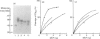Abstract
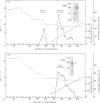
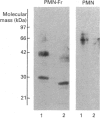
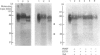
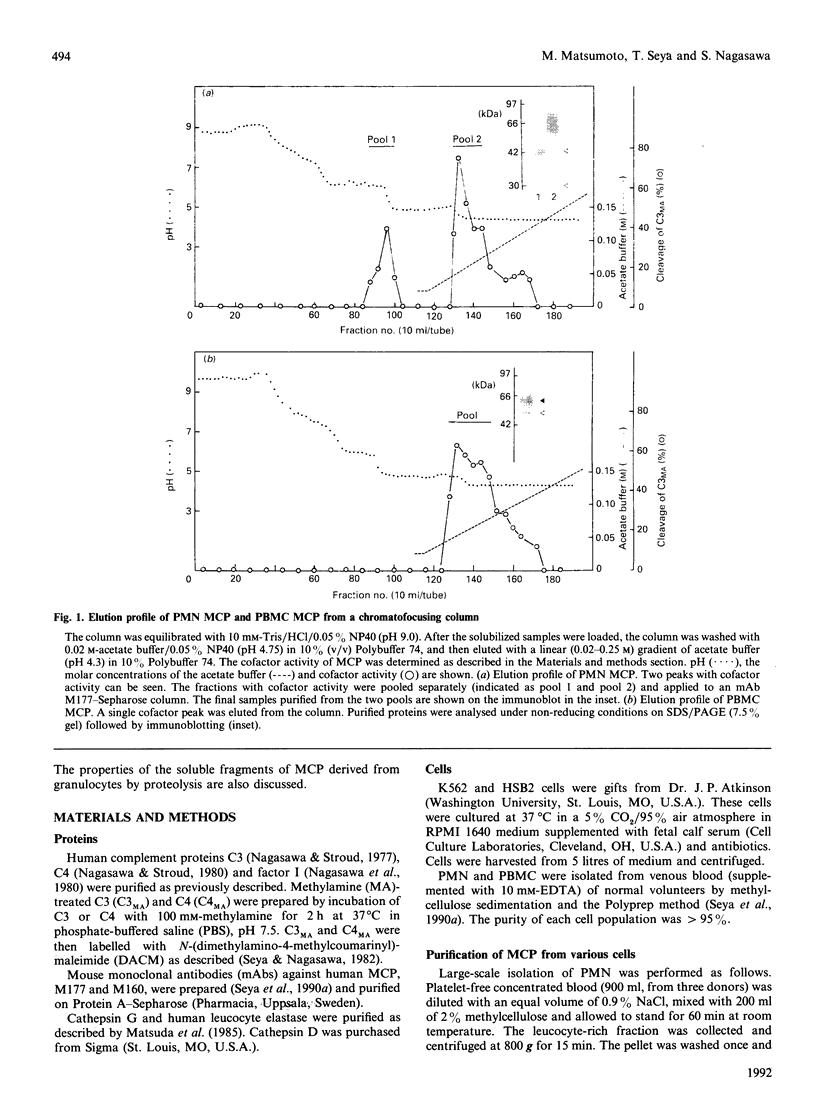
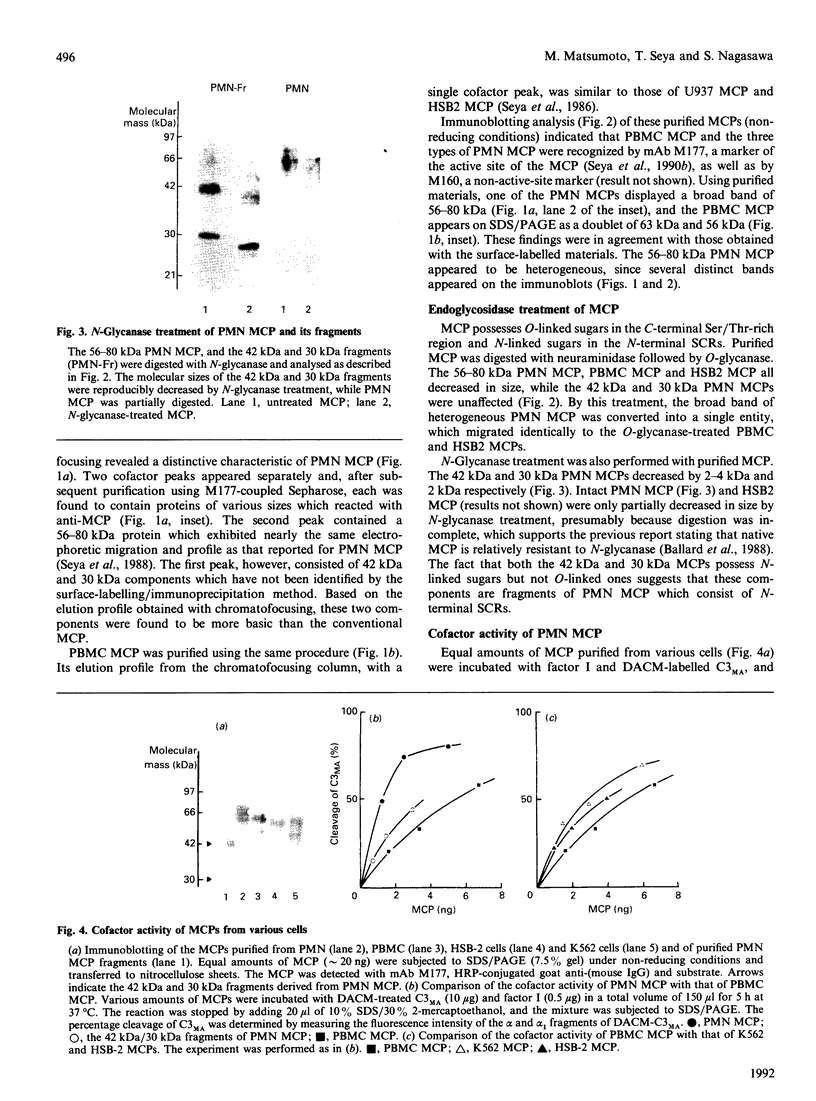
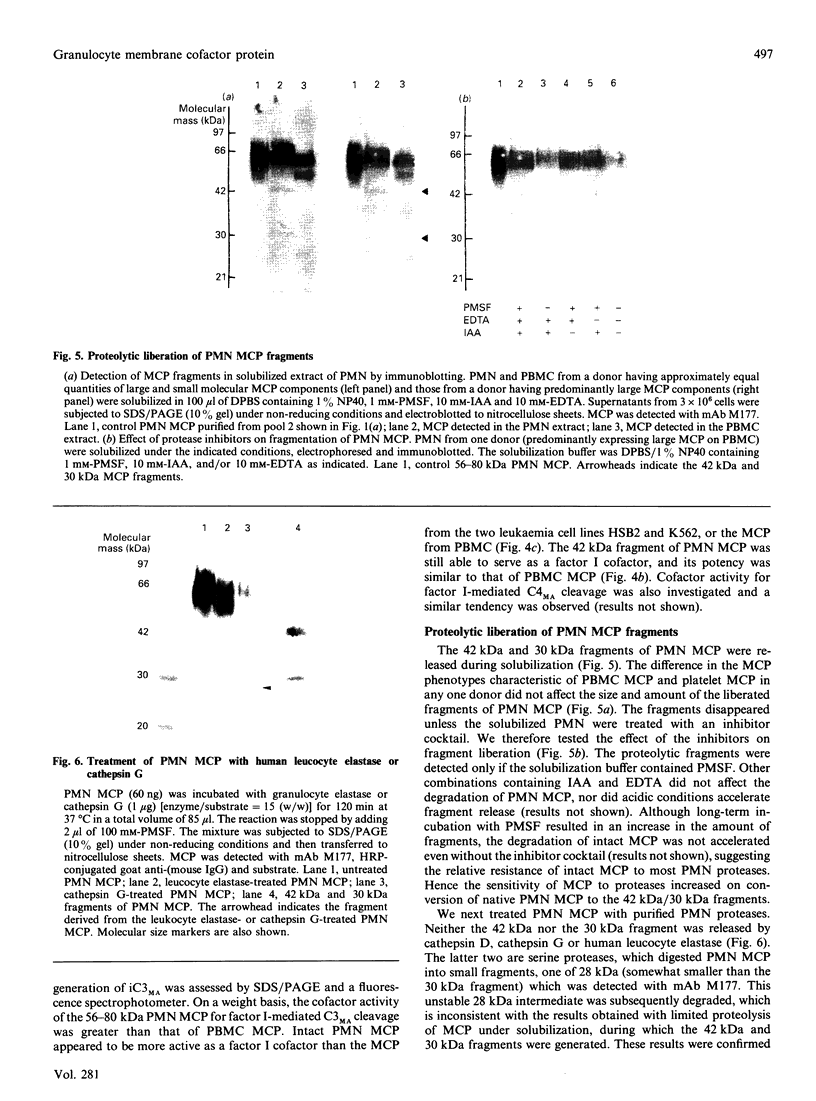
Human granulocytes (polymorphonuclear leucocytes, PMN) possess a membrane cofactor protein (MCP, CD46), which is structurally and functionally distinct from the MCPs of other cell types: it shows a single broad band of 56-80 kDa (without the doublet pattern characteristic of MCP) on SDS/PAGE and has less affinity for complement component C3b. We purified PMN MCP using monoclonal antibodies in order to study the molecular differences between it and other MCPs. Several forms of PMN MCP with size heterogeneity were noted on SDS/PAGE and by immunoblotting. O-Glycanase treatment decreased this heterogeneity, yielding a fast-migrating component identical in position on SDS/PAGE to the O-glycanase-treated MCP of other cells. The cell-specific variation of MCP, therefore, arises from post-translational glycosylation and not from a difference in primary structure. The Factor I cofactor activity of PMN MCP was more efficient in cleaving the methylamine-treated complement components C4/C3 than was MCP from other cells, which shared a similar potency of cofactor activity on a weight basis. Two types of small-form PMN MCP were identified during purification. These were 42 kDa and 30 kDa in size; the former was recognized by M177 (a monoclonal antibody against the active site marker), possessed N-linked sugars [located on the short consensus repeats (SCRs)] but not O-linked ones (on the Ser/Thr-rich region), and retained cofactor activity for C3b/C4b cleavage, similar in potency to that of other MCPs. The functionally active soluble form of MCP was observed specifically in PMN. Protease inhibitors did not inhibit liberation of the fragments, although the generated fragments became susceptible to serine proteases. The findings show that the SCRs are the