Abstract
Background:
Parkinson’s disease (PD) is characterized by striatal dopamine deficiency. Since dopamine cannot cross the digestive and blood-brain barriers, its precursor, levodopa (L-DOPA), remains the mainstay of treatment. However, the significant pharmacokinetic (Pk) and pharmacodynamic (Pd) limitations of L-DOPA, combined with the severity of PD, may trigger motor and non-motor complications, for which continuous dopaminergic delivery therapies have been developed.
Objective:
The aim of this study was to review the literature on the Pk/Pd limitations of L-DOPA and how current treatments of continuous dopaminergic administration ameliorate these problems, in order to identify the need for new therapeutic avenues.
Methods:
A comprehensive literature search was carried out using PubMed and 75 articles were initially extracted. Following independent screening by two reviewers and consideration of eligibility, 10 articles were chosen for further analysis. Information concerning the Pk/Pd of L-DOPA was classified for each article.
Results:
Pk/Pd problems notably include: (i) restricted digestive and cerebral absorption; (ii) unnecessary peripheral distribution; (iii) short half-life; (iv) age- and PD-induced decline of central aromatic L-amino acid decarboxylase; (v) misdistribution in many cells; and (vii) pulsatile stimulation of dopaminergic receptors. Current treatments only slightly ameliorate some of these problems.
Conclusions:
Many Pk/Pd constraints are not resolved by existing continuous dopaminergic delivery therapies. This highlights the significant gap between these treatments and the ideal of continuous dopaminergic stimulation.
Keywords: Parkinson’s disease, dopamine, continuous dopaminergic stimulation, motor fluctuations and dyskinesia, apomorphine
INTRODUCTION
Parkinson’s disease (PD) is a complex neuropsychiatric condition characterized by progressive degeneration of the nigro-striatal pathway, responsible for managing automatic motor and non-motor functions [1, 2]. The degeneration of dopamine-producing neurons in the substantia nigra pars compacta leads to reduced dopamine concentrations in the striatum. Symptoms of the disease generally appear when at least 80% of the dopamine has been reduced [3]. Since its introduction in the late 1960 s, L-DOPA (L-3,4-dihydroxyphenylalanine), a precursor of the dopamine (dihydroxy-3,4-phenyl-ethylamine) has proven to be incredibly effective in managing the motor symptoms, dramatically improving the lives of many patients worldwide. L-DOPA response is now a diagnostic criterion [4–7].
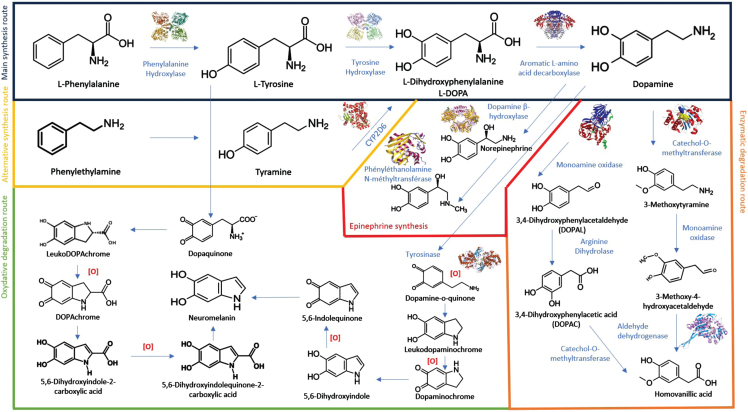
L-DOPA then undergoes metabolization into dopamine, mediated by dopamine-decarboxylase (aromatic L-amino acid decarboxylase). Within adrenergic and noradrenergic neurons, dopamine can be further converted into noradrenaline via the action of dopamine β-hydroxylase. Finally, dopamine is catabolized by many enzymatic and non-enzymatic pathways, and notably the consecutive actions of two enzymes: monoamine oxidases A and B and catecholamine O-methyl transferase (COMT). Both of these enzymes are targeted by anti-parkinsonian drugs combined with L-DOPA, to extend its effects by roughly 30% (entacapone and tolcapone for COMT inhibitors) (Fig. 1).
Fig. 1.
Complex metabolization of L-DOPA and dopamine through many enzymatic and non-enzymatic pathways.
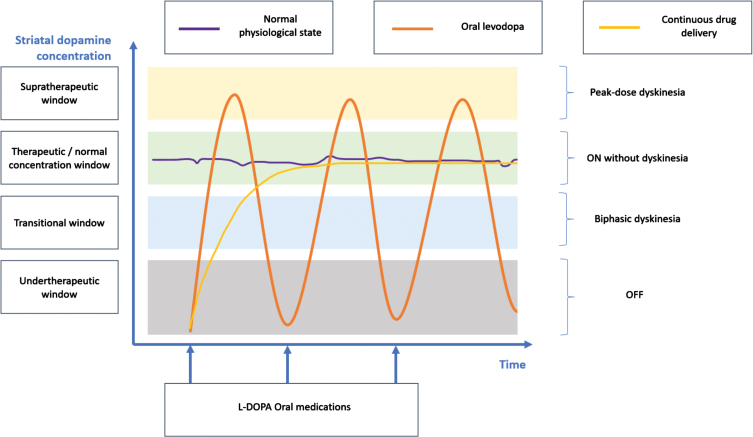
Due to its pharmacokinetic (Pk) and pharmacodynamic (Pd) limitations, L-DOPA triggers L-DOPA-related complications (LDRC), including fluctuating efficacy such as motor fluctuations, wearing off, and L-DOPA-induced dyskinesia (LID) (1), affecting 50% of patients within 5 years and 80% of patients after 10 years of disease progression [8]. Peak-dose dyskinesia is the most common type of dyskinesia [9]. Off-period typically arises early in the morning, before the first dose of L-DOPA. Biphasic dyskinesia results from fluctuating plasma concentrations of L-DOPA and may occur 10–15 min post-dosing (Fig. 2). The earlier and more severe the degeneration of dopaminergic neurons, the more likely these complications will occur [10]. L-DOPA may also give rise to non-motor complications [11]. In older patients, the adverse effects more frequently include confusion, hallucinations, delusions, psychosis, and agitation.
Fig. 2.
L-DOPA related complications as a function of the striatal dopamine concentration (i.e., reflecting the consequences of the pharmacokinetic and pharmacodynamic limitations). This diagram represents a patient’s diurnal period with regular medication intake.
To address the Pk/Pd limitations that give rise to LDRC, the concept of continuous dopaminergic stimulation has been proposed and continuous dopaminergic delivery therapies have been developed together with preclinical validation in 6 OH-dopamine rats [12] and MPTP monkeys [13, 14]. It is currently possible to administer dopaminergic therapies via two continuous administration routes, either subcutaneous or intrajejunal via gastrostomy, with two therapeutic principles: apomorphine, a dopaminergic agonist, levodopa/carbidopa prodrug (foslevodopa) or levodopa/carbidopa gel with or without entacapone.
