Abstract
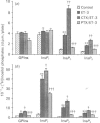
Endothelin-3 (ET-3) stimulated phosphoinositide metabolism and synthesis of prostaglandins in cultured rat Kupffer cells. ET-3-induced hydrolysis of phosphoinositides was characterized by the production of various inositol phosphates and of glycerophosphoinositol. The mechanism of ET-3-stimulated metabolism of phosphoinositides and synthesis of prostaglandins appeared to be distinct from the effect of platelet-activating factor (PAF) on these processes described previously [Gandhi, Hanahan & Olson (1990) J. Biol. Chem. 265, 18234-18241]. On a molar basis ET-3 was significantly more potent than PAF in stimulating phosphoinositide metabolism, e.g. ET-3-induced hydrolysis of phosphoinositides occurred at 1 pM, whereas PAF was ineffective at concentrations less than 1 nM. Upon challenging Kupffer cells with both ET-3 and PAF, an additive stimulation of phosphoinositide metabolism was observed, suggesting that the actions of these factors may be exerted on separate phosphoinositide pools. Treatment of Kupffer cells with pertussis toxin resulted in an inhibition of ET-3-induced phospholipase C activation; in contrast, cholera toxin treatment caused potentiation of ET-3-stimulated phospholipase C activity. Both toxins, however, inhibited PAF-stimulated phospholipase C activity. The present results suggest that the stimulatory effects of ET-3 and PAF on the phosphodiesteric metabolism of phosphoinositides in Kupffer cells require different guanine-nucleotide-binding proteins. Furthermore, the effects of bacterial toxins on ET-3- and PAF-induced phosphoinositide metabolism were not mediated by cyclic AMP. ET-3-induced metabolism of phosphoinositides was inhibited completely in Kupffer cells pretreated with ET-3, suggesting homologous ligand-induced desensitization of the ET-3 receptors. In contrast, similar experiments using PAF showed only a partial desensitization of subsequent PAF-induced phosphoinositide metabolism. In contrast to the increased production of prostaglandins E2 and D2 observed upon stimulation of Kupffer cells with PAF, ET-3 stimulated the biosynthesis of prostaglandin E2 only. Consistent with their additive effects on phosphoinositide metabolism, PAF and ET-3 exhibited an additive stimulation of the synthesis of prostaglandin E2.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreis P. G., Whitfield J. F., Armato U. Stimulation of DNA synthesis and mitosis of hepatocytes in primary cultures of neonatal rat liver by arachidonic acid and prostaglandins. Exp Cell Res. 1981 Aug;134(2):265–272. doi: 10.1016/0014-4827(81)90425-0. [DOI] [PubMed] [Google Scholar]
- Bartfai T. Preparation of metal-chelate complexes and the design of steady-state kinetic experiments involving metal nucleotide complexes. Adv Cyclic Nucleotide Res. 1979;10:219–242. [PubMed] [Google Scholar]
- Bilibin A. F., Gracheva N. M. Lekarstvennaia bolezn' v klinike infektsionnykh zabolevanii. Sov Med. 1974 Jul;0(7):3–10. [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Putney J. W., Jr Inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate formation in Ca2+-mobilizing-hormone-activated cells. Biochem J. 1985 Nov 15;232(1):237–243. doi: 10.1042/bj2320237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton D. B., Fisher R. A., Hanahan D. J., Olson M. S. Platelet-activating factor-mediated vasoconstriction and glycogenolysis in the perfused rat liver. J Biol Chem. 1986 Jan 15;261(2):644–649. [PubMed] [Google Scholar]
- Chao W., Liu H., Hanahan D. J., Olson M. S. Regulation of platelet-activating factor receptors in rat Kupffer cells. J Biol Chem. 1989 Dec 5;264(34):20448–20457. [PubMed] [Google Scholar]
