amputee Prosthetics Project- Prosthesis Satisfaction Inventory, Child Amputee Prosthetics Project- Functional Scale Index, and Lower Limb Function Questionnaire) had data on psychometric properties for children who use LLP.
CONCLUSIONS:
Few studies report psychometric data for assessing the overall HRQoL, function, and/or satisfaction for children who use LLP. Further research is needed to validate or create new outcome measures that assess the HRQoL, satisfaction, and/or function of children who use LLP.
Keywords: Outcome measures, psychometric properties, children, amputation, artificial limbs
1. Introduction
Approximately 1.6 million people are living with limb loss or limb difference (LLLD) in the United States [1]. Children with LLLD account for 1.6% of this total, or about 25,000 individuals [1]. The rapid growth and development of children necessitates that the treatment plan and componentry of prostheses be routinely and longitudinally evaluated. Outcome measures provide clinicians with information that is essential to this evaluation process. Not only do outcome measures inform the medical team, patient, and caregiver of changes in function, but they may also aid in determining prosthetic componentry.
Outcome measures are standardized instruments that collect observable information on function, satisfaction, or health related quality of life (HRQoL) constructs through patient reported, parent-proxy reported, or clinician-assessed information. The rapid change and development of children’s language and physical capabilities highlight the need for outcome measures to be psychometrically examined before use in children who use lower limb prostheses (LLP). Several validated outcome measures exist for adult LLP users [2], but it is unknown how many exist for children who use LLP. A lack of psychometrically-sound outcomes measures prevents rapid progress in understanding treatment outcomes in children with LLP.
Due to the lack of population-specific outcome measures, clinicians and researchers are faced with having to decide on the suitability of the outcome measure for their pediatric patients with LLP. This lack also likely results in practitioners using a measure that is not validated for pediatrics or not validated in LLP users, or not using a measure at all. Consequently, there is the potential for misinterpretation of outcomes, use of instruments that are not appropriate for children or not sensitive to change in children who use LLP, or a lack of routine standardized outcome measurement for these patients. Clinicians and researchers could benefit from a resource to aid in choosing outcome measures to assess their pediatric LLP patients.
The purpose of this review was (1) to identify standardized outcome measures that measure function, HRQoL, and/or treatment satisfaction in children who use LLP, and (2) to report on the availability of data on the reliability, validity, and responsiveness of those measures. Scoping review methods were undertaken for this work because it involved a broad investigation of the literature and identification of research gaps. In addition, outcomes measures for children who use LLP are an emerging research topic. As such, there are not enough studies reporting data on these measures to inform the certainty of evidence related to their psychometric properties. This scoping review instead aimed to provide groundwork for future research on outcome measure refinement, use, and development in this patient population. It may also serve as a resource to aid clinicians and researchers when selecting among available measures to assess outcomes in their pediatric LLP patients.
2. Methods
2.1. Overview
A scoping literature review was conducted based on methods described by Peters and colleagues [3–5] and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) reporting guidelines. The protocol for this scoping review was not registered.
2.2. Information sources
A search of PubMed, Cumulated Index to Nursing and Allied Health Literature (CINAHL), and Web of Science was performed on August 4, 2023, to identify a list of outcome measures used in published research with children using LLP. Using the same databases, secondary searches were performed to find published articles describing psychometric assessment(s) of each identified outcome measure with children who use LLP.
2.3. Search strategies
To find pediatric LLP research publications, the author team developed search strategies for each database using terms related to amputation, prostheses, and pediatrics (Appendix 1). Following the extraction of outcome measures from eligible articles, secondary search strategies were developed to find published data about psychometric properties for each outcome measure in children who use LLP. Search terms for the secondary search included the name of the outcome measure, terms related to psychometric properties, and terms related to prostheses and pediatrics (Appendix 2). Eligible articles from the secondary search were subjected to forward and backward citation searches to ensure that all published articles with psychometric data for each measure of interest were identified. Results from primary and secondary searches were exported to an Endnote (Clarivate Analytics, Philadelphia, PA) library, and duplicate publications were removed.
2.4. Selection
Following both the primary (to identify outcome measures used in pediatric LLP research) and the secondary (to identify published data on psychometric properties for each outcome measure) searches, two authors independently screened search results based on title, abstract, and full text review. Articles deemed eligible by at least one reviewer during title and abstract reviews were included in the next round. If the two reviewers did not reach consensus for eligibility in the full text review round, a third, independent reviewer was consulted. A forward and backward search of each included article’s references was conducted to ensure no eligible articles were missed. However, no additional articles were found during this search.
2.5. Eligibility criteria
For the primary search of pediatric LLP research, article eligibility criteria included (1) participants were children (<18 years old) who used LLP and had amputation or limb loss at or above the ankle, (2) researchers used standardized outcome measure(s) that assessed function, HRQoL, and/or satisfaction with treatment, and (3) the article was original research or a research protocol published in 2001 or after.
For the secondary search to identify psychometric properties in pediatric LLP samples, article eligibility criteria included (1) participants were children (<18 years old) who used LLP and had amputation or limb loss at or above the ankle, and (2) the article reported psychometric properties for one or more of the outcome measures identified in the primary search. Articles identified in either the primary or secondary rounds were excluded if they did not include human participants or were only published as a poster or abstract.
2.6. Data extraction and synthesis of results
Data from articles deemed eligible in the primary search of pediatric LLP research were extracted into an Excel spreadsheet (Microsoft, Redmond, WA) by a single reviewer and checked for accuracy by a second reviewer. Extracted data from primary round articles included the outcome measures used in each study, as well as study characteristics (e.g., sample size, age range, level of amputation, and study design) to characterize the body of research in pediatric LLP. Data extraction forms were tested and edited prior to use.
Data extracted from eligible articles that assessed measurement properties in samples of children who use LLP included characteristics of the studies (e.g., sample size, age range, level of amputation, and study design) and information relating to the report of the measurement’s psychometric properties. Extracted data were checked for accuracy by a second independent reviewer and summarized in tables. Study characteristics, such as demographic information, were collected to assess the generalizability of psychometric data for measurement of children who use LLP. Psychometric data in children who use LLP, when available, were collected. Examples of articles that assess psychometric properties in related populations (e.g., people who use LLP or children) were noted, but details about these studies were not extracted as this information was outside of the scope of this article.
2.7. Deviations from the a priori protocol
Methods deviated from the a priori protocol in article eligibility criteria. Specifically, non-English articles, gray literature, and research protocols were originally excluded but ultimately included in the final publication in response to reviewer suggestions.
3. Results
3.1. Outcome measures used in pediatric prosthetic research publications
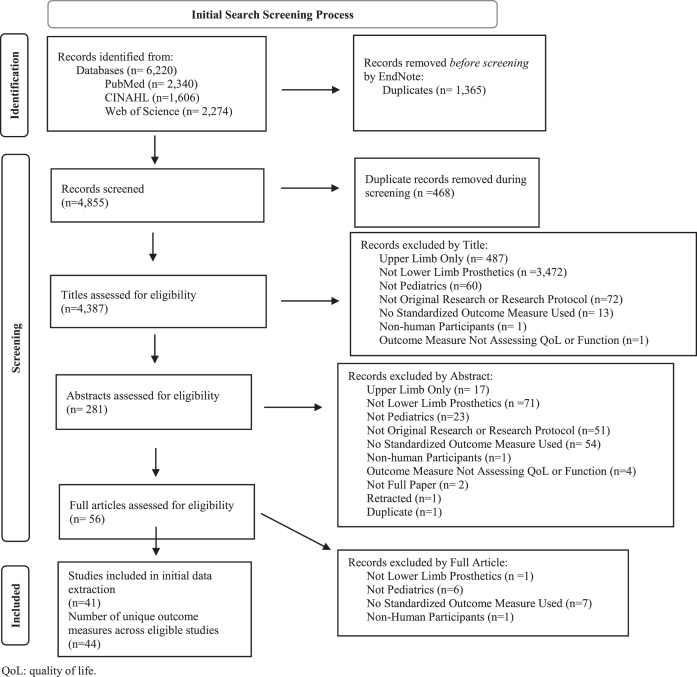
The primary search identified 4,387 articles after removing duplicates (Fig. 1). A total of 44 different standardized outcome measures were used to assess children who used LLP across the 41 articles that met eligibility criteria. Collectively, the standardized outcome measures assessed function (19 measures), HRQoL (13 measures), or satisfaction (1 measure) or were multi-domain instruments (11 measures) that included assessments of function, HRQoL, or satisfaction as one of the subdomains (Table 1). Over 80% of the standardized measures used in the 20-year period of this review were described in only one or two research publications each.
Fig. 1.
PRISMA 2020 Flow Diagram [98] for the primary search. The aim of the primary search was to identify outcome measures that were used in pediatric lower limb prosthetics research from 2001-present. The primary search resulted in the identification of 41 studies that described use of 44 unique outcome measures.
Table 1.