Subcutaneous administration of apomorphine, a non-narcotic morphine derivative, enables continuous administration with an external pump and avoids the hepatic first-pass effect [15, 16]. Apomorphine administration enhances motor symptoms and quality of life [17], but does not entirely remove the need for oral treatments, and as a result, their Pk challenges persist [17–19]. Levodopa/carbidopa intestinal gel infusion (LCIG) enables continuous administration of L-DOPA into the proximal jejunum via a percutaneous gastrostomy tube. This technique can completely replace oral treatment, but is hampered by poor ergonomics (cumbersome external pump and daily replacement of L-DOPA/carbidopa cassettes), limiting its use to the most advanced patients [20–22]. An identical strategy combines L-DOPA/carbidopa gel with entacapone (Lecigon®) to reduce the dose of L-DOPA by 20% [23]. Foslevodopa/foscarbidopa (FL/FC) continuous subcutaneous infusion (ABBV-951 therapy, prodrug of L-DOPA) with an external pump has proven to be effective in a recent clinical trial [24]. A similar approach with a continuous subcutaneous L-DOPA/carbidopa delivery system has demonstrated its feasibility and safety [25].
We carried out a literature review of the Pk/Pd limitations of L-DOPA, in order to assess how these challenges might be addressed by current and emerging continuous dopaminergic administration treatments, and highlight potential areas for improving symptomatic dopaminergic therapies.
METHODS
Data source and search strategy
This review was conducted in alignment with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. However, certain deviations from these guidelines were undertaken due to the exclusive pharmacological focus of this literature review, for example, there was no statistical analysis or systematization of the extraction of quantitative results from the studies.
The bibliographic search was conducted using the PubMed database. While Medline and Embase were also explored, the returned articles were essentially identical to those found on PubMed.
To achieve the most effective search algorithm, multiple iterative searches were conducted incorporating key terms such as: pharmacokinetics, L-DOPA, and apomorphine. This ensured the number of articles retrieved was manageable and maintained a high degree of relevance. The final search algorithm employed was: (“pharmacokinetic” [Title] OR “pharmacokinetical” [Title] OR “pharmacokinetics” [Title]) AND (“Levodopa” [Title] OR “L-dopa” [Title] OR “Apomorphin” [Title] OR “Apomorphine” [Title]).
The literature search was conducted on November 1, 2023. Any articles published more than 20 years ago were not included in the selection. Additionally, articles in languages other than French or English were automatically excluded by the search algorithm.
Inclusion and exclusion criteria
Regarding the inclusion criteria, the selected papers needed to delineate the pharmacology, as well as the Pk/Pd of oral L-DOPA administration, LCIG infusion, or continuous subcutaneous apomorphine administration.
In terms of exclusion criteria, papers that did not primarily focus on the Pk/Pd of the aforementioned molecules were not included. Additionally, articles examining non-general cases regarding the form of the drug, administration route, patient population, or associated pathologies were disregarded. Studies involving healthy volunteers or those investigating the bioequivalence of generics were also excluded.
There were no inclusion or exclusion parameters pertaining to the type of articles, which ranged from clinical trials and literature reviews to pharmacology articles.
Selection of articles
All titles and abstracts of the identified studies were initially screened by one reviewer (AD) in accordance with the inclusion and exclusion criteria. From this preliminary screening, full texts deemed potentially eligible were obtained and independently reviewed by reviewers (AD, CM and DD). Any discrepancies encountered were discussed and resolved.
Data extraction and synthesis
One reviewer (AD) carried out the data extraction process, with a second reviewer (DD) conducting an independent reliability check. Data, including lead author, publication date, journal, abstract, and other pertinent information, were extracted systematically into a standardized form.
RESULTS
Selection of articles
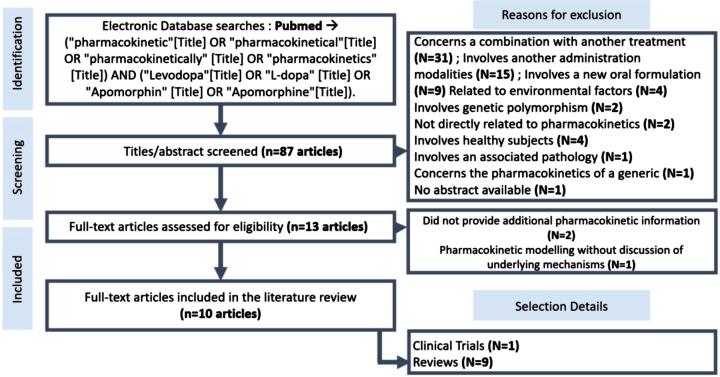
The selected articles are in the Table 1. As depicted in Fig. 3, the search of the PubMed database returned a total of 87 articles. Among those not selected, 31 were related to the Pk/Pd of L-DOPA in conjunction with other drugs, primarily COMT inhibitors. 15 articles were related to L-DOPA administration methods other than those under review, while nine articles compared the Pk of different oral L-DOPA formulations, such as conventional and sustained-release forms. Four articles investigated the influence of environmental factors on L-DOPA Pk, including the dietary intake of the subjects. Two articles analyzed the impact of genetic polymorphisms, particularly in relation to genes encoding enzymes involved in the conversion of L-DOPA to dopamine. Another four articles focused on healthy subjects, two were unrelated to L-DOPA Pk or the apomorphine molecule, and one article explored patients with another pathology concomitant with PD. One article compared the Pk profiles of a generic and a brand name oral L-DOPA formulation. Lastly, one article was not available; the initial selection was based solely on the article’s title and abstract.
Table 1.
