Abstract
Numerous studies have demonstrated the clinical value of continuous glucose monitoring (CGM) in type 1 diabetes (T1D) and type 2 diabetes (T2D) populations. However, the eligibility criteria for CGM coverage required by the Centers for Medicare & Medicaid Services (CMS) ignore the conclusive evidence that supports CGM use in various diabetes populations that are currently deemed ineligible. In an earlier article, we discussed the limitations and inconsistencies of the agency’s CGM eligibility criteria relative to current scientific evidence and proposed practice solutions to address this issue and improve the safety and care of Medicare beneficiaries with diabetes. Although Medicaid is administered through CMS, there is no consistent Medicaid policy for CGM coverage in the United States. This article presents a rationale for modifying and standardizing Medicaid CGM coverage eligibility across the United States.
Keywords: type 1 diabetes, type 2 diabetes, Medicaid, continuous glucose monitoring, insulin
Introduction
The prevalence of diabetes among all adults in the United States was 13.0% in 2018. 1 However, prevalence is disproportionally higher in Native American (14.5%), Hispanic (11.8%), and black populations (12.1%) compared with white individuals (7.4%), particularly in those with low socioeconomic status.1,2 Individuals with less than a high school education (16.6%) 1 and/or low socioeconomic status are also at significantly greater risk of diabetes complications regardless of race or ethnicity.2,3
According to the Centers for Medicare & Medicaid Services (CMS), 87 384 715 individuals are enrolled in Medicaid and Children’s Health Insurance Programs (CHIP) 4 ; more than half are under 21 years of age. 5 Black (32.0%) and Hispanic (30.0%) beneficiaries comprise the largest percentage of the Medicaid population. 6
Despite advances in medications and diabetes technologies, the median percentage of Medicaid/CHIP beneficiaries with HbA1c levels > 9.0% within the 31 states that report this measure is estimated to be 39.0%. 7 Only 12% of Medicaid beneficiaries have achieved the recommended HbA1c target of < 7.0% 8 compared with those covered by commercial health plans (20%) or Medicare (26%). 9 Higher rates of disability, depression, and comorbidities among Medicaid beneficiaries compared with individuals covered by Medicare or commercial health plans have also been reported, 10 all of which can impact treatment adherence and clinical outcomes.
Frequent glucose monitoring is recommended by all major diabetes organizations11 -14; it is considered essential to glycemic management in individuals with type 1 diabetes (T1D) and insulin-treated type 2 diabetes (T2D). Although fingerstick blood glucose monitoring (BGM) is the most common method for testing, a growing number of patients have adopted continuous glucose monitoring (CGM). Large, randomized trials and real-world studies have shown CGM to be safe and effective in improving HbA1c, lowering hypoglycemia risk, and reducing diabetes-related hospitalizations in patients treated with insulin.15 -21 The current optimal care for persons with T1D and insulin-requiring T2D is with an insulin pump with automated insulin delivery (AID). These systems require CGM connectivity and input for determination of pump-delivered insulin doses. A recent study showed equal benefit among publically insured users compared with those with private insurance. 22
Many Medicaid beneficiaries do not have access to CGM, due, primarily, to overly restrictive eligibility criteria. For example, two states (Georgia and Alabama) only provide CGM coverage for pediatric patients. 23 Because lower socioeconomic status and race/ethnicity are strong predictors for the development of diabetes-related complications and mortality,24,25 it is important that Medicaid reconsider their eligibility criteria for CGM coverage.
In an earlier article, we discussed the limitations and inconsistencies of CMS’s CGM eligibility criteria relative to current scientific evidence and proposed practical solutions to address this issue and improve the safety and care of Medicare beneficiaries with diabetes. 26 Table 1 presents a summary of our recommendations for modifying the eligibility requirements. These recommendations closely align with the agency’s proposed changes to CGM eligibility 27 (Table 2). The purpose of this article is to present a rationale for applying and standardizing these recommendations across all state Medicaid programs.
Table 1.
Recommendations for Modifying Medicare CGM Eligibility Requirements. 26
| Criterion | Supporting evidence |
|---|---|
| 1. Diagnosed with T1D. |
CGM use confers: • Significant reductions in HbA1c.15,17,28 -36 • Significant reductions in severe hypoglycemia events.18,32,33,37 • Significant increases in %TIR.17,20,30,36,38 • Significant decreases in %TBR.17,30,36 • Significant reductions in diabetes-related hospitalizations.18,32,33,39 |
| 2. Diagnosed with T2D and treated with any insulin therapy. |
CGM use confers: • Significant reductions in HbA1c.29,31,40 -47 • Significant increases in %TIR.29,40,47 • Significant decreases in %TBR.19,48 • Significant decreases in %TAR. 47 • Significant reductions in severe hypoglycemia events. 37 • Significant reductions in diabetes-related hospitalizations.39,49 |
| 3. Diagnosed with T2D and documented problematic hypoglycemia regardless of diabetes therapy. This would include a history of at least one of the following conditions: • Level 2 (moderate) hypoglycemia—characterized by glucose levels ≤ 54 mg/dL. • Level 3 (severe) hypoglycemia—characterized by physical/mental dysfunction requiring third-party assistance. Nocturnal hypoglycemia. |
Older diabetes patients are at increased hypoglycemia risk: • T2D patients treated with antihyperglycemic medications (eg, insulin and sulfonylureas) are at higher risk for hypoglycemia than those treated with non-hypoglycemia medications (eg, metformin). 50 • T2D patients ≥65 years treated with basal insulin (typically one injection per day) are at increased risk for severe hypoglycemia. 51 • A key driver of hypoglycemia risk is impaired hypoglycemia awareness.52,53 CGM use confers: • Significant reductions in diabetes-related hospitalizations, including severe hypoglycemia events.39,49 • Significant reductions in severe hypoglycemia events. 37 • Significant reductions in hypoglycemia fear and increases in patient confidence in avoiding/treating hypoglycemia,28,54 thereby supporting treatment adherence.55,56 |
| 4. Chronic kidney disease (CKD). |
CGM use facilitates: • More frequent treatment changes and improved glycemic control without increased risk of hypoglycemia. 57 • Effective monitoring and managing glycemic levels in patients without diabetes with ESRD undergoing dialysis. 58 |
| 5. In-person or telemedicine consultation with the prescribing healthcare provider prior to CGM initiation and every 6 months thereafter while continuing CGM therapy. |
Use of telemedicine consults: • Significantly reduces HbA1c.59 -64 • Reduces the incidence of severe hypoglycemic events. 63 • Significantly reduces diabetes-related distress. 65 • Significantly improves medication adherence. 66 • Effectively addresses the obstacles caused by the COVID-19 pandemic.67 -71 • Are more effective for patients who are residents of cities and using the websites as their intervention method. 61 Use of downloaded CGM data into standardized reports: • Supports patient education. 72 • Enhances patient engagement in their self-management. 72 |
Abbreviations: CGM, continuous glucose monitoring; %TIR, percentage time in range; %TBR, percentage time below range; HbA1c, glycated hemoglobin; %TAR, percentage time above range; ESRD, end-stage renal disease.
Table 2.
CMS Proposed LCD for Glucose Monitors. 27
| To be eligible for coverage of a CGM and related supplies, the beneficiary must meet all of the following initial coverage criteria 1-5: 1. The beneficiary has diabetes mellitus (Refer to the ICD-10 code list in the LCD-related Policy Article for applicable diagnoses); and, 2. The beneficiary’s treating practitioner has concluded that the beneficiary (or beneficiary’s caregiver) has sufficient training using the CGM prescribed as evidenced by providing a prescription; and, 3. The CGM is prescribed in accordance with its FDA indications for use; and, 4. The beneficiary for whom a CGM is being prescribed, to improve glycemic control, meets at least one of the criteria below: (A) The beneficiary is insulin-treated with at least one daily administration of insulin; or, (B) The beneficiary has a history of problematic hypoglycemia with documentation of at least one of the following: • Recurrent level 2 hypoglycemic events (glucose < 54 mg/dL (3.0 mmol/L) that persist despite multiple (two or more) attempts to adjust medication(s) and/or modify the diabetes treatment plan; or A history of one level 3 hypoglycemic event (glucose < 54 mg/dL (3.0 mmol/L) characterized by altered mental and/or physical state requiring third-party assistance for treatment of hypoglycemia 5. Within 6 months prior to ordering the CGM, the treating practitioner has an in-person or Medicare-approved telehealth visit with the beneficiary to evaluate their diabetes control and determined that criteria 1-4 above are met. |
Abbreviations: CMS, Centers for Medicare & Medicaid Services; LCD, Local Coverage Determination; CGM, continuous glucose monitoring; ICD, International Classification of Diseases.