- Cozza E. N., Vila M. C., Gomez-Sanchez C. E. ET-1 receptors in C-6 cells: homologous down-regulation and modulation by protein kinase C. Mol Cell Endocrinol. 1990 Apr 17;70(2):155–164. doi: 10.1016/0303-7207(90)90155-2. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Anoxia and endothelium-dependent reactivity of the canine femoral artery. J Physiol. 1983 Feb;335:65–74. doi: 10.1113/jphysiol.1983.sp014519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Heterogeneous behavior of the canine arterial and venous wall. Importance of the endothelium. Circ Res. 1982 Oct;51(4):439–447. doi: 10.1161/01.res.51.4.439. [DOI] [PubMed] [Google Scholar]
- Dean N. M., Moyer J. D. Separation of multiple isomers of inositol phosphates formed in GH3 cells. Biochem J. 1987 Mar 1;242(2):361–366. doi: 10.1042/bj2420361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieter P., Schulze-Specking A., Decker K. Differential inhibition of prostaglandin and superoxide production by dexamethasone in primary cultures of rat Kupffer cells. Eur J Biochem. 1986 Sep 15;159(3):451–457. doi: 10.1111/j.1432-1033.1986.tb09907.x. [DOI] [PubMed] [Google Scholar]
- Fisher R. A., Shukla S. D., Debuysere M. S., Hanahan D. J., Olson M. S. The effect of acetylglyceryl ether phosphorylcholine on glycogenolysis and phosphatidylinositol 4,5-bisphosphate metabolism in rat hepatocytes. J Biol Chem. 1984 Jul 25;259(14):8685–8688. [PubMed] [Google Scholar]
- Gandhi C. R., Hanahan D. J., Olson M. S. Two distinct pathways of platelet-activating factor-induced hydrolysis of phosphoinositides in primary cultures of rat Kupffer cells. J Biol Chem. 1990 Oct 25;265(30):18234–18241. [PubMed] [Google Scholar]
- Gandhi C. R., Ross D. H. Characterization of a high-affinity Mg2+-independent Ca2+-ATPase from rat brain synaptosomal membranes. J Neurochem. 1988 Jan;50(1):248–256. doi: 10.1111/j.1471-4159.1988.tb13257.x. [DOI] [PubMed] [Google Scholar]
- Gandhi C. R., Stephenson K., Olson M. S. Endothelin, a potent peptide agonist in the liver. J Biol Chem. 1990 Oct 15;265(29):17432–17435. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hansen C. A., Mah S., Williamson J. R. Formation and metabolism of inositol 1,3,4,5-tetrakisphosphate in liver. J Biol Chem. 1986 Jun 25;261(18):8100–8103. [PubMed] [Google Scholar]
- Hirata Y., Fukuda Y., Yoshimi H., Emori T., Shichiri M., Marumo F. Specific receptor for endothelin in cultured rat cardiocytes. Biochem Biophys Res Commun. 1989 May 15;160(3):1438–1444. doi: 10.1016/s0006-291x(89)80165-2. [DOI] [PubMed] [Google Scholar]
- Koseki C., Imai M., Hirata Y., Yanagisawa M., Masaki T. Autoradiographic distribution in rat tissues of binding sites for endothelin: a neuropeptide? Am J Physiol. 1989 Apr;256(4 Pt 2):R858–R866. doi: 10.1152/ajpregu.1989.256.4.R858. [DOI] [PubMed] [Google Scholar]
- Kuraja I. J., Woodcock E. A. Endothelin stimulates inositol phosphate accumulation in rat right and left atria: a comparison with noradrenaline. Clin Exp Pharmacol Physiol. 1990 Apr;17(4):275–280. doi: 10.1111/j.1440-1681.1990.tb01320.x. [DOI] [PubMed] [Google Scholar]
- Lagente V., Chabrier P. E., Mencia-Huerta J. M., Braquet P. Pharmacological modulation of the bronchopulmonary action of the vasoactive peptide, endothelin, administered by aerosol in the guinea-pig. Biochem Biophys Res Commun. 1989 Feb 15;158(3):625–632. doi: 10.1016/0006-291x(89)92767-8. [DOI] [PubMed] [Google Scholar]
- Lin W. W., Lee C. Y., Chuang D. M. Comparative studies of phosphoinositide hydrolysis induced by endothelin-related peptides in cultured cerebellar astrocytes, C6-glioma and cerebellar granule cells. Biochem Biophys Res Commun. 1990 Apr 30;168(2):512–519. doi: 10.1016/0006-291x(90)92351-y. [DOI] [PubMed] [Google Scholar]
