Abstract
Immune escape from cytotoxic T-lymphocyte (CTL) responses has been shown to occur not only by changes within the targeted epitope but also by changes in the flanking sequences which interfere with the processing of the immunogenic peptide. However, the frequency of such an escape mechanism has not been determined. To investigate whether naturally occurring variations in the flanking sequences of an immunodominant human immunodeficiency virus type 1 (HIV-1) Gag CTL epitope prevent antigen processing, cells infected with HIV-1 or vaccinia virus constructs encoding different patient-derived Gag sequences were tested for recognition by HLA-A*0201-restricted, p17-specific CTL. We found that the immunodominant p17 epitope (SL9) and its variants were efficiently processed from minigene expressing vectors and from six HIV-1 Gag variants expressed by recombinant vaccinia virus constructs. Furthermore, SL9-specific CTL clones derived from multiple donors efficiently inhibited virus replication when added to HLA-A*0201-bearing cells infected with primary or laboratory-adapted strains of virus, despite the variability in the SL9 flanking sequences. These data suggest that escape from this immunodominant CTL response is not frequently accomplished by changes in the epitope flanking sequences.
Cytotoxic T lymphocytes (CTL) that recognize viral antigen presented by major histocompatibility complex (MHC) class I antigens have been shown to control viral replication in vivo in animal models (16, 21, 30). In human immunodeficiency type 1 (HIV-1) infection, CTL specific for HIV-1-derived, human lymphocyte antigen (HLA) class I-restricted epitopes are also believed to be an important part of the host response against this virus (50). However, despite the persistently high turnover of this extremely variable virus in the presence of a vigorous HIV-1-specific CTL response, the development of sequence variation within targeted epitopes is inconsistent, and thus the effectiveness of CTL responses in vivo remains questionable (5, 8, 27, 29, 36). There are a few well-documented examples of HIV-1 escape from CTL recognition in vivo (4, 17, 25, 29, 37), although this is not a consistent finding (5). In all reported instances of escape, the viral mutations have been located within the CTL epitope, which theoretically could have led to (i) reduced binding of the epitope to the restricting HLA class I molecule, (ii) nonrecognition by the T-cell receptor (TCR) of the CTL, (iii) abrogated processing of the epitope from the precursor protein, or (iv) antagonism (12, 24, 35).
Another proposed mechanism for escape from CTL immune surveillance is variation of the amino acids in the sequences flanking the epitope. Such changes have the potential to prevent antigen presentation on the MHC class I molecule by abrogating effective processing of the epitope (reviewed in references 41 and 42). While it has been shown in a murine cytomegalovirus model that changes in the flanking region of the immunodominant epitope can prevent the generation of a protective CTL response after vaccinia virus-based vaccination, no similar reports exist for the human system (13). Furthermore, there is limited information available regarding the sequence requirements for proteasome-mediated processing (14) and TAP-dependent transport of viral peptides (11, 44, 51), possibly reflecting relatively low sequence specificity of the antigen-processing machinery. However, there may be some important residues for proper antigen processing of MHC class I restricted epitopes, which, if changed, inhibit antigen processing (11, 14, 44, 51).
In this study, we assessed the effects of sequence variation in the flanking regions of an immunodominant viral epitope. We focused on the HLA-A*0201-restricted CTL response against the well-characterized immunodominant HIV-Gag p17-derived epitope SLYNTVATL (SL9, Gag p17, amino acids 77 to 85 [5, 18, 20]). Our previous studies have shown that some individuals maintain a strong CTL response to this epitope in the setting of a high viral load without developing escape variants within the epitope (5). Thus, it was important to determine whether variation in flanking residues might have the potential to prevent the processing and presentation of this epitope. Minigenes expressing episomal vectors, vaccinia virus constructs encoding different Gag sequences, and common laboratory-adapted HIV-1 strains, as well as clinical viral isolates, were used for these analyses (46, 48, 54). Despite various changes in the epitope flanking sequences, no evidence was observed for the occurrence of immune escape by changes in the epitope flanking sequences.
MATERIALS AND METHODS
Subjects.
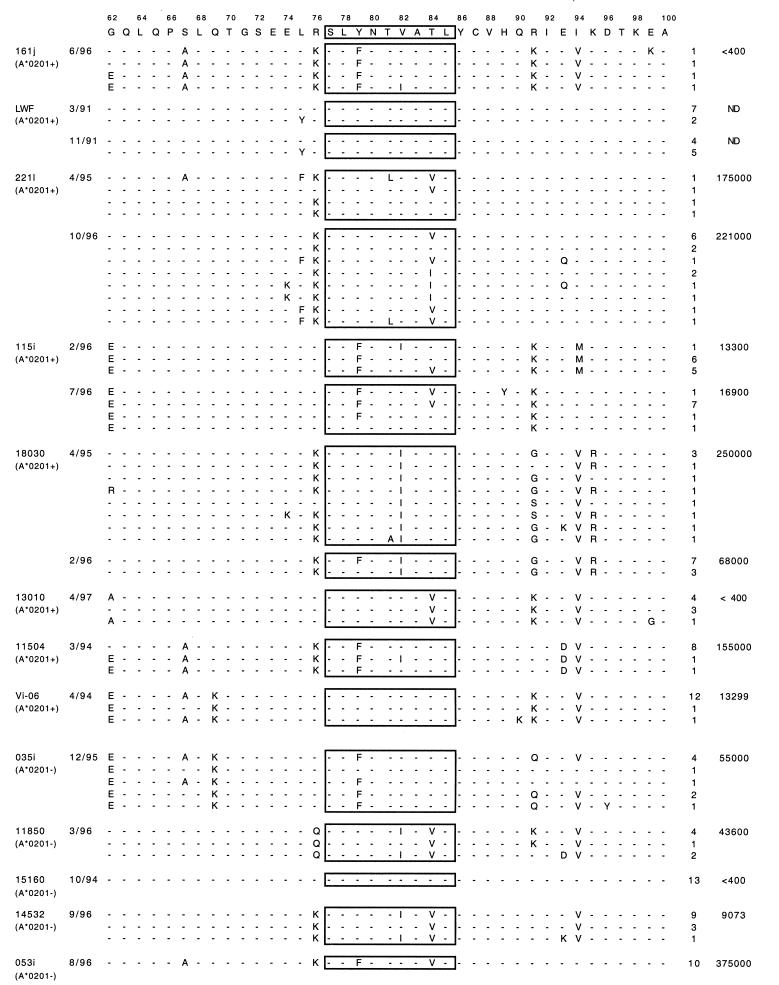
Thirteen HIV-1-infected individuals (eight HLA-A*0201 positive and five HLA-A*0201 negative), with a duration of infection ranging from 4 to 17 years, were included in this study. Six subjects are part of the San Francisco City Clinic Cohort (39), and six subjects (221L, 161j, 115, 035i, VI-06, and 53i) are from the Boston area. One subject (LWF) was accidentally infected with HIV-1 IIIB (47) and his HIV-1-specific CTL responses have previously been reported (40). The viral load was measured by the Roche Amplicor assay (Roche Molecular Systems, Branchburg, N.J.), with a lower detection limit of 400 viral copies/ml. The subjects had not received antiviral treatment by the time the samples were obtained except for subjects VI-06 (viral load, 12,300/ml; AZT/Nev/ddI) and 53i (450,000/ml, AZT/Delaviridine). All subjects gave written informed consent for these studies.