Outcome measures identified in pediatric lower limb prosthetics (LLP) publications and the populations for whom psychometric data is available
| Name of outcome measure | # Times used in pediatric LLP research | Psychometric data available | ||||
| Pediatrics | Prosthetics | Pediatric prosthetics | ||||
| Function | Performance-based | 6-minute walk test (6MWT) | 6 [14, 27–31] | Yes [23] | Yes [24] | No |
| 10-meter walk test (10mWT) | 2 [31, 32] | Yes [33] | Yes [34] | No | ||
| 20-meter walk test (20mWT) | 1 [29] | No | No | No | ||
| Alberta Infant Motor Scale (AIMS) | 1 [35] | Yes [36] | No | No | ||
| Amputee Mobility Predictor (AMP) | 2 [37, 38] | No | Yes [39] | No | ||
| Community Balance and Mobility Scale (CB&M) | 1 [31] | Yes [40] | No | No | ||
| Functional Level Determining Scale | 1 [41] | No | No | No | ||
| Functional Mobility Assessment (FMA) | 2 [9, 42] | Yes [9] | Yes [9] | Yes [9] | ||
| L test of functional mobility (L-Test) | 1 [32] | Yes [43] | Yes [44] | No | ||
| Muscle Power Sprint Test (MPST) | 1 [45] | Yes [46] | No | No | ||
| Special Interest Group in Amputee Medicine- Mobility (SIGAM) | 2 [47, 48] | No | Yes [49] | No | ||
| Star Excursion Balance Test (SEBT) | 1 [30] | Yes [50] | No | No | ||
| Timed Up and Down Stairs (TUDS) | 2 [30, 45] | Yes [51] | No | No | ||
| Timed Up and Go (TUG) | 1 [14] | Yes [52] | Yes [53] | No | ||
| Patient-reported | Child Amputee Prosthetics Project- Functional Scale Index | 1 [54] | Yes [11] | Yes [11] | Yes [11] | |
| Locomotor Capabilities Index (LCI-5) | 3 [29, 55, 56] | No | Yes [57] | No | ||
| Lower Limb Function Questionnaire (LLFQ) | 2 [14, 29] | Yes [14] | Yes [14] | Yes [14] | ||
| Nottingham Extended Activities of Daily Living Scale (NEADLS) | 1 [58] | No | No | No | ||
| Toronto Extremity Salvage Scores (TESS) | 2 [59, 60] | Yes [61] | No | No | ||
| Health related quality of life | Patient-reported | Amputee Body Image Scale (ABIS) | 1 [62] | No | Yes [63] | No |
| Behavior Assessment System for Children (BASC) | 1 [64] | Yes [65] | No | No | ||
| Cancer Institute Quality of Life (CI-QoL) | 1 [27] | No | No | No | ||
| DISABKIDS Chronic Generic Measure (DCGM-37) | 1 [66] | Yes [67] | No | No | ||
| Impact of Pain on Daily Living and Behaviour Scale | 1 [47] | No | No | No | ||
| KIDSCREEN 52 | 1 [68] | Yes [69] | No | No | ||
| Piers Harris Children’s Self Concept Scale | 1 [64] | Yes [70] | No | No | ||
| Patient Reported Outcome Measure Information System (PROMIS) Numerical Pain Scale | 1 [71] | Yes [72] | Yes [73] | No | ||
| Patient Reported Outcome Measure Information System (PROMIS) Pain Behavior | 1 [71] | Yes [74] | No | No | ||
| Patient Reported Outcome Measure Information System (PROMIS) Pain Interference | 1 [71] | Yes [74] | Yes [73] | No | ||
| Patient Reported Outcome Measure Information System (PROMIS) Pain Quality- Affective | 1 [71] | Yes [72] | No | No | ||
| Patient Reported Outcome Measure Information System (PROMIS) Pain Quality- Sensory | 1 [71] | Yes [72] | No | No | ||
| Wong &Baker FACES Pain Scale (FACES) | 1 [9] | Yes [75] | No | No | ||
| Satisfaction | Patient-reported | Child Amputee Prosthetics Project- Prosthesis Satisfaction Inventory (CAPP-PSI) | 1 [64] | Yes [10] | Yes [10] | Yes [10] |
| Multiple domains | Patient-reported | Childhood Health Assessment Questionnaire (CHAQ) | 1 [28] | Yes [76] | No | No |
| Children’s Assessment of Participation and Enjoyment (CAPE) | 1 [68] | Yes [77] | No | No | ||
| Experienced | 1 [45] | No | No | No | ||
| Competence and Cosmesis of prosthesis for Children with a Limb Deficiency (Co-Co ChiLD) | ||||||
| Gait Outcomes Assessment List for Lower Limb Differences questionnaire (GOAL-LD) | 1 [6] | Yes [6] | Yes [6] | Yes [6] | ||
| Pediatric Outcomes Data Collection Instrument (PODCI) | 4 [78–81] | Yes [82] | No | No | ||
| Pediatric Quality of Life Inventory (Peds QL) | 6 [28, 48, 62, 64, 83, 84] | Yes [85] | No | No | ||
| Prosthesis Evaluation Questionnaire (PEQ) | 3 [29, 86, 87] | No | Yes [88] | No | ||
| Short Form Health Survey (SF-36) | 2 [60, 89] | Yes [90] | Yes [91] | No | ||
| Short Musculoskeletal Function Assessment | 1 [92] | No | No | No | ||
| Trinity Amputation and Prosthesis Experience Scale (TAPES) | 3 [55, 62, 78] | No | Yes [93] | No | ||
| Performance-based | Musculoskeletal Tumor Society (MSTS) | 6 [27, 59, 60, 94–96] | Yes [97] | No | No | |
3.2. Psychometric properties of pediatric LLP outcome measures
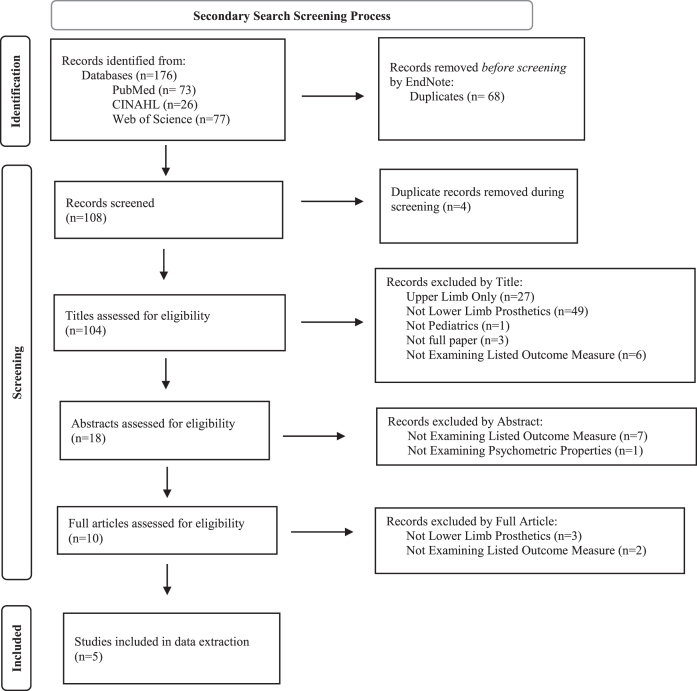
Secondary searches identified publications that reported on the psychometric properties of outcome measures extracted from articles found in the primary search. These secondary searches resulted in a total of 104 articles after removing duplicates. Following title and abstract review, 10 articles were retained for full-text review. Five articles examining five separate outcome measures met eligibility criteria and were included in the full data extraction (Fig. 2, Table 2). Measure and psychometric properties of the five measures are summarized below.
Fig. 2.
PRISMA 2020 Flow Diagram [98] for the secondary search. The aim of the secondary search was to identify articles that describe the psychometric assessment of outcome measures in children who use lower limb prostheses. The secondary search resulted in the identification of five eligible articles that described psychometric properties for five unique outcome measures.
Table 2.
Study characteristics for publications that assessed psychometric properties of outcome measures for pediatric participants who use lower limb prostheses (LLP)
| Study characteristics | Sample characteristics | |||||||
| Measure | Article | Study design | Total sample size | LLP user sample size | Mean/Age range (years) | Level of amputation/limb difference | Prosthesis design classification | Etiology |
| Gait Outcomes Assessment List for Lower Limb Differences (GOAL-LD)a | Dermott et al. [6] | Prospective cohort study | 25 | 1 | 13.7/9–18 | Transtibial | Not reported | Fibular hemimelia (16) Post-traumatic growth arrest (5) Developmental (4) |
| Functional Mobility Assessment (FMA)b | Pierce et al. [9] | Prospective cohort study | 37 | 25 | 12.4/8–19 | Symes (8) Knee disarticulation (6) Transtibial (5) Transfemoral (2) Rotationplasty (2) | Below knee Above knee | Congenital (16) Osteosarcoma (4) Infection (3) Trauma (1) Other (1) |
| Child amputee prosthetics project-prosthesis satisfaction inventoryc | Pruitt et al. [10] | Retrospective Cohort Study | 97 | 57 | 8.1/1–17 | Not reported | Not reported | Congenital (76) Acquired (21) |
| Child Amputee Prosthetics Project- Functional Scale Index (CAPP-FSI)c | Pruitt et al. [11] | Retrospective Cohort Study | 65 | 43 | Not reported/8–17 | Not reported | Not reported | Congenital (49) Acquired (16) |
| Lower limb function questionnaired | Funk et al. [14] | Prospective Cohort Study: Validity Evaluations | 40 | 9 | 16.3/13–25 | Not reported | Below knee Above knee | Not reported |
aFunding provided by the Government of Ontario, the Canadian Institutes of Health Research, the University of Toronto Open Fellowship, SickKids Continuing Professional Development Fund, and Holland Bloorview Graduate Student Support. bFunding source not reported. cFunding provided by grants from the Shriners Hospitals for Crippled Children Research Fund and the Milo Brooks Foundation for Limb Deficient Children. dFunding provided through “private fundraising by individual researchers.”