Rationale for Modification and Standardization of Medicare Coverage
Current Medicaid Eligibility Criteria for CGM Coverage Is Inconsistent
Although Medicaid is administered through CMS, there is no consistent Medicaid policy for CGM coverage in the United States. According to the latest industry data (Abbott Diabetes Care, data on file), seven states have published no CGM coverage criteria except through medical necessity. Among states that provide coverage there are significant variations in eligibility criteria. Whereas some states cover CGM for individuals with T1D and T2D, 22 others cover T1D beneficiaries only. In addition to differences in the type of diabetes covered for CGM, state Medicaid programs also differ in other ways, including age, prior fingerstick testing frequency, type of insulin therapy, prescriber requirements, and how beneficiaries receive their supplies. Table 3 illustrates how state Medicaid programs can vary in their eligibility requirements; states were selected to demonstrate the wide variability. A state-by-state listing of the most current requirements is presented in Supplementary Table 1.
Table 3.
CGM Eligibility Criteria for Selected States.
| Criteria | T1D | T2D | GDM | ≥3 daily injections or insulin pump a | BGM ≥4 times daily | HbA1c ≥7% b | Frequent severe hypo (<50mg/dL) | Hypoglycemia unawareness | History of hyperglycemia c | Nocturnal hypoglycemia | DKA | Preprandial-postprandial hyperglycemia | Dawn phenomenon | Benefit |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arkansas | Yes | Yes | Yes | Yes | Yes | DME | ||||||||
| Georgiad,e | Yes | Yes | Yes | Yes | Yes | Yes | Yes | DME | ||||||
| Idaho | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | DME | |||
| Kentucky a | Yes | Yes | Rx | |||||||||||
| Michigan | Yes | Yes | Yes | Yes | Yes | DME | ||||||||
| Missouri | Yes | Yes f | Yes f | Yes a | Rx | |||||||||
| Nevada | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Rx | ||||||
| New Hampshire | Yes | Yes | Yes | Yes | Yes | Yes | Rx | |||||||
| New York e | Yes | Yes | Yes | Yes | Yes | Rx | ||||||||
| Oklahoma | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Rx | ||||
| Rhode Island | Yes | Yes | Yes | Yes | Yes | DME |
Abbreviations: CGM, continuous glucose monitoring; GDM, gestational diabetes; BGM, blood glucose monitoring; HbA1c, glycated hemoglobin; DKA, diabetic ketoacidosis; DME, Durable Medical Equipment; Rx, pharmacy benefit.
≥3 times daily or insulin pump which may require frequent adjustments.
Or not achieving target HbA1c.
Including unexplained hyperglycemia.
Pediatric coverage only.
Prescription by an endocrinologist.
Use of rapid-acting insulin is required.
Strong Evidence Supports CGM Use in Various Diabetes Populations
Unlike BGM, which only provides a single, point-in-time value, CGM continuously measures glucose levels and automatically transmits the data the user’s smartphone or dedicated reader in numerical and graphical formats. This immediate access to glucose data enables users to more accurately determine insulin dosages and take immediate action to mitigate current or impending glycemic events (eg, severe hypoglycemia and hyperglycemia). Some CGM systems also feature a predictive low glucose alert that notifies the user when severe hypoglycemia is predicted to occur within the next 20 minutes. Moreover, current CGM systems can be programmed to transmit users’ data to their clinicians for in-depth analysis and treatment recommendations.
The clinical efficacy of CGM has been demonstrated in numerous studies of individuals with T1D and those with T2D who are treated with intensive insulin therapy regardless of insulin delivery method.15,17,19,20,28 -30,32,36,37,40,43 -45,47,48,50,73 -75 Benefits of CGM use in this population include reductions in HbA1c,15,17,28 -35,36,37,76 fewer severe hypoglycemia events,18,32,33,37 less hypoglycemia fear,18,77 reductions in diabetic ketoacidosis (DKA), 37 increased time within target glucose range (TIR),17,20,30,38,78 and reductions in time below range.17,30,78 Large observational registry and database studies have also shown an association between CGM use and significant reductions in hospitalizations for severe hypoglycemia and DKA.18,32,33,39 The clinical benefits of CGM are not limited to individuals treated with intensive insulin regimens. Several recent studies have demonstrated improvements in glycemic control, reduction in hypoglycemia, and lower rates of hospitalizations in and health resource utilization.47,79,80 The use of CGM in children and young adults has become the standard of care treatment as stated in the diabetes treatment guidelines given the overwhelming evidence indicating favorable outcomes with CGM use. 81
The value of CGM use within the Medicaid/Chip population is underscored by recent data from Addala et al 82 who assessed the impact of continued and interrupted CGM use on HbA1c within a cohort of young adolescents (age 12.9 ± 4.2 years) who were enrolled in public insurance plans. Investigators reported improvements in HbA1c among those patients who were provided uninterrupted access to CGM; whereas HbA1c levels increased in patients whose access was interrupted due to insurance-related issues. Use of CGM in persons with T1D and T2D is critical for diabetes management with AID systems. Individuals covered by Medicare or Medicaid showed equal benefit from use of at least one AID pump/CGM system. 22
An additional advantage of CGM technology is the ability to automatically share and discuss glucose data with healthcare professionals in real time. Use of virtual telehealth visits in conjuction with remote monitoring of CGM data has been shown to improve glycemic control,59 -64 reduce diabetes-related distress, 65 and enhance treatment adherence, 66 with increased cost and time efficiences compared with in-clinic diabetes visits.59,83 -85 Use of these technologies proved extremely valuable and effective in overcoming many of the obstacles encountered throughout the COVID-19 pandemic.67 -69,71,86
Racial/Socioeconomic Disparities Impact Access and Treatment
Medicaid beneficiaries have greater difficulty accessing specialists, such as endocrinologists, than those with private insurance. 87 This is significant because many primary care providers are challenged to provide adequate diabetes care to individuals treated with intensive insulin therapy and often delay intensifying insulin therapy due to the complexity of these regimens. 88
Importantly, as recently reported by the American Diabetes Association, Medicaid beneficiaries who are treated with insulin are 2-5 times less likely to use a CGM than those covered by commercial insurance; however, this gap in coverage is less pronounced when considering only white beneficiaries. 89
Pihoker et al 90 found that younger T1D patients who are covered by Medicaid are more likely to be treated with less-intensive insulin therapy and receive fewer changes to their current insulin regimens than those with private insurance, a disparity that is particularly pervasive among black and Hispanic patients.
In a cross-sectional, multicenter analysis of patient- and chart-reported variables, Agarwal et al 91 investigated racial/ethnic disparities within a cohort of 300 young adults (20 years) with T1D: 33% white, 32% black, and 34% Hispanic. Investigators reported that significantly fewer black (28%) and Hispanic (37%) patients had ever used a CGM device compared with white patients (71%), P < 0.001. Additionally, they found that young black and Hispanic participants had lower annual household incomes, less education, and higher neighborhood poverty. Lai et al 92 reported similar findings of racial disparities in CGM initiation and continued use.
Similar disparities in CGM use were reported in a retrospective review of 227 adult T1D patients. 93 Among the 68 (30%) patients who used CGM, differences in the proportions of users were notable: 47% white, 22% Hispanic, and 14% black. 93 Patients covered by government health insurance had lower odds of using technology (odds ratio [OR], 0.43) compared with patients with private health insurance.