Cell lines.
Epstein-Barr virus (EBV)-transformed B lymphoblastoid cell lines were maintained in RPMI 1640 medium containing 20% (vol/vol) heat-inactivated fetal calf serum (FCS), 10 mM HEPES buffer, 50 U of penicillin per ml, 50 μg of streptomycin per ml, and 2 mM l-glutamine (46). T-cell lines and clones were maintained in the same medium containing 10% FCS (designated R10) supplemented with 50 U of recombinant interleukin-2 (IL-2) (designated R10-50) per ml. Recombinant IL-2 was a kind gift from M. Gately and Hoffman-La Roche.
Sequencing of viral DNA.
Proviral DNA was extracted from frozen peripheral blood mononuclear cell (PBMC) pellets and used in serial dilutions in a nested PCR reaction as described earlier (5). The lowest detectable target sequence copy number in the endpoint diluted sample was used for nested PCR amplification. The same internal primers were used to sequence the resulting 642-bp PCR product in both directions. Sequence data are available from GenBank under accession numbers AF017813 to AF017828, AF017841 to AF017980, AF060031 to AF060073, and AF073382 to AF073441. The SL9 epitope sequences without flanking residues have been reported previously for the HLA-A*0201-negative individuals and in part for the second time points of patients 115I, 18030, and 221L (5).
Cytotoxicity assays using vaccinia virus and episomal vectors.
Infections with recombinant vaccinia virus expressing various Gag sequences and transfections with episomal vectors were performed as previously described (45, 54). Briefly, autologous EBV-transformed B cells were infected at a multiplicity of infection (MOI) of 3 with vaccinia virus and incubated overnight in 2 ml of R20 medium in 24-well plates. The cells were harvested, pulsed with 51Cr, and used as target cells in cytotoxicity assays at 10,000 cells/well. The recombinant vaccinia viruses used expressed either the entire HIV-1 Gag sequence (constructs 11-102, 22-102, 22-104, 22-105, 22-202, and 22-204), the patient LWF-derived p17 sequence with (p17-L75Y) or without (p17-wt) a leucine-to-tyrosine change at position 75, or the SL9 sequence only with an additional N-terminal methionine residue (VV-met-SL9). HMY-A2 cells were used for the transfection with the episomal vectors expressing either the SL9 epitope only or the SL9 epitope with the additional three N-terminal flanking amino acids Glu-Leu-Arg and Glu-Tyr-Arg, respectively (48, 54). In peptide titration assays, peptides were titrated directly in the assay at final concentrations ranging from 100 μg/ml to 10 pg/ml and were incubated with 51Cr-labeled target cells alone for 45 min prior to the addition of effector cells. CTL clones specific for epitopes in p24 and specific for SL9 were used at indicated effector/target cells ratios.
Inhibition of viral replication.
CTL-mediated inhibition of HIV-1 laboratory-adapted virus strains or patient-derived primary isolates were tested as previously described (48). T1 cells (HLA-A*0201 positive), H9 cells (HLA-A*0201 negative), and CD4+ T cells form patients VI-06 and 035i were infected with HIV-1 IIIB and HIV-1 NL4-3, respectively (MOI ranging from 2 × 10−3 to 10−1 50% tissue culture infective doses/cell [48]). After infection, the cells were seeded in a 24-well plate at 5 × 105 cells/well, in a total volume of 2 ml of R10-50 in the presence of SL9-specific CTL clones at effector/target ratios ranging from 0.25:1 to 2:1. At 3- to 4-day intervals, 1 ml was removed for HIV-1 p24 antigen quantitative enzyme-linked immunosorbent assay measurement (DuPont, Boston, Mass.) and replaced with fresh medium.
RESULTS
SL9 flanking sequences in HLA-A*0201-positive and HLA-A*0201-negative individuals.
Sequence variation within regions flanking CTL epitopes have been reported to contribute to immune evasion (9, 13). To determine whether such a mechanism of escape plays a role in HIV-1 infection, we examined the sequences flanking the immunodominant, HLA-A*0201-restricted CTL epitope SL9 in HIV-1 Gag p17. This epitope was selected because our previous studies indicated that escape variation within the SL9 epitope is not more frequent in HLA-A*0201-positive than in HLA-A*0201-negative individuals and that the presence of potential escape variations did not correlate with viral load during chronic infection (8). Since sequence variation can also mediate escape from CTL recognition by altering peptide processing (2, 3, 13–15, 33, 34, 52), the SL9 flanking regions of virus isolates from eight HLA-A*0201-positive individuals with detectable SL9 CTL responses were compared to sequences from five HLA-A*0201-negative, HIV-1-infected individuals (Table 1). We focused on changes within 15 amino acids of the SL9 epitope, since other studies have shown that residues close to the epitope can influence processing (2, 3, 13–15, 33, 34, 52). Although four other potential CTL epitopes have been reported in this region (restricted by HLA-A1, -A11, -B8, and -B62), only two of the HLA-A*0201-positive individuals express one of these alleles (HLA-A11 in subject LWF and HLA-B8 in 221L) (6).
