Abstract
Background:
Emerging evidence underscores the high prevalence of neurobehavioral difficulties like ADHD, ASD and OCD, in patients with Duchenne muscular dystrophy (DMD). The substantial impact of these complex behavioral challenges in addition to motor function decline on the well-being of affected individuals and their families is increasingly evident. However, a uniform approach for effective screening, assessment and management of the neurobehavioral symptoms remains elusive.
Objective:
We explored strategies used by healthcare professionals with clinical expertise in DMD to address neurobehavioral symptoms, in order to uncover diverse practices and to identify potential directions for clinical approaches in managing DMD neurobehavioral symptoms.
Methods and results:
Twenty-eight respondents from 16 different countries completed an online survey. Only 35% of the centers systematically screened for neurobehavioral difficulties in their DMD population. Predominant screening methods included history taking and clinical observation. Common neurobehavioral difficulties encompassed learning challenges, dependency from adults, anxiety, concentration difficulties, and social deficits. The participating centers frequently employed parental counseling and liaison with psychosocial healthcare professionals for psychosocial intervention.
Conclusion:
This study underscores the complex behavioral landscape in DMD, highlighting the need for validated screening, assessment and management strategies and collaborative efforts in implementing these. We advocate for international consensus recommendations for screening, assessment and management of neurobehavioral difficulties in DMD to enhance patient care and communication across healthcare settings.
Keywords: Duchenne muscular dystrophy, behavior, psychology, surveys and questionnaires
INTRODUCTION
Duchenne muscular dystrophy (DMD) is a severe neuromuscular disorder caused by a mutation in the dystrophin gene, resulting in progressive muscle atrophy and weakness [1]. In addition, an accumulating body of evidence underscores the complex overlap with cognitive and behavioral comorbidities, referred to as neurobehavioral difficulties in this paper [2–8]. These neurobehavioral symptoms are recognized as intrinsic components of the disease, manifesting an important impact on the overall quality of life of affected individuals and their caregivers [2–8]. Patients with DMD are at elevated risk of developing psychiatric disorders, including attention deficit and hyperactivity disorder (ADHD), autism spectrum disorder (ASD), obsessive-compulsive disorder (OCD), as well as anxiety and depressive disorders [9, 10]. Moreover, research has shown that patients may exhibit multiple psychiatric diagnoses or display combinations of disruptive behavioral patterns that do not neatly fit into conventional psychiatric classifications [4, 6, 11, 12]. This diversity in neurobehavioral difficulties has been attributed to the multifactorial nature of DMD, including the interplay of genetic, environmental, and psychosocial factors [5]. Despite growing scientific knowledge, a noticeable gap persists between the management of the physical symptoms and the neurobehavioral aspects associated with DMD. This discordance mirrors within clinical guidelines, which provide detailed guidance for the physical facets but offer less explicit direction regarding the neurobehavioral problems [13, 14].
The evolving therapeutic landscape, particularly with the emergence of gene therapy, introduces an additional layer to contemplate. These therapies predominantly target the muscular challenges linked to DMD, with potential extension of patient lifespan [15]. However, the neurobehavioral dimensions remain relatively under-addressed. With prolonged life expectancy, behavioral challenges will become even more important to address in order to enhance the overall quality of life for both patients and their families [16].
This study investigated how healthcare professionals in diverse countries, screen, assess and manage neurobehavioral symptoms in the clinical care for DMD patients, and which neurobehavioral symptoms are frequently reported. By gaining insights from a multidisciplinary cohort of healthcare professionals across different centers in different countries, we aimed to chart current practices to learn which next steps are essential to more effectively address the neurobehavioral difficulties interwoven with DMD.