3.2.1. Gait Outcomes Assessment List for Lower Limb Differences Questionnaire (GOAL-LD)
One article [6] examined psychometric properties of the GOAL-LD, a patient-reported outcome measure assessing six domains including gait function/mobility, pain/discomfort/fatigue, physical activities/games/recreation, gait appearance, use of braces/assistive devices, and body image/self-esteem. The GOAL-LD was adapted from the original Gait Outcomes Assessment List (GOAL) [7] for use with children who have lower limb differences. The article included 25 children, aged 9–18 years, who were recruited from limb reconstruction clinics [6]. However, only one participant used LLP (Table 2). This study reported on face and content validity, which were assessed through cognitive interviews with children who had lower limb differences and their parents, and through online surveys with healthcare professionals (i.e., pediatric orthopaedic surgeons, physical therapists, and nurse practitioners). Items in the questionnaire with at least 90% acceptance by respondents were included with a total of 45 items in the final instrument (Table 3). Further psychometric assessments (e.g., test-retest reliability and construct validity) were reported in a follow-up article but were not included in this review because the sample for the follow-up article did not include children who use LLP [8].
Table 3.
Measurement characteristics for publications that assessed psychometric properties of outcome measures for pediatric participants who use lower limb prostheses (LLP)
| Study characteristics | Measurement characteristics | ||||
| Measure | Article | Construct measured | Type of measure | Report of validity | Report of reliability |
| Gait Outcomes Assessment List for Lower Limb Differences (GOAL-LD) | Dermott et al. [6] | Multiple domain • Gait function/mobility • Pain/discomfort/fatigue • Physical activities, games, recreation • Gait appearance • Use of braces and assistive devices • Body image/self-esteem |
Patient-reported | Face and content validity: • Assessed through cognitive interviews with children with LLP, surveys to healthcare providersa |
Not assesseda |
| Functional Mobility Assessment (FMA) | Pierce et al. [9] | Functional mobility | Performance-based and patient-reported | Construct validity: • Known groups: • Children with and without LLP different in total FMA scores (p < .001) and performance-based FMA sub-scores (p < .001) |
Not assessed |
| Child Amputee Prosthetics Project- Prosthesis Satisfaction Inventory (CAPP-PSI) | Pruitt et al. [10] | Prosthesis satisfaction • Subscales: • Parent satisfaction • (Parent rating of) child satisfaction • Satisfaction with service |
Proxy-reported | Face validity: • Review by expert clinicians • Construct validity: • Convergent validity- CAPP-PSI prosthesis satisfaction subscales correlated with prosthesis wear and use: r = .37-.56 • Convergent validity- CAPP-PSI prosthesis satisfaction subscales correlated with prosthesis satisfaction with appearance visual analogue scale (VAS): r = .5 • Divergent validity- CAPP-PSI satisfaction with service low to no correlation with wear time or prosthesis satisfaction with appearance VAS |
Internal consistency • Item-to-item (Cronbach’s alpha = .8-.9) • Item-total correlations (r = .5-.8) |
| Child Amputee Prosthetics Project- Functional Scale Index (CAPP-FSI) | Pruitt et al. [11] | Two Scales: • Upper extremity function • Lower extremity function |
Proxy-reported | Face validity: • Review by expert clinicians • Construct validity: • Known groups- children with limb loss or limb difference were significantly more likely to endorse “uses prosthesis” for lower extremity items on the CAPP-FSI (p < .0001) compared to children with upper limb loss or difference |
Internal consistency • Item-to-item (Cronbach’s alpha = 0.58–0.96) • Item-total correlations (r > .25) |
| Lower Limb Function Questionnaire (LLFQ) | Funk et al. [14] | Lower limb function | Patient-reported | Construct validity: • Convergent validity- • 6-minute walk test: not correlated • Obstacle test: r = .62 • Energy expenditure: not correlated • Timed Up and Go test: r=-.45 • Spatiotemporal parameters: r > 0.6 (cadence and base of support not correlated) |
Test-retest reliability (intraclass correlation = 0.79) |
aAdditional validity assessments in participants with lower limb differences, but no evidence that children who used LLP were among the participants in this study sample [8].
3.2.2. Functional Mobility Assessment (FMA)
One article [9] examined the psychometric properties of the FMA, a performance-based outcome measure with three patient-reported items examining functional mobility. The FMA was originally developed for patients with osteosarcoma aged 13 years or older [9]. Scores from the Timed Up and Go (TUG), Timed Up and Down Stairs (TUDS), and the 9-minute walk test (9MWT), as well as heart rate (HR) during each activity, Borg Rating of Perceived Exertion (RPE), Wong and Baker FACES pain scale, assistive device use, and other patient-reported items are combined in the FMA to produce a maximum score of 70. Authors of this study also collected physiological cost index (PCI) to compare each item of the FMA for the control and LLP user groups to assess known groups validity. The study included 37 participants, aged 8–19 years, 25 of whom had amputations or limb differences at varied levels. They reported good ability to discriminate between control and LLP user groups in overall FMA score and the individual sub-scores of the TUG, TUDS, and 9MWT items (p < 0.01), providing evidence of known groups validity. No difference was found between the two groups in FACES, RPE, or PCI for any item of the FMA (Tables 2, 3).
3.2.3. Child Amputee Prosthetics Project- Prosthesis Satisfaction Inventory (CAPP-PSI)
One article examined the psychometric properties of the CAPP-PSI parent-proxy reported outcome measure assessing HRQoL [10]. This measure is one of the scales within the CAPP and looks specifically at satisfaction with the prosthesis and service. CAPP-PSI has three subscales: parent-rated child satisfaction with the prosthesis (proxy measure), parent satisfaction with the prosthesis, and parent satisfaction with service. The study included 97 upper and lower limb prosthesis users, aged 1–17 years, with 57 participants using LLP. This study reported on face validity, construct validity, and internal consistency (i.e., item-to-item and item-total correlations). Face validity was assessed by expert clinician review. For convergent construct validity, child and parent satisfaction scales one year later were significantly correlated with parent ratings of prosthesis appearance on a visual analogue scale when the device was new (r = 0.52 and r = 0.48, respectively). However, only parent satisfaction was significantly correlated to their rating of prosthesis appearance one year later (r = 0.40). Parent and child satisfaction with prosthesis scales were correlated with prosthesis wear and use (r = 0.37–0.56). For divergent construct validity, satisfaction with service was not well correlated with prosthesis wear and use. Internal consistency reliability was reported for item-to-item correlations (Cronbach’s alpha = 0.8–0.9) and item-total correlations (r = 0.5–0.8) (Tables 2, 3).
3.2.4. CAPP- Functional Scale Index (CAPP-FSI)
One article examined the psychometric properties of the CAPP-FSI parent-proxy reported outcome measure assessing function [11]. This measure is another scale within the CAPP; it looks specifically at the function of children who use a prosthesis. Items in the instrument assess the extent to which the child engages in a range of activities and whether they use their prosthesis to complete the activity. The study included 65 upper and lower limb prosthesis users, aged 8–17, with 43 participants using LLP. CAPP-FSI has two subscales: upper extremity function and lower extremity function (Table 2). This study reported on face validity, construct validity, and internal consistency (i.e., item-to-item and item-total correlations). Face validity was assessed by expert clinician review. Known groups construct validity was assessed by examining prosthesis use for a given task in a category (upper or lower extremity) between upper and lower limb loss or difference groups. Children with LLLD were significantly more likely to endorse “uses prosthesis” for lower extremity items on the CAPP-FSI (p < 0.0001) compared to children with upper limb loss or difference. With respect to internal consistency reliability assessments, the measure had moderate to good task-to-task consistency (Cronbach’s alpha = 0.58–0.96), and all item-total correlations for retained items were r > 0.25 (Table 3). Two subsequent measures were derived from the CAPP-FSI specifically for toddlers [12] and preschoolers [13].
3.2.5. Lower Limb Function Questionnaire (LLFQ)
One article examined construct validity and test-retest reliability of the LLFQ, a patient-reported outcome measure of overall function [14]. This study included 40 participants, aged 13–25 years, nine of whom used LLP. Convergent construct validity was assessed by comparing the LLFQ to other functional performance tests (i.e., 6-Minute Walk Test (6MWT), TUG, obstacle course distance traveled, oxygen consumption during the 6MWT and obstacle course, and gait parameters during the TUG). LLFQ mean score was significantly correlated with distance traveled on an obstacle course (r = 0.62) and some of the TUG gait parameters: velocity (r = 0.66), stride length (r = 0.76), stance phase percent of gait cycle (r=–0.699), single-support phase percent of gait cycle (r = 0.70), and double-support phase percent of gait cycle (r=– 0.695). LLFQ mean score was not significantly correlated with 6MWT scores, overall TUG scores, or oxygen consumption during the obstacle course or 6MWT. Test-retest reliability was reported to have an intraclass correlation coefficient (ICC) of 0.79 (Tables 2, 3).