Main results of the pharmacokinetic and pharmacodynamic analyses of L-DOPA in the selected articles (articles about CSAI, LCIG, and other continuous dopaminergic stimulation are also included)
| Article | Date | Study description | Information of interest |
| Lang and Lozano [1] | 1998 | Review | •Progressive degeneration of the nigro-striatal pathway, responsible for managing automatic motor and non-motor functions. •Dopamine scarcity manifests as physical symptoms, such as tremors, rigidity, and movement difficulties, including walking, but can also lead to cognitive and psychiatric disorders. |
| LeWitt [36] | 2004 | Review | •Pharmacokinetics and pharmacology of subcutaneously administered apomorphine. •Metabolism of apomorphine: oxidation, N-demethylation, metabolism by COMT, glucuronidation and sulfation. |
| Stocchi [46] | 2006 | Review | •Controlled-release L-DOPA preparations are not efficacious in reducing the incidence of motor fluctuations. •Adjunct therapies can be used to control symptoms and a variety of drugs can be employed to reduce OFF time, but do not address the underlying problem. |
| Khor and Hsu [37] | 2007 | Review | •Absorption of L-DOPA is via the saturable LNAA transport system for large amino acids. •A high protein diet may compete with the uptake of L-DOPA into the brain, therefore, may result in reduced L-DOPA effects. |
| Nutt [47] | 2008 | Review | •The motor fluctuations are not entirely a pharmacokinetic problem although continuous delivery certainly improves motor fluctuations by reducing ‘off time’. •Intermittent dopaminergic stimulation may also be important in the development of dyskinesia. |
| Contin and Martinelli [38] | 2010 | Review | •High pre-systemic metabolism of dopamine in the gut by the enzyme L-AADC. •Rapid intestinal absorption by a saturable facilitated transport system shared with other LNAA systems. •Facilitated transport from plasma to the brain (by the LNAA system). |
| Müller [9] | 2013 | Review | •Long-term L-DOPA use is complicated by the development of LDRC, with the short half-life of L-DOPA playing a central role in the development of these motor complications. •Pharmacokinetic investigations of three possibilities to solve the pharmacokinetic problems of L-DOPA: ∘ Sustained-release L-DOPA formulations: these did not delay onset of LDRC. The slower rise in plasma L-DOPA concentrations and reduced peak plasma concentration may prolong the duration of low, subtherapeutic concentrations of L-DOPA in patients with complex wearing-off. ∘ L-DOPA infusion systems: more stable plasma L-DOPA concentrations compared to intermittent standard-release L-DOPA formulations. •L-DOPA/carbidopa/entacapone: the longer half-life of L-DOPA with coadministration of entacapone can account for the longer durations of clinical effect of single L-DOPA/dopadecarboxylase inhibitor doses (26–56%). |
| LeWitt [26] | 2015 | Review | •Production of TOPA and TOPA-quinone à excitotoxic properties. •Production of 5-cysteinyldopa (a substrate for decarboxylation to form 5-S-cysteinyldopamine, a pharmacologically active compound that induces oxidative stress in dopaminergic neurons). •Challenges of L-DOPA transport to the brain. |
| Stoessl [43] | 2015 | Review | •This review provides in vivo validation for the hypothesis that pulsatile stimulation of dopamine receptors plays a critical role in the emergence of long-term motor complications of therapy. |
| Classen et al. [11] | 2017 | Review | •L-DOPA may lead to non-motor complications: fatigue, sleep disorders, psychiatric disorders (including mood disorders and anxiety), dysautonomia, pain, and cognitive disorders. |
| Othman et al. [39] | 2017 | Clinical Trial | •LCIG results in lower variability and fluctuations in L-DOPA and carbidopa plasma concentrations compared to LC-oral. •LCIG helps to bypass the impact of intra-subject variability in gastric emptying rate. •Even with stable plasma L-DOPA concentrations achieved with LCIG infusion, there are still many uncontrolled factors that contribute to altering the Pk/Pd response of L-DOPA, such as transport across the blood-brain barrier, enzymatic conversion of L-DOPA to dopamine, the storage capacity for dopamine in the dopaminergic nerve terminals, dopamine release at the effect site, and changes in pre- and post-synaptic dopamine receptor sensitivity. |
| Beckers et al. [35] | 2022 | Review | •Two mechanisms of peripheral L-DOPA resistance: •Excessive bacterial production of the enzyme TDC à SIBO. TDC is an enzyme which normally digests dietary tyrosine. However, TDC, produced by certain gut bacteria, can also decarboxylate L-DOPA, reducing its bioavailability. •Systemic induction of the enzyme aromatic L-AADC. |
| Added articles on LDRC | |||
| Juncos et al. [12] | 1989 | Review | •Validation of continuous dopaminergic stimulation in rats with unilateral 6-hydroxydopamine lesions. |
| Langston [2] | 2006 | Review | •Progressive degeneration of the nigro-striatal pathway, responsible for managing automatic motor and non-motor functions. •Dopamine scarcity manifests as physical symptoms such as tremors, rigidity, and movement difficulties, including walking, but can also lead to cognitive and psychiatric disorders. |
| Manson et al. [8] | 2012 | Review | •L-DOPA-induced dyskinesias affect 50% of patients within 5 years and 80% of patients after 10 years of disease progression. |
| Smith et al. [10] | 2012 | Review | •Rapid onset of Parkinsonian symptoms is linked to rapid degeneration of dopaminergic neurons (imaging of the nigrostriatal dopaminergic system). |
| Sato et al. [27] | 2018 | Case report | •Parkinsonian patients experience drug retention in the esophagus or the epiglottic vallecula. This retention can lower the peak plasma concentration of L-DOPA. |
| Added articles on the preclinical validation of continuous delivery therapies on LDRC | |||
| Smith et al. [13] | 2005 | Preclinical trial | •Validation of continuous dopaminergic stimulation in parkinsonian primates. |
| Bibbiani et al. [14] | 2005 | Preclinical trial | •Validation of continuous dopaminergic stimulation in parkinsonian primates. |
| Added articles on clinical validation of continuous delivery therapies on LDRC | |||
| Deleu et al. [15] | 2004 | Review | •Apomorphine shows effectiveness in improving motor scores and reducing ‘off’ periods, with continuous infusion also reducing dyskinesias and offering a L-DOPA-sparing effect. •The subcutaneous route facilitates the continuous administration of apomorphine. |
| Devos et al. [20] | 2009 | Clinical Trial | •LCIG seems to be an effective last-line therapy for motor complications in PD. Technical problems are commonplace and improvements should be considered. |
| Nyholm [22] | 2012 | Review | •The large majority of studies have reported that LCIG is clinically effective at relieving the symptoms of advanced PD and improving QoL in comparison to oral therapy. |
| Olanow et al. [21] | 2014 | Clinical Trial | •12-week, randomized, double-blind, double-dummy, double-titration trial, enrolled adults with advanced PD and motor complications. •2 arms: treatment with immediate-release oral levodopa-carbidopa plus placebo intestinal gel infusion or levodopa-carbidopa intestinal gel infusion plus oral placebo. •LCIG therapy results in an average increase of 1.9 h of dyskinesia-free “on” time, compared to optimized oral treatment. |
| Trenkwalder et al. [16] | 2015 | Review | •Subcutaneous apomorphine is recognized as an effective therapy for PD patients who experience “off” periods despite optimized oral medication. |
| Drapier et al. [17] | 2016 | Observational Study | •Assessment of QoL improvement by CSAI in patients with advanced PD. •At 6 months, their HR-QoL had significantly improved (p = 0.011), as had their total UPDRS score (p < 0.001). |
| Katzenschlager et al. [18] | 2018 | Clinical Trial | •Randomized, placebo-controlled, double-blind, multicenter trial •Apomorphine infusion (mean final dose 4 · 68 mg/h (SD: 1.5)) significantly reduced off time compared with placebo (–2.47 h per day (SD: 3.70) in the apomorphine group vs –0 · 58 h per day (SD: 2.80) in the placebo group; difference –1 · 89 h per day [95% CI: –3 · 16 to –0 · 62; p = 0 · 0025). |
| Antonini et al. 19] | 2018 | Medical Opinion | •Apomorphine administration enhances motor symptoms and patients’ quality of life but does not entirely remove the need for oral treatments. |
| Bergquist et al. [54] | 2022 | Clinical trial: randomized, 3-period cross-over, open-label multicenter trial | •Continuous subcutaneous and intravenous infusion with a continuously buffered acidic levodopa/carbidopa solution yields steady state plasma concentrations of levodopa that are equivalent in magnitude, and non-inferior in variability, to those obtained with LCIG in patients with advanced PD. |
| Added articles on deep brain stimulation as another second-line treatment of LDRC | |||
| Perestelo-Pérez et al. [50] | 2014 | Meta analysis | •Meta-analysis of RCTs describes the efficacy of DBS in improving motor signs, functionality and QoL of PD patients. •RCTs that compared DBS plus medication versus medication (alone or plus sham DBS) in PD patients were included. •More than 50% of the oral treatment is maintained under subthalamic stimulation. |
| Bratsos et al. [49] | 2018 | Review | •Systematic search including RCT comparing DBS to BMT in PD patients. •DBS was superior to BMT at improving impairment/disability, QoL and reducing medication doses •More than 50% of the oral treatment is maintained under subthalamic stimulation. |
| Added article on continuous subcutaneous foslevodopa-foscarbidopa | |||
| Soileau et al. [24] | 2022 | Clinical trial | •12-week randomized, double-blind, double-dummy, active-controlled study. •Foslevodopa-foscarbidopa improved motor fluctuations, with benefits in both ‘on time’ without troublesome dyskinesia and ‘off time’. •Average increase in dyskinesia-free “On” periods of 1.7 h. |
| Added articles on intracerebral administration of anaerobic dopamine | |||
| Laloux et al. [51] | 2017 | Preclinical trial | •A-dopamine restored motor function and induced a dose dependent increase of nigro-striatal tyrosine hydroxylase positive neurons in mice (MPTP treated) after 7 days. •The safety profile is highly favorable as A-dopamine did not induce dyskinesia or behavioral sensitization as observed with peripheral L-DOPA treatment. |
| Moreau et al. [52] | 2020 | Preclinical trial | •60 days of a continuous circadian i.c.v. of A-dopamine improved motor symptoms of MPTP treated primates without tachyphylaxis. No dyskinesia was observed even with very high doses. |
PD, Parkinson’s disease; L-DOPA, levodopa; L-AADC, L-amino acid decarboxylase; COMT, catechol-O-methyl transferase; LCIG, levodopa-carbidopa intestinal gel; LNAA, large neutral amino acids; SIBO, small-intestinal bacterial overgrowth; TDC, tyrosine decarboxylase; TOPA, 2,4,5-trihydroxyphenylalanine; Pk, pharmacokinetics; Pd, pharmacodynamics; LDRC, L-DOPA-related complications; QoL, quality of life; CSAI, Continuous Subcutaneous Apomorphine Infusion; SD, standard deviation; DBS, deep brain stimulation; RCT, randomized controlled trial; BMT, Best Medical treatment; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
Fig. 3.