As reported by Pihoker et al, younger T1D patients covered by Medicaid are more likely to be treated with less-intensive insulin therapy and receive fewer changes to their current insulin regimens than those with private insurance. This disparity is particularly striking among black and Hispanic patients. 90 Numerous studies have shown that children/adolescents with T1D who are of lower socioeconomic status and covered by public health plans have higher HbA1c values, greater incidence of DKA, and diminished quality of life.94 -97
Use of CGM Can Reduce Healthcare Resource Utilization and Associated Costs
Early data have shown higher hospitalization rates for DKA over time for Medicaid beneficiaries compared with individuals covered by commercial health plans. 98 Analyses of 2012 Medicaid claims data 99 and the MarketScan multistate Medicaid database 100 also revealed significantly higher costs for adults and children/adolescents with diabetes (with and without a disability) compared with individuals without diabetes. As reported by Ng et al, 99 diabetes-related costs were significantly higher among adults with diabetes ($9530) and no disability compared with no diabetes or disability ($4545). Shrestha et al 100 reported similar findings, with even greater cost disparities between children/adolescents with diabetes ($24 093) and those with no diabetes ($14 149).
Disparities in healthcare resource utilization and costs are likely related to differences in access to care between individuals living in low-income vs high-income communities. 87 As reported by Nguyen et al, 101 individuals living in low-income urban and rural areas are more likely to have fewer primary care providers in their communities (0.5% and 7.4%, respectively) than those living in higher socioeconomic communities.
Given the demonstrated impacts of CGM use in improving overall glycemic control15,17,20,28 -35,38,76,78 and reducing incidence of DKA and severe hypoglycemia events,18,32,33,39 increasing beneficiary access to this vital technology has the potential to improve their health and well-being while reducing the long-term costs of diabetes.
Because diabetes-related costs differ from state to state, it is difficult to assess the total diabetes-related costs among Medicaid beneficiaries. However, program administrators can calculate the potential savings associated with CGM use in their state based on findings from a recent large, multicenter prospective observational cohort study of T1D adults (n = 515). 18 The study showed that use of CGM during an observation period was associated with significant reductions in the number of patients with severe hypoglycemia and/or DKA hospitalizations, which decreased by 73% (from 11% to 3%) and 80% (from 5% to 1%), respectively, after 1 year.
Current Eligibility Criteria Are Overly Restrictive in Most States
Coverage requires history of frequent fingerstick testing
Although the medical community traditionally relies on high-quality scientific evidence when developing clinical guidelines for managing diabetes and other conditions, state Medicaid programs tend to ignore the evidence when establishing coverage eligibility criteria for CGM. For example, in many states, beneficiaries must document a history of prior fingerstick testing. (Supplemental Table 1) This requirement is both unduly restrictive and medically unfounded. 26
As reported in the DIAMOND study, only 48% of the rtCGM users (T1D and T2D) were preforming fingerstick testing ≥4 times per day at baseline; however, there was no association between Hb1c reductions at study end and baseline fingerstick frequency. 29 A similar absence of association between previous BGM frequency and positive clinical outcomes with rtCGM use has been observed in other large, randomized trials.
In a study of adult T2D patients, the mean self-reported fingerstick frequency at baseline for the BGM and rtCGM and BGM groups was 3.2 and 3.3, respectively. 40 The mean change in HbA1c at 6 months, was significantly greater in the rtCGM group (−1.0) compared with BGM users (−0.6%), P = 0.005. Again, there was no association between baseline BGM frequency and rtCGM outcomes. Similar findings that showed no association between fingerstick testing frequency and glycemic outcomes were observed in a post hoc analysis of the REPLACE study (Table 4). 19 Results from a recent retrospective claims data analysis also showed no association between prior fingerstick frequency and reductions in acute diabetes events (ADE). 39
Table 4.
Change in Glycemic and Patient-Reported Outcomes Among CGM Users by Baseline BGM Frequency in the REPLACE Study: ≥4 vs <4 Tests/Day. 102
| BGM change from baseline | Adjusted mean change from baseline | Difference in adjusted means | P value | |||
|---|---|---|---|---|---|---|
| BGM frequency/day | BGM frequency/day | |||||
| ≥4 (n = 90) | <4 (n = 59) | ≥4 (n = 90) | <4 (n = 59) | |||
| HbA1c (%) | −0.21 | −0.37 | −0.29 | −0.24 | −0.05 | .6891 |
| %Time < 70 mg/dL (%) | −3.44 | −2.23 | −3.01 | −2.90 | −0.11 | .8497 |
| %Time < 55 mg/dL (%) | −1.77 | −1.53 | −1.63 | −1.73 | 0.10 | .7012 |
| Number of hypos < 70 mg/dL | −0.32 | −0.19 | −0.27 | −0.26 | −0.01 | .9050 |
| Number of hypos < 55 mg/dL | −0.20 | −0.18 | −0.18 | −0.22 | 0.04 | .3222 |
| Treatment satisfaction | 13.54 | 13.65 | 13.42 | 13.48 | −0.06 | .9444 |
Abbreviations: CGM, continuous glucose monitoring; BGM, blood glucose monitoring; HbA1c, glycated hemoglobin.
Findings from a recent retrospective claims data analysis have also shown no association between prior BGM frequency and reductions in ADE associated with CGM use. A cohort of 12 521 individuals with T1D and T2D experienced reductions in ADE from 0.245 to 0.132 events/patient-year (P < 0.001), with similar reductions observed in patients testing <4 and ≥4 times per day. 39
Coverage is limited to intensive insulin therapy
Given that the vast majority of individuals with T1D are currently treated with either multiple daily insulin injections (MDI) or insulin pump therapy, the CGM requirement of intensive insulin therapy specifically targets those with T2D. Studies have demonstrated that use of CGM by T2D patients confers significant reductions in HbA1c levels,29,31,40 -45 significant increases in time above range (%TIR),29,40 significant decreases in time below range (%TBR),19,48 and significant reductions in diabetes-related hospitalizations39,49 regardless of insulin regimen. Although a substantial number of T2D Medicare beneficiaries are treated with less-intensive insulin regimens or non-insulin medications, they are at higher risk diabetes-related events (eg, hospitalizations and emergency room visits) than younger patients.50 -53
The requirement for a documented history of frequent insulin dosage adjustment based on BGM values is burdensome for both healthcare providers and patients, and there is no evidence demonstrating its value as a predictor of successful CGM use. Moreover, this requirement ignores the broader utility of the CGM, which is the automated alarm/alert feature which warns patients of current or impending hypoglycemia/hyperglycemia, enabling them to take immediate remedial action. Finally, the specific wording of the requirement for “injecting” insulin fails to address other options for insulin administration (eg, insulin infusion using a pump and inhaled insulin).
“Deadlines” imposed for improved glycemic control
Some states require beneficiaries to show improved glycemic control within a specific time period. Given the numerous socioeconomic obstacles that challenge the Medicaid population, glycemic improvements may take longer in some patients. Moreover, the metrics of improvement will vary on an individual basis and may extend beyond glycemic control alone. The decision to continue CGM should be left to the clinical judgment of the prescribing healthcare provider.
Patient care must be provided by endocrinologists
Another significant obstacle to CGM access is the requirement for prescription from a board-certified endocrinologist. Of the 43 states and the District of Columbia that publish eligibility criteria for CGM coverage, seven require endocrinologists to prescribe or to provide consultation on a prescription (California, Georgia, Maryland, New York, South Carolina, South Dakota, Wisconsin). 23 This requirement does not consider the logistical obstacles patients may face if they have to travel long distances to receive care. For example, in Georgia, the vast majority of the practicing endocrinologists are located in the northwestern portion of the state. Moreover, there are only 65 board-certified endocrinologists who are enrolled to provide care to Medicaid beneficiaries, mostly in the large urban metropolitan area of Atlanta. This creates significant access limitations for patients living in rural areas or the southern portion of Georgia where diabetes prevalence is highest (Figure 1).
Figure 1.