MATERIAL AND METHODS
To answer these research goals, an explorative study was conducted through an online survey directed to clinical DMD experts. The survey consisted of 20 questions to be completed via the online tool “Google Forms” (Appendix 1). All questions were composed and approved by a team of paediatric neurologists (LDW and NG) and child behavioral specialists (SG and JL) with expertise in DMD. The first ten questions were about the occupational context of the participating healthcare professionals, followed by five questions concerning the methodologies they use to screen for behavioral difficulties in boys with DMD within their respective clinical setting. Further, one question probed how frequently 45 different types of behavior were seen in their DMD population, one question asked how they screen, assess, and manage neurobehavioral symptoms in their clinical practice and another question evaluated their opinion on how neurobehavioral symptoms in DMD should be approached. The last two questions were open questions for comments or to express their interest to collaborate around this topic.
A link to this survey was emailed to 64 clinicians working with children and adults with DMD or research experts who had published about brain, cognition, or behavior in DMD. Eligible participants were selected by NG based on the participant list of the 2018 World Muscle Society congress. A first email was sent on 13 June 2019 and a reminder email was sent on 5 July 2019. The survey was closed on 15 July 2019.
Before starting the survey, participants had to indicate if they gave permission to use their answers for research and publication goals. All results were processed anonymously.
Data analysis
Data analysis was explorative and therefore descriptive. Frequencies and percentages of responses were calculated using SPSS v28 [17]. Four questions existed of a 5-point Likert-scale (question 14, 16, 17 and 18). The responses of those questions were as follows:
-
-
Question 14: Never; Rarely; When indicated; Systematically; Always
-
-
Question 16 & 17: Never; Rarely; Sometimes; Often; Very often
-
-
Question 18: I strongly disagree; I disagree; Neutral; I agree; I strongly agree
RESULTS
Participants
Twenty-eight DMD experts (43,75%) completed the survey. Table 1 gives an overview of the main characteristics of the participants. We have representation of 4 different continents: Europe (n = 20), North America (n = 5), South America (n = 2) and Australia (n = 1). Most of the responders were working in a hospital (n = 23), a rehabilitation center (n = 3), a university (n = 1) or a research facility (n = 1). All participants indicated to be part of a multidisciplinary team.
Table 1.
Demographical variables
| n | |
| Total participants | 28 |
| Different countriesa | 16 |
| What is your discipline? | |
| (Child) Neurologist | (15) 18 |
| General pediatrician | 4 |
| Rehabilitation specialist | 3 |
| Psychologist | 1 |
| General practitioner | 1 |
| Social worker | 1 |
| How many patients with DMD do you treat? | |
| >200 | 8 |
| 150–200 | 3 |
| 100–150 | 4 |
| 50–100 | 6 |
| 0–50 | 7 |
| How many years of experience do you have with DMD? | |
| >10 years | 24 |
| 5–10 years | 3 |
| 0–5 years | 1 |
| What kind of patients do you see? | |
| Children &adolescents | 17 |
| Children, adolescents &adults | 9 |
| Adults | 2 |
aBelgium (n = 4), United States of America (n = 2), Czech Republic (n = 1), Italy (n = 1), The Netherlands (n = 3), United Kingdom (n = 5), Turkey (n = 1), Argentina (n = 1), Canada (n = 3), Germany (n = 1), Norway (n = 1), France (n = 1), Switzerland (n = 1), Denmark (n = 1), Australia (n = 1), Chile (n = 1).
Screening for behavioral difficulties
Thirty-five percent of the participants responded that screening for behavioral difficulties in boys with DMD is performed systematically. The majority of respondents (54%) stated that active screening for such difficulties is conducted, albeit without a standardized approach. Two participants reported to solely engage in screening upon parental request, while one participant does not perform screening at all. Seventy-eight percent of the participants (n = 22) reported the lack of a dedicated protocol to screen for neurobehavioral functioning in boys with DMD. The responsibility to screen lies with the medical specialist (n = 19), followed by the psychologist within the team (n = 15), a social worker (n = 6), a nurse (n = 3), or a physiotherapist (n = 1). Forty percent of the participants do not have a psychologist or behavioral specialist in their team and even more participants (about 65%) reported to not have a speech therapist in their team. Further insights into the multidisciplinary composition of the multidisciplinary teams of the participants is presented in Table 2.
Table 2.