functional domain of MCP and that the MCP proteolysis found only in PMN may modulate the properties of PMN MCP. In conclusion, the structural features of PMN MCP largely reflect a variability in the O-linked sugars, and the decreased affinity for C3b may be in part attributable to proteolysis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard L. L., Bora N. S., Yu G. H., Atkinson J. P. Biochemical characterization of membrane cofactor protein of the complement system. J Immunol. 1988 Dec 1;141(11):3923–3929. [PubMed] [Google Scholar]
- Ballard L., Seya T., Teckman J., Lublin D. M., Atkinson J. P. A polymorphism of the complement regulatory protein MCP (membrane cofactor protein or gp45-70). J Immunol. 1987 Jun 1;138(11):3850–3855. [PubMed] [Google Scholar]
- Caras I. W., Davitz M. A., Rhee L., Weddell G., Martin D. W., Jr, Nussenzweig V. Cloning of decay-accelerating factor suggests novel use of splicing to generate two proteins. Nature. 1987 Feb 5;325(6104):545–549. doi: 10.1038/325545a0. [DOI] [PubMed] [Google Scholar]
- Cole J. L., Housley G. A., Jr, Dykman T. R., MacDermott R. P., Atkinson J. P. Identification of an additional class of C3-binding membrane proteins of human peripheral blood leukocytes and cell lines. Proc Natl Acad Sci U S A. 1985 Feb;82(3):859–863. doi: 10.1073/pnas.82.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5867–5871. doi: 10.1073/pnas.76.11.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Gigli I., Nussenzweig V. Human C4-binding protein. II. Role in proteolysis of C4b by C3b-inactivator. J Exp Med. 1978 Oct 1;148(4):1044–1051. doi: 10.1084/jem.148.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga T. W., van der Schoot C. E., Jost C., Klaassen R., Kleijer M., von dem Borne A. E., Roos D., Tetteroo P. A. The PI-linked receptor FcRIII is released on stimulation of neutrophils. Nature. 1988 Jun 16;333(6174):667–669. doi: 10.1038/333667a0. [DOI] [PubMed] [Google Scholar]