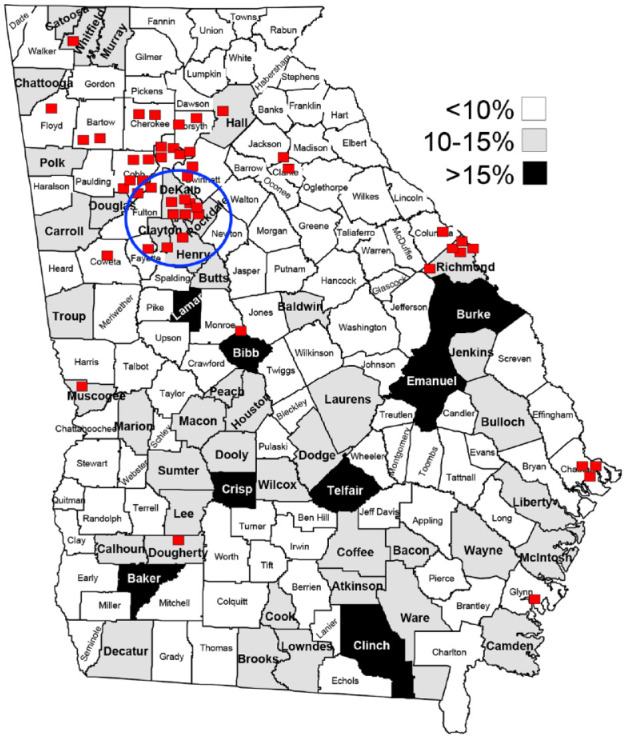
Endocrinologists enrolled in Medicaid vs diabetes prevalence by county. 103 Red squares—endocrinology office; blue circle—greater Atlanta metropolitan area.
Moreover, this requirement does not align with the growing shortage of endocrinologists nationwide. As predicted by Vigersky et al, 104 the shortage of adult endocrinologists will increase to ~2700 by 2025 in the absence of any intervention. It will be up to primary care clinicians to fill the widening supply-demand gap. Fortunately, efforts are currently in place at various medical institutions to provide CGM training to primary care physicians (PCP) through fellowship programs. 105 The implementation of such measures will further assist in increasing access and ensuring delivery of adequate care for patients with diabetes using these technologies.
Documentation requirements are onerous and potentially harmful
Changes in documentation requirements are needed. In addition to restrictive eligibility requirements, access to CGM is further hindered by the onerous documentation that healthcare providers are required to submit in order to obtain coverage for their patients. In a 2017 survey conducted by the American Medical Association (AMA), 92% of the 1000 clinicians surveyed reported that the documentation required to obtain authorization for medications and medical devices both delays patient treatment and negatively affects clinical outcomes. 106
Recommendations for Reducing Patient/Provider Burden
Provide coverage for FDA-approved AID devices
Clinical evidence supporting the efficacy and safety of automated insulin delivery (AID) systems has grown over the last 5 years with the introduction of commercially available, and soon to become available, AID systems. AID systems utilize a sophisticated algorithm that continuously modifies insulin delivery in response to glucose values obtained by CGM, residual insulin action, and other inputs, such as meal intake and exercise announcement. Numerous clinical trials and real-world studies have shown that use of AID systems significantly improve overall glycemic control and reduce severe hypoglycemia events in adults and children/adolescents with T1D.107 -118 Importantly, Medicaid currently provides insulin pump coverage for eligible beneficiaries. Without CGM, patients are not able to use AID pumps, which are the current and future best methods for management of insulin delivery in individuals treated with intensive insulin therapy. 22
Simplify and streamline documentation
The focus of CGM eligibility documentation must be on simplifying administrative tasks and providing clear guidance to durable medical equipment (DME) suppliers and pharmacy coverage administrators. We recommend that state Medicaid programs develop and standardize a simple checklist to document each patient’s diagnosed disease (T1D, T2D, CKD/ESRD) using ICD-9 and ICD-10 codes. A complete list of all relevant ICD codes should be included as a supplement to the checklist. Several options for documentation of problematic hypoglycemia should also be considered. (Table 5) Documentation of initial and follow-up consultations with healthcare providers would be documented as “yes” or “no.”
Table 5.
Considerations for Documenting Problematic Hypoglycemia.
| Presence of ≥1 glucose value indicating Level 2 (Severe) or nocturnal hypoglycemia events from available BGM data (prior 30 days). |
| Presence of ≥7 glucose values indicating frequent Level 1 (Moderate) hypoglycemia events from available BGM data (prior 30 days). |
| In the absence of BGM records, self-reported incidence/severity of hypoglycemia events. |
| Presence of Level 2/nocturnal hypoglycemia events and/or frequent Level 1 hypoglycemia events obtained from professional (short-term) CGM use. |
Abbreviations: BGM, blood glucose monitoring; CGM, continuous glucose monitoring.
Promote CGM acquisition as a pharmacy benefit
Most Medicaid patients currently receive their CGM devices through DME suppliers. However, they often encounter significant delays in processing their requests for CGM devices. These delays are multifactorial and detrimental to improving diabetes management. Many patients have competing needs and logistical barriers to receiving care, leading to recurrent hospitalizations for diabetes-related emergencies (DKA and hypoglycemia). During the time awaiting CGM approval, many of these patients remain at risk for diabetes-related admissions and readmissions, adding to the already existing disparities in care and health outcomes.
Moreover, the ongoing process for maintaining supplies through DME companies is difficult to navigate, especially for those coping with limited finances and multiple social pressures to maintain health and diabetes care. In the setting of these competing life demands (often including food insecurity), handling the process of ongoing DME requests or approvals required to get their supplies often results in intermittent use of CGM.
The opportunity to obtain their CGM supplies through pharmacy channels—a process that is more streamlined and improves continued access to devices—would have a significant impact on their ability to improve their glycemic control and clinical outcomes.
Summary
Diabetes continues to be a significant and growing health concern that threatens to overwhelm both public and private health systems. Because the prevalence of diabetes and its comorbidities is highest in people of color and/or low socioeconomic status, it is critical that these individuals have access to high-quality care for their diabetes.
Although a substantial and growing body of evidence demonstrates the clinical benefits of CGM in individuals with T1D and T2D regardless of their current therapy and prior glucose monitoring frequency,19,39,41,42,46,49,102,119 CGM use is disproportionally low among individuals in racial/ethnic and low socioeconomic populations.91,92
Inappropriate, medically unfounded Medicaid eligibility criteria for CGM coverage deny access to CGM within a substantial population of beneficiaries with diagnosed diabetes, and further worsen disparities—particularly among minorities or patients in rural areas. Moreover, current policies are inconsistent with the established literature and current standards of care.11,12
Limiting access to CGM achieves neither cost-efficiencies nor clinical efficacies. We believe our evidence-based recommendations for modifying current eligibility criteria both streamlines the administrative processes for documenting medical necessity and expands access to our most vulnerable diabetes population.
Supplemental Material
Supplemental material, sj-jpg-1-dst-10.1177_19322968221144052 for Increase Access, Reduce Disparities: Recommendations for Modifying Medicaid CGM Coverage Eligibility Criteria by Rodolfo J. Galindo, Grazia Aleppo, Christopher G. Parkin, David A. Baidal, Anders L. Carlson, Eda Cengiz, Gregory P. Forlenza, Davida F. Kruger, Carol Levy, Janet B. McGill and Guillermo E. Umpierrez in Journal of Diabetes Science and Technology
Footnotes
Abbreviations: %TAR, percentage time above range; %TBR, percentage time below range; %TIR, percentage time in range; ADE, acute diabetes events; AID, automated insulin delivery; AMA, American Medical Association; BGM, blood glucose monitoring; CGM, continuous glucose monitoring; CKD, chronic kidney disease; CMS, Centers for Medicare & Medicaid Services; COVID-19, Coronavirus Disease 2019; DME, Durable Medical Equipment; DKA, diabetic ketoacidosis; ESRD, end-stage renal disease; GDM, gestational diabetes; HbA1c, glycated hemoglobin; ICD, International Classification of Diseases; LCD, Local Coverage Determination; OR, odds ratio; Rx, pharmacy benefit; T1D, type 1 diabetes; T2D, type 2 diabetes.