Rate of disciplines represented in neuromuscular teams
| Discipline | n (%) |
| Physiotherapist | 28 (100%) |
| Nurse | 26 (93%) |
| Occupational therapist | 19 (68%) |
| Dietician | 19 (68%) |
| Social worker | 18 (64%) |
| Psychologist | 17 (61%) |
| Speech therapist | 10 (36%) |
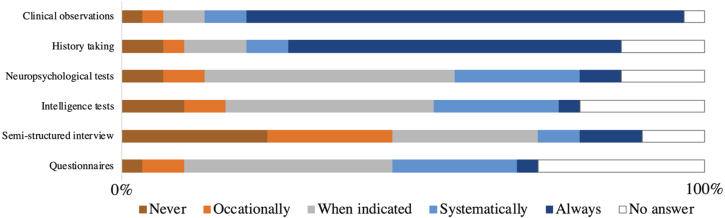
History taking and clinical observation emerged as predominant techniques for screening (Fig. 1). Behavioral questionnaires, intelligence tests, and other neuropsychological assessments are primarily employed on an as-needed basis. However, one participant consistently incorporates these tools, while six participants report to administer them across a wide age range, but not systematically following a protocol. Semi-structured interviews are predominantly only conducted when deemed appropriate.
Fig. 1.
Which instrument do you use to screen for behavioral problems? This figure represents the number of answers that were given to this question by experienced clinical experts in the DMD field ( n = 28).
Reported neurobehavioral symptoms
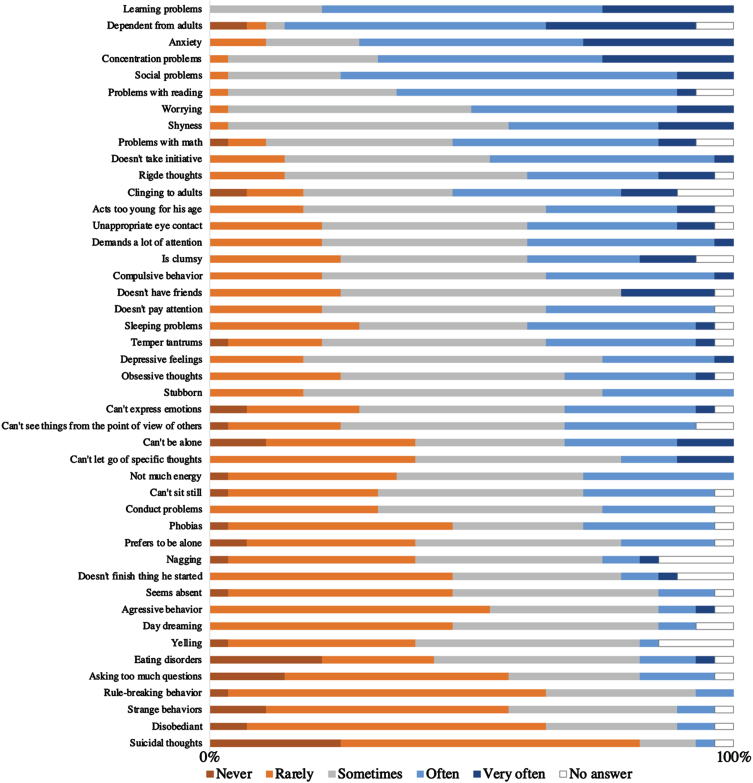
Figure 2 depicts the types of neurobehavioral symptoms consistently reported by experts as being most frequently observed in boys with DMD within their clinical practice. Predominant neurobehavioral problems included learning difficulties, reliance on adults, manifestations of anxiety, concentration issues, and social challenges. Between 65% and 85% of participants indicated that they encounter these neurobehavioral problems often or very often during their clinical practice. Occurrence of suicidal thoughts was exceptionally rare, with 85% of participants noting these instances as occasionally or never encountered.
Fig. 2.
How often do you see this type of behavior in boys with DMD in your clinical practice? This figure represents the number of answers that were given to this question by experienced clinical experts in the DMD field ranked from most frequent to less frequent reported, based on average scores (n = 28).