TABLE 1.
In vivo proviral sequences in the SL9 flanking region from HLA-A*0201-positive and -negative patients
The consensus sequence is derived from the HIV-1 HXBR2 isolate and includes HIV-1 Gag residues 62 to 100. Numbering is according to reference 31. ND, not done.
n, number of identical sequences.
Viral load is expressed as the number of viral RNA copies per milliliter of plasma.
Of the 39 amino acid positions analyzed, 17 were found to exhibit variability compared to the HIV-1 HXB2R sequence. The most frequent variation was an arginine-to-lysine change at position 76 (R76K), but this variant was seen in both HLA-A*0201-positive and -negative individuals and in persons with high (>10,000 viral particles/ml) and low viral loads. Although 16 changes were exclusively found in HLA-A*0201-positive individuals, none of these changes was associated exclusively with high viral load, and these variants never comprised more than two-thirds of the viral population in vivo. In subject LWF, who was accidentally infected with HIV-1 IIIB, only a single change was found in Gag p17 in samples obtained at two time points approximately 6 years after infection. This consisted of a change from leucine to tyrosine at position 75 (L75Y) and is unique among our cohort; it has not been described in other individuals (26). These data indicate that there are no consistent SL9 flanking sequence changes which are exclusively found in HLA-A*0201-positive individuals with high viral loads.
Processing of HIV-1 Gag sequences expressed by recombinant vaccinia viruses.
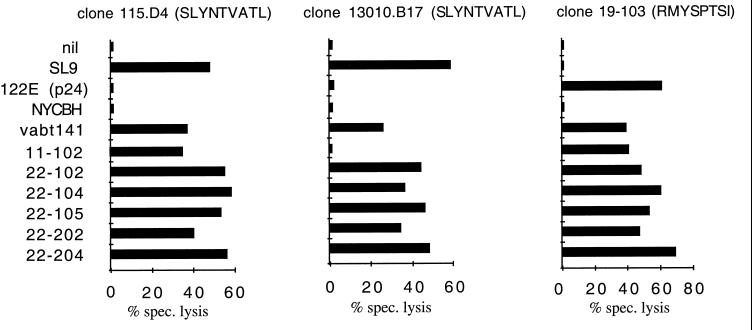
To define more precisely the effect of sequence variation in flanking residues on CTL recognition, a series of HIV-1 Gag vaccinia virus constructs from patient isolates (Table 2) was tested for recognition by distinct HLA-A*0201-restricted, SL9-specific CTL clones derived from different chronically infected donors (Fig. 1). To control for the expression and processing of the Gag protein from the various vaccinia virus constructs, specific CTL clones that recognize an epitope in HIV-1 Gag p24 presented on the same target cells were used. When the different recombinant vaccinia virus constructs were tested for CTL sensitization, all were recognized by at least two SL9-specific CTL clones. An exception was construct VV-Gag 11-102, which was not recognized by CTL clone 13010.B17. As determined by peptide titration assays, this lack of recognition was due to a mutation within the SL9 epitope of construct VV-Gag 11-102 (Y79F) which specifically interfered with recognition by clone 13010.B17 (data not shown). However, the epitope variant in VV-Gag 11-102 was clearly appropriately presented, since cells infected with VV-Gag 11-102 were efficiently lysed by the SL9-specific clone 115.D4. Fluorescence-activated cell sorter analyses demonstrated that the amount of cell lysis was associated with the amount of intracellular HIV-1 Gag-p24 produced (data not shown), indicating that the observed minor differences in cell lysis induced by infection with the different vaccinia virus constructs were due to the amount of antigen produced rather than to different efficiencies in the processing of the variant p17 sequences. These data indicate that none of the flanking region changes listed in Table 2, including the most frequent R76K mutation, prevented the processing of SL9 from the HIV-1 Gag protein.
TABLE 2.
SL9 flanking sequences of HIV-1 HXB2 and recombinant vaccinia virus Gag constructs
| Origin | Amino acid sequence (HIV-1 Gag, p17 amino acids 62–100)a |
|---|---|
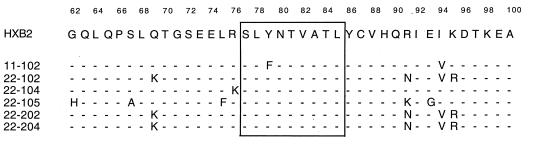 | |
The consensus sequence is derived from the HIV-1 HXB2 isolate and includes HIV-1 Gag residues 62 to 100. Numbering is according to reference 31.
FIG. 1.
Recognition of the SL9 epitope expressed by different vaccinia virus constructs. HLA-A*0201-positive B-LCL cells were infected overnight with vaccinia virus constructs or pulsed with optimal peptides (SL9 for clones 115.D4 and 13010.B17) or, in this experiment, with the HLA-B52 restricted peptide 122E (Gag p24, RMYSPTSI, amino acids 275 to 282) recognized by clone 19-203 for 90 min. The effector/target ratio was 5:1. This experiment was repeated three times with different CTL clones and target cell lines from three different donors, yielding the same pattern of recognition.
Effect of the unique L75Y mutation on SL9 processing from vaccinia virus constructs and episomal vectors.