4. Discussion
Results from this scoping review demonstrated that 44 self-reported and performance-based measures have been used to assess function, HRQoL, and satisfaction in recent pediatric LLP research. There were almost as many outcome measures as the number of pediatric LLP research articles (41), indicating a wide variety of measures in use. The variation in standardized outcome measures used across these research publications presents challenges to clinicians and researchers who attempt to synthesize findings across pediatric LLP research studies. Such synthesis is particularly important for clinicians determining outcomes and treatment decisions for children who use LLP, given that sample sizes are often small in each individual research study. Consistency in the standardized outcome measures used across research studies would facilitate synthesis of pediatric LLP research information to support evidence-based practice. This is highlighted by the recently published recommendation from the International Society of Prosthetics and Orthotics, which provided recommendations for specific standardized outcome measures to be used in assessing adults with lower limb absence [15]. A potential opportunity then arises for the development of core outcome sets or consensus in outcomes of importance in pediatric LLP research. A lack of validated outcome measures is seen in multiple medical disciplines (e.g., pediatric orthopedics [16]) and diagnoses (e.g., cerebral palsy [17]).
Of the 44 outcome measures identified, only five had publications that described the assessment of validity and/or reliability in children who use LLP. However, the data provided in these studies did not demonstrate a complete psychometric assessment for any of these standardized outcome measures, meaning that the publications did not describe validity and/or reliability assessments that could guide clinical use of these measures. Furthermore, the articles that assessed measurement properties for these instruments included relatively few children who use LLP. The age range of the participants was typically skewed towards older pediatric participants. No data of responsiveness or normative data were found for any measure examined.
There are many reasons for this dearth of publications in pediatric LLP outcome measures, which may include recruiting difficulties, few clinicians and researchers specializing in pediatric LLP, heterogeneity of this population, and a lack of emphasis on outcome measure use. The relatively small number of pediatric LLP users available to participate in these studies may increase the difficulty in recruiting participants for validation studies. This limitation is compounded by even fewer pediatric specialized clinicians and researchers with the experience and expertise to conduct these studies. Much of the research in outcome measures for LLP users has been funded by the Department of Veterans Affairs and Department of Defense, which understandably prioritize research for adults who use LLP. These factors contribute both to the paucity of literature in general and to the lack of outcome measures that appropriately assess children who use LLP and that provide appropriate assessment by clinicians who care for them. For example, while multiple measures are available to assess comfort or pain in children, only two studies [9, 18] used single-domain outcome measures specific to comfort or pain. This is surprising given how integral comfort and pain are to overall quality of life and how frequently they are given as reasons for visits to the prosthetist’s office. Further, condition-specific assessments of comfort, such as the Socket Comfort Score (SCS) [19, 20], were not found through the initial search. Assessment of socket comfort is a critical aspect of prosthetic care. Thus, measures like the SCS could provide valuable clinical information when treating pediatric LLP patients. However, further research is needed to support use of these condition-specific measures for children who use LLP.
Additionally, the demographics and causes of limb loss are different and more varied in children than adults. Congenital limb deficiency, trauma, and cancer diagnoses are the leading causes for amputation in children, while vascular disease is the primary etiology resulting in amputation in adults [1]. Congenital limb difference in children is noted in 0.079% of overall births and varies greatly in cause [21]. Many of these are idiopathic in nature and present clinically in a number of different ways [22]. These etiological differences of amputation can be associated with distinctions in overall activity level and functionality. Children typically have a higher activity level and physically change more rapidly than adults. Because of this preconception, insurance more readily covers prostheses for children as they outgrow them. Consequently, the expectation for adults is that prostheses are replaced at most every five years with the need for additional medical justification, unless significant functional or physical change has occurred sooner. This stereotypical difference in activity level and standardized insurance coverage may have led to a deemphasis of outcome measures in the pediatric LLP population.
This review highlights the need for further testing and/or development of standardized outcome measures to support the holistic and multidisciplinary care of children who use LLP. Routine assessment using standardized performance-based and patient-reported outcome measures of function, HRQoL, and satisfaction may identify areas for improvement in prosthetic treatment and overall health that might otherwise go unnoticed. Standardized outcome measures that assess patients, both clinically and scientifically, should have evidence of psychometric testing (reliability, validity, and responsiveness) in the population of interest to assure that they are appropriate for clinical use. Normative data would also be necessary when interpreting results. This study demonstrates the variability in standardized outcome measures used and the lack of psychometric evidence to support the use of outcome measures to assess children who use LLP.
Until this evidence is developed, other factors need to be considered. When faced with limitations in standardized outcome measures well-suited for use with children who use LLP, some clinicians and researchers will consider using outcome measures validated only in pediatric patients not using LLP or only in adults with LLP. For example, the psychometric properties of the 6MWT have been evaluated in various pediatric populations [23] and in adult LLP users [24]. It could be argued that the 6MWT may be appropriate for use in a child who uses LLP, can follow instructions, and has adequate attention span, even though there is not yet psychometric evidence supporting its use in this specific population. The fact that there is sufficient evidence in not just one but two similar populations may be sufficient to support its use clinically for children who use LLP. It also gives substantial support for a future validation study in children who use LLP. Limited research funding for this population may require careful consideration of which outcome measures should be studied for further validation. If a measure has sufficient evidence available in pediatric populations or adult LLP users, then it may be more advantageous to focus future studies on measures with less evidence overall.
In some cases, it is reasonable that prosthetic-specific measures designed for adults may be applied to children who use LLP. Measures like the Amputee Mobility Predictor (AMP) may be considered for future research with older children (i.e., teenagers) who use LLP to determine age-specific applicability of the measure. Researchers must be careful in choosing their methods of assessment based on the evidence available. Clinicians must use good clinical judgement (e.g., Does my patient have the cognitive capacity to understand the instructions?, Is my patient tall enough to stand and sit in the chair?) when deciding which outcome measures are best for their specific patients in the absence of evidence. Although the AMP does not have psychometric evidence in children who use LLP, a practitioner who is familiar with and comfortable administering the measure may decide to use it to assess older children who use LLP in order to track longitudinal changes in function and areas for improvement.
4.1. Limitations
This scoping review has limitations. First, a scoping review protocol was developed a priori but not registered. Registering a scoping review a priori is important to mitigate the risk for reporting bias. All deviations from the a priori protocol were reported in the methods section to increase transparency and minimize this risk. The lack of standardized language across the pediatric LLP literature made the initial search and screening process difficult. Terms that have been used to describe an internal prosthesis (e.g., endoprosthesis) were mixed and inconsistently used. Since this article examined only the use of external LLP, this inconsistent language was difficult to navigate. In addition, the wording of some article titles and abstracts may have caused them to be misunderstood as not meeting inclusion criteria. To reduce the impact of this inconsistent language on the results, two reviewers independently screened each article at each phase of the screening process. In addition, a conservative approach was taken to be as inclusive as possible in the title and abstract rounds of the screening process. The use of controlled vocabulary was used in the search strategy to be as inclusive as possible. Another limitation of this article is that it only examined the outcome measures used in published literature over the past 20 years. Thus, measures that are only being used clinically but not in recent research were not included in the review. This decision was made because outcome measures that are being used in the literature are likely to have the most evidence for use in pediatric LLP. This also provided objective and repeatable criteria to help determine inclusion or exclusion of a measure for further examination. New standardized outcome measures are being developed that may be well-suited to children who use LLP (e.g., LimbQ Kids [25], Pedi CHAMP [26]). However, these instruments were not yet published at the time of writing this article. These measures, and future ones yet to be developed, will hopefully begin to close the gap in the literature on outcome measures well-suited to measurement in children who use LLP.
5. Conclusion
The amount of literature assessing children using LLP is limited overall, as is the literature presenting psychometric data for outcome measures assessing this population. Further research is needed to validate the current outcome measures in pediatric LLP. Clinicians may be especially interested in those measures that are separately validated in both pediatric non-LLP and adult LLP users. If a measure is not found to be valid in children who use LLP, then the development of new measures may be warranted to ensure that clinicians and researchers are able to assess the constructs of interest in this population. Once the necessary evidence is available, a set of outcome measures for children who use LLP can be developed to help clinicians and researchers make informed decisions about the measure they choose to assess their patients.