Management of neurobehavioral problems
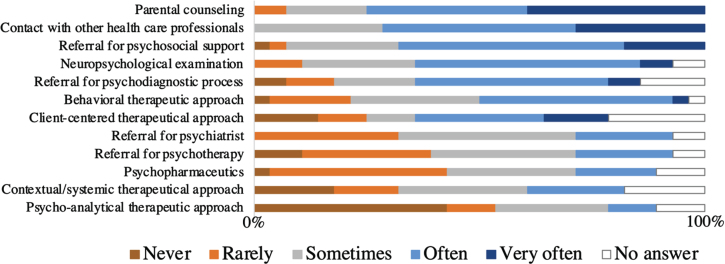
Among interventions to address neurobehavioral problems in boys with DMD, participants commonly resort to parental counseling, with 78% indicating frequent utilization (Fig. 3). Additional interventions include predominantly liaising with other healthcare professionals (71% often to very often), referring for psychosocial support (68% often to very often), and conducting neuropsychological assessments (62% often to very often). Behavioral therapeutic strategies appear to be more frequently used compared to other forms of psychotherapeutic interventions. Psychopharmacological approaches are occasionally implemented among participants.
Fig. 3.
Which types of interventions do you use and how often? This figure represents the number of answers that were given to this question by experienced clinical experts in the DMD field ( n = 28).
Opinion about management of behavioral issues in DMD
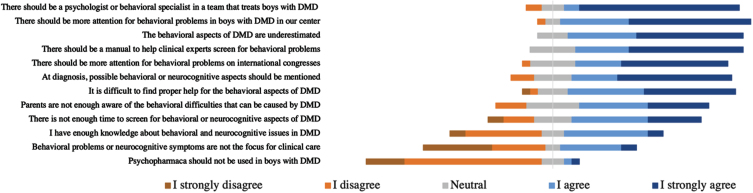
In the survey, participants were asked to give their opinion on 12 statements about management of behavioral issues in the field of DMD. Figure 4 shows these statements with a rate of agreement of the participants on those statements. Most participants (75%) totally agreed with the statement that there should be a behavioral specialist in a team taking care of boys with DMD. Almost all participants agreed or totally agreed with the statements that there should be more attention for behavioral problems in boys with DMD in their center (96%) and that those behavioral aspects are generally underestimated (100%).
Fig. 4.
Do you agree with following statements on the management of behavioral issues in boys with DMD? This figure represents the number of answers that were given to this question by experienced clinical experts in the DMD field ( n = 28).
DISCUSSION
The present study sheds a first light on which neurobehavioral difficulties in individuals with DMD are most often encountered by different DMD experts in different countries and healthcare institutions. Further, it explores how screening, assessment and management is performed in different neuromuscular centers. In the majority of participating healthcare centers, the responsibility to screen for neurobehavioral symptoms primarily falls upon medical specialists, primarily pediatricians. This seems intuitively sensible given their regular and proximate interactions with patients and their families. Nevertheless, previous research in the context of other medical conditions has showed that many doctors and pediatricians often encounter substantial barriers in fulfilling this role [18]. They report several constraints such as limited time, inadequate training, insufficient expertise, and diminished confidence when it comes to screening for neurobehavioral issues and counselling for patients and their families on these difficulties [19]. This reflects in the current study, where nearly half of the respondents report insufficient knowledge about neurobehavioral difficulties in DMD. Furthermore, almost all participants strongly agree on the need for behavioral specialists, guidelines, and tools to address neurobehavioral difficulties in DMD. These findings are of particular relevance, especially considering the fact that the complexity of care for DMD patients increases with time, and a multifaceted approach is essential to address all aspects of their well-being [13, 14].