- Iida K., Nussenzweig V. Complement receptor is an inhibitor of the complement cascade. J Exp Med. 1981 May 1;153(5):1138–1150. doi: 10.1084/jem.153.5.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Berg E. L., Butcher E. C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989 Sep 15;245(4923):1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lublin D. M., Liszewski M. K., Post T. W., Arce M. A., Le Beau M. M., Rebentisch M. B., Lemons L. S., Seya T., Atkinson J. P. Molecular cloning and chromosomal localization of human membrane cofactor protein (MCP). Evidence for inclusion in the multigene family of complement-regulatory proteins. J Exp Med. 1988 Jul 1;168(1):181–194. doi: 10.1084/jem.168.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T., Nagasawa S., Koide T., Koyama J. Limited proteolysis of a chemically modified third component of human complement, C3, by cathepsin G of human leukocytes. J Biochem. 1985 Jul;98(1):229–236. doi: 10.1093/oxfordjournals.jbchem.a135262. [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Nagaki K., Kitamura H., Kuramitsu S., Nagasawa S., Seya T. Probing the C4-binding site on C1s with monoclonal antibodies. Evidence for a C4/C4b-binding site on the gamma-domain. J Immunol. 1989 Apr 15;142(8):2743–2750. [PubMed] [Google Scholar]
- Matsumoto M., Sugita Y., Seya T. Alternative complement pathway-mediated myeloid cell cytotoxicity: repertoire of membrane factors participating in regulation of C3 deposition and cytolysis. Eur J Immunol. 1991 Aug;21(8):1787–1792. doi: 10.1002/eji.1830210802. [DOI] [PubMed] [Google Scholar]
- McNearney T., Ballard L., Seya T., Atkinson J. P. Membrane cofactor protein of complement is present on human fibroblast, epithelial, and endothelial cells. J Clin Invest. 1989 Aug;84(2):538–545. doi: 10.1172/JCI114196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Lublin D. M., Holers V. M., Ayers D. J., Getty R. R., Leykam J. F., Atkinson J. P., Tykocinski M. L. Cloning and characterization of cDNAs encoding the complete sequence of decay-accelerating factor of human complement. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2007–2011. doi: 10.1073/pnas.84.7.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Roberts W. L., Haas R., Rosenberry T. L. Decay accelerating factor of complement is anchored to cells by a C-terminal glycolipid. Biochemistry. 1986 Nov 4;25(22):6740–6747. doi: 10.1021/bi00370a003. [DOI] [PubMed] [Google Scholar]