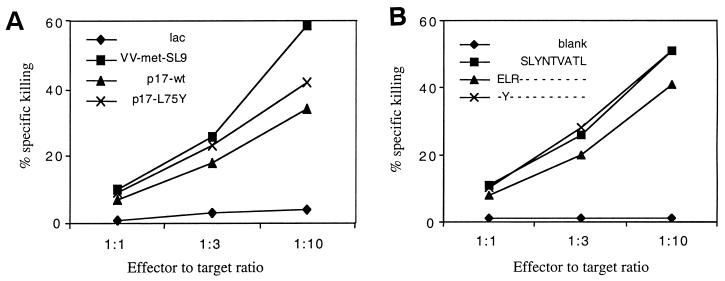
Patient LWF, a laboratory worker who was infected with HIV-1 IIIB, showed strong and persistent SL9-specific CTL activity in PBMC for at least 8 years (40). The autologous viral population showed remarkable stability, and only one change from the infecting virus was observed in p17 6 years after infection (Table 1). We therefore evaluated whether a leucine-to-tyrosine change at position 75 could prevent SL9 processing. It should be noted that there are two CTL epitopes described that contain a tyrosine residue at position 75 (26), and CTL responses against these epitopes could have induced this change. However, both epitopes are restricted by HLA alleles not expressed by patient LWF, which makes it unlikely that this mutation occurred under CTL pressure. To assess whether the change could be a result of immune pressure against the SL9 epitope, different expression systems were used to analyze the effect of this variation on SL9 processing. First, vaccinia virus constructs expressing either the SL9 epitope alone or the two different p17 sequences found in patient LWF (with or without the L75Y change) were compared for sensitization of SL9-specific CTL clones. Figure 2A shows that cells infected with all three constructs were well recognized by SL9-specific CTL lines, indicating that L75Y does not block antigen processing. These findings were confirmed by transfecting HLA-A*0201-positive HMY-A2 cells with episomal vectors expressing the SLYNTVATL, ELRSLYNTVATL, and EYRSLYNTVATL sequences, respectively (Fig. 2B). All three vectors were able to sensitize target cells for SL9-specific CTL lysis, indicating that the SL9 peptide was efficiently presented by these constructs. Furthermore, expression of the SL9 epitope from the L75Y mutated precursor protein resulted in slightly enhanced recognition with both expression systems. These data suggest that the unique L75Y change observed in patient LWF did not abrogate the processing of SL9.
FIG. 2.
Processing of SL9 from patient LWF-derived Gag sequences expressed as vaccinia virus minigene constructs and episomal vectors. (A) Vaccinia virus construct expression system. HLA-A*0201-positive B-LCL cells were infected overnight with vaccinia virus constructs expressing patient LWF-derived p17 sequences or the SL9 epitope only. (B) Episomal vector expression system. Alternatively, HMY-A2 cells were stably transfected with episomal vectors expressing SL9 with or without three additional N-terminal amino acid residues. B-LCL and HMY-A2 target cells were 51Cr labeled for 90 min and incubated with a polyclonal SL9-specific CTL line at the indicated effector/target ratios for 4 h in a standard cytotoxicity assay.
Processing and presentation of the SL9 peptide in HIV-1-infected cells.
The recognition of vaccinia virus-expressed Gag by SL9-specific CTL indicates that the observed changes do not prevent processing in recombinant vaccinia virus-infected cells. To rule out possible artifacts, such as vaccinia virus construct-mediated superphysiological expression of recombinant antigens (10, 23, 28), we determined whether flanking sequence changes can alter CTL recognition in HIV-1-infected cells (49). Laboratory-adapted virus strains and patient isolates were used in an in vitro replication inhibition assay described previously (48). These experiments allowed for the analysis of very common sequences present in replication-competent virus strains (HIV-IIIB, NL4-3, and JR-CSF) and their impact on SL9 processing in a more physiological setting than with the vaccinia virus constructs (Table 3). The inclusion of two patient isolates allowed testing of additional, common changes in the SL9 flanking region.
TABLE 3.
SL9 flanking sequences expressed by replication-competent HIV-1 strains and patient isolates
| Isolate | Gag-p17 region amino acid sequence (amino acids 62–100)a |
|---|---|
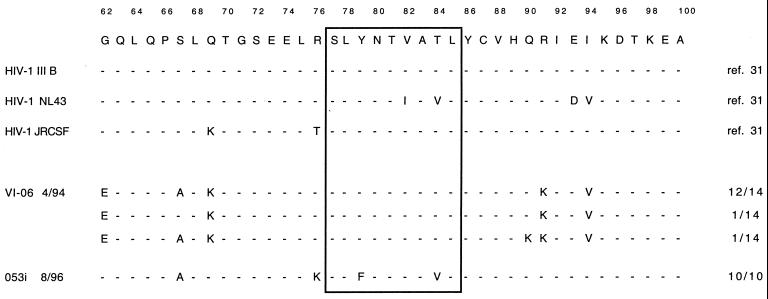 | |
The consensus sequence is derived from the HIV-1 HXB2 isolate and includes HIV-1 Gag residues 62 to 100. Numbering is according to reference 31.
Figure 3 shows the results for inhibition of HIV-1 isolates with flanking sequence changes. Both patient-derived primary isolates (Table 3) were efficiently inhibited by clone 115D4, indicating efficient processing of the SL9 epitope from these sequences (Fig. 3a and b). Replication of the laboratory strain IIIB was likewise efficiently inhibited by two CTL clones tested (Fig. 3c), but only when the infected cells expressed HLA-A*0201 (Fig. 3d). Inhibition of the molecular clone NL4-3 was less efficient (Fig. 3e), but peptide titration assays showed that this was due to impaired recognition of sequences changes (V82I/T84V) within the epitope (Fig. 3f). The differences between the inhibition of HIV-1 IIIB and NL4-3 could therefore be due to impaired interaction of the TCR with the peptide-MHC complex rather than to differences in antigen processing. It is noteworthy that clone 115D4, which showed a 2-log difference in the peptide titration assays, had a clearly diminished ability to control NL4-3 replication. For clone 161j.XA/14, the recognition of the V82I/T84V was reduced by 5 logs, which may be the reason for the almost complete loss of inhibition of replication for strain NL4-3 by this clone.
FIG. 3.
