Abstract
The absence of 5'-deoxy-5'-methylthioadenosine phosphorylase (MTAase) activity in malignant cells, and the putative localization of its gene, suggest that this enzyme deficiency might be due to a genomic alteration also involving a tumour-suppressor gene. We studied the possible occurrence of inactive forms of the protein in two MTAase-negative cell lines, namely K562 and Jurkat, by immunochemical methods. Two highly specific antisera, directed against different epitopes of the phosphorylase [Della Ragione, Oliva, Gragnaniello, Russo, Palumbo & Zappia (1990) J. Biol. Chem. 265, 6241-6246], were used to carry out immunotitration and immunoblotting analyses, as well as to investigate the biosynthesis of the enzyme. No MTAase protein was detected by Western-blotting technique performed under conditions where all the phosphorylase-positive samples gave a clear band at the MTAase subunit molecular mass. No cross-reacting material was observed by a sensitive immunotitration method which permitted the detection of as low as 0.5 ng of protein. Moreover, the results obtained by [35S]methionine-labelling experiments ruled out phosphorylase biosynthesis in the negative cell lines. Altogether, these data suggest that an alteration at the gene level hampering the specific mRNA biosynthesis or resulting in an untranslatable mRNA is the cause of the enzyme deficiency in the MTAase-negative cell lines studied.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backlund P. S., Jr, Smith R. A. Methionine synthesis from 5'-methylthioadenosine in rat liver. J Biol Chem. 1981 Feb 25;256(4):1533–1535. [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Bigner S. H., Mark J., Burger P. C., Mahaley M. S., Jr, Bullard D. E., Muhlbaier L. H., Bigner D. D. Specific chromosomal abnormalities in malignant human gliomas. Cancer Res. 1988 Jan 15;48(2):405–411. [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Call K. M., Glaser T., Ito C. Y., Buckler A. J., Pelletier J., Haber D. A., Rose E. A., Kral A., Yeger H., Lewis W. H. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell. 1990 Feb 9;60(3):509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- Carrera C. J., Eddy R. L., Shows T. B., Carson D. A. Assignment of the gene for methylthioadenosine phosphorylase to human chromosome 9 by mouse-human somatic cell hybridization. Proc Natl Acad Sci U S A. 1984 May;81(9):2665–2668. doi: 10.1073/pnas.81.9.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan J. M., Halaban R., Francke U. Cytogenetic analysis of melanocytes from premalignant nevi and melanomas. J Natl Cancer Inst. 1988 Sep 21;80(14):1159–1164. doi: 10.1093/jnci/80.14.1159. [DOI] [PubMed] [Google Scholar]
- Della Ragione F., Cartenì-Farina M., Gragnaniello V., Schettino M. I., Zappia V. Purification and characterization of 5'-deoxy-5'-methylthioadenosine phosphorylase from human placenta. J Biol Chem. 1986 Sep 15;261(26):12324–12329. [PubMed] [Google Scholar]
- Della Ragione F., Cartenì-Farina M., Porcelli M., Cacciapuoti G., Zappia V. High-performance liquid chromatographic analysis of 5'-methylthioadenosine in rat tissues. J Chromatogr. 1981 Nov 13;226(1):243–249. doi: 10.1016/s0378-4347(00)84229-2. [DOI] [PubMed] [Google Scholar]
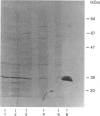
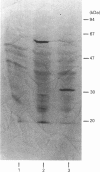
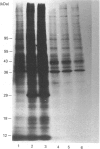
- Della Ragione F., Oliva A., Gragnaniello V., Russo G. L., Palumbo R., Zappia V. Physicochemical and immunological studies on mammalian 5'-deoxy-5'-methylthioadenosine phosphorylase. J Biol Chem. 1990 Apr 15;265(11):6241–6246. [PubMed] [Google Scholar]