- Marcheselli V. L., Rossowska M. J., Domingo M. T., Braquet P., Bazan N. G. Distinct platelet-activating factor binding sites in synaptic endings and in intracellular membranes of rat cerebral cortex. J Biol Chem. 1990 Jun 5;265(16):9140–9145. [PubMed] [Google Scholar]
- Marsden P. A., Danthuluri N. R., Brenner B. M., Ballermann B. J., Brock T. A. Endothelin action on vascular smooth muscle involves inositol trisphosphate and calcium mobilization. Biochem Biophys Res Commun. 1989 Jan 16;158(1):86–93. doi: 10.1016/s0006-291x(89)80180-9. [DOI] [PubMed] [Google Scholar]
- Moon D. G., Horgan M. J., Andersen T. T., Krystek S. R., Jr, Fenton J. W., 2nd, Malik A. B. Endothelin-like pulmonary vasoconstrictor peptide release by alpha-thrombin. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9529–9533. doi: 10.1073/pnas.86.23.9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S. P., Schulman E. S., Liu M. C., Hayes E. C., Lichtenstein L. M. Separation of major prostaglandins, leukotrienes, and monoHETEs by high performance liquid chromatography. J Immunol Methods. 1983 Nov 25;64(3):335–343. doi: 10.1016/0022-1759(83)90441-6. [DOI] [PubMed] [Google Scholar]
- Powell W. S. Rapid extraction of arachidonic acid metabolites from biological samples using octadecylsilyl silica. Methods Enzymol. 1982;86:467–477. doi: 10.1016/0076-6879(82)86218-6. [DOI] [PubMed] [Google Scholar]
- Resink T. J., Scott-Burden T., Bühler F. R. Activation of multiple signal transduction pathways by endothelin in cultured human vascular smooth muscle cells. Eur J Biochem. 1990 Apr 30;189(2):415–421. doi: 10.1111/j.1432-1033.1990.tb15504.x. [DOI] [PubMed] [Google Scholar]
- Resink T. J., Scott-Burden T., Bühler F. R. Activation of phospholipase A2 by endothelin in cultured vascular smooth muscle cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):279–286. doi: 10.1016/s0006-291x(89)80209-8. [DOI] [PubMed] [Google Scholar]
- Reynolds E. E., Mok L. L., Kurokawa S. Phorbol ester dissociates endothelin-stimulated phosphoinositide hydrolysis and arachidonic acid release in vascular smooth muscle cells. Biochem Biophys Res Commun. 1989 Apr 28;160(2):868–873. doi: 10.1016/0006-291x(89)92515-1. [DOI] [PubMed] [Google Scholar]
- Samuelsson B., Goldyne M., Granström E., Hamberg M., Hammarström S., Malmsten C. Prostaglandins and thromboxanes. Annu Rev Biochem. 1978;47:997–1029. doi: 10.1146/annurev.bi.47.070178.005025. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Serradeil-Le Gal C., Jouneaux C., Sanchez-Bueno A., Raufaste D., Roche B., Préaux A. M., Maffrand J. P., Cobbold P. H., Hanoune J., Lotersztajn S. Endothelin action in rat liver. Receptors, free Ca2+ oscillations, and activation of glycogenolysis. J Clin Invest. 1991 Jan;87(1):133–138. doi: 10.1172/JCI114962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba R., Yanagisawa M., Miyauchi T., Ishii Y., Kimura S., Uchiyama Y., Masaki T., Goto K. Elimination of intravenously injected endothelin-1 from the circulation of the rat. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S98–S102. doi: 10.1097/00005344-198900135-00024. [DOI] [PubMed] [Google Scholar]
- Shukla S. D., Buxton D. B., Olson M. S., Hanahan D. J. Acetylglyceryl ether phosphorylcholine. A potent activator of hepatic phosphoinositide metabolism and glycogenolysis. J Biol Chem. 1983 Sep 10;258(17):10212–10214. [PubMed] [Google Scholar]
- Simonson M. S., Dunn M. J. Cellular signaling by peptides of the endothelin gene family. FASEB J. 1990 Sep;4(12):2989–3000. doi: 10.1096/fasebj.4.12.2168326. [DOI] [PubMed] [Google Scholar]