Author Contributions: R.J.G., G.A., C.G.P., and J.B.M. wrote the manuscript. D.B., A.L.C., E.C. G.F., D.F.K., C.L., and G.E.U. provided input on the draft. All authors reviewed the final draft and approved its submission.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: R.J.G. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) under award numbers 2P30DK1110246 and K23DK123384-3. R.J.G. received research support to Emory University for investigator-initiated studies from Novo Nordisk, Dexcom, and Eli-Lilly and consulting fees from Sanofi, Eli-Lilly, Boehringer, Pfizer, and Weight Watchers, outside of this work. G.A. has received research support to Northwestern University from Astra Zeneca, Dexcom, Eli-Lilly, Emmes, Fractyl Health, Insulet, Novo Nordisk, and WellDoc. G.A. has received consulting fees from Eli-Lilly, Dexcom, and Insulet outside of this work. C.G.P. has received consulting fees from Abbott Diabetes Care, CeQur, Dexcom, LifeScan, Mannkind, Roche Diabetes Care, and ProventionBio. D.A.B. has no disclosures associated with this manuscript. A.L.C. has received research support from UnitedHealthcare, Abbott, Dexcom, Eli-Lilly, Insulet, Medtronic, Novo Nordisk, and Sanofi, and is a consultant for Medtronic and Insulet, with all financial support going to his institution. In addition, ALC has a patent, Treatment of Hypoglycemia Unawareness with Intranasal Insulin, pending to HealthPartners Institute. EC is a scientific advisor for Eli-Lilly, Novo Nordisk, Adocia, and Arecor. G.P.F. received research support from Medtronic, Dexcom, Abbott, Tandem, Insulet, Beta Bionics, and Lilly, and has served as a consultant, speaker, and advisory board member for Medtronic, Dexcom, Abbott, Tandem, Insulet, Beta Bionics, and Lilly. D.F.K. institution has received research support from NIH, Helmsley Foundation, Novo Nordisk, Abbott Diabetes, and Dexcom. D.F.K. has received consulting fees from Novo Nordisk, Abbott Diabetes, Mannkind, CeQur, Sanofi, Medical Module, ProventionBio, and Pendulum. D.F.K. is the speaker for Dexcom, Novo Nordisk, Sanofi-Aventis, Xeris, and CeQur. D.F.K. has stock options not redeemed in Pendulum. C.J.L. has received research support by the NIDDK and Helmsley Foundation and industry support paid to the Icahn School of Medicine at Mount Sinai from Abbott Diabetes, Dexcom, Insulet, Novo Nordisk, Senseonics, and Tandem. C.J.L. has received consulting fees from Eli-Lilly, and Dexcom outside of this work. J.B.M. has received research support from the NIH, Helmsley Foundation, JDRF, Novo Nordisk, and Beta Bionics. J.B.M. has received consulting fees from Bayer, Boehringer Ingelheim and Lilly, Mannkind, Novo Nordisk, and Thermo Fisher outside of this work. G.E.U. is partly supported by research grants from the National Institutes of Health (NIH/NATS UL 3UL1TR002378-05S2) from the Clinical and Translational Science Award program, and from National Institutes of Health and National Center for Research Resources (NIH/NIDDK 2P30DK111024-06). G.E.U. has received research support (to Emory University) from Astra Zeneca, Bayer, Abbott, and Dexcom.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Abbott Diabetes Care provided funding for editorial assistance in developing this article.
ORCID iDs: Rodolfo J. Galindo  https://orcid.org/0000-0002-9295-3225
https://orcid.org/0000-0002-9295-3225
Grazia Aleppo  https://orcid.org/0000-0003-1644-5947
https://orcid.org/0000-0003-1644-5947
Christopher G. Parkin  https://orcid.org/0000-0001-6838-5355
https://orcid.org/0000-0001-6838-5355
Anders L. Carlson  https://orcid.org/0000-0002-5738-2818
https://orcid.org/0000-0002-5738-2818
Eda Cengiz  https://orcid.org/0000-0001-7992-9506
https://orcid.org/0000-0001-7992-9506
Gregory P. Forlenza  https://orcid.org/0000-0003-3607-9788
https://orcid.org/0000-0003-3607-9788
Carol Levy  https://orcid.org/0000-0002-3492-2578
https://orcid.org/0000-0002-3492-2578
Guillermo E. Umpierrez  https://orcid.org/0000-0002-3252-5026
https://orcid.org/0000-0002-3252-5026
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020: estimates of diabetes and Its burden in the United States. Date unknown. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed February 29, 2020.
- 2. Brown AF, Ettner SL, Piette J, et al. Socioeconomic position and health among persons with diabetes mellitus: a conceptual framework and review of the literature. Epidemiol Rev. 2004;26:63-77. [DOI] [PubMed] [Google Scholar]
- 3. Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. 2021;44(1):258-279. doi: 10.2337/dci20-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Medicaid.gov. August 2022. Medicaid & CHIP enrollment data highlights. Date unknown. https://www.medicaid.gov/medicaid/program-information/medicaid-and-chip-enrollment-data/report-highlights/index.html. Accessed May 20, 2022.
- 5. Centers for Medicare & Medicaid Services. 2020. Medicaid and CHIP beneficiary profile: characteristics, health status, access, utilization, expenditures, and experience. https://www.medicaid.gov/medicaid/quality-of-care/downloads/beneficiary-profile-2021.pdf. Published 2021. Accessed May 23, 2022.
- 6. Kaiser Family Foundation. Medicaid coverage rates for the nonelderly by race/ethnicity. Date unknown. https://www.kff.org/medicaid/state-indicator/nonelderly-medicaid-rate-by-raceethnicity/?currentTimeframe=0&selectedRows=%7B%22states%22:%7B%22all%22:%7B%7D%7D,%22wrapups%22:%7B%22united-states%22:%7B%7D%7D%7D&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed May 12, 2021.
- 7. Centers for Medicare & Medicaid Services. Medicaid.gov. Comprehensive diabetes care: hemoglobin A1c (HbA1c) poor control (>9.0%): ages 18 to 75. Date unknown. https://www.medicaid.gov/state-overviews/scorecard/comprehensive-diabetes-care/index.html. Accessed December 18, 2020.
- 8. American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S83-S96. [DOI] [PubMed] [Google Scholar]
- 9. Pettus JH, Zhou FL, Shepherd L, et al. Incidences of severe hypoglycemia and diabetic ketoacidosis and prevalence of microvascular complications stratified by age and glycemic control in U.S. adult patients with type 1 diabetes: a real-world study. Diabetes Care. 2019;42(12):2220-2227. doi: 10.2337/dc19-0830. [DOI] [PubMed] [Google Scholar]
- 10. Garfield SS, Xenakis JJ, Bastian A, McBride M. Experiences of people with diabetes by payer type: an analysis of the roper diabetes data set. Diabetes Ther. 2015;6(2):113-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American Diabetes Association. 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S97-S112. [DOI] [PubMed] [Google Scholar]
- 12. Grunberger G, Sherr J, Allende M, et al. American association of clinical endocrinology clinical practice guideline: the use of advanced technology in the management of persons with diabetes mellitus. Endocr Pract. 2021;27(6):505-537. [DOI] [PubMed] [Google Scholar]
- 13. National Institute for Health and Care Excellence. Type 1 diabetes in adults: diagnosis and management. https://www.nice.org.uk/guidance/ng17. Published 2015. Accessed December 1, 2022. [PubMed]
- 14. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. NICE guideline NG28. https://www.nice.org.uk/guidance/ng28. Published 2017. Accessed December 3, 2018.