Flowchart showing the search for articles in the PubMed database.
DISCUSSION
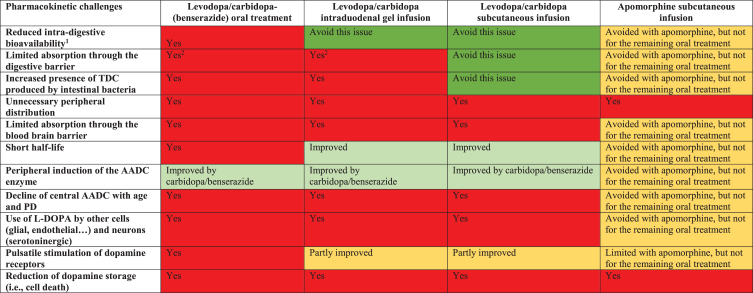
The Pk/Pd challenges stemming from oral L-DOPA administration are outlined alongside the effects of the two commercially available continuous dopaminergic delivery therapies, namely LCIG and the apomorphine infusion pump. The various Pk limitations of L-DOPA and how continuous delivery therapies can address them are summarized in Table 2.
Table 2.
Pharmacokinetic problems with oral L-DOPA and the value of continuous dopaminergic administration therapies in resolving these problems
The color code represents the degree of impact of pharmacological bias (essentially pharmacokinetic) on the dopaminergic strategy. Red: significant impact; orange: partial impact (because 50–70% of the oral treatment is maintained), with a light vs. dark gradation if it is significant; green: therapeutic strategy not affected, with a light vs. dark gradation if it is significant. PD, Parkinson’s disease; TDC, tyrosine decarboxylase; AADC, amino acid decarboxylase.
Short half-life of L-DOPA
L-DOPA has a half-life of about 90 min [26], requiring patients to take doses very frequently (every 2–3 h) throughout the day to maintain consistent control over motor symptoms. The intermittent intake of L-DOPA requires the neurons in the substantia nigra to store the compound. As the disease advances, the number of these neurons diminishes, reducing this storage capacity. This progression compels patients to decrease the intervals between doses, creating greater Pk inconsistencies and resulting in motor fluctuations. The short half-life, combined with the increased dosing frequency leads to abnormal, large-scale, and intermittent activation of dopaminergic neurons.
The Pk challenges arising from the short half-life of L-DOPA are significantly alleviated by LCIG and FL/FC due to its continuous delivery mechanism. Additionally, the subcutaneous infusion of apomorphine mitigates this issue by lessening the dependence on oral L-DOPA administration.
Variable intragastric bioavailability
One of the challenges with oral L-DOPA administration is its variable intragastric bioavailability, which can be influenced by several factors.
Dysphagia, characterized by difficulty in swallowing saliva, liquids, or solid food, becomes prevalent with the progression of certain diseases. It is primarily caused by bradykinesia of the posterior region of the tongue and the jaw. Such a condition can result in aspiration, potentially leading to pulmonary infections. Additionally, it can cause dehydration and malnutrition. Importantly, dysphagia can also influence the response to oral medications, causing phenomena like “delayed-on” (a prolonged latency in response to a dose) and “no-on” (total dose ineffectiveness). Various clinical cases have identified instances where patients experience drug retention in the esophagus or the epiglottic vallecula. This retention can lower the peak plasma concentration of L-DOPA. However, reports in the literature have shown that treating dysphagia effectively, including through speech therapy, can revert to a more standard Pk profile for oral L-DOPA [27].
Currently available continuous dopamine delivery therapies offer solutions to these challenges. LCIG and FL/FC directly bypass the issues arising from dysphagia, ensuring more consistent drug bioavailability. Subcutaneous infusion of apomorphine provides a partial solution by diminishing the need for oral treatments, thus reducing the complications associated with dysphagia.
Gastrointestinal transit disturbances [28]
Constipation stands out as a prevalent non-motor symptom in PD, affecting more than 80% of patients [29, 30]. Often, this symptom is attributed to impairment of the autonomic nervous system, resulting in reduced digestive tract motility. Interestingly, constipation can manifest up to a decade before the emergence of motor symptoms. Various factors influence intestinal mobility and the movement of its contents, such as neurohumoral influences, an imbalance in the intestinal microbiota, with evidence suggesting the efficacy of probiotics as a therapeutic approach [31], intestinal inflammation, medication effects, and overall lifestyle habits.
Beyond the pronounced effect on a patient’s well-being and quality of life, gastroparesis, and its consequent delay in gastric emptying, hampers optimal absorption and the efficacy of orally administered drugs, like L-DOPA [32–34]. This results in increased pre-systemic decarboxylation, further diminishing the drug’s therapeutic potential.
LCIG and apomorphine improve this challenge. FL/FC overcomes this challenge.
Alterations in the gut microbiota and the role of tyrosine decarboxylase (TDC) production
It is important to highlight the changes in composition of the gut microbiota when discussing the Pk challenges of L-DOPA. Beckers et al. [35] reviewed multiple studies that indicated an elevated presence of specific bacterial genera in PD patients, notably Akkermansia (observed in 12 studies), Lactobacillus (10 studies), and Bifidobacterium (four studies). This increased bacterial presence could be attributed to the compromised intestinal motility inherent to PD. Interestingly, certain bacteria within these genera, specifically Enterococcus faecalis, Enterococcus farcium, Lactobacillus brevis, and Providencia rettgeri are known to produce tyrosine decarboxylase (TDC). While TDC primarily facilitates dietary tyrosine digestion, it also has the capability to decarboxylate L-DOPA. Intriguingly, there is a positive correlation between jejunal expression levels of TDC encoding genes and the required daily L-DOPA dosage for symptom control in PD patients. Conversely, there is a negative correlation with circulating plasma L-DOPA levels.
