Abstract
Objective:
There is increasing use of open-source artificial pancreas systems (APS) in the management of Type 1 diabetes. Our aim was to assess the safety and efficacy of the automated insulin delivery system AndroidAPS (AAPS), compared with stand-alone pump therapy in people with type 1 diabetes. The primary outcome was the difference in the percentage of time in range (TIR, 70-180 mg/dL). Secondary aims included mean sensor glucose value and percent continuous glucose monitor (CGM) time below range (TBR, <70 mg/dL).
Research Design and Methods:
This open-label single-center randomized crossover study (ANZCTR, Australian New Zealand clinical trial registry, ANZCTR-ACTRN12620001191987) comprised 20 participants with type 1 diabetes on established pump therapy, assigned to either stand-alone insulin pump therapy or the open-source AAPS hybrid closed-loop system for four weeks, with crossover to the alternate arm for the following four weeks. The CGM outcome parameters were measured by seven-day CGM at baseline and the final week of each four-week study arm.
Results:
Twenty participants were recruited (60% women), aged 45.8 ± 15.9 years, with mean diabetes duration of 23.9 ± 13.2 years, baseline glycated hemoglobin (HbA1c) 7.5% ± 0.5% (58 ± 6 mmol/mol) and mean TIR 62.3% ± 12.9%. The change in TIR from baseline for AAPS compared with stand-alone pump therapy was 18.6% (11.4-25.9), (P < .001), TIR 76.6% ± 11.7%, 58.0% ± 15.6%, for AAPS and stand-alone pump, respectively. Time glucose <54 mg/dL was not increased (mean = −2.0%, P = .191). No serious adverse events or episodes of severe hypoglycemia were recorded.
Conclusions:
This clinical trial of the open-source AAPS hybrid closed-loop system performed in an at-home setting demonstrated comparable safety to stand-alone pump therapy. The glycemic outcomes of AAPS were superior with improved TIR, and there was no significant difference in TBR compared with stand-alone pump therapy.
Keywords: artificial pancreas systems, continuous glucose monitoring, insulin pump therapy, time in range
Background
The combination of continuous glucose monitoring (CGM) with insulin pumps has resulted in improved glycemic control in people with type 1 diabetes. 1 The addition of safety features, including low glucose suspend and predictive low glucose suspend have facilitated improved glycemic control with lower rates of hypoglycemia.2,3 Recent years have seen the emergence of closed-loop technologies, known as Artificial Pancreas Systems (APS). These delivery systems consist of a control algorithm that receives and processes real-time continuous sensor glucose data and automatically adjusts insulin delivery via an insulin pump.
The #WeAreNotWaiting movement has emerged with the intent of increasing the availability and affordability of advanced closed-loop technology, with the development of open-source “do-it-yourself” (DIY) APS—such as “OpenAPS” or “AndroidAPS” (AAPS). This movement has enabled many people with type 1 diabetes to build their own automated insulin delivery systems using existing technology. The AAPS is one of these systems consisting of an application on a device that works as a hybrid closed-loop system using a model predictive control (MPC) algorithm. The algorithm adjusts basal insulin delivery rate based on current glucose levels, insulin on board, carbohydrate consumption, and prediction of future glucose changes based on modelled insulin-glucose dynamics. 4
The number of people with type 1 diabetes utilizing these uncertified products is increasing, with more than 2720 users worldwide as of July 2022. 5 There is currently limited glycemic control, safety, and quality of life data available using the AAPS system. A retrospective analysis of 20 Open APS users reported significant improvements in glucose levels, time in range (TIR), and HbA1c. 6 Similarly, a UK-based observational study concluded that Open APS resulted in reduced HbA1c and increased TIR compared with stand-alone pump therapy. 7 However, there is a need to directly compare the safety and efficacy of this system with standard therapy, with prospective randomized controlled studies.8 -10
The use of OpenAPS is limited by a number of factors, including lack of regulatory approval and safety and efficacy data from prospective randomized clinical trials. We, therefore, aimed to assess the safety and efficacy of the hybrid closed-loop open-source AAPS system compared with stand-alone insulin pump therapy in patients with established type 1 diabetes. A YpsoPump OPN was used with AAPS source code 2.5, with the incorporation of a pump driver, which was developed to interface with the YpsoPump OPN.
Research Design and Methods
Study Design and Population
This was an open-label single-center randomized crossover trial with participants recruited between November 2020 and October 2021 from people attending the diabetes service at the Baker Heart and Diabetes Institute. Inclusion criteria were a diagnosis of type 1 diabetes >6 months duration, use of insulin pump therapy for >6 months duration, and HbA1c <10.0%. Exclusion criteria were pregnancy, current use of real-time CGM within the previous 3 months, hospitalization for severe hypoglycemia or ketoacidosis in the past 6 months, chronic kidney disease (estimated glomerular filtration rate [eGFR] <45 mL/min/1.73 m2), planned international travel during the study period, and use of oral hypoglycemic agents or non-insulin injectable agents within past 4 weeks. All successfully screened participants received CGM and pump education, and pump settings were optimized at baseline. All participants provided written informed consent, and ethics approval was obtained from Bellberry Human Research Ethics Committee (HREC; 2019-07-645) and the research governance officer of Baker Heart and Diabetes Institute. The clinical trial was registered with the Australian New Zealand clinical trial registry (ANZCTR-ACTRN12620001191987).