Inhibition of viral replication by SL9-specific CTL clones and peptide titration of SL9 and the NL4-3 encoded variant V821/T84V. (a and b) HLA-A*0201-expressing CD4 cells were infected with viral isolates from subjects VI-06 or 053i and cultured in the presence (◊) or absence (⧫) of the SL9-specific CTL cone 115D4. (c and d) HLA-A*0201-positive T1 cells (c) and HLA-A*0201-negative H9 cells (d) were infected with HIV-1 IIIB and cultured in the presence of SL9-specific CTL clone 115D4 (◊) or clone 161jXA/14 (▵) or without CTL (⧫). (e) T1 cells were infected with HIV-1 NL4-3 isolate and cultured with the same clones as for panels c and d. The production of p24 was measured after 3 to 15 days. (f) For peptide titrations, clones 115.D4 and 161j.XA/14 were both tested on the SL9 sequences expressed by HIV-1 HXB2 (HIV-1 IIIB, SL9 consensus sequence) and by HIV-1 NL4-3 (SL9 variant V82I/T84V). Peptide titration was carried out with HLA-A*0201-positive EBV-transformed B-LCL cells as target cells in a standard 51Cr release assay.
These data indicate that none of the changes observed in these virus strains influence the processing of the SL9 peptide or its variants; rather, the only reduction in recognition was due to changes within the epitope.
DISCUSSION
We previously reported that the immunodominant CTL response to the HLA-A*0201-restricted SL9 epitope in HIV-1 Gag p17 does not readily induce changes within the epitope that would lead to escape from CTL recognition (5). Here, we have extended these studies to the sequences flanking the presented nonameric epitope by analyzing the impact of changes in the SL9 flanking sequences on the processing and the presentation of this epitope. We find no evidence that commonly occurring flanking residue changes adversely affect CTL recognition. All the vaccinia virus constructs and the episomal vectors were recognized by a panel of CTL clones when they contained the SL9 consensus sequence or an SL9 variant recognized by the CTL clones used. Furthermore, viral strains with a variety of naturally occurring flanking sequence variations are inhibited by SL9-specific CTL clones. These results demonstrate that SL9 and its variants were efficiently processed from all of these sequences and also show an association between the degree of inhibition of viral replication and recognition of the SL9 variant, suggesting that inhibition was dependent on TCR recognition rather than on the processing of the SL9 epitope.
The data presented here suggest that viral escape from epitope processing may be difficult to achieve. Because the processing machinery has limited polymorphism and must be versatile enough to supply peptides for multiple HLA alleles, one would expect rather nonspecific protease activity, which should make escape from processing difficult (41). Despite this presumably unspecific antigen processing, some amino acid residues have been described to be important in proteasome-mediated processing and TAP-dependent peptide transport into the endoplasmic reticulum (1, 3, 11, 13–15, 32, 34, 35, 41–44, 51, 52). However, a close analysis of all the sequences presented here did not reveal changes that have been described to affect antigen processing, such as the substitution of residues immediately flanking the epitope by charged amino acids (35) or by glycine or proline (3, 13–15, 34, 41, 42, 52). In addition, substitution of lysine residues involved in ubiquitin-dependent antigen degradation (14, 43) did not affect the processing of the K87R variants, since vaccinia virus constructs 22-102, 22-202, and 22-204, which express this variant, were well recognized.
Furthermore, changes of residues preferred by the TAP heterodimer (hydrophobic residues in the third position, hydrophobic and charged residues in position 2, and hydrophobic and acidic residues at the C-terminal end) did not occur (15, 34). Thus, it also seems unlikely that the TAP-allele polymorphism could have an impact on the occurrence of escape variants, which is also underscored by the fact that in our experiments B-cell lines and CD4 T cells from different subjects expressing different TAP1 and TAP2 alleles were able to process the HIV-Gag protein and to present SL9 (data not shown). Furthermore, in a cohort of 13 chronically HIV-1-infected, HLA-A*0201-positive patients, TAP-allele polymorphism is not correlated with viral load, although it may be possible that TAP polymorphism becomes relevant for certain variants (data not shown).
Given the high viral turnover of HIV-1 in vivo, one might expect the virus to rapidly develop effective SL9 escape variations, which either abrogate recognition by TCR or profoundly impair antigen processing. Accordingly, a lack of escape may be due to constraints in viral fitness. The variations in the in vivo flanking sequences shown in Table 1 are likely to represent replication-competent in vivo sequences. Conceivably, some changes may not be tolerated due to viral fitness constraints, making it impossible for the virus to escape. Analysis of the sequences listed in the Los Alamos HIV Database show that the C-terminal end of SL9 and the C-terminal flanking sequences are indeed very conserved (31). This region has also been described as important for correct protein folding, p17 trimer complex formation, and viral replication (7). Consequently, the lack of escape variation may not be due to the lack of CTL-mediated immune pressure but rather due to the limited tolerance for changes around the SL9 epitope. However, in the presence of strong CTL pressure, such inability to escape should allow the host to inhibit viral replication.
Our studies do not address whether amino acid substitutions different from the ones that have been found in in vivo sequences could influence antigen processing, but they do indicate that the specific changes we observed in natural HIV-1 variants did not prevent epitope processing. It remains possible that changes in the epitope flanking region may partially but not completely inhibit the efficient processing of the epitope. Although we cannot completely rule out that this may explain the different levels of cell lysis observed with the various vaccinia virus constructs used, several lines of evidence speak against it. First, reports that describe successful escape from antigen processing demonstrate not only partial but often complete inhibition of cell lysis, indicating that processing escape variants can evade processing completely (13, 52). Second, the linear association between the amount of expressed antigen and the degree of cell lysis observed (data not shown) suggests that the limiting factor for cell lysis observed here is antigen availability rather than variable efficiency in epitope processing. This suggests that the variants that were tested with the different vaccinia virus constructs were comparably processed and presented.