While a multitude of multidisciplinary teams boast the inclusion of physical health specialists such as physiotherapists and nurses, access to psychologists remains relatively scarce, with fewer than two-thirds of the teams having direct access to this service. A notable disparity was found between the perceived necessity for psychologist involvement and their reported presence within multidisciplinary teams. This incongruity might stem from gaps in the distribution of resources. Some centers may have the resources to integrate psychosocial professionals into their neuromuscular teams, while others may rely on networks of mental healthcare providers for referrals in cases of significant neurobehavioral difficulties. Unfortunately, some centers may lack access to these resources entirely. Consequently, DMD patients may receive different care for the neurobehavioral aspect of their disease based on geographical location, rather than clinical need. This discrepancy contradicts clinical practice guidelines advocating for a standardized approach across countries, regions, and centers. While referral for profound neurobehavioral problems is a crucial first step, the integration of psychosocial care within a team enables early interventions that can help in the prevention of the development of psychopathological behavior. In general, our findings corroborate existing literature, pointing out the need for systematic screening, assessment and management strategies tailored to the specific needs of this population [20].
Future directions
Our findings underscore the diverse nature of behavioral issues in DMD, including learning difficulties, reliance on adults, manifestations of anxiety, concentration issues, and social challenges. Undoubtedly, not every behavior represents a neurobehavioral difficulty, and certain behaviors may align with typical developmental stages. However, the survey questions were designed to gather input from experts regarding the behaviors most commonly encountered in their clinical practice. We operated under the assumption that behaviors discussed or perceived as significant by experts are indicative of a notable burden or challenge. It follows that normal, non-disturbing behaviors would be less frequently mentioned in a clinical context. Consequently, our study captures a selective group of behaviors deemed significant by clinicians, reflecting the challenges encountered in the care of individuals with DMD. The current study aimed to inventory specific behaviors without categorizing them into domains of functioning or psychiatric classification models. However, the findings suggest the importance of future research in this area.
The diverse nature of these neurobehavioral difficulties emphasizes the need for a systematic approach with proper screening and assessment, and multidisciplinary management. However, they also reveal a strong agreement that neurobehavioral problems in DMD are still underestimated by parents and clinicians, and they receive inadequate attention in clinical practice and in research. Despite a consensus that neurobehavioral problems should be a focus point in clinical care, our results align with previous findings that clinicians face challenges such as time constraints, lack of suitable instruments, and an insufficient network for psychosocial referrals [18]. Furthermore, the variations in reported screening tools, interventions, and perceptions related to psychological healthcare professionals call for a collective effort to establish uniformity, standardized assessment tools, and evidence-based guidelines [21–23]. Achieving such consensus within the DMD community would not only ensure comprehensive biopsychosocial patient care, but also facilitate coherent communication across diverse healthcare and research settings.
Exemplifying this need, initiatives in the Human Immunodeficiency Virus (HIV) and Tuberous Sclerosis Complex (TSC) communities demonstrate how international collaboration can elevate awareness and standardize communication, resulting in consensus recommendations for managing neurobehavioral difficulties in these populations [24–30]. Notably, the successful implementation of the concept TSC-Associated Neuropsychiatric Disorders (TAND) and related initiatives in the TSC community have provided a unified nomenclature, improving clinical and research communication and emphasizing the importance of addressing complex neurobehavioral dimensions in TSC [31]. Inspired by these efforts, we propose the term DuMAND (Duchenne Muscular Dystrophy-Associated Neurobehavioral Difficulties) as umbrella term for the full range of manifestations associated with DMD that relate to behavior, psychiatric disorders, and intellectual, academic, neuropsychological, and psychosocial disabilities. Next steps should involve the identification of all manifestations specific to DMD and the development of an international consensus on a more uniform clinical care approach for screening, assessment and management of these DuMAND symptoms.