- Diaz M. O., Ziemin S., Le Beau M. M., Pitha P., Smith S. D., Chilcote R. R., Rowley J. D. Homozygous deletion of the alpha- and beta 1-interferon genes in human leukemia and derived cell lines. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5259–5263. doi: 10.1073/pnas.85.14.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G., Brizuela L., Potashkin J., Beach D. Identification of p34 and p13, human homologs of the cell cycle regulators of fission yeast encoded by cdc2+ and suc1+. Cell. 1987 Jul 17;50(2):319–325. doi: 10.1016/0092-8674(87)90227-3. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Kamatani N., Nelson-Rees W. A., Carson D. A. Selective killing of human malignant cell lines deficient in methylthioadenosine phosphorylase, a purine metabolic enzyme. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1219–1223. doi: 10.1073/pnas.78.2.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamatani N., Yu A. L., Carson D. A. Deficiency of methylthioadenosine phosphorylase in human leukemic cells in vivo. Blood. 1982 Dec;60(6):1387–1391. [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Miyakoshi J., Dobler K. D., Allalunis-Turner J., McKean J. D., Petruk K., Allen P. B., Aronyk K. N., Weir B., Huyser-Wierenga D., Fulton D. Absence of IFNA and IFNB genes from human malignant glioma cell lines and lack of correlation with cellular sensitivity to interferons. Cancer Res. 1990 Jan 15;50(2):278–283. [PubMed] [Google Scholar]
- Murphy S. B., Raimondi S. C., Rivera G. K., Crone M., Dodge R. K., Behm F. G., Pui C. H., Williams D. L. Nonrandom abnormalities of chromosome 9p in childhood acute lymphoblastic leukemia: association with high-risk clinical features. Blood. 1989 Jul;74(1):409–415. [PubMed] [Google Scholar]
- Rose E. A., Glaser T., Jones C., Smith C. L., Lewis W. H., Call K. M., Minden M., Champagne E., Bonetta L., Yeger H. Complete physical map of the WAGR region of 11p13 localizes a candidate Wilms' tumor gene. Cell. 1990 Feb 9;60(3):495–508. doi: 10.1016/0092-8674(90)90600-j. [DOI] [PubMed] [Google Scholar]
- SCHLENK F., EHNINGER D. J. OBSERVATIONS ON THE METABOLISM OF 5'-METHYLTHIOADENOSINE. Arch Biochem Biophys. 1964 Jul 20;106:95–100. doi: 10.1016/0003-9861(64)90161-4. [DOI] [PubMed] [Google Scholar]
- Sager R. Tumor suppressor genes: the puzzle and the promise. Science. 1989 Dec 15;246(4936):1406–1412. doi: 10.1126/science.2574499. [DOI] [PubMed] [Google Scholar]
- Seidenfeld J., Wilson J., Williams-Ashman H. G. Androgenic regulation of 5'-deoxy-5'-methylthioadenosine concentrations and methylthioadenosine phosphorylase activity in relation to polyamine metabolism of rat prostate. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1861–1868. doi: 10.1016/s0006-291x(80)80116-1. [DOI] [PubMed] [Google Scholar]
- Toohey J. I. Methylthio group cleavage from methylthioadenosine. Description of an enzyme and its relationship to the methylthio requirement of certain cells in culture. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1273–1280. doi: 10.1016/0006-291x(77)91430-9. [DOI] [PubMed] [Google Scholar]
- Traweek S. T., Riscoe M. K., Ferro A. J., Braziel R. M., Magenis R. E., Fitchen J. H. Methylthioadenosine phosphorylase deficiency in acute leukemia: pathologic, cytogenetic, and clinical features. Blood. 1988 Jun;71(6):1568–1573. [PubMed] [Google Scholar]
- Weinberg R. A. Positive and negative controls on cell growth. Biochemistry. 1989 Oct 17;28(21):8263–8269. doi: 10.1021/bi00447a001. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Seidenfeld J., Galletti P. Trends in the biochemical pharmacology of 5'-deoxy-5'-methylthioadenosine. Biochem Pharmacol. 1982 Feb 1;31(3):277–288. doi: 10.1016/0006-2952(82)90171-x. [DOI] [PubMed] [Google Scholar]
- Zappia V., Oliva A., Cacciapuoti G., Galletti P., Mignucci G., Cartení-Farina M. Substrate specificity of 5'-methylthioadenosine phosphorylase from human prostate. Biochem J. 1978 Dec 1;175(3):1043–1050. doi: 10.1042/bj1751043. [DOI] [PMC free article] [PubMed] [Google Scholar]