Since many of the in vivo SL9 variants are recognized (5) and since epitope processing does not seem to be abrogated, this indicates that the strong SL9-specific CTL responses that are detectable in vitro may not exert a strong selection pressure in vivo, either due to (i) the functional impairment of the CTL in vivo, (ii) the plasticity of TCR recognition, or (iii) the existence of immune privileged sites where the virus is not accessible to CTL. Alternatively, the virus may not be able to escape CTL pressure since potential escape variants influence viral fitness in a negative way. This would also explain why the majority of HLA-A*0201-positive individuals have SL9-specific CTL precursors even after years of chronic infection and would also offer an explanation as to why the SL9-specific responses are considered “immunodominant” (5, 18). However, this would also imply that these SL9-specific CTL are unable to control viral replication, despite a lack of escape variants, again favoring the possibility that SL9-specific CTL in vivo are functionally impaired, e.g., that they may lack help from CD4 T cells (19, 22, 38). While such functionally impaired CTL have been described recently in a murine model (53), the human system may require longitudinal studies that follow acutely infected individuals with preserved HIV-1-specific CD4 T-cell responses to answer the question as to what degree functional impairment of CTL responses or variability of viral sequences is responsible for persistent high viral loads found in untreated patients.
ACKNOWLEDGMENTS
We thank Barbara Wilkes for the p24 specific CTL clone 19-103, Debbie Ruhl for performing the inhibition assays with subject 53i, and Andreas Suhrbier for helpful discussions.
This work was supported by a Burroughs Wellcome Fund/Infectious Disease Society of America Young Investigators Award to R.P.J.; by the Pediatric AIDS Foundation and a Public Health Service grant (HD31756); by grants AI33327, AI33314, AI39966, AI28568, and AI30914 from the National Institutes of Health and R64/CCV 912541 from The Centers for Disease Control; and by a grant from the Schweizerische Stiftung fuer Medizinisch Biologische Stipendien to C.B.
REFERENCES
- 1.Androlewicz M J, Cresswell P. How selective is the transporter associated with antigen processing? Immunity. 1996;5:1–5. doi: 10.1016/s1074-7613(00)80304-0. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann C C, Tong L, Cua R, Sensintaffar J, Stohlman S. Differential effects of flanking residues on presentation of epitopes from chimeric peptides. J Virol. 1994;68:5306–5310. doi: 10.1128/jvi.68.8.5306-5310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmann C C, Yao Q, Ho C, Buckwold S L. Flanking residues alter antigenicity and immunogenicity of multi-unit CTL epitopes. J Immunol. 1996;157:3242–3249. [PubMed] [Google Scholar]
- 4.Borrow P, Lewicki H, Wei X, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B, Shaw G M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 5.Brander C, Hartman K E, Trocha A K, Jones N G, Johnson R P, Korber B, Wentworth P, Buchbinder S P, Wolinsky S, Walker B D, Kalams S A. Lack of strong immune selection pressure by the immunodominant, HLA-A*0201-restricted CTL response in chronic HIV-1 infection. J Clin Investig. 1998;101:2559–2566. doi: 10.1172/JCI2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brander C, Walker B D. The HLA class I restricted CTL response in HIV infection: systematic identification of optimal epitopes. In: Korber B, Brander C, Walker B, Koup R, Moore J, Haynes B, Meyers G, editors. HIV molecular immunology database. Los Alamos National Laboratory: Theoretical Biology and Biophysics, Los Alamos, N.Mex. http://hiv-web.lanl.gov/immunology/index.html. [6 October 1999, last date accessed.] 1996. [Google Scholar]
- 7.Cannon P M, Matthews S, Clark N, Byles E D, Iourin O, Hockley D J, Kingsman S M, Kingsman A J. Structure-function studies of the human immunodeficiency virus type 1 matrix protein, p17. J Virol. 1997;71:3474–3483. doi: 10.1128/jvi.71.5.3474-3483.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Z W, Shen L, Miller M D, Ghim S H, Hughes A L, Letvin N L. Cytotoxic T lymphocytes do not appear to select for mutations in an immunodominant epitope of simian immunodeficiency virus gag. J Immunol. 1992;149:4060–4066. [PubMed] [Google Scholar]
- 9.Couillin I, Culmann-Penciolelli B, Gomard E, Choppin J, Levy J-P, Guillet J-G, Saragosti S. Impaired cytotoxic T lymphocyte recognition due to genetic variations in the main immunogenic region of the human immunodeficiency virus 1 NEF protein. J Exp Med. 1994;180:1129–1134. doi: 10.1084/jem.180.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cucchiarini M, Barcellini-Couget S, Lefebvre J C, Doglio A. T cells chronically infected with HIV-1 do not contain sufficient Nef to promote CD4 downmodulation in the absence of envelope mediated effects. J Acquir Immune Defic Syndr. 1998;17:112–119. doi: 10.1097/00042560-199802010-00003. [DOI] [PubMed] [Google Scholar]
- 11.Daniel S, Caillat-Zucman S, Hammer J, Bach J F, van Endert P M. Absence of functional relevance of human transporter associated with antigen processing polymorphism for peptide selection. J Immunol. 1997;159:2350–2357. [PubMed] [Google Scholar]
- 12.Davenport M P. Antagonists or altruists: do viral mutants modulate T cell responses? Immunol Today. 1995;16:432–437. doi: 10.1016/0167-5699(95)80020-4. [DOI] [PubMed] [Google Scholar]
- 13.Del Val M, Schlicht H-J, Ruppert T, Reddehase M J, Koszinowski U. Efficient processing of an antigenic sequence for presentation by MHC class I molecules depends on its neighbouring residues in the protein. Cell. 1991;66:1145–1153. doi: 10.1016/0092-8674(91)90037-y. [DOI] [PubMed] [Google Scholar]