Appendix 1 – Search Strategy Table for Primary Search
| PubMed | ||
| Search | Field Code | Search term(s) |
| 1 | MeSH Terms | artificial limb OR amputation |
| 2 | Title/Abstract | “limb deficiency” OR “limb difference” OR “congenital difference” OR “congenital deficiency” OR “limb deficiencies” OR “limb differences” OR “congenital differences” OR “congenital deficiencies” OR prosthesis OR prosthetic* OR prostheses |
| 3 | 1 OR 2 | |
| 4 | MeSH Terms | pediatric OR children |
| 5 | Title/Abstract | child OR kid OR kids OR toddler* OR infant* OR teen* OR adolescent* OR “young adult” OR “young adults” |
| 6 | 4 OR 5 | |
| 7 | Title/Abstract | “total hip” OR “total joint” OR valve* OR dental OR teeth OR tooth OR penile OR ocular OR cochlear OR ossicular OR stapes OR palate OR eye OR eyes OR ear OR ears OR arthroplasty OR endoprosthesis OR endoprostheses OR animal* OR veterinary |
| 8 | 3 AND 6 NOT 7 | |
| CINAHL (EBSCO) | ||
| Search | Field Code | Search term(s) |
| 1 | SU | artificial limb OR artificial limbs OR amputation OR limb prosthesis |
| 2 | TI | “limb deficiency” OR “limb difference” OR “congenital difference” OR “congenital deficiency” OR “limb deficiencies” OR “limb differences” OR “congenital differences” OR “congenital deficiencies” OR prosthesis OR prosthetic* OR prostheses OR “artificial limb” OR “artificial limbs” |
| 3 | AB | “limb deficiency” OR “limb difference” OR “congenital difference” OR “congenital deficiency” OR “limb deficiencies” OR “limb differences” OR “congenital differences” OR “congenital deficiencies” OR prosthesis OR prosthetic* OR prostheses OR “artificial limb” OR “artificial limbs” |
| 4 | 1 OR 2 OR 3 | |
| 5 | SU | pediatrics OR children |
| 6 | TI | child OR kid OR kids OR toddler* OR infant* OR teen* OR adolescent* OR “young adult” OR “young adults” OR pediatrics OR children |
| 7 | AB | child OR kid OR kids OR toddler* OR infant* OR teen* OR adolescent* OR “young adult” OR “young adults” OR pediatrics OR children |
| 8 | 5 OR 6 OR 7 | |
| 9 | TI | “total hip” OR “total joint” OR valve* OR dental OR teeth OR tooth OR penile OR ocular OR cochlear OR ossicular OR stapes OR palate OR eye OR eyes OR ear OR ears OR arthroplasty OR endoprosthesis OR endoprostheses OR animal* OR veterinary |
| 10 | AB | “total hip” OR “total joint” OR valve* OR dental OR teeth OR tooth OR penile OR ocular OR cochlear OR ossicular OR stapes OR palate OR eye OR eyes OR ear OR ears OR arthroplasty OR endoprosthesis OR endoprostheses OR animal* OR veterinary |
| 11 | 9 OR 10 | |
| 12 | 4 AND 8 NOT 11 | |
| Web of Science | ||
| Search | Field Code | Search term(s) |
| 1 | TI | artificial limb OR artificial limbs OR Amputation OR “limb deficiency” OR “limb difference” OR “congenital difference” OR “congenital deficiency” OR “limb deficiencies” OR “limb differences” OR “congenital differences” OR “congenital deficiencies” OR prosthesis OR prosthetic* OR prostheses |
| 2 | AB | artificial limb OR artificial limbs OR “limb deficiency” OR “limb difference” OR “congenital difference” OR “congenital deficiency” OR “limb deficiencies” OR “limb differences” OR “congenital differences” OR “congenital deficiencies” OR prosthesis OR prosthetic* OR prostheses |
| 3 | 1 OR 2 | |
| 4 | TI | pediatric* OR children OR child OR kid OR kids OR toddler* OR infant* OR teen* OR adolescent* OR “young adult” OR “young adults” |
| 5 | AB | pediatric* OR children OR child OR kid OR kids OR toddler* OR infant* OR teen* OR adolescent* OR “young adult” OR “young adults” |
| 6 | 4 OR 5 | |
| 7 | TI | “total hip” OR “total joint” OR valve* OR dental OR teeth OR tooth OR penile OR ocular OR cochlear OR ossicular OR stapes OR palate OR eye OR eyes OR ear OR ears OR arthroplasty OR endoprosthesis OR endoprostheses OR animal* OR veterinary |
| 8 | AB | “total hip” OR “total joint” OR valve* OR dental OR teeth OR tooth OR penile OR ocular OR cochlear OR ossicular OR stapes OR palate OR eye OR eyes OR ear OR ears OR arthroplasty OR endoprosthesis OR endoprostheses OR animal* OR veterinary |
| 9 | 7 OR 8 | |
| 10 | 3 AND 6 NOT 9 | |
Appendix 2 – Search Strategy Tables for Secondary Search
| Pediatric Prosthetics | ||
| PubMed | ||
| Search | Field Code | Search term(s) |
| 1 | Title/Abstract | [name of outcome measure] |
| 2 | Title/Abstract | psychometric* OR valid* OR reliab* |
| 3 | 1 AND 2 | |
| 4 | MeSH Terms | artificial limb OR amputation |
| 5 | Title/Abstract | “limb deficiency” OR “limb difference” OR “congenital difference” OR “congenital deficiency” OR “limb deficiencies” OR “limb differences” OR “congenital differences” OR “congenital deficiencies” OR prosthesis OR prosthetic* OR prostheses |
| 6 | 4 OR 5 | |
| 7 | MeSH Terms | pediatric OR children |
| 8 | Title/Abstract | child OR kid OR kids OR toddler* OR infant* OR teen* OR adolescent* OR “young adult” OR “young adults” |
| 9 | 7 OR 8 | |
| 10 | 3 AND 6 AND 9 | |
| CINAHL (EBSCO) | ||
| Search | Field Code | Search term(s) |
| 1 | TI | [name of outcome measure] |
| 2 | AB | [name of outcome measure] |
| 3 | 1 OR 2 | |
| 4 | SU | psychometrics |
| 5 | TI | psychometric* OR valid* OR reliab* |
| 6 | AB | psychometric* OR valid* OR reliab* |
| 7 | 4 OR 5 OR 6 | |
| 8 | 3 AND 7 | |
| 9 | SU | artificial limb OR artificial limbs OR amputation OR limb prosthesis |
| 10 | TI | “limb deficiency” OR “limb difference” OR “congenital difference” OR “congenital deficiency” OR “limb deficiencies” OR “limb differences” OR “congenital differences” OR “congenital deficiencies” OR prosthesis OR prosthetic* OR prostheses OR “artificial limb” OR “artificial limbs” |
| 11 | AB | “limb deficiency” OR “limb difference” OR “congenital difference” OR “congenital deficiency” OR “limb deficiencies” OR “limb differences” OR “congenital differences” OR “congenital deficiencies” OR prosthesis OR prosthetic* OR prostheses OR “artificial limb” OR “artificial limbs” |
| 12 | 9 OR 10 OR 11 | |
| 13 | SU | pediatrics OR children |
| 14 | TI | child OR kid OR kids OR toddler* OR infant* OR teen* OR adolescent* OR “young adult” OR “young adults” OR pediatrics OR children |
| 15 | AB | child OR kid OR kids OR toddler* OR infant* OR teen* OR adolescent* OR “young adult” OR “young adults” OR pediatrics OR children |
| 16 | 13 OR 14 OR 15 | |
| 17 | 8 AND 12 AND 16 | |
| Web of Science | ||
| Search | Field Code | Search term(s) |
| 1 | TI | [name of outcome measure] |
| 2 | AB | [name of outcome measure] |
| 3 | 1 OR 2 | |
| 4 | TI | psychometric* OR valid* OR reliab* |
| 5 | AB | psychometric* OR valid* OR reliab* |
| 6 | 4 OR 5 | |
| 7 | 3 AND 6 | |
| 8 | TI | artificial limb OR artificial limbs OR Amputation OR “limb deficiency” OR “limb difference” OR “congenital difference” OR “congenital deficiency” OR “limb deficiencies” OR “limb differences” OR “congenital differences” OR “congenital deficiencies” OR prosthesis OR prosthetic* OR prostheses |
| 9 | AB | artificial limb OR artificial limbs OR “limb deficiency” OR “limb difference” OR “congenital difference” OR “congenital deficiency” OR “limb deficiencies” OR “limb differences” OR “congenital differences” OR “congenital deficiencies” OR prosthesis OR prosthetic* OR prostheses |
| 10 | 8 OR 9 | |
| 11 | TI | pediatric* OR children OR child OR kid OR kids OR toddler* OR infant* OR teen* OR adolescent* OR “young adult” OR “young adults” |
| 12 | AB | pediatric* OR children OR child OR kid OR kids OR toddler* OR infant* OR teen* OR adolescent* OR “young adult” OR “young adults” |
| 13 | 11 OR 12 | |
| 14 | 7 AND 10 AND 13 | |
Funding
Funding for this work was provided by the generous donors of Gillette Children’s Specialty Healthcare.
Conflict of interest
The authors have no conflict of interest to report.
Ethical considerations
This study, as a literature review, is exempt from Institutional Review Board approval.