- 15. Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317:371-378. [DOI] [PubMed] [Google Scholar]
- 16. Heinemann L, Guido Freckmann G, Gabriele Faber-Heinemann G, et al. Benefits of continuous glucose monitoring use in adults with type 1 diabetes and impaired hypoglycaemia awareness and/or severe hypoglycaemia treated with multiple daily insulin injections: results of the multicentre, randomised controlled HypoDE study. Lancet. 2018;391(10128):1367-1377. [DOI] [PubMed] [Google Scholar]
- 17. Šoupal J, Petruželková L, Grunberger G, et al. Glycemic outcomes in adults with T1D are impacted more by continuous glucose monitoring than by insulin delivery method: 3 years of follow-up from the COMISAIR study. Diabetes Care. 2020;43(1):37-43. [DOI] [PubMed] [Google Scholar]
- 18. Charleer S, Mathieu C, Nobels F, et al. Effect of continuous glucose monitoring on glycemic control, acute admissions, and quality of life: a real-world study. Clin Endocrinol Metab. 2018;103(3):1224-1232. [DOI] [PubMed] [Google Scholar]
- 19. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Use of flash glucose sensing technology for 12 months as a replacement for blood glucose monitoring in insulin-treated type 2 diabetes. Diabetes Ther. 2017;8:573-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388:2254-2263. [DOI] [PubMed] [Google Scholar]
- 21. Bergenstal RM, Kerr MSD, Roberts GJ, Souto D, Nabutovsk Y, Hirsch IB. Flash CGM is associated with reduced diabetes events and hospitalizations in insulin-treated type 2 diabetes. J Endocr Soc. 2021;5(4):bvab013. doi: 10.1210/jendso/bvab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Forlenza GP, Carlson AL, Galindo RJ, et al. Real-world evidence supporting tandem control-IQ hybrid closed-loop success in the Medicare and Medicaid type 1 and type 2 diabetes populations. Diabetes Technol Ther. 2022;24:814-823. doi: 10.1089/dia.2022.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Center for Healthcare Strategies. Expanding Medicaid access to continuous glucose monitors. https://www.chcs.org/media/Expanding-Medicaid-Access-to-Continuous-Glucose-Monitors_011222.pdf. Published 2022. Accessed December 1, 2022.
- 24. Secrest AM, Costacou T, Gutelius B, et al. Association of socioeconomic status with mortality in type 1 diabetes: the Pittsburgh epidemiology of diabetes complication (EDC) study. Ann Epidemiol. 2011;21(5):367-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Secrest AM, Costacou T, Gutelius B, Miller RG, Songer TJ, Orchard TJ. Associations between socioeconomic status and major complications in type 1 diabetes: the Pittsburgh epidemiology of diabetes complication (EDC) study. Ann Epidemiol. 2011;21(5):374-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galindo RJ, Parkin CG, Aleppo G, et al. What’s wrong with this picture? a critical review of current centers for Medicare & Medicaid services coverage criteria for continuous glucose monitoring. Diabetes Technol Ther. 2021;23:652-660. doi: 10.1089/dia.2021.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Centers for Medicare & Medicaid Services. Glucose monitors: proposed local coverage determination (LCD). Date unknown. https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=39473&ver=16. Accessed October 25, 2022.
- 28. Lind M, Polonsky W, Hirsch IB. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317(4):379-387. [DOI] [PubMed] [Google Scholar]
- 29. Ruedy KJ, Parkin CG, Riddlesworth TD, Graham C; for the DIAMOND Study Group. Continuous glucose monitoring in older adults with type 1 and type 2 diabetes using multiple daily injections of insulin: results from the DIAMOND trial. J Diabetes Sci Technol. 2017;11(6):1138-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Šoupal J, Petruželková L, Flekač M, et al. Comparison of different treatment modalities for type 1 diabetes, including sensor-augmented insulin regimens, in 52 weeks of follow-up: a COMISAIR study. Diabetes Technol Ther. 2016;18(9):532-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11(1):279-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Charleer S, De Block C, Van Huffel L, et al. Quality of life and glucose control after 1 year of nationwide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (FUTURE): a prospective observational real-world cohort study. Diabetes Care. 2020;43(2):389-397. [DOI] [PubMed] [Google Scholar]
- 33. Fokkert M, van Dijk P, Edens M, et al. Improved well-being and decreased disease burden after 1-year use of flash glucose monitoring (FLARE-NL4). BMJ Open Diabetes Res Care. 2019;7(1):e000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tyndall V, Stimson RH, Zammitt NN, et al. Marked improvement in HbA1c following commencement of flash glucose monitoring in people with type 1 diabetes. Diabetologia. 2019;62(8):1349-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paris I, Henry C, Pirard F, Gérard A-C, Colin IM. The new FreeStyle Libre flash glucose monitoring system improves the glycaemic control in a cohort of people with type 1 diabetes followed in real-life conditions over a period of one year. Endocrinol Diabetes Metab. 2018;1:e00023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6354746/. Accessed May 6, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leelarathna L, Evans ML, Neupane S, et al. Intermittently scanned continuous glucose monitoring for type 1 diabetes. N Engl J Med. 2022;387(16):1477-1487. doi: 10.1056/NEJMoa2205650. [DOI] [PubMed] [Google Scholar]
- 37. Beck SE, Kelly C, Price DA; COACH Study Group. Non-adjunctive continuous glucose monitoring for control of hypoglycaemia (COACH): results of a post-approval observational study. Diabet Med. 2022;39(2):e14739. doi: 10.1111/dme.14739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Beers CA, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol. 2016;4(11):893-902. [DOI] [PubMed] [Google Scholar]
- 39. Hirsch IB, Kerr MSD, Roberts GJ, et al. Utilization of continuous glucose monitors is associated with reduction in inpatient and outpatient emergency acute diabetes events regardless of prior blood test strip usage. Diabetes. 2020;69(suppl 1):875-P. [Google Scholar]
- 40. Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. [DOI] [PubMed] [Google Scholar]
- 41. Miller E, Brandner L, Wright E. 84-LB: HbA1c reduction after initiation of the FreeStyle Libre system in type 2 diabetes patients on long-acting insulin or noninsulin therapy. Diabetes. 2020;69(suppl 1):84-LB. [Google Scholar]
- 42. Wright E, Kerr MSD, Reyes I, Nabutovsky Y, Miller E. 78-LB: HbA1c reduction associated with a FreeStyle Libre system in people with type 2 diabetes not on bolus insulin therapy. Diabetes. 2020;69:78-LB. [Google Scholar]
- 43. Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Majithia AR, Kusiak CM, Lee AA, et al. Glycemic outcomes in adults with type 2 diabetes participating in a continuous glucose monitor-driven virtual diabetes clinic: prospective trial. J Med Internet Res. 2020;22(8):e21778. doi: 10.2196/21778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bergenstal RM, Layne JE, Zisser H, et al. Remote application and use of real-time continuous glucose monitoring by adults with type 2 diabetes in a virtual diabetes clinic. Diabetes Technol Ther. 2021;23(2):128-132. doi: 10.1089/dia.2020.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Manning JP, Halford J, Sulik B, Sulik M, Parkin CG, Liljenquist DR. Use of continuous glucose monitoring is acceptable and potentially beneficial in older T2DM patients treated with basal insulin therapy: a pilot study. Infusystems USA. 2014;11(1):1-5. [Google Scholar]
- 47. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325(22):2262-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miller E, Kerr MSD, Roberts GJ, Souto D, Nabutovsky Y, Wright E. FreeStyle Libre® system use associated with reduction in acute diabetes events and all-cause hospitalizations in patients with type 2 diabetes without bolus insulin. Diabetes. 2020;69(suppl 1):85-LB. [Google Scholar]
- 50. Gehlaut RR, Dogbey GY, Schwartz FL, Marling CR, Shubrook JH. Hypoglycemia in type 2 diabetes—more common than you think: a continuous glucose monitoring study. J Diabetes Sci Technol. 2015;9(5):999-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hollander PA, Kiljanski J, Spaepen E, Harris CJ. Risk of clinically relevant hypoglycaemia in patients with type 2 diabetes self-titrating insulin glargine U-100. Diabetes Obes Metab. 2019;21(11):2413-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weinstock RS, DuBose SN, Bergenstal RM, et al. Risk factors associated with severe hypoglycemia in older adults with type 1 diabetes. Diabetes Care. 2016;39(4):603-610. [DOI] [PubMed] [Google Scholar]