- 14.Eggers M, Boes-Fabian B, Ruppert T, Kloetzel P M, Koszinowski U H. The cleavage preference of the proteasome governs the yield of antigenic peptides. J Exp Med. 1995;182:1865–1870. doi: 10.1084/jem.182.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenlohr L C, Yewdell J W, Bennink J R. Flanking sequences influence the presentation of an endogenously synthesized peptide to cytotoxic T lymphocytes. J Exp Med. 1992;175:481–487. doi: 10.1084/jem.175.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallimore A, Cranage M, Cook N, Almond N, Bootman J, Rud E, Silvera P, Dennis M, Corcoran T, Stott J, et al. Early suppression of SIV replication by CD8+ nef-specific cytotoxic T cells in vaccinated macaques. Nat Med. 1995;1:1167–1173. doi: 10.1038/nm1195-1167. [DOI] [PubMed] [Google Scholar]
- 17.Goulder P J, Phillips R E, Colbert R A, McAdam S, Ogg G, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael A J, Rowland-Jones S. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 18.Goulder P J R, Sewell A K, Lalloo D G, Price D A, Whelan J A, Evans J, Taylor G P, Luzzi G, Giangrande P, Phillips R E, McMichael A J. Patterns of immunodominance in HIV-1-specific cytotoxic T lymphocyte responses on two human histocompatibility leukocyte antigen identical siblings with HLA-A*0201 are influenced by epitope mutation. J Exp Med. 1997;185:1423–1433. doi: 10.1084/jem.185.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hay C M, Ruhl D J, Basgoz N, Wilson C C, Billingsley J M, DePasquale M P, D’Aquila R, Wolinsky S, Crawford J M, Montefiori D, Walker B D. Lack of viral escape and defective in vivo activation of HIV-1-specific CTL in rapidly progressive infection. J Virol. 1999;73:5509–5519. doi: 10.1128/jvi.73.7.5509-5519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson R P, Trocha A, Yang L, Mazzara G P, Panicali D L, Buchanan T M, Walker B D. HIV-1 gag-specific cytotoxic T lymphocytes recognize multiple highly conserved epitopes. Fine specificity of the gag-specific response defined by using unstimulated peripheral blood mononuclear cells and cloned effector cells. J Immunol. 1991;147:1512–1521. [PubMed] [Google Scholar]
- 21.Kaegi D, Ledermann B, Buerki K, Zinkernagel R, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–232. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- 22.Kalams S A, Buchbinder S P, Rosenberg E S, Billingsley J M, Colbert D S, Jones N G, Shea A K, Trocha A K, Walker B D. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 Infection. J Virol. 1999;73:6715–6720. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karacostas V, Wolffe E J, Nagashima K, Gonda M A, Moss B. Overexpression of the HIV-1 gag-pol polyprotein results in intracellular activation of HIV-1 protease and inhibition of assembly and budding of virus-like particles. Virology. 1993;193:661–671. doi: 10.1006/viro.1993.1174. [DOI] [PubMed] [Google Scholar]
- 24.Klenerman P, Rowland-Jones S, McAdam S, Edwards J, Daenke S, Lalloo D, Koppe B, Rosenberg W, Boyd D, Edwards A, Giagrande P, Phillips R E, McMichael A J. Naturally occurring HIV-1 gag variants antagonise cytotoxic T cell activity. Nature. 1994;369:403–410. doi: 10.1038/369403a0. [DOI] [PubMed] [Google Scholar]
- 25.Koenig S, Conley A J, Brewah Y A, Jones G M, Leath S, Boots L J, Davey V, Pantaleo G, Demarest J F, Carter C, Wannebo C, Yanelli J R, Rosenberg S A, Lane H C. Transfer of HIV-1-specific cytotoxic T lymphocytes to an AIDS patient leads to selection for mutant HIV variants and subsequent disease progression. Nat Med. 1995;1:330–336. doi: 10.1038/nm0495-330. [DOI] [PubMed] [Google Scholar]
- 26.Korber B, Walker B, Brander C, Koup R, Moore J, Haynes B, Meyers G. HIV molecular immunology database 1998. Los Alamos National Laboratory: Theoretical Biology and Biophysics. N.Mex: Los Alamos; 1998. [Google Scholar]
- 27.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marti W R, Zajac P, Spagnoli G, Haberer M, Oertli D. Nonreplicating recombinant vaccinia virus encoding human B7 molecules elicits effective costimulation of naive and memory CD4 T lymphocytes in vitro. Cell Immunol. 1997;179:146–152. doi: 10.1006/cimm.1997.1158. [DOI] [PubMed] [Google Scholar]
- 29.McMichael A. T cell responses and viral escape. Cell. 1998;93:673–676. doi: 10.1016/s0092-8674(00)81428-2. [DOI] [PubMed] [Google Scholar]
- 30.McMichael A J, Michie C A, Gotch F M, Smith G L, Moss B. Recognition of influenza A virus nucleoprotein by human cytotoxic T lymphocytes. J Gen Virol. 1986;67:719–726. doi: 10.1099/0022-1317-67-4-719. [DOI] [PubMed] [Google Scholar]
- 31.Meyers G, Foley B, Mellors J W, Korber B, Jeang K, Wain-Hobson S. Human retroviruses and AIDS 1996: a compilation and analysis of nucleic acid and amino acid sequences. Los Alamos: Los Alamos National Laboratories Theoretical Biology; 1996. http://hiv-web.lanl.gov , N.Mex. http://hiv-web.lanl.gov. [6 October 1999, last date accessed.] . [6 October 1999, last date accessed.] [Google Scholar]
- 32.Momburg F, Roelse J, Howard J C, et al. Selectivity of MHC-encoded peptide transporters from human, mouse and rat. Nature. 1994;367:648–651. doi: 10.1038/367648a0. [DOI] [PubMed] [Google Scholar]
- 33.Neisig A, Roelse J, Sijts A J, Ossendorp F, Feltkamp M C, Kast W M, Melief C J, Neefjes J J. Major differences in transporter associated with antigen presentation (TAP)-dependent translocation of MHC class I-presentable peptides and the effect of flanking sequences. J Immunol. 1995;154:1273–1279. [PubMed] [Google Scholar]