References
- [1]. Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422–9. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- [2]. Condie E, Scott H, Treweek SP. Lower Limb Prosthetic Outcome Measures: A Review of the Literature 1995 to 2005. J Prosthet Orthot. 2006;18(6):P13–P45. doi: 10.1097/00008526-200601001-00004. [DOI] [Google Scholar]
- [3]. Peters MDJ, Marnie C, Tricco AC, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. 2020;18(10):2119–26. doi: 10.11124/jbies-20-00167. [DOI] [PubMed] [Google Scholar]
- [4]. Peters MDJ, Marnie C, Colquhoun H, et al. Scoping reviews: reinforcing and advancing the methodology and application. Syst Rev. 2021;10(1):263. doi: 10.1186/s13643-021-01821-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Peters MDJ, Godfrey C, McInerney P, Munn Z, Tricco AC, Khalil H Chapter 11: Scoping Reviews version) (2020 version). In: Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis. JBI; 2020. [Google Scholar]
- [6]. Dermott JA, Wright FV, Salbach NM, Narayanan UG. Development of the gait outcomes assessment list for lower-limb differences (GOAL-LD) questionnaire: a child and parent reported outcome measure. Health Qual Life Outcomes. 2021;19(1):139. doi: 10.1186/s12955-021-01775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Narayanan U, Davidson B, Weir S. Gait outcomes assessment list (The GOAL): developing a meaningful outcome measure for ambulatory children with cerebral palsy. Dev Med Child Neurol. 2011;53(S5):79. doi: 10.1111/dmcn.13732. [DOI] [Google Scholar]
- [8]. Griffiths AL, Donnan LT, Iobst CA, Kelley SP, Bouchard M, Narayanan UG. The Gait Outcomes Assessment List for Children With Lower Limb Difference (GOAL-LD): Assessment of Reliability and Validity. J Pediatr Ortho. 2021;41(7):450–456. doi: 10.1097/bpo.0000000000001866. [DOI] [PubMed] [Google Scholar]
- [9]. Pierce S, Fergus A, Brady B, Wolff-Burke M. Examination of the functional mobility assessment tool for children and adolescents with lower extremity amputations. Pediatr Phys Ther. 2011;23(2):171–7. doi: 10.1097/PEP.0b013e318218f0b7. [DOI] [PubMed] [Google Scholar]
- [10]. Pruitt SD, Varni JW, Seid M, Setoguchi Y. Prosthesis satisfaction outcome measurement in pediatric limb deficiency. Arch Phys Med Rehabil. 1997;78(7):750–4. doi: 10.1016/s0003-9993(97)90084-8. [DOI] [PubMed] [Google Scholar]
- [11]. Pruitt SD, Varni JW, Setoguchi Y. Functional status in children with limb deficiency: development and initial validation of an outcome measure. Arch Phys Med Rehabil. 1996;77(12):1233–8. doi: 10.1016/s0003-9993(96)90185-9. [DOI] [PubMed] [Google Scholar]
- [12]. Pruitt SD, Seid M, Varni JW, Setoguchi Y. Toddlers with limb deficiency: conceptual basis and initial application of a functional status outcome measure. Arch Phys Med Rehabil. 1999;80(7):819–24. doi: 10.1016/s0003-9993(99)90233-2. [DOI] [PubMed] [Google Scholar]
- [13]. Pruitt SD, Varni JW, Seid M, Setoguchi Y. Functional status in limb deficiency: development of an outcome measure for preschool children. Arch Phys Med Rehabil. 1998;79(4):405–11. doi: 10.1016/s0003-9993(98)90141-1. [DOI] [PubMed] [Google Scholar]
- [14]. Funk L, Thiessen D, Wright V, Andrysek J, Rispin K. Reliability and validity of the Lower Limb Function Questionnaire when completed by young adult orthotic and prosthetic device users. Disabil Rehabil Assist Technol. 2017;12(3):262–71. doi: 10.3109/17483107.2015.1129458. [DOI] [PubMed] [Google Scholar]
- [15]. Tan JM, Halford GRJ, Lukin M, Kohler F. Recommendations from the ISPO lower-limb COMPASS: Patient-reported and performance-based outcome measures. Prosthet Orthot Int. 2023;47(1):13–25. doi: 10.1097/pxr.0000000000000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Truong WH, Price MJ, Agarwal KN, et al. Utilization of a Wide Array of Nonvalidated Outcome Scales in Pediatric Orthopaedic Publications: Can’t We All Measure the Same Thing? J Pediatr Ortho. 2019;39(2):e153–e8. doi: 10.1097/bpo.0000000000001263. [DOI] [PubMed] [Google Scholar]
- [17]. Gilbertson T, Bjornson KF, McDonald CL, Hafner BJ. Clinical Gait Measures for Ambulatory Children with Cerebral Palsy: A Review. J Prosthet Orthot. 2016;28(1):2–12. doi: 10.1097/JPO.0000000000000080. [DOI] [Google Scholar]
- [18]. Bjorklund KA, Alexander J, Tulchin-Francis K, et al. Targeted Muscle Reinnervation for Limb Amputation to Avoid Neuroma and Phantom Limb Pain in Patients Treated at a Pediatric Hospital. Plast Reconstr Surg Glob Open. 2023;11(4):e4944. doi: 10.1097/gox.0000000000004944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Hanspal RS, Fisher K, Nieveen R. Prosthetic socket fit comfort score. Disabil Rehabil. 2003;25(22):1278–80. doi: 10.1080/09638280310001603983. [DOI] [PubMed] [Google Scholar]
- [20]. Morgan SJ, Askew RL, Hafner BJ. Measurements of Best, Worst, and Average Socket Comfort Are More Reliable Than Current Socket Comfort in Established Lower Limb Prosthesis Users. Arch Phys Med Rehabil. 2022;103(6):1201–4. doi: 10.1016/j.apmr.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Gold NB, Westgate M-N, Holmes LB. Anatomic and etiological classification of congenital limb deficiencies. Am J Med Genetics Part A. 2011;155(6):1225–35. doi: 10.1002/ajmg.a.33999. [DOI] [PubMed] [Google Scholar]
- [22]. Shivers E, Day S. A literature review of the causes of congenital limb deficiencies over the last 20 years. J Prosthet Orthot. 2022;36(1):e8–317. doi: 10.1097/JPO.0000000000000444. [DOI] [Google Scholar]
- [23]. Li AM, Yin J, Yu CC, et al. The six-minute walk test in healthy children: reliability and validity. Eur Respir J. 2005;25(6):1057–60. doi: 10.1183/09031936.05.00134904. [DOI] [PubMed] [Google Scholar]
- [24]. Lin SJ, Bose NH. Six-minute walk test in persons with transtibial amputation. Arch Phys Med Rehabil. 2008;89(12):2354–9. doi: 10.1016/j.apmr.2008.05.021. [DOI] [PubMed] [Google Scholar]
- [25]. Chhina H, Klassen A, Bade D, Kopec J, Cooper A. Establishing content validity of LIMB-Q Kids: a new patient-reported outcome measure for lower limb deformities. Qual Life Res. 2022;31(9):2805–18. doi: 10.1007/s11136-022-03140-z. [DOI] [PubMed] [Google Scholar]
- [26]. Tulchin-Francis K, Ulman S. Correlations between functional testing and the Pedi-CHAMP agility test in youth athletes. Orthop J Sports Med. 2021;9(7 suppl3). doi: 10.1177/2325967121s00131. [DOI] [Google Scholar]
- [27]. Satish MS, Vijay S, Raja A, Anitha D. Rehabilitation interventions and outcomes for a person with rotationplasty. Indian J Physiother Occup Ther. 2018;12(4):107. doi: 10.5958/0973-5674.2018.00089.8. [DOI] [Google Scholar]
- [28]. Tofts LJ, Hamblin N. C-Leg® improves function and qualityof life in an adolescent traumatic trans-femoral amputee: a casestudy. Prosthet Orthot Int. 2014;38(5):413–7. doi: 10.1177/0309364613502354. [DOI] [PubMed] [Google Scholar]
- [29]. Andrysek J, Wright FV, Rotter K, et al. Long-term clinical evaluation of the automatic stance-phase lock-controlled prosthetic knee joint in young adults with unilateral above-knee amputation. Disabil Rehabil Assist Technol. 2017;12(4):378–84. doi: 10.3109/17483107.2016.1173730. [DOI] [PubMed] [Google Scholar]
- [30]. Morris EJ, Tofts L, Patterson M, et al. Physical performance of children with longitudinal fibular deficiency (fibular hemimelia). Disabil Rehabil. 2022;44(12):2763–2773. doi: 10.1080/09638288.2020.1849420. [DOI] [PubMed] [Google Scholar]
- [31]. Feick E, Hamilton PR, Luis M, et al. A pilot study examining measures of balance and mobility in children with unilateral lower-limb amputation. Prosthet Orthot Int. 2016;40(1):65–74. doi: 10.1177/0309364614560941. [DOI] [PubMed] [Google Scholar]
- [32]. García-García Ó, Mosteiro S, Suárez-Iglesias D, Ayán C. Exercise training program in children with lower-limbamputation. Rev Assoc Med Bras. 2021;67(2):277–81. doi: 10.1590/1806-9282.67.02.20200723. [DOI] [PubMed] [Google Scholar]
- [33]. Graser JV, Letsch C, van Hedel HJA. Reliability of timed walking tests and temporo-spatial gait parameters in youths with neurological gait disorders. BMC Neurol. 2016;16(1):15. doi: 10.1186/s12883-016-0538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Datta D, Ariyaratnam R, Hilton S. Timed walking test — an all-embracing outcome measure for lower-limb amputees? Clin Rehabil. 1996;10(3):227–32. doi: 10.1177/026921559601000307. [DOI] [Google Scholar]