Participants were randomized (1:1 allocation through a random number generator by the research team), without restrictions, to either AAPS or stand-alone pump therapy for the first four weeks, followed by four weeks of the alternate treatment arm, separated by a one-week washout period in between to prevent any carryover effects. Participants were monitored in an inpatient research ward for 24 hours after initiation of AAPS and discharged after safety assessments and review by a clinical research team member. Patients were reviewed by an endocrinologist, diabetes educator, and research coordinator at weeks one, two, and four of each study arm. Blinded CGM (iPro2) was inserted at baseline and at week four of each study arm to measure glycemic outcomes. All participants in the AAPS arm were commenced on Dexcom G5 (San Diego, CA, USA) and followed with the Dexcom Follow application and a Nightscout account for the four weeks of AAPS. In the event of prolonged hyperglycemia, hypoglycemia, or loss of signal (>4 hours), participants were contacted to ensure safety. All the participants and caregivers had access to a 24-hour telephone helpline for the research team.
AAPS System
AAPS comprises three components:
Dexcom G5 CGM: The CGM data were transferred in real-time through Bluetooth to Dexcom G5 mobile App.
Upgraded YpsoPump (Ypsomed, Burgdorf, Switzerland): An upgraded version of YpsoPump with bidirectional communication capability was specifically developed for this study to enable communication with the AAPS interface.
The AAPS App: an application on the study phone (Samsung Galaxy) with the controller algorithm.
A standard YpsoPump, without modification, was used for the stand-alone pump therapy arm of the trial. During this trial phase, participants performed capillary blood glucose testing and did not use CGM. The YpsoPump OPN pump was used for the AAPS arm. This pump had the same hardware as the regular YpsoPump, with enhancements made to the Bluetooth interface to allow hands-free control from the host device. Bluetooth protocol documentation was supplied by Ypsomed, and a driver was then created. In the lead-up to the trial, a copy of the APS 2.5 source code was frozen, and a pump driver was developed to interface with the YpsoPump OPN pump. The driver was subjected to in-silico testing before progressing to further functional testing. During this testing, the YpsoPump OPN pump was run in parallel with a copy of the existing AAPS software, using an Accu-Chek Spirit Combo pump for one week. Dosing records were compared in detail to verify that the YpsoPump OPN pump would deliver insulin as expected. Users were issued with locked-down handsets, with both the operating system and AAPS app configurations locked down to prevent any alterations. The AAPS app data (including CGM and insulin pump data) were uploaded to the Nightscout cloud server in real-time. Clinicians could log in using an encrypted connection to Nightscout to monitor CGM and insulin delivery data. All configuration changes were made by clinicians remotely or during study visits. In case of any AAPS or Dexcom CGM failures, the YpsoPump OPN pumps were programmed to automatically return to a safe basal delivery mode that delivered insulin according to preset basal delivery rates.
Basal insulin delivery rates were adjusted every five minutes based on varying glucose levels. Pump output was altered by temporary basal rate adjustment using the OpenAPS reference design 1 (oref1) algorithm. 11 A target blood glucose of 110 mg/dL was set for all participants. The default maximum basal rate was set at 8 units/h or five times the profile basal rate (whichever was smaller), and the maximum allowed bolus was 15 units. For meal boluses, participants entered the meal’s carbohydrate content into the AAPS app, and the integrated bolus calculator activated a command to YpsoPump to deliver the dose.
Stand-alone pump therapy involved using YpsoPump with regular blood glucose monitoring and standard insulin pump management. The YpsoPump was connected to the mylife app (Figure 1). The mylife app imported the therapy data from the YpsoPump through Bluetooth. Participants entered finger-prick capillary glucose levels and carbohydrate counting data into the app and the integrated bolus calculator enabled them to calculate mealtime or correctional bolus insulin doses.
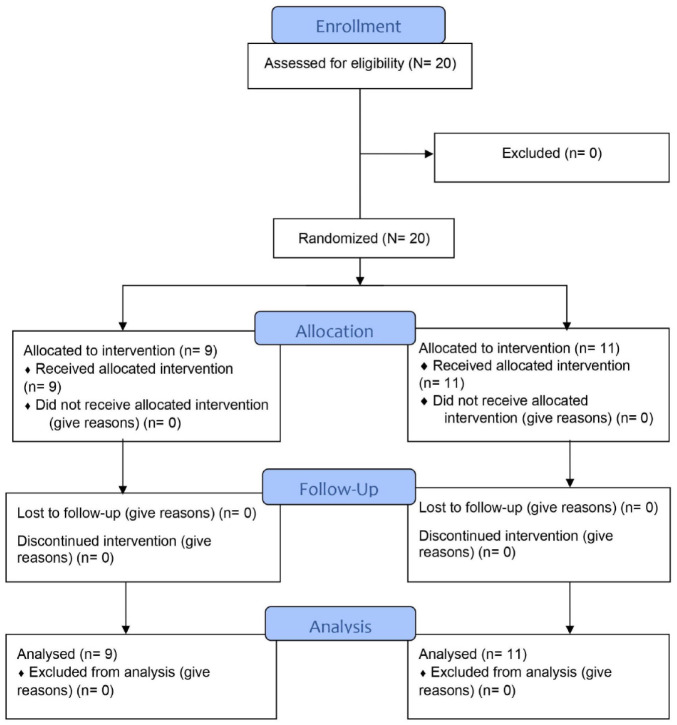
Figure 1.
CONSORT flow diagram