- Simonson M. S., Dunn M. J. Endothelin-1 stimulates contraction of rat glomerular mesangial cells and potentiates beta-adrenergic-mediated cyclic adenosine monophosphate accumulation. J Clin Invest. 1990 Mar;85(3):790–797. doi: 10.1172/JCI114505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolarics Z., Tanács B., Garzó T., Mandl J., Mucha I., Antoni F., Machovich R., Horváth I. Prostaglandin and thromboxane synthesizing activity in isolated murine hepatocytes and nonparenchymal liver cells. Prostaglandins Leukot Med. 1984 Dec;16(3):379–388. doi: 10.1016/0262-1746(84)90194-x. [DOI] [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Sugiura M., Inagami T., Kon V. Endotoxin stimulates endothelin-release in vivo and in vitro as determined by radioimmunoassay. Biochem Biophys Res Commun. 1989 Jun 30;161(3):1220–1227. doi: 10.1016/0006-291x(89)91372-7. [DOI] [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Yanagisawa M., Yamashita K., Masaki T. A novel vasoactive peptide endothelin stimulates mitogenesis through inositol lipid turnover in Swiss 3T3 fibroblasts. J Biol Chem. 1989 May 15;264(14):7856–7861. [PubMed] [Google Scholar]
- Takuwa Y., Kasuya Y., Takuwa N., Kudo M., Yanagisawa M., Goto K., Masaki T., Yamashita K. Endothelin receptor is coupled to phospholipase C via a pertussis toxin-insensitive guanine nucleotide-binding regulatory protein in vascular smooth muscle cells. J Clin Invest. 1990 Mar;85(3):653–658. doi: 10.1172/JCI114488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa Y., Ohue Y., Takuwa N., Yamashita K. Endothelin-1 activates phospholipase C and mobilizes Ca2+ from extra- and intracellular pools in osteoblastic cells. Am J Physiol. 1989 Dec;257(6 Pt 1):E797–E803. doi: 10.1152/ajpendo.1989.257.6.E797. [DOI] [PubMed] [Google Scholar]
- Terashita Z., Shibouta Y., Imura Y., Iwasaki K., Nishikawa K. Endothelin-induced sudden death and the possible involvement of platelet activating factor (PAF). Life Sci. 1989;45(20):1911–1918. doi: 10.1016/0024-3205(89)90545-6. [DOI] [PubMed] [Google Scholar]
- Turk J., Wolf B. A., McDaniel M. L. Glucose-induced accumulation of inositol trisphosphates in isolated pancreatic islets. Predominance of the 1,3,4-isomer. Biochem J. 1986 Jul 1;237(1):259–263. doi: 10.1042/bj2370259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte P. M., Katusic Z. S. Endothelium-derived contracting factor: endothelin and/or superoxide anion? Trends Pharmacol Sci. 1988 Jul;9(7):229–230. doi: 10.1016/0165-6147(88)90146-0. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Chignard M., Benveniste J., Lefort J., Wal F. Background and present status of research on platelet-activating factor (PAF-acether). Ann N Y Acad Sci. 1981;370:119–137. doi: 10.1111/j.1749-6632.1981.tb29727.x. [DOI] [PubMed] [Google Scholar]
- Vigne P., Marsault R., Breittmayer J. P., Frelin C. Endothelin stimulates phosphatidylinositol hydrolysis and DNA synthesis in brain capillary endothelial cells. Biochem J. 1990 Mar 1;266(2):415–420. doi: 10.1042/bj2660415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Joseph S. K., Thomas A. P. Inositol trisphosphate and diacylglycerol as intracellular second messengers in liver. Am J Physiol. 1985 Mar;248(3 Pt 1):C203–C216. doi: 10.1152/ajpcell.1985.248.3.C203. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Masaki T. Molecular biology and biochemistry of the endothelins. Trends Pharmacol Sci. 1989 Sep;10(9):374–378. doi: 10.1016/0165-6147(89)90011-4. [DOI] [PubMed] [Google Scholar]
- Yoshimi H., Hirata Y., Fukuda Y., Kawano Y., Emori T., Kuramochi M., Omae T., Marumo F. Regional distribution of immunoreactive endothelin in rats. Peptides. 1989 Jul-Aug;10(4):805–808. doi: 10.1016/0196-9781(89)90117-4. [DOI] [PubMed] [Google Scholar]
- de Nucci G., Thomas R., D'Orleans-Juste P., Antunes E., Walder C., Warner T. D., Vane J. R. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]