LCIG is not entirely exempt from these challenges but, compared to oral treatments, its susceptibility is diminished. The administration of apomorphine subcutaneously sidesteps gastrointestinal absorption, thus ameliorating, to a degree, the Pk challenges, although residual oral treatments might still present complications [36].
FL/FC overcomes this challenge.
Selective absorption challenges of L-DOPA in the digestive system
Absorption of L-DOPA is selective and intricate. The molecule depends on sodium-driven active transport mechanisms for its passage across both the digestive and blood-brain barriers. Furthermore, the transport of neutral L-amino acids, like L-DOPA, is restricted to a specific segment, primarily the duodenum and the initial portion of the jejunum. These unique Pk traits lead to an absorption rivalry between certain dietary components, specifically L-neutral amino acids from dietary proteins and orally administered L-DOPA [37]. As a result, after consuming a meal, patients might experience motor blockade episodes or notice variations in the Pk of their oral medications based on the composition of their meal. Given these challenges, the common recommendation is for patients to ingest their L-DOPA dose at least 45 min prior to eating, which reduces but does not eliminate the lower biodistribution.
LCIG does not overcome this challenge. The administration of apomorphine subcutaneously sidesteps gastrointestinal absorption, thus ameliorating, to a degree, the Pk challenges, although residual oral treatments might still present complications. FL/FC overcomes this challenge.
Peripheral distribution
The majority of orally-administered L-DOPA undergoes first-pass hepatic metabolism or is distributed in the skeletal muscle [26]. This molecule exhibits a pronounced peripheral distribution, as various organs and tissues, including the stomach, liver, kidneys, and even endothelial cells of blood vessels, possess dopaminergic receptors. Such widespread distribution has dual implications. On the one hand, it triggers side-effects such as nausea or hypotension (the latter results from the activation of D1-like receptors causing vasodilation). On the other, it significantly restricts the amount of L-DOPA reaching the blood-brain barrier. Highlighting the scope of peripheral L-DOPA decarboxylation, it is notable that nearly 45% of the body’s total dopamine is synthesized within the epithelial cells of the gastrointestinal tract. L-DOPA is routinely co-administered with a COMT inhibitor, which plays a role in converting L-DOPA to 3-O-methyldopa. In clinical scenarios, this inhibitor is believed to extend the effect of L-DOPA by approximately 20–30 min per dose. As noted previously, peripheral dopa decarboxylase activity inhibitors like carbidopa or benserazide are also employed to enhance the bioavailability of L-DOPA.
LCIG, FL/FC and apomorphine do not overcome this challenge.
Peripheral induction of the amino acid decarboxylase enzyme
One of the challenges with the Pk of oral L-DOPA stems from activation of the aromatic L- amino acid decarboxylase (L-AADC) enzyme. Triggered by decarboxylase inhibitors, this activation prompts the early conversion of L-DOPA to dopamine peripherally. This, in turn, significantly diminishes the bioavailability of L-DOPA in the brain. Studies indicate that when administered alongside peripheral AADC inhibitors such as carbidopa or benserazide, the bioavailability of oral L-DOPA increases threefold [38]. It is noteworthy that while the apomorphine pump reduces issues related to peripheral distribution, it does not fully eliminate them. Likewise, LCIG, despite its broad peripheral distribution, which is not needed for its neurological effects, presents similar challenges [39].
LCIG and FL/FC do not overcome this challenge. The administration of apomorphine subcutaneously ameliorates this challenge, although residual oral treatments may still cause complications.
Stimulation of different dopamine receptors
Dopamine, which is stimulated by the administration of L-DOPA in PD patients, can bind to various receptors. The five types of dopamine receptors differ in their central and peripheral locations, genes coding for their protein, affinity for dopamine, and the nature of the response their stimulation elicits. They are divided into two categories: D1-like receptors and D2-like receptors, depending on the type of G protein to which they couple. Stimulation of D1 and D5 receptors, which belong to the D1-like category and are located exclusively at the postsynaptic level, results in the production of cyclic AMP and depolarization of the neuronal membrane due to their association with the Gs protein. Conversely, D2-like receptors, which can be both presynaptic and postsynaptic, reduce the amount of cyclic AMP produced and induce hyperpolarization when activated, leading to a decrease in the number of neurotransmitters released.
While apomorphine is categorized as a dopamine agonist, it exhibits characteristics that differentiate it from other oral dopamine agonists. Its catechol moiety enables apomorphine to interact with a broad spectrum of D1- and D2-like receptors, including D1, D2 S, D2 L, D3, D4, and D5 [5, 20, 40]. However, the mechanisms by which apomorphine and dopamine influence dopaminergic neurotransmission are not identical. Dopamine neurons can oscillate between two distinct activity states: slow tonic firing and phasic burst firing [41]. While apomorphine can partially reinstate slow tonic firing through the stimulation of postsynaptic receptors, it lacks the capability to be taken up by the dopamine transporter. This means that, unlike dopamine, apomorphine does not enhance presynaptic dopamine availability and cannot potentiate phasic dopamine signals, a mechanism crucial for memory functions [42].
Variability and constraints of L-DOPA absorption across the blood-brain barrier
Similar to its interaction with the digestive barrier, the passage of L-DOPA through the blood-brain barrier depends on a saturable sodium-dependent transporter. This setup places L-DOPA in competition with other L-neutral amino acids that originate from protein degradation and various dietary amino acid sources. As a result, access of L-DOPA to the brain is both constrained and unpredictable [26].
LCIG and FL/FC do not overcome this challenge. The administration of apomorphine subcutaneously ameliorates this challenge, although residual oral treatments may still present complications.
Distribution of L-DOPA in serotoninergic neurons, astrocytes, and endothelial cells
After entering the central nervous system, L-DOPA is taken up by various cell types other than dopaminergic neurons through the L-type amino acid transporter [43]. These cells, which include serotoninergic neurons, glial cells, and endothelial cells, express L-AADC, enabling them to convert L-DOPA into dopamine and store it within vesicles [44]. Notably, these cell types lack the dopamine transporter DAT, rendering them incapable of reuptake of the synthesized dopamine, resulting in unregulated dopamine production. Research has indicated a link between serotoninergic neurons and L-DOPA-induced dyskinesia. Specifically, experiments employing 5,7-dihydroxytryptamine, a selective neurotoxin targeting serotonergic neurons, have led to the cessation of these L-DOPA-triggered side-effects.
LCIG and FL/FC do not overcome this challenge. The administration of apomorphine subcutaneously ameliorates this challenge, although residual oral treatments may still present complications.