- 53. Bremer JP, Jauch-Chara K, Hallschmid M, Schmid S, Schultes B. Hypoglycemia unawareness in older compared with middle-aged patients with type 2 diabetes. Diabetes Care. 2009;32:1513-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ólafsdóttir AF, Polonsky W, Bolinder J, et al. A randomized clinical trial of the effect of continuous glucose monitoring on nocturnal hypoglycemia, daytime hypoglycemia, glycemic variability, and hypoglycemia confidence in persons with type 1 diabetes treated with multiple daily insulin injections (GOLD-3). Diabetes Technol Ther. 2018;20(4):274-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barnard K, Thomas S, Royle P, Noyes K, Waugh N. Fear of hypoglycaemia in parents of young children with type 1 diabetes: a systematic review. BMC Pediatr. 2010;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Haugstvedt A, Wentzel-Larsen T, Graue M, Sovik O, Rokne B. Fear of hypoglycaemia in mothers and fathers of children with type 1 diabetes is associated with poor glycaemic control and parental emotional distress: a population-based study. Diabet Med. 2010;27(1):72-78. [DOI] [PubMed] [Google Scholar]
- 57. Joubert M, Fourmy C, Henri P, Ficheux M, Lobbedez T, Reznik Y. Effectiveness of continuous glucose monitoring in dialysis patients with diabetes: the DIALYDIAB pilot study. Diabetes Res Clin Pract. 2015;107(3):348-354. [DOI] [PubMed] [Google Scholar]
- 58. Sobngwi E, Ashuntantang G, Ndounia E, et al. Continuous interstitial glucose monitoring in nondiabetic subjects with end-stage renal disease undergoing maintenance haemodialysis. Diabetes Res Clin Pract. 2010;90(1):22-25. [DOI] [PubMed] [Google Scholar]
- 59. Faruque LI, Wiebe N, Ehteshami-Afshar A, et al. Effect of telemedicine on glycated hemoglobin in diabetes: a systematic review and meta-analysis of randomized trials. CMAJ. 2017;189:E341-E364. doi: 10.1503/cmaj.150885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tchero H, Kangambega P, Briatte C, Brunet-Houdard S, Retali GR, Rusch E. Clinical effectiveness of telemedicine in diabetes mellitus: a meta-analysis of 42 randomized controlled trials. Telemed J E Health. 2019;25(7):569-583. [DOI] [PubMed] [Google Scholar]
- 61. Salehi S, Olyaeemanesh A, Mobinizadeh M, Nasli-Esfahani E, Riazi H. Assessment of remote patient monitoring (RPM) systems for patients with type 2 diabetes: a systematic review and meta-analysis. J Diabetes Metab Disord. 2020;19:115-127. doi: 10.1007/s40200-019-00482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang X, Shu W, Du J, et al. Mobile health in the management of type 1 diabetes: a systematic review and meta-analysis. BMC Endocr Disord. 2019;19:21. doi: 10.1186/s12902-019-0347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Charpentier G, Benhamou PY, Dardari D, et al. The Diabeo software enabling individualized insulin dose adjustments combined with telemedicine support improves HbA1c in poorly controlled type 1 diabetic patients: a 6-month, randomized, open-label, parallel-group, multicenter trial (TeleDiab 1 Study). Diabetes Care. 2011;34(3):533-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dixon RF, Zisser H, Layne JE, et al. A virtual type 2 diabetes clinic using continuous glucose monitoring and endocrinology visits. J Diabetes Sci Technol. 2019;14:908-911. doi: 10.1177/1932296819888662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Polonsky WH, Layne JE, Parkin CG, et al. Impact of participation in a virtual diabetes clinic on diabetes-related distress in individuals with type 2 diabetes. Clin Diabetes. 2020;38(4):357-362. doi: 10.2337/cd19-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: an analysis of third-party managed care claims data. Clin Ther. 2006;28(10):1712-1725. [DOI] [PubMed] [Google Scholar]
- 67. Keesara S, Jonas A, Schulman K. Covid-19 and healthcare’s digital revolution. N Engl J Med. 2020;382:e82. doi: 10.1056/NEJMp2005835. [DOI] [PubMed] [Google Scholar]
- 68. Galindo RJ, Aleppo G, Klonoff DC, et al. Implementation of continuous glucose monitoring in the hospital: emergent considerations for remote glucose monitoring during the COVID-19 pandemic. J Diabetes Sci Technol. 2020;14(4):822-832. doi: 10.1177/1932296820932903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jones MS, Goley AL, Alexander BE, Keller SB, Caldwell MM, Buse JB. Inpatient transition to virtual care during COVID-19 pandemic. Diabetes Technol Ther. 2020;22(6):444-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Using telehealth to expand access to essential health services during the COVID-19 pandemic. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html. Published June 10, 2020. Accessed June 26, 2020.
- 71. Peters AL, Garg SK. The silver lining to COVID-19: avoiding diabetic ketoacidosis admissions with telehealth. Diabetes Technol Ther. 2020;22(6):449-453. [DOI] [PubMed] [Google Scholar]
- 72. Matthaei S. Assessing the value of the Ambulatory Glucose Profile in clinical practice. Br J Diabetes Vasc Dis. 2014;14:148-152. [Google Scholar]
- 73. Aleppo G, Ruedy KJ, Riddlesworth TD, et al. REPLACE-BG: a randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well-controlled type 1 diabetes. Diabetes Care. 2017;40(4):538-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Beck RW, Riddlesworth TD, Ruedy KJ, et al. Effect of initiating use of an insulin pump in adults with type 1 diabetes using multiple daily insulin injections and continuous glucose monitoring (DIAMOND): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(9):700-708. [DOI] [PubMed] [Google Scholar]
- 75. Oskarsson P, Antuna R, Geelhoed-Duijvestijn P, Krӧger J, Weitgasser R, Bolinder J. Impact of flash glucose monitoring on hypoglycaemia in adults with type 1 diabetes managed with multiple daily injection therapy: a pre-specified subgroup analysis of the IMPACT randomised controlled trial. Diabetologia. 2018;61(3):539-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Eeg-Olofsson K, Svensson A-M, Franzén S, Ismail HA, Levrat-Guillen F. Sustainable HbA1c decrease at 12 months for adults with type 1 and type 2 diabetes using the FreeStyle Libre® system: a study within the National Diabetes Register in Sweden. Diabetes. 2020;69(suppl 1):74-LB. [Google Scholar]
- 77. Klak A, Mańczak M, Owoc J, Olszewski R. Impact of continuous glucose monitoring on improving emotional well-being among adults with type 1 diabetes mellitus: a systematic review and meta-analysis. Pol Arch Intern Med. 2021;131(9):808-818. [DOI] [PubMed] [Google Scholar]
- 78. Sandig D, Grimsmann J, Reinauer C, et al. Continuous glucose monitoring in adults with type 1 diabetes: real-world data from the German/Austrian prospective diabetes follow-up registry. Diabetes Technol Ther. 2020;22(8):602-612. doi: 10.1089/dia.2020.0019. [DOI] [PubMed] [Google Scholar]
- 79. Miller D, Kerr MSD, Roberts GJ, Souto D, Nabutovsky Y, Wright D., Jr. FreeStyle Libre® system use associated with reduction in acute diabetes events and all-cause hospitalizations in patients with type 2 diabetes without bolus insulin. Am J Manag Care. 2021;27(11):e372-e377. doi: 10.37765/ajmc.2021.88780. [DOI] [PubMed] [Google Scholar]
- 80. Wright EE, Kerr MSD, Reyes IJ, Nabutovsky Y, Miller M. Use of flash continuous glucose monitoring is associated with A1c reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34:184-189. doi: 10.2337/ds20-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cengiz D, Danne T, Ahmad T, et al. ISPAD clinical practice consensus guidelines 2022: insulin treatment in children and adolescents with diabetes. Date unknown. https://cdn.ymaws.com/www.ispad.org/resource/dynamic/forums/20220810_094404_31885.pdf. Accessed December 1, 2022. [DOI] [PubMed]
- 82. Addala A, Maahs DM, Scheinker D, Chertow S, Leverenz B, Prahalad P. Uninterrupted continuous glucose monitoring access is associated with a decrease in HbA1c in youth with type 1 diabetes and public insurance. Pediatr Diabetes. 2020;21(7):1301-1309. doi: 10.1111/pedi.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Su D, Zhou J, Kelley MS, et al. Does telemedicine improve treatment outcomes for diabetes? a meta-analysis of results from 55 randomized controlled trials. Diabetes Res Clin Pract. 2016;116:136-148. [DOI] [PubMed] [Google Scholar]
- 84. Marcolino MS, Maia JX, Alkmim MB, Boersma E, Ribeiro AL. Telemedicine application in the care of diabetes patients: systematic review and meta-analysis. PLoS ONE. 2013;8(11):e79246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Heitkemper EM, Mamykina L, Travers J, et al. Do health information technology self-management interventions improve glycemic control in medically underserved adults with diabetes? a systematic review and meta-analysis. J Am Med Inform Assoc. 2017;24:1024-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Welsh JB, Thomas R. Continuous glucose monitoring: an emerging standard of care. Am J Manag Care. 2019;25(4 spec no):SP116-SP119. [PubMed] [Google Scholar]
- 87. Nguyen KH, Sommers BD. Access and quality of care by insurance type for low-income adults before the affordable care act. Am J Public Health. 2016;106(8):1409-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bin rsheed A, Chenoweth I. Barriers that practitioners face when initiating insulin therapy in general practice settings and how they can be overcome. World J Diabetes. 2017;8(1):28-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. American Diabetes Association. Health equity and diabetes technology: a study of access to continuous glucose monitors by payer and race executive summary. Date unknown. https://diabetes.org/sites/default/files/2021-10/ADA%20CGM%20Utilization%20White%20Paper.pdf. Accessed November 20, 2022.