Several limitations warrant consideration when interpreting the findings of this study. Firstly, the sample selection, although comprised of prominent and experienced DMD centers, might limit its representativeness for smaller or less experienced institutions. We took a deliberate approach in selecting participants for our study, ensuring invitations were extended only to experts affiliated with recognized neuromuscular centers engaged in international collaboration and participation in international congresses. Despite varying backgrounds, the majority of respondents (26 out of 28) were trained as medical specialists. Furthermore, most participants possess over a decade of experience in the DMD field, with 75% of them treating more than 50 patients annually. While a larger sample size would have been favorable, DMD remains a rare disease, with expertise concentrated in specific centers within each country. Nevertheless, we believe we have successfully reached a substantial portion of the most renowned DMD experts globally. Secondly, as the study’s methodology relied on a survey approach, responses could be influenced by recall bias or misunderstanding of certain wordings. Due to the reliance on self-reporting, the potential for social desirability bias cannot be entirely dismissed. Finally, the scope of our investigation was confined to a specific (pre-COVID-19) time frame, and therefore longitudinal insights or dynamic changes over time may not have been fully captured. The recent interest in scientific literature focusing on neurobehavioral difficulties suggests an increased awareness for this aspect of the disease, potentially leading to advancements in clinical care. However, the need for consistent screening methods remains and consensus regarding the standard of care for management of these difficulties is still missing.
In conclusion, our study underlines the complex and diverse nature of neurobehavioral difficulties in DMD and the challenge of addressing these in clinical practice. Our findings emphasize the urgent need for international collaboration in developing unified protocols that enhance the quality of care for individuals and their families struggling with the complex behavioral dimensions of DMD.
Supplementary Material
ACKNOWLEDGMENTS
We genuinely thank all participants for contributing to this study.
This research project was supported financially by the Rondou Fund and the Kan-Go! Fund, KU Leuven, Leuven; Belgium.
This work is generated within the European Reference Network for Rare Neuromuscular Diseases ERN EURO-NMD; Project ID No. 739543.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JND-230251.
AUTHOR CONTRIBUTIONS
SG, JL, NG and LDW were involved in the conceptualization and design of the study. SG was involved in the acquisition and analysis of the data. SG, JL, NG, ND and LDW played an important role in the interpretation of the results. SG drafted a significant proportion of the manuscript. All authors reviewed the final manuscript.
CONFLICT OF INTEREST
No conflict of interest was reported.
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available within the article and/or its supplementary material.
REFERENCES
- [1]. Deenen JC, Horlings CG, Verschuuren JJ, Verbeek AL, van Engelen BG. The Epidemiology of Neuromuscular Disorders: A Comprehensive Overview of the Literature. J Neuromuscul Dis. 2015;2:73–85. [PubMed] [Google Scholar]
- [2]. Saito Y, Takeshita E, Komaki H, Nishino I, Sasaki M. Determining neurodevelopmental manifestations in Duchenne muscular dystrophy using a battery of brief tests. J Neurol Sci. 2022;440:120340. doi: 10.1016/j.jns.2022.120340 [DOI] [PubMed] [Google Scholar]
- [3]. Battini R, Chieffo D, Bulgheroni S, Piccini G, Pecini C, Lucibello S, et al. Cognitive profile in Duchenne muscular dystrophy boys without intellectual disability: The role of executive functions. Neuromuscular Disorders. 2018;28:122–8. doi: 10.1016/j.nmd.2017.11.018 [DOI] [PubMed] [Google Scholar]