- [35]. Strączyńska A, Radzimińska A, Weber-Rajek M, Strojek K, Piekorz Z, Goch A. Rehabilitation of infants after transtibial amputation due to thrombosis in the perinatal period. Case report. Wiad Lek. 2019;72(7):1408–12. doi: 10.36740/WLek201907131. [DOI] [PubMed] [Google Scholar]
- [36]. Blanchard Y, Neilan E, Busanich J, Garavuso L, Klimas D. Interrater reliability of early intervention providers scoring the alberta infant motor scale. Pediatr Phys Ther. 2004;16(1):13–8. doi: 10.1097/01.Pe0000113272.34023.56. [DOI] [PubMed] [Google Scholar]
- [37]. Ulger O, Sener G. Functional outcome after prosthetic rehabilitation of children with acquired and congenital lower limb loss. J Pediatr Orthop B. 2011;20(3):178–83. doi: 10.1097/BPB.0b013e3283449362. [DOI] [PubMed] [Google Scholar]
- [38]. Ülger Ö, Şener G. Mobility grade and energy consumptionin congenital and acquired child amputees. Fiz Rehabil. 2008;19(3), 110–6. [Google Scholar]
- [39]. Gailey RS, Roach KE, Applegate EB, et al. The amputee mobility predictor: an instrument to assess determinants of the lower-limb amputee’s ability to ambulate. Arch Phys Med Rehabil. 2002;83(5):613–27. doi: 10.1053/ampr.2002.32309. [DOI] [PubMed] [Google Scholar]
- [40]. Wright FV, Ryan J, Brewer K. Reliability of the Community Balance and Mobility Scale (CB&M) in high-functioning school-aged children and adolescents who have an acquired brain injury. Brain Inj. 2010;24(13-14):1585–94. doi: 10.3109/02699052.2010.523045. [DOI] [PubMed] [Google Scholar]
- [41]. Ülger Ö, Topuz S, Bayramlar K, Şener G. Preparing thequestionnaire to determine the funcitional level in childrens withlimb loss/deficiency and pre-outcomes. Turk J Physiother Rehabil. 2015;26(3):101–6. doi: 10.7603/s40680-015-0016-2. [DOI] [Google Scholar]
- [42]. Corr AM, Liu W, Bishop M, et al. Feasibility and functional outcomes of children and adolescents undergoing preoperative chemotherapy prior to a limb-sparing procedure or amputation. Rehabil Oncol. 2017;35(1):38–45. doi: 10.1097/01.REO.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Cetin SY, Erel S. Investigation of the validity and reliability of the L test in children with cerebral palsy. Physiother Theory Pract. 2022;38(1):182–8. doi: 10.1080/09593985.2020.1731894. [DOI] [PubMed] [Google Scholar]
- [44]. Deathe AB, Miller WC. The L test of functional mobility: measurement properties of a modified version of the timed “up & go” test designed for people with lower-limb amputations. Phys Ther. 2005;85(7):626–35. doi: 10.1093/ptj/85.7.626. [DOI] [PubMed] [Google Scholar]
- [45]. Verheul FJM, Verschuren O, Zwinkels M, et al. Effectiveness of a crossover prosthetic foot in active children with a congenital lower limb deficiency: an explorative study. Prosthet Orthot Int. 2020;44(5):305–13. doi: 10.1177/0309364620912063. [DOI] [PubMed] [Google Scholar]
- [46]. Douma-van Riet D, Verschuren O, Jelsma D, Kruitwagen C, Smits-Engelsman B, Takken T. Reference values for the muscle power sprint test in 6- to 12-year-old children. Pediatr Phys Ther. 2012;24(4):327–32. doi: 10.1097/PEP.0b013e3182694a4c. [DOI] [PubMed] [Google Scholar]
- [47]. Prigent M, Brochard S, Thepaut M, et al. Improvement in activities and participation in an adolescent following secondary foot amputation. J Pediatr Surg Case Rep.. 2020;59:101544. doi: 10.1016/j.epsc.2020.101544. [DOI] [Google Scholar]
- [48]. Calder P, Shaw S, Roberts A, et al. A comparison of functional outcome between amputation and extension prosthesis in the treatment of congenital absence of the fibula with severe limb deformity. J Child Ortho. 2017;11(4):318–25. doi: 10.1302/1863-2548.11.160264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Ryall NH, Eyres SB, Neumann VC, Bhakta BB, Tennant A. The SIGAM mobility grades: a new population-specific measure for lower limb amputees. Disabil Rehabil. 2003;25(15):833–44. doi: 10.1080/0963828021000056460. [DOI] [PubMed] [Google Scholar]
- [50]. Kim DH, An DH, Yoo WG. Reliability, standard error of measurement, and minimal detectable change of the star excursion balance test in children with cerebral palsy. J Back Musculoskelet Rehabil. 2020;33(6):909–12. doi: 10.3233/bmr-170863. [DOI] [PubMed] [Google Scholar]
- [51]. Chrysagis N, Skordilis EK, Koutsouki D. Validity and clinical utility of functional assessments in children with cerebral palsy. Arch Phys Med Rehabil. 2014;95(2):369–74. doi: 10.1016/j.apmr.2013.10.025. [DOI] [PubMed] [Google Scholar]
- [52]. Williams EN, Carroll SG, Reddihough DS, Phillips BA, Galea MP. Investigation of the timed ‘up & go’ test in children. Dev Med Child Neurol. 2005;47(8):518–24. doi: 10.1017/s0012162205001027. [DOI] [PubMed] [Google Scholar]
- [53]. Schoppen T, Boonstra A, Groothoff JW, de Vries J, Göeken LNH, Eisma WH. The timed “up and go” test: Reliability and validity in persons with unilateral lower limb amputation. Arch Phys Med Rehabil. 1999;80(7):825–8. doi: 10.1016/S0003-9993(99)90234-4. [DOI] [PubMed] [Google Scholar]
- [54]. Ülger Ö, Topuz S, Bayramlar K, Şener G. Determining the functional state in acquired and congenital child amputees. Fiz Rehabil. 2007;18(3), 187–93. [Google Scholar]
- [55]. Morrison SG, Thomson P, Lenze U, Donnan LT. Syme Amputation: Function, Satisfaction, and Prostheses. J Pediatr Ortho. 2020;40(6):e532–e6. doi: 10.1097/bpo.0000000000001430. [DOI] [PubMed] [Google Scholar]
- [56]. Kant P, Koh SH, Neumann V, Elliot C, Cotter D. Treatment of longitudinal deficiency affecting the femur: comparing patient mobility and satisfaction outcomes of Syme amputation against extension prosthesis. J Pediatr Ortho. 2003;23(2), 236–42. [PubMed] [Google Scholar]
- [57]. Franchignoni F, Orlandini D, Ferriero G, Moscato TA. Reliability, validity, and responsiveness of the locomotor capabilities index in adults with lower-limb amputation undergoing prosthetic training. Arch Phys Med Rehabil. 2004;85(5):743–8. doi: 10.1016/j.apmr.2003.06.010. [DOI] [PubMed] [Google Scholar]
- [58]. Onat SS, Ünsal-Delialioğlu S, Özel S. The importance oforthoses on activities of daily living in patients with unilaterallower limb amputations. J Back Musculoskelet Rehabil. 2017;30(4):829–33. doi: 10.3233/bmr-160532. [DOI] [PubMed] [Google Scholar]
- [59]. Fuchs B, Kotajarvi BR, Kaufman KR, Sim FH. Functional outcome of patients with rotationplasty about the knee. Clin Orthop Relat Res. 2003;415:52–8. doi: 10.1097/01.blo.0000093896.12372.c1. [DOI] [PubMed] [Google Scholar]
- [60]. Hopyan S, Tan JW, Graham HK, Torode IP. Function and upright time following limb salvage, amputation, and rotationplasty for pediatric sarcoma of bone. J Pediatr Ortho. 2006;26(3):405–8. doi: 10.1097/01.bpo.0000203016.96647.43. [DOI] [PubMed] [Google Scholar]
- [61]. Davis AM, Wright JG, Williams JI, Bombardier C, Griffin A, Bell RS. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res. 1996;5(5):508–16. doi: 10.1007/bf00540024. [DOI] [PubMed] [Google Scholar]
- [62]. Demirdel S, Ülger Ö. Body image disturbance, psychosocialadjustment and quality of life in adolescents with amputation. Disabil Health J. 2021;14(3):101068. doi: 10.1016/j.dhjo.2021.101068. [DOI] [PubMed] [Google Scholar]
- [63]. Gallagher P, Horgan O, Franchignoni F, Giordano A, MacLachlan M. Body image in people with lower-limb amputation: a Rasch analysis of the Amputee Body Image Scale. Am J Phys Med Rehabil. 2007;86(3):205–15. doi: 10.1097/PHM.0b013e3180321439. [DOI] [PubMed] [Google Scholar]
- [64]. Birch JG, Paley D, Herzenberg JE, et al. Amputation Versus Staged Reconstruction for Severe Fibular Hemimelia: Assessment of Psychosocial and Quality-of-Life Status and Physical Functioning in Childhood. JB JS Open Access. 2019;4(2):e0053. doi: 10.2106/jbjs.Oa.18.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65]. Doyle A, Ostrander R, Skare S, Crosby RD, August GJ. Convergent and criterion-related validity of the Behavior Assessment System for Children-Parent Rating Scale. J Clin Child Psychol. 1997;26(3):276–84. doi: 10.1207/s15374424jccp2603_6. [DOI] [PubMed] [Google Scholar]
- [66]. Ylimäinen K, Nachemson A, Sommerstein K, Stockselius A, Norling Hermansson L. Health-related quality of life in Swedish children and adolescents with limb reduction deficiency. Acta Paediatr. 2010;99(10):1550–5. doi: 10.1111/j.1651-2227.2010.01855.x. [DOI] [PubMed] [Google Scholar]