- 90. Pihoker C, Badaru A, Anderson A, et al. Insulin regimens and clinical outcomes in a type 1 diabetes cohort: the SEARCH for diabetes in youth study. Diabetes Care. 2013;36(1):27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Agarwal S, Kanapka LG, Raymond JK, et al. Racial-ethnic inequity in young adults with type 1 diabetes. J Clin Endocrinol Metab. 2020;105(8):e2960-e2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lai CW, Lipman TH, Willi SM, Hawkes CP. Racial and ethnic disparities in rates of continuous glucose monitor initiation and continued use in children with type 1 diabetes. Diabetes Care. 2021;44(1):255-257. [DOI] [PubMed] [Google Scholar]
- 93. Wirunsawanya K. Racial differences in technology use among type 1 diabetes in a safety-net hospital. J Endocr Soc. 2020;4(suppl 1):OR30-03. [Google Scholar]
- 94. Prahalad P, Addala A, Buckingham BA, Wilson DM, Maahs DM. Sustained continuous glucose monitor use in low-income youth with type 1 diabetes following insurance coverage supports expansion of continuous glucose monitor coverage for all. Diabetes Technol Ther. 2018;20(9):632-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cengiz E, Xing D, Wong JC, et al. Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D Exchange clinic registry. Pediatr Diabetes. 2013;14(6):447-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Govan L, Maietti E, Torsney B, et al. The effect of deprivation and HbA1c on admission to hospital for diabetic ketoacidosis in type 1 diabetes. Diabetologia. 2012;55(9):2356-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hassan K, Loar R, Anderson BJ, Heptulla RA. The role of socioeconomic status, depression, quality of life, and glycemic control in type 1 diabetes mellitus. J Pediatr. 2006;149(4):526-531. [DOI] [PubMed] [Google Scholar]
- 98. Benoit SR, Zhang Y, Geiss LS, Gregg EW, Albright A. Trends in diabetic ketoacidosis hospitalizations and in-hospital mortality — United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2018;67(12):362-365. https://www.cdc.gov/mmwr/volumes/67/wr/mm6712a3.htm. Accessed February 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ng BP, Laxy M, Shrestha SS, et al. Prevalence and medical expenditures of diabetes-related complications among adult Medicaid enrollees with diabetes in eight U.S. states. J Diabetes Complications. 2021;35(3):107814. doi: 10.1016/j.jdiacomp.2020.107814. [DOI] [PubMed] [Google Scholar]
- 100. Shrestha SS, Zhang P, Thompson TJ, Gregg EW, Albright A, Imperatore G. Medical expenditures associated with diabetes among youth with Medicaid coverage. Med Care. 2017;55(7):646-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nguyen CA, Chernew ME, Ostrer I, Beaulieu ND. Comparison of healthcare delivery systems in low- and high-income communities. AMJC. 2019;7(4):11-18. https://www.ajmc.com/view/comparison-of-healthcare-delivery-systems-in-low-and-highincome-communities. Accessed April 22, 2021. [Google Scholar]
- 102. Anderson JE, Gavin JR, Kruger DF. Current eligibility requirements for CGM coverage are harmful, costly, and unjustified. Diabetes Technol Ther. 2020;22(3):169-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Centers for Disease Control and Prevention. Diabetes data and statistics. Date unknown. https://www.cdc.gov/diabetes/data/index.html. Accessed December 1, 2022.
- 104. Vigersky RA, Fish L, Hogan P, et al. The clinical endocrinology workforce: current status and future projections of supply and demand. J Clin Endocrinol Metab. 2014;99(9):3112-3121. [DOI] [PubMed] [Google Scholar]
- 105. Shubrook JH, Ramirez BF, Healy AM, et al. Primary care diabetes fellowship programs: developing national standards. Clin Diabetes. 2021;39(1):88-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. American Medical Association. 2017. AMA prior authorization physician survey. Date unknown. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/arc/prior-auth-2017.pdf. Accessed March 1, 2021.
- 107. Bergenstal RM, Nimri R, Beck RW, et al. A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet. 2021;397(10270):208-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Collyns OJ, Meier RA, Betts ZL, et al. Improved glycemic outcomes with Medtronic MiniMed advanced hybrid closed-loop delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes Care. 2021;44(4):969-975. doi: 10.2337/dc20-2250. [DOI] [PubMed] [Google Scholar]
- 109. Isganaitis E, Raghinaru D, Ambler-Osborn L, et al. Closed-loop insulin therapy improves glycemic control in adolescents and young adults: outcomes from the international diabetes closed-loop trial. Diabetes Technol Ther. 2021;23(5):342-349. doi: 10.1089/dia.2020.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Breton MD, Kanapka LG, Beck RW, et al. A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med. 2020;383:836-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. McAuley SA, Lee MH, Paldus B, et al. Six months of hybrid closed-loop versus manual insulin delivery with fingerprick blood glucose monitoring in adults with type 1 diabetes: a randomized, controlled trial. Diabetes Care. 2020;43:3024-3033. [DOI] [PubMed] [Google Scholar]
- 112. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet. 2018;392:1321-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Benhamou PY, Franc S, Reznik Y, et al. Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. Lancet Digit Health. 2019;1(1):e17-e25. [DOI] [PubMed] [Google Scholar]
- 115. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316:1407-1408. [DOI] [PubMed] [Google Scholar]
- 116. Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(3):155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Carlson AL, Sherr JL, Shulman DI, et al. Safety and glycemic outcomes during the MiniMed™ advanced hybrid closed-loop system pivotal trial in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2022;24:178-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Brown SA, Forlenza GP, Bode BW, et al. Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care. 2021;44:1630-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Roussel R, Bruno Guerci B, Vicaut E, et al. Dramatic drop in ketoacidosis rate after FreeStyle Libre™ system initiation in type 1 and type 2 diabetes in France, especially in people with low self-monitoring of blood glucose (SMBG): a nationwide study. Diabetes. 2020;69(suppl 1):68-OR. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-dst-10.1177_19322968221144052 for Increase Access, Reduce Disparities: Recommendations for Modifying Medicaid CGM Coverage Eligibility Criteria by Rodolfo J. Galindo, Grazia Aleppo, Christopher G. Parkin, David A. Baidal, Anders L. Carlson, Eda Cengiz, Gregory P. Forlenza, Davida F. Kruger, Carol Levy, Janet B. McGill and Guillermo E. Umpierrez in Journal of Diabetes Science and Technology