- Nagasawa S., Ichihara C., Stroud R. M. Cleavage of C4b by C3b inactivator: production of a nicked form of C4b, C4b', as an intermediate cleavage product of C4b by C3b inactivator. J Immunol. 1980 Aug;125(2):578–582. [PubMed] [Google Scholar]
- Nagasawa S., Stroud R. M. Mechanism of action of the C3b inactivator: requirement for a high molecular weight cofactor (C3b-C4bINA cofactor) and production of a new C3b derivative (C3b'). Immunochemistry. 1977 Nov-Dec;14(11-12):749–756. doi: 10.1016/0019-2791(77)90345-7. [DOI] [PubMed] [Google Scholar]
- Nagasawa S., Stroud R. M. Purification and characterization of a macromolecular weight cofactor for C3b-inactivator, C4bC3bINA-cofactor, of human plasma. Mol Immunol. 1980 Nov;17(11):1365–1372. doi: 10.1016/0161-5890(80)90005-x. [DOI] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Porteu F., Nathan C. Shedding of tumor necrosis factor receptors by activated human neutrophils. J Exp Med. 1990 Aug 1;172(2):599–607. doi: 10.1084/jem.172.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell D. F., McKenzie I. F., Lublin D. M., Johnson P. M., Atkinson J. P., Oglesby T. J., Deacon N. J. The human cell-surface glycoproteins HuLy-m5, membrane co-factor protein (MCP) of the complement system, and trophoblast leucocyte-common (TLX) antigen, are CD46. Immunology. 1990 Jun;70(2):155–161. [PMC free article] [PubMed] [Google Scholar]
- Ripoche J., Sim R. B. Loss of complement receptor type 1 (CR1) on ageing of erythrocytes. Studies of proteolytic release of the receptor. Biochem J. 1986 May 1;235(3):815–821. doi: 10.1042/bj2350815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayama K., Shiraishi S., Shirakata Y., Kobayashi Y., Seya T., Miki Y. Expression and characterization of membrane co-factor protein (MCP) in human skin. J Invest Dermatol. 1991 Oct;97(4):722–724. doi: 10.1111/1523-1747.ep12484155. [DOI] [PubMed] [Google Scholar]