Decline of central L-AADC with advancing age and PD progression
As PD progresses and with increasing age, there is a notable decline in the activity of L-AADC within dopaminergic neurons. This decline results in decreased conversion of L-DOPA into dopamine over time. While some research suggests that, even in the later stages of the disease, the activity of the enzyme remains sufficiently robust for L-DOPA to dopamine conversion [26], other studies have highlighted potential therapeutic avenues. For instance, gene therapy aimed at enhancing the expression of L-AADC has been shown to improve Parkinson’s symptoms [45].
LCIG and FL/FC do not overcome this challenge. The administration of apomorphine subcutaneously ameliorates this challenge, although residual oral treatments may still present complications.
Pd issues with the pulsatile stimulation of dopamine receptors
One of the core challenges faced in the treatment of PD stems from the pulsatile stimulation of dopamine receptors [46, 47], which can be attributed to the short half-life of L-DOPA and the Pk challenges associated with its oral, and to a lesser extent, enteral administration. Such unpredictable and uneven stimulation from L-DOPA often results in motor complications, including peak dose fluctuations, biphasic dyskinesias, the wearing-off phenomenon, and circadian off periods [48]. This has given rise to the idea of “continuous dopaminergic stimulation” aiming for steady L-DOPA administration to achieve more consistent dopamine receptor activation. Yet, a more precise term might be “continuous dopaminergic administration” because the treatments do not genuinely offer uninterrupted stimulation. Moreover, the stimulation of dopamine receptors is complex and multiple with at least a low and continuous tonic stimulation (i.e., inhibition) and a phasic one (i.e., activation). The inherent nature of L-DOPA and its misdistribution to cells other than dopaminergic neurons remain a significant hurdle, irrespective of administration continuity. Only direct dopamine administration could possibly sidestep this misdistribution challenge.
LCIG, FL/FC and apomorphine do not overcome this challenge.
Decline in dopamine storage due to neuronal death
As PD advances, the gradual death of dopamine neurons significantly impacts the Pk of L-DOPA. Imaging studies have indicated that with disease progression, there is a heightened release of dopamine in the brain following a L-DOPA dose, potentially explaining L-DOPA-induced dyskinesias. This can be attributed to multiple factors: the diminished capacity for vesicular dopamine storage, a decrease in dopamine reuptake sites due to vanishing DATs, and the unintended utilization of L-DOPA by non-dopaminergic cells. Continuous dopaminergic administration may reduce the impact of this problem on treatment pharmacokinetics. However, being only symptomatic treatments, LCIG, FL/FC, and apomorphine do not overcome this challenge.
How can continuous dopaminergic delivery therapies be improved?
While continuous dopaminergic delivery therapies can address some of the Pk challenges posed by oral L-DOPA, they cannot address many pharmacological challenges. This may explain why pivotal therapeutic trials showed only a 1.9-h improvement in On without troublesome dyskinesias compared to the placebo group with LCIG (22) or an apomorphine pump (18), and 1.7 h with FL/FC [24]. In fact, there was an improvement of around 2 h in the placebo group and less than 2 additional hours in the treated groups in patients who had an average of 6 h of non-control LDRC. It therefore appears that these therapeutic strategies, while clearly effective, offer only an incomplete solution to LDRC. Interestingly, brain stimulation does not improve the situation any further, with 3.25 h without placebo group (i.e., it was also around 4 h including the placebo effect for continuous dopaminergic delivery therapies). This may be explained by the fact that more than 50% of the oral treatment is maintained under subthalamic stimulation [49, 50]. This underlines the importance of progressing towards innovative continuous dopaminergic treatments, whether through new molecules, formulations, or administration methods, while taking these Pk/Pd challenges into account.
One of the most obvious explanations is that these LDRCs are intimately linked to L-DOPA use, and that as long as L-DOPA is used, it causes dyskinesias, even if LDRC and dyskinesias are less pronounced with continuous administration, they persist due to all the Pd mechanisms involved. Interestingly, there is one therapeutic innovation that could potentially overcome these mechanisms, namely the direct administration of dopamine at the intracerebral level. This strategy has never been developed previously, for fear of rapid and major oxidation of dopamine. However, a dopamine that is synthesized, stored, and administered anaerobically (A-dopamine) has recently been developed. This continuous cerebral infusion of A-dopamine involves surgical implantation of a thin catheter in the 3rd ventricle just proximal to the bilateral striatum, connected to a programmable pump under the abdominal skin. This telemetry-controlled pump continuously delivers an anaerobic solution of dopamine hydrochloride directly into the cerebrospinal fluid, enabling doses to be precisely adapted to circadian needs. Preclinical results in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) lesioned mice, 6 OH-dopamine lesioned rats, and MPTP lesioned monkeys demonstrated that intracerebral administration of A-dopamine induced a strong and continuous motor improvement. The most astounding preclinical finding was that even at very high doses of dopamine, no dyskinesia was observed in any of the three models. Conversely, oral L-DOPA induced dyskinesia even with the smallest effective dose [51, 52]. A Phase I/IIB clinical trial involving 12 patients is in progress (ClinicalTrials.gov ID: NCT04332276) [53]. Although clinical data and further Pk analysis are required, it is consistent to hypothesize that central administration of A-dopamine could limit peripheral and central Pk problems and Pd issues (i.e., pulsatile stimulation and dopamine misdistribution).
Conclusion
L-DOPA, combined with an AADC inhibitor, has improved the lives of millions of patients suffering from PD and remains a pivotal treatment. However, the many Pk/Pd limitations of L-dopa have a major impact on the lives of patients with the induction of severe LDRC. We have seen that most L-DOPA formulations and delivery strategies retain the many Pk/Pd limitations. This should encourage researchers and manufacturers to develop new dopaminergic stimulation strategies without L-DOPA, such as dopamine administration.
ACKNOWLEDGMENTS
We would like to thank Everpharma for its financial support and Val Hopwood, BSc, PhD, for proofreading the manuscript.
FUNDING
This review was funded by EverPharma.
CONFLICT OF INTEREST
AD is a former employee of InBrain Pharma. DD and CM are co-founders of InBrain Pharma and InVenis Biotherapies, in which they have an equity stake. CM is CMO of Feetme. DD and CM have provided consulting services or served on the scientific boards of Abbvie, Alterity, Orkyn, Air Liquide, Apopharma, Lundbeck, EverPharma, and Boston Scientific.
DATA AVAILABILITY
All data is available on request by e-mail from david.devos@chu-lille.fr.