- 34.Niedermann G, Butz S, Ihlenfeldt H G, Grimm R, Lucchiari M, Hoschutzky H, Jung G, Maier B, Eichmann K. Contribution of proteasome-mediated proteolysis to the hierarchy of epitopes presented by major histocompatibility complex class I molecules. Immunity. 1995;2:289–299. doi: 10.1016/1074-7613(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 35.Ossendorp F, Eggers M, Neisig A, Ruppert T, Groettrup M, Sijts A, Mengede E, Kloetzel P, Neefjies J, Koszinowski U, Melief C. A single residue exchange within a viral CTL epitope alters proteasome-mediated degradation resulting in lack of antigen presentation. Immunity. 1996;5:115–124. doi: 10.1016/s1074-7613(00)80488-4. [DOI] [PubMed] [Google Scholar]
- 36.Pantaleo G, Demarest J F, Soudeyns H, Graziosi C, Denis F, Adelsberger J W, Borrow P, Saag M S, Shaw G M, Sekaly R P, et al. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature. 1994;370:463–467. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- 37.Price D A, Goulder P J R, Klenerman P, Sewell A, Easterbrook P J, Troop M, Bangham C R M, Phillips R E. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc Natl Acad Sci USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1 specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 39.Rutherford G W, Lifson A R, Hessol N A, Darrow W W, O’Malley P M, Buchbinder S P, Barnhart J L, Bodecker T W, Cannon L, Doll L S, et al. Course of HIV-1 infection in a cohort of homosexual and bisexual men: an 11 year follow up study. Br Med J. 1990;301:1183–1188. doi: 10.1136/bmj.301.6762.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sipsas N V, Kalams S A, Trocha A, He S, Blattner W A, Walker B D, Johnson R P. Identification of type-specific cytotoxic T lymphocyte responses to homologous viral proteins in laboratory workers accidentally infected with the human immunodeficiency virus-1. J Clin Investig. 1997;99:752–762. doi: 10.1172/JCI119221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suhrbier A. Multi-epitope DNA vaccines. Immunol Cell Biol. 1997;75:402–408. doi: 10.1038/icb.1997.63. [DOI] [PubMed] [Google Scholar]
- 42.Thomson S A, Elliott S L, Sherritt M A, Sproat K W, Coupar B E, Scalzo A A, Forbes C A, Ladhams A M, Mo X Y, Tripp R A, Doherty P C, Moss D J, Suhrbier A. Recombinant polyepitope vaccines for the delivery of multiple CD8 cytotoxic T cell epitopes. J Immunol. 1996;157:822–826. [PubMed] [Google Scholar]
- 43.Townsend A, Bastin J, Gould K, Brownlee G, Andrew M, Coupar B, Boyle D, Chan S, Smith G. Defective presentation to class I restricted cytotoxic T lymphocytes in vaccinia infected cells is overcome by enhanced degradation of antigen. J Exp Med. 1988;168:1211–1224. doi: 10.1084/jem.168.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Endert P, Riganelli D, Greco G, Fleischbauer K, Sidney J, Sette A, Bach J. The peptide-binding motif for the human transporter associated with antigen processing. J Exp Med. 1995;182:1883–1895. doi: 10.1084/jem.182.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker B D, Chakrabarti S, Moss B, Paradis T J, Flynn T, Durno A G, Blumberg R S, Kaplan J C, Hirsch M S, Schooley R T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987;328:345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- 46.Walker B D, Flexner C, Paradis T J, Fuller T C, Hirsch M S, Schooley R T, Moss B. HIV-1 reverse transcriptase is a target for cytotoxic T lymphocytes in infected individuals. Science. 1988;240:64–66. doi: 10.1126/science.2451288. [DOI] [PubMed] [Google Scholar]
- 47.Weiss S H, Goedert J J, Gartner S, Popovic M, Waters D, Marham P, di Marzo Veronese F, Gail M H, Barkley W E, Gibbons J, Gill F A, Leuther M, Shaw G M, Gallo R C, Blattner W A. Risk of human immunodeficiency virus (HIV-1) infection among laboratory workers. Science. 1988;239:68–71. doi: 10.1126/science.3336776. [DOI] [PubMed] [Google Scholar]
- 48.Yang O O, Kalams S A, Trocha A, Cao H, Luster A, Johnson R P, Walker B D. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class 1-restricted triggering of cytolytic and noncytolytic mechanisms. J Virol. 1997;71:3120–3128. doi: 10.1128/jvi.71.4.3120-3128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang O O, Trocha A C, Kalams S A, Johnson R P, Roberts M R, Walker B D. Lysis of HIV-1 infected cells and inhibition of viral replication by universal receptor T cells. Proc Natl Acad Sci USA. 1997;94:11478–11483. doi: 10.1073/pnas.94.21.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang O O, Walker B D. CD8 cells in human immunodeficiency virus type 1 pathogenesis: cytolytic and noncytolytic inhibition of viral replication. Adv Immunol. 1997;66:273–311. doi: 10.1016/s0065-2776(08)60600-8. [DOI] [PubMed] [Google Scholar]
- 51.Yellen-Shaw A J, Laughlin C E, Metrione R M, Eisenlohr L C. Murine transporter associated with antigen presentation (TAP) preferences influence class I-restricted T cell responses. J Exp Med. 1997;186:1655–1662. doi: 10.1084/jem.186.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yellen-Shaw A J, Wherry E J, Dubois G C, Eisenlohr L C. Point mutation flanking a CTL epitope ablates in vitro and in vivo recognition of a full-length viral protein. J Immunol. 1997;158:3227–3234. [PubMed] [Google Scholar]
- 53.Zajac A J, Blattman J N, Murali-Krishna K, Sourdive D J, Suresh M, Altman J D, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:71–82. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zweerink H J, Gammon M C, Utz U, Sauma S Y, Harrer T, Hawkins J C, Johnson R P, Sirotina A, Hermes J D, Walker B D, et al. Presentation of endogenous peptides to MHC class I-restricted cytotoxic T lymphocytes in transport deletion mutant T2 cells. J Immunol. 1993;150:1763–1771. [PubMed] [Google Scholar]