- [4]. Darmahkasih AJ, Rybalsky I, Tian C, Shellenbarger KC, Horn PS, Lambert JT, et al. Neurodevelopmental, behavioral, and emotional symptoms common in Duchenne muscular dystrophy. Muscle Nerve. 2020;61:466–74. doi: 10.1002/mus.26803 [DOI] [PubMed] [Google Scholar]
- [5]. Doorenweerd N. Combining genetics, neuropsychology and neuroimaging to improve understanding of brain involvement in Duchenne muscular dystrophy – a narrative review. Neuromuscul Disord. 2020;30:437–42. doi: 10.1016/j.nmd.2020.05.001 [DOI] [PubMed] [Google Scholar]
- [6]. Ricotti V, Mandy WP, Scoto M, Pane M, Deconinck N, Messina S, et al. Neurodevelopmental, emotional, andbehavioural problems in Duchenne muscular dystrophy in relation to underlying dystrophin gene mutations. Dev MedChild Neurol. 2016;58:77–84. doi: 10.1111/dmcn.12922 [DOI] [PubMed] [Google Scholar]
- [7]. Pane M, Lombardo ME, Alfieri P, D’Amico A, Bianco F, Vasco G, et al. Attention Deficit Hyperactivity Disorder and Cognitive Function in Duchenne Muscular Dystrophy: Phenotype-Genotype Correlation. The Journal of Pediatrics. 2012;161:705–9.e1. doi: 10.1016/j.jpeds.2012.03.020 [DOI] [PubMed] [Google Scholar]
- [8]. Sienko S, Buckon C, Fowler E, Bagley A, Staudt L, Sison-Williamson M, et al. Prednisone and Deflazacort in Duchenne Muscular Dystrophy: Do They Play a Different Role in Child Behavior and Perceived Quality of Life? PLoS Curr. 2016;8. doi: 10.1371/currents.md.7628d9c014bfa29f821a5cd19723bbaa [DOI] [PMC free article] [PubMed]
- [9]. Banihani R, Smile S, Yoon G, Dupuis A, Mosleh M, Snider A, et al. Cognitive and Neurobehavioral Profile in Boys With Duchenne Muscular Dystrophy. J Child Neurol. 2015;30:1472–82. doi: 10.1177/0883073815570154 [DOI] [PubMed] [Google Scholar]
- [10]. Hendriksen JG, Vles JS. Neuropsychiatric disorders in males with duchenne muscular dystrophy: Frequency rate ofattention-deficit hyperactivity disorder (ADHD), autism spectrum disorder, and obsessive—compulsive disorder. JChild Neurol. 2008;23:477–81. doi: 10.1177/0883073807309775 [DOI] [PubMed] [Google Scholar]
- [11]. Weerkamp PMM, Geuens S, Collin P, Goemans N, Vermeulen RJ, De Waele L, et al. Psychopharmaceutical treatment for neurobehavioral problems in Duchenne muscular dystrophy: A descriptive study using real-world data. Neuromuscul Disord. 2023;33:619–26. doi: 10.1016/j.nmd.2023.05.011 [DOI] [PubMed] [Google Scholar]
- [12]. Colombo P, Nobile M, Tesei A, Civati F, Gandossini S, Mani E, et al. Assessing mental health in boys with Duchenne muscular dystrophy: Emotional, behavioural and neurodevelopmental profile in an Italian clinical sample. European Journal of Paediatric Neurology. 2017;21:639–47. doi: 10.1016/j.ejpn.2017.02.007. [DOI] [PubMed] [Google Scholar]
- [13]. Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, et al. Diagnosis and management of Duchenne muscular dystrophy, part Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17:251–67. doi: 10.1016/s1474-4422(18)30024-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Colvin MK, et al. Diagnosis and management of Duchenne muscular dystrophy, part 3: Primary care, emergency management, psychosocial care, and transitions of care across the lifespan. The Lancet Neurology. 2018;17:445–55. doi: 10.1016/s1474-4422(18)30026-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Sun C, Shen L, Zhang Z, Xie X. Therapeutic Strategies for Duchenne Muscular Dystrophy: An Update. Genes (Basel). 2020;11(8):837. doi: 10.3390/genes11080837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Duan D. Duchenne Muscular Dystrophy Gene Therapy in 2023: Status, Perspective, and Beyond. Hum Gene Ther. 2023;34:345–9. doi: 10.1089/hum.2023.29242.ddu [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Corp. I, IBM SPSS Statistics. 2021: Armonk, NY.