REFERENCES
- [1]. Lang AE, Lozano AM (1998) Parkinson’s disease. N Engl J Med 39, 1130–1143. [DOI] [PubMed] [Google Scholar]
- [2]. Langston JW (2006) The Parkinson’s complex: Parkinsonism is just the tip of the iceberg. Ann Neurol 59, 591–596. [DOI] [PubMed] [Google Scholar]
- [3]. Gröger A, Kolb R, Schäfer R, Klose U (2014) Dopamine reduction in the substantia nigra of Parkinson’s disease patients confirmed by magnetic resonance spectroscopic imaging. PLoS One 9, e84081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow CW, Tanner C, Marek K; Parkinson Study Group (2004) Levodopa and the progression of Parkinson’s disease. N Engl J Med 351, 2498–2508. [DOI] [PubMed] [Google Scholar]
- [5]. Fahn S (1999) Parkinson disease, the effect of levodopa, and the ELLDOPA trial. Earlier vs Later L-DOPA. Arch Neurol 56, 529–535. [DOI] [PubMed] [Google Scholar]
- [6]. Malek N, Kanavou S, Lawton MA, Pitz V, Grosset KA, Bajaj N, Barker RA, Ben-Shlomo Y, Burn DJ, Foltynie T, Hardy J, Williams NM, Wood N, Morris HR, Grosset DG; PRoBaND clinical consortium (2019) L-dopa responsiveness in early Parkinson’s disease is associated with the rate of motor progression. Parkinsonism Relat Disord 65, 55–61. [DOI] [PubMed] [Google Scholar]
- [7]. Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30, 1591–1601. [DOI] [PubMed] [Google Scholar]
- [8]. Manson A, Stirpe P, Schrag A (2012) Levodopa-induced-dyskinesias clinical features, incidence, risk factors, management and impact on quality of life. J Parkinsons Dis 2, 189–198. [DOI] [PubMed] [Google Scholar]
- [9]. Müller T (2013) Pharmacokinetic considerations for the use of levodopa in the treatment of Parkinson disease: Focus on levodopa/carbidopa/entacapone for treatment of levodopa-associated motor complications. Clin Neuropharmacol 36, 84–91. [DOI] [PubMed] [Google Scholar]
- [10]. Smith Y, Wichmann T, Factor SA, DeLong MR (2012) Parkinson’s disease therapeutics: New developments and challenges since the introduction of levodopa. Neuropsychopharmacology 37, 213–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Classen J, Koschel J, Oehlwein C, Seppi K, Urban P, Winkler C, Wüllner U, Storch A (2017) Nonmotor fluctuations: Phenotypes, pathophysiology, management, and open issues. J Neural Transm (Vienna) 124, 1029–1036. [DOI] [PubMed] [Google Scholar]
- [12]. Juncos JL, Engber TM, Raisman R, Susel Z, Thibaut F, Ploska A, Agid Y, Chase TN (1989) Continuous and intermittent levodopa differentially affect basal ganglia function. Ann Neurol 25, 473–478. [DOI] [PubMed] [Google Scholar]
- [13]. Smith LA, Jackson MJ, Al-Barghouthy G, Rose S, Kuoppamaki M, Olanow W, Jenner P (2005) Multiple small doses of levodopa plus entacapone produce continuous dopaminergic stimulation and reduce dyskinesia induction in MPTP-treated drug-naïve primates. Mov Disord 20, 306–314. [DOI] [PubMed] [Google Scholar]
- [14]. Bibbiani F, Costantini LC, Patel R, Chase TN (2005) Continuous dopaminergic stimulation reduces risk of motor complications in parkinsonian primates. Exp Neurol 192, 73–78. [DOI] [PubMed] [Google Scholar]
- [15]. Deleu D, Hanssens Y, Northway MG (2004) Subcutaneous apomorphine: An evidence-based review of its use in Parkinson’s disease. Drugs Aging 21, 687–709. [DOI] [PubMed] [Google Scholar]
- [16]. Trenkwalder C, Chaudhuri KR, García Ruiz PJ, LeWitt P, Katzenschlager R, Sixel-Döring F, Henriksen T, Sesar Á, Poewe W; Expert Consensus Group for Use of Apomorphine in Parkinson’s Disease; Baker M, Ceballos-Baumann A, Deuschl G, Drapier S, Ebersbach G, Evans A, Fernandez H, Isaacson S, van Laar T, Lees A, Lewis S, Martínez Castrillo JC, Martinez-Martin P, Odin P, O’Sullivan J, Tagaris G, Wenzel K (2015) Expert Consensus Group report on the use of apomorphine in the treatment of Parkinson’s disease–Clinical practice recommendations. Parkinsonism Relat Disord 21, 1023–1030. [DOI] [PubMed] [Google Scholar]
- [17]. Drapier S, Eusebio A, Degos B, Vérin M, Durif F, Azulay JP, Viallet F, Rouaud T, Moreau C, Defebvre L, Fraix V, Tranchant C, Andre K, Courbon CB, Roze E, Devos D (2016) Quality of life in Parkinson’s disease improved by apomorphine pump: The OPTIPUMP cohort study. J Neurol 263, 1111–1119. [DOI] [PubMed] [Google Scholar]
- [18]. Katzenschlager R, Poewe W, Rascol O, Trenkwalder C, Deuschl G, Chaudhuri KR, Henriksen T, van Laar T, Spivey K, Vel S, Staines H, Lees A (2018) Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): A multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol 17, 749–759. [DOI] [PubMed] [Google Scholar]
- [19]. Antonini A, Stoessl AJ, Kleinman LS, Skalicky AM, Marshall TS, Sail KR, Onuk K, Odin PLA (2018) Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: A multi-country Delphi-panel approach. Curr Med Res Opin 34, 2063–2073. [DOI] [PubMed] [Google Scholar]
- [20]. Devos D, French DUODOPA Study Group (2009) Patient profile, indications, efficacy and safety of duodenal levodopa infusion in advanced Parkinson’s disease. Mov Disord 24, 993–1000. [DOI] [PubMed] [Google Scholar]
- [21]. Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, Vanagunas A, Othman AA, Widnell KL, Robieson WZ, Pritchett Y, Chatamra K, Benesh J, Lenz RA, Antonini A; LCIG Horizon Study Group (2014) Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: A randomised, controlled, double-blind, double-dummy study. Lancet Neurol 13, 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Nyholm D (2012) Duodopa® treatment for advanced Parkinson’s disease: A review of efficacy and safety. Parkinsonism Relat Disord 18, 916–929. [DOI] [PubMed] [Google Scholar]
- [23]. Senek M, Nielsen EI, Nyholm D (2017) Levodopa-entacapone-carbidopa intestinal gel in Parkinson’s disease: A randomized crossover study. Mov Disord 32, 283–286. [DOI] [PubMed] [Google Scholar]
- [24]. Soileau MJ, Aldred J, Budur K, Fisseha N, Fung VS, Jeong A, Kimber TE, Klos K, Litvan I, O’Neill D, Robieson WZ, Spindler MA, Standaert DG, Talapala S, Vaou EO, Zheng H, Facheris MF, Hauser RA (2022) Safety and efficacy of continuous subcutaneous foslevodopa-foscarbidopa in patients with advanced Parkinson’s disease: A randomised, double-blind, active-controlled, phase 3 trial. Lancet Neurol 21, 1099–1109. [DOI] [PubMed] [Google Scholar]
- [25]. Olanow CW, Espay AJ, Stocchi F, Ellenbogen AL, Leinonen M, Adar L, Case RJ, Orenbach SF, Yardeni T, Oren S, Poewe W; 006 study group (2021) Continuous subcutaneous levodopa delivery for Parkinson’s disease: A randomized study. J Parkinsons Dis 11, 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. LeWitt PA (2015) Levodopa therapy for Parkinson’s disease: Pharmacokinetics and pharmacodynamics. Mov Disord 30, 64–72. [DOI] [PubMed] [Google Scholar]
- [27]. Sato H, Yamamoto T, Sato M, Furusawa Y, Murata M (2018) Dysphagia causes symptom fluctuations after oral L-DOPA treatment in a patient with Parkinson disease. Case Rep Neurol 10, 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Xu J, Wang L, Chen X, Le W (2022) New understanding on the pathophysiology and treatment of constipation in Parkinson’s disease. Front Aging Neurosci 14, 917499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Fasano A, Visanji NP, Liu LWC, Lang AE, Pfeiffer RF (2015) Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol 14, 625–639. [DOI] [PubMed] [Google Scholar]
- [30]. Ivan IF, Irincu VL, Diaconu Ştefania, Falup-Pecurariu O, Ciopleiaş B, Falup-Pecurariu C (2021) Gastro-intestinal dysfunctions in Parkinson’s disease (Review). Exp Ther Med 22, 1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Tan AH, Hor JW, Chong CW, Lim S (2020) Probiotics for Parkinson’s disease: Current evidence and future directions. JGH Open 5, 414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Mukherjee A, Biswas A, Das SK (2016) Gut dysfunction in Parkinson’s disease. World J Gastroenterol 22, 5742–5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Doi H, Sakakibara R, Sato M, Masaka T, Kishi M, Tateno A, Tateno F, Tsuyusaki Y, Takahashi O (2012) Plasma levodopa peak delay and impaired gastric emptying in Parkinson’s disease. J Neurol Sci 319, 86–88. [DOI] [PubMed] [Google Scholar]
- [34]. Müller T, Erdmann C, Bremen D, Schmidt WE, Muhlack S, Woitalla D, Goetze O (2006) Impact of gastric emptying on levodopa pharmacokinetics in Parkinson disease patients. Clin Neuropharmacol 29, 61–67. [DOI] [PubMed] [Google Scholar]
- [35]. Beckers M, Bloem BR, Verbeek MM (2022) Mechanisms of peripheral levodopa resistance in Parkinson’s disease. NPJ Parkinsons Dis 8, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. LeWitt PA (2004) Subcutaneously administered apomorphine: Pharmacokinetics and metabolism. Neurology 62(6 Suppl 4), S8–11. [DOI] [PubMed] [Google Scholar]
- [37]. Khor SP, Hsu A (2007) The pharmacokinetics and pharmacodynamics of levodopa in the treatment of Parkinson’s disease. Curr Clin Pharmacol 2, 234–243. [DOI] [PubMed] [Google Scholar]
- [38]. Contin M, Martinelli P (2010) Pharmacokinetics of levodopa. J Neurol 257(Suppl 2), S253–261. [DOI] [PubMed] [Google Scholar]
- [39]. Othman AA, Rosebraugh M, Chatamra K, Locke C, Dutta S (2017) Levodopa-carbidopa intestinal gel pharmacokinetics: Lower variability than oral levodopa-carbidopa. J Parkinsons Dis 7, 275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Carbone F, Djamshidian A, Seppi K, Poewe W (2019) Apomorphine for Parkinson’s disease: Efficacy and safety of current and new formulations. CNS Drugs 33, 905–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Goto Y, Grace AA (2005) Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci 8, 805–812. [DOI] [PubMed] [Google Scholar]
- [42]. Breitenstein C, Korsukewitz C, Flöel A, Kretzschmar T, Diederich K, Knecht S (2006) Tonic dopaminergic stimulation impairs associative learning in healthy subjects. Neuropsychopharmacology 31, 2552–2564. [DOI] [PubMed] [Google Scholar]
- [43]. Stoessl AJ (2015) Central pharmacokinetics of levodopa: Lessons from imaging studies. Mov Disord 30, 73–79. [DOI] [PubMed] [Google Scholar]
- [44]. Sampaio-Maia B, Serrão MP, Soares-da-Silva P (2001) Regulatory pathways and uptake of L-DOPA by capillary cerebral endothelial cells, astrocytes, and neuronal cells. Am J Physiol Cell Physiol 280, C333–342. [DOI] [PubMed] [Google Scholar]
- [45]. Mittermeyer G, Christine CW, Rosenbluth KH, Baker SL, Starr P, Larson P, Kaplan PL, Forsayeth J, Aminoff MJ, Bankiewicz KS (2012) Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson’s disease. Hum Gene Ther 23, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Stocchi F (2006) The levodopa wearing-off phenomenon in Parkinson’s disease: Pharmacokinetic considerations. Expert Opin Pharmacother 7, 1399–1407. [DOI] [PubMed] [Google Scholar]
- [47]. Nutt JG (2008) Pharmacokinetics and pharmacodynamics of levodopa. Mov Disord 23 Suppl 3, S580–584. [DOI] [PubMed] [Google Scholar]
- [48]. Olanow W, Schapira AH, Rascol O (2000) Continuous dopamine-receptor stimulation in early Parkinson’s disease. Trends Neurosci 23(10 Suppl), S117–126. [DOI] [PubMed] [Google Scholar]
- [49]. Bratsos S, Karponis D, Saleh SN (2018) Efficacy and safety of deep brain stimulation in the treatment of Parkinson’s disease: A systematic review and meta-analysis of randomized controlled trials. Cureus 10, e3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Perestelo-Pérez L, Rivero-Santana A, Pérez-Ramos J, Serrano-Pérez P, Panetta J, Hilarion P (2014) Deep brain stimulation in Parkinson’s disease: Meta-analysis of randomized controlled trials. J Neurol 261, 2051–2060. [DOI] [PubMed] [Google Scholar]
- [51]. Laloux C, Gouel F, Lachaud C, Timmerman K, Do Van B, Jonneaux A, Petrault M, Garcon G, Rouaix N, Moreau C, Bordet R, Duce JA, Devedjian JC, Devos D (2017) Continuous cerebroventricular administration of dopamine: A new treatment for severe dyskinesia in Parkinson’s disease? Neurobiol Dis 103, 24–31. [DOI] [PubMed] [Google Scholar]
- [52]. Moreau C, Rolland AS, Pioli E, Li Q, Odou P, Barthelemy C, Lannoy D, Demailly A, Carta N, Deramecourt V, Auger F, Kuchcinski G, Laloux C, Defebvre L, Bordet R, Duce J, Devedjian JC, Bezard E, Fisichella M, Devos D (2020) Intraventricular dopamine infusion alleviates motor symptoms in a primate model of Parkinson’s disease. Neurobiol Dis 139, 104846. [DOI] [PubMed] [Google Scholar]
- [53]. Moreau C, Odou P, Demailly A, Touzet G, Reyns N, Carta N, Barthelemy C, Lannoy D, Palas B, Gouges B, Devedjian JC, Defebvre L, Fisichella M, Devos D (2022) Continuous circadian intracerebroventricular administration of anaerobically preserved dopamine greatly reduces severe L-dopa-related complications in Parkinson’s disease [abstract]. Mov Disord 37(suppl 2), https://www.mdsabstracts.org/abstract/continuous-circadian-intracerebroventricular-administration-of-anaerobically-preserved-dopamine-greatly-reduces-severe-l-dopa-related-complications-in-parkinsons-disease/ [Google Scholar]
- [54]. Bergquist F, Ehrnebo M, Nyholm D, Johansson A, Lundin F, Odin P, Svenningsson P, Hansson F, Bring L, Eriksson E, Dizdar N (2022) Pharmacokinetics of intravenously (DIZ101), subcutaneously (DIZ102), and intestinally (LCIG) infused levodopa in advanced Parkinson disease. Neurology 99, e965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is available on request by e-mail from david.devos@chu-lille.fr.