- Seya T., Atkinson J. P. Functional properties of membrane cofactor protein of complement. Biochem J. 1989 Dec 1;264(2):581–588. doi: 10.1042/bj2640581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seya T., Ballard L. L., Bora N. S., Kumar V., Cui W., Atkinson J. P. Distribution of membrane cofactor protein of complement on human peripheral blood cells. An altered form is found on granulocytes. Eur J Immunol. 1988 Aug;18(8):1289–1294. doi: 10.1002/eji.1830180821. [DOI] [PubMed] [Google Scholar]
- Seya T., Farries T., Nickells M., Atkinson J. P. Additional forms of human decay-accelerating factor (DAF). J Immunol. 1987 Aug 15;139(4):1260–1267. [PubMed] [Google Scholar]
- Seya T., Hara T., Matsumoto M., Akedo H. Quantitative analysis of membrane cofactor protein (MCP) of complement. High expression of MCP on human leukemia cell lines, which is down-regulated during cell differentiation. J Immunol. 1990 Jul 1;145(1):238–245. [PubMed] [Google Scholar]
- Seya T., Hara T., Matsumoto M., Sugita Y., Akedo H. Complement-mediated tumor cell damage induced by antibodies against membrane cofactor protein (MCP, CD46). J Exp Med. 1990 Dec 1;172(6):1673–1680. doi: 10.1084/jem.172.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seya T., Nagasawa S. A fluorometric method for determination of C3b inactivator. Clin Chim Acta. 1982 Mar 12;119(3):189–196. doi: 10.1016/0009-8981(82)90331-x. [DOI] [PubMed] [Google Scholar]
- Seya T., Turner J. R., Atkinson J. P. Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J Exp Med. 1986 Apr 1;163(4):837–855. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern P. L., Beresford N., Thompson S., Johnson P. M., Webb P. D., Hole N. Characterization of the human trophoblast-leukocyte antigenic molecules defined by a monoclonal antibody. J Immunol. 1986 Sep 1;137(5):1604–1609. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976 Nov 2;144(5):1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]