- [18]. Olson AL, Kelleher KJ, Kemper KJ, Zuckerman BS, Hammond CS, Dietrich AJ. Primary care pediatricians’ roles and perceived responsibilities in the identification and management of depression in children and adolescents. Ambul Pediatr. 2001;1:91–8. doi: [DOI] [PubMed] [Google Scholar]
- [19]. Horwitz SM, Storfer-Isser A, Kerker BD, Szilagyi M, Garner A, O’Connor KG, et al. Barriers to the Identification and Management of Psychosocial Problems: Changes From 2004 to 2013. Acad Pediatr. 2015;15:613–20. doi: 10.1016/j.aca2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Colvin MK, Poysky J, Kinnett K, Damiani M, Gibbons M, Hoskin J, et al. Psychosocial Management of the Patient With Duchenne Muscular Dystrophy. Pediatrics. 2018;142:S99–109. doi: 10.1542/peds.2018-0333L [DOI] [PubMed] [Google Scholar]
- [21]. Weerkamp P, Chieffo D, Collin P, Moriconi F, Papageorgiou A, Vainieri I, et al. Psychological test usage in duchenne muscular dystrophy: An EU multi-centre study. Eur J Paediatr Neurol. 2023;46:42–7. doi: 10.1016/j.ejpn.2023.06.007 [DOI] [PubMed] [Google Scholar]
- [22]. Weerkamp PM, Mol EM, Sweere DJ, Schrans DG, Vermeulen RJ, Klinkenberg S, et al. Wechsler Scale Intelligence Testing in Males with Dystrophinopathies: A Review and Meta-Analysis. Brain Sciences. 2022;12:1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Hellebrekers DMJ, Lionarons JM, Faber CG, Klinkenberg S, Vles JSH, Hendriksen JGM. Instruments for the Assessment of Behavioral and Psychosocial Functioning in Duchenne and Becker Muscular Dystrophy; a Systematic Review of the Literature. Journal of Pediatric Psychology. 2019;44:1205–23. doi: 10.1093/jpepsy/jsz062 [DOI] [PubMed] [Google Scholar]
- [24]. Cervi F, Saletti V, Turner K, Peron A, Bulgheroni S, Taddei M, et al. The TAND checklist: A useful screening tool in children with tuberous sclerosis and neurofibromatosis type 1. Orphanet J Rare Dis. 2020;15:237. doi: 10.1186/s13023-020-01488-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. de Vries PJ, Belousova E, Benedik MP, Carter T, Cottin V, Curatolo P, et al. Natural clusters of tuberous sclerosis complex (TSC)-associated neuropsychiatric disorders (TAND): New findings from the TOSCA TAND research project. J Neurodev Disord. 2020;12:24. doi: 10.1186/s11689-020-09327-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. de Vries PJ, Belousova E, Benedik MP, Carter T, Cottin V, Curatolo P, et al. TSC-associated neuropsychiatric disorders (TAND): Findings from the TOSCA natural history study. Orphanet J Rare Dis. 2018;13:157. doi: 10.1186/s13023-018-0901-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. de Vries PJ, Leclezio L, Gardner-Lubbe S, Krueger D, Sahin M, Sparagana S, et al. Multivariate data analysis identifies natural clusters of Tuberous Sclerosis Complex Associated Neuropsychiatric Disorders (TAND). Orphanet J Rare Dis. 2021;16:447. doi: 10.1186/s13023-021-02076-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. de Vries PJ, Wilde L, de Vries MC, Moavero R, Pearson DA, Curatolo P. A clinical update on tuberous sclerosis complex-associated neuropsychiatric disorders (TAND). Am J Med Genet C Semin Med Genet. 2018;178:309–20. doi: 10.1002/ajmg.c.31637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Heunis TM, Bissell S, Byars AW, Capal JK, Chambers N, Cukier S, et al. Empowering Families Through Technology: A Mobile-Health Project to Reduce the TAND Identification and Treatment Gap (TANDem). Front Psychiatry. 2022;13:834628. doi: 10.3389/fpsyt.2022.834628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Heunis TM, Chambers N, Vanclooster S, Bissell S, Byars AW, Capal JK, et al. Development and Feasibility of theSelf-Report Quantified Tuberous Sclerosis Complex-Associated Neuropsychiatric Disorders Checklist (TAND-SQ). Pediatr Neurol. 2023;147:101–23. doi: 10.1016/j.pediatrneurol.2023.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Marcinkowska AB, Tarasewicz A, Jáźwiak S, Dębska-Ślizień A, Szurowska E. Tuberous sclerosis complexassociated neuropsychiatric disorders. Psychiatr Pol. 2022:1-20. doi: 10.12740/PP/OnlineFirst/146265 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the article and/or its supplementary material.