- [67]. af Sandeberg M, Johansson EM, Hagell P, Wettergren L. Psychometric properties of the DISABKIDS Chronic Generic Module (DCGM-37) when used in children undergoing treatment for cancer. Health Qual Life Outcomes. 2010;8:109. doi: 10.1186/1477-7525-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Michielsen A, van Wijk I, Ketelaar M. Participation and health-related quality of life of Dutch children and adolescents with congenital lower limb deficiencies. J Rehabil Med. 2011;43(7):584–9. doi: 10.2340/16501977-0825. [DOI] [PubMed] [Google Scholar]
- [69]. Ravens-Sieberer U, Gosch A, Rajmil L, et al. KIDSCREEN-52 quality-of-life measure for children and adolescents. Expert Rev Pharmacoecon Outcomes Res. 2005;5(3):353–64. doi: 10.1586/14737167.5.3.353. [DOI] [PubMed] [Google Scholar]
- [70]. Alexopoulos DS, Foudoulaki E. Construct validity of the Piers-Harris Children’s Self-concept Scale. Psychol Rep. 2002;91(3 Pt 1):827–38. doi: 10.2466/pr0.2002.91.3.827. [DOI] [PubMed] [Google Scholar]
- [71]. Bjorklund KA, Alexander J, Tulchin-Francis K, et al. Targeted Muscle Reinnervation for Limb Amputation to Avoid Neuroma and Phantom Limb Pain in Patients Treated at a Pediatric Hospital. Plast Reconstr Surg Glob Open. 2023;11(4):e4944. doi: 10.1097/gox.0000000000004944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72]. Jacobson CJ, Kashikar-Zuck S, Farrell J, et al. Qualitative Evaluation of Pediatric Pain Behavior, Quality, and Intensity Item Candidates and the PROMIS Pain Domain Framework in Children With Chronic Pain. J Pain. 2015;16(12):1243–55. doi: 10.1016/j.jpain.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Hafner BJ, Morgan SJ, Askew RL, Salem R. Psychometric evaluation of self-report outcome measures for prosthetic applications. J Rehabil Res Dev. 2016;53(6):797–812. doi: 10.1682/jrrd.2015.12.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74]. Mitchell SL, McLaughlin KH, Bachmann KR, Sponseller PD, Reider LM. Construct Validity of Pediatric PROMIS Computerized Adaptive Testing Measures in Children With Adolescent Idiopathic Scoliosis. J Pediatr Ortho. 2022;42(7):e720–e6. doi: 10.1097/bpo.0000000000002190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14(1):9–17. [PubMed] [Google Scholar]
- [76]. Morales NM, Funayama CA, Rangel VO, et al. Psychometric properties of the Child Health Assessment Questionnaire (CHAQ) applied to children and adolescents with cerebral palsy. Health Qual Life Outcomes. 2008;6:109. doi: 10.1186/1477-7525-6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77]. King GA, Law M, King S, et al. Measuring children’s participation in recreation and leisure activities: construct validation of the CAPE and PAC. Child Care Health Dev. 2007;33(1):28–39. doi: 10.1111/j.1365-2214.2006.00613.x. [DOI] [PubMed] [Google Scholar]
- [78]. Dabaghi A, Haces F, Capdevila R. Is There a Difference in Function Outcome, Satisfaction, and Adjustment to Having a Prosthesis in Primary Transtibial Amputations Versus Multiple Previous Reconstructive Procedures Prior to Amputation? J Prosthet Orthot. 2015;27(2):40–3. doi: 10.1097/JPO.0000000000000054. [DOI] [Google Scholar]
- [79]. McQuerry J, Gammon L, Carpiaux A, et al. Effect of Amputation Level on Quality of Life and Subjective Function in Children. J Pediatr Ortho. 2019;39(7):e524–e30. doi: 10.1097/bpo.0000000000001321. [DOI] [PubMed] [Google Scholar]
- [80]. Jeans KA, Karol LA, Cummings D, Singhal K. Comparison of gait after Syme and transtibial amputation in children: factors that may play a role in function. J Bone Joint Surg Am. 2014;96(19):1641–7. doi: 10.2106/jbjs.N.00192. [DOI] [PubMed] [Google Scholar]
- [81]. Floccari LV, Jeans KA, Herring JA, Johnston CE, Karol LA. Comparison of Outcomes by Reconstructive Strategy in Patients with Prostheses for Proximal Femoral Focal Deficiency. J Bone Joint Surg Am. 2021;103(19):1817–25. doi: 10.2106/jbjs.20.02001. [DOI] [PubMed] [Google Scholar]
- [82]. McCarthy ML, Silberstein CE, Atkins EA, Harryman SE, Sponseller PD, Hadley-Miller NA. Comparing reliability and validity of pediatric instruments for measuring health and well-being of children with spastic cerebral palsy. Dev Med Child Neurol. 2002;44(7):468–76. doi: 10.1017/s0012162201002377. [DOI] [PubMed] [Google Scholar]
- [83]. Johansen H, Dammann B, Øinæs Andersen L, Andresen IL. Children with congenital limb deficiency in Norway: issues related to school life and health-related quality of life. A cross-sectional study. Disabil Rehabil. 2016;38(18):1803–10. doi: 10.3109/09638288.2015.1107770. [DOI] [PubMed] [Google Scholar]
- [84]. Lim AH, Thomas D, Graham KH. Cross-sectional study to investigate the health-related quality of life in children with severe lower limb trauma in Victoria. J Paediatr Child Health. 2013;49(2):131–7. doi: 10.1111/jpc.12090. [DOI] [PubMed] [Google Scholar]
- [85]. Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37(2):126–39. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- [86]. Chu CK, Wong MS. Comparison of prosthetic outcomes between adolescent transtibial and transfemoral amputees after Sichuan earthquake using Step Activity Monitor and Prosthesis Evaluation Questionnaire. Prosthet Orthot Int. 2016;40(1):58–64. doi: 10.1177/0309364614556837. [DOI] [PubMed] [Google Scholar]
- [87]. Firth GB, Masquijo JJ, Kontio K. Transtibial Ertl amputation for children and adolescents: a case series and literature review. J Child Ortho. 2011;5(5):357–62. doi: 10.1007/s11832-011-0364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88]. Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931–8. doi: 10.1016/s0003-9993(98)90090-9. [DOI] [PubMed] [Google Scholar]
- [89]. Harris JD, Trinh TQ, Scharschmidt TJ, Mayerson JL. Exceptional functional recovery and return to high-impact sports after Van Nes rotationplasty. Orthopedics. 2013;36(1):e126–31. doi: 10.3928/01477447-20121217-32. [DOI] [PubMed] [Google Scholar]
- [90]. Jörngården A, Wettergen L, von Essen L. Measuringhealth-related quality of life in adolescents and young adults:Swedish normative data for the SF-36 and the HADS, and the influenceof age, gender, and method of administration. Health Qual LifeOutcomes. 2006;4:91. doi: 10.1186/1477-7525-4-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91]. Resnik L, Borgia M. Reliability of outcome measures for people with lower-limb amputations: distinguishing true change from statistical error. Phys Ther. 2011;91(4):555–65. doi: 10.2522/ptj.20100287. [DOI] [PubMed] [Google Scholar]
- [92]. Oberc A, Sułko J. Fibular hemimelia - diagnostic management, principles, and results of treatment. J Pediatr Orthop B. 2013;22(5):450–6. doi: 10.1097/BPB.0b013e32836330dd. [DOI] [PubMed] [Google Scholar]
- [93]. Gallagher P, Franchignoni F, Giordano A, MacLachlan M. Trinity amputation and prosthesis experience scales: a psychometric assessment using classical test theory and rasch analysis. Am J Phys Med Rehabil. 2010;89(6):487–96. doi: 10.1097/PHM.0b013e3181dd8cf1. [DOI] [PubMed] [Google Scholar]
- [94]. Mavrogenis AF, Abati CN, Romagnoli C, Ruggieri P. Similar survival but better function for patients after limb salvage versus amputation for distal tibia osteosarcoma. Clin Orthop Relat Res. 2012;470(6):1735–48. doi: 10.1007/s11999-011-2238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95]. Agarwal M, Puri A, Anchan C, Shah M, Jambhekar N. Rotationplasty for bone tumors: is there still a role? Clin Orthop Relat Res. 2007;459:76–81. doi: 10.1097/BLO.0b013e31805470f0. [DOI] [PubMed] [Google Scholar]
- [96]. Benedetti MG, Okita Y, Recubini E, Mariani E, Leardini A, Manfrini M. How Much Clinical and Functional Impairment do Children Treated With Knee Rotationplasty Experience in Adulthood? Clin Orthop Relat Res. 2016;474(4):995–1004. doi: 10.1007/s11999-016-4691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97]. Lee SH, Kim DJ, Oh JH, Han HS, Yoo KH, Kim HS. Validation of a functional evaluation system in patients with musculoskeletal tumors. Clin Orthop Relat Res. 2003;(411):217–26. doi: 10.1097/01.blo.0000069896.31220.33. [DOI] [PubMed] [Google Scholar]
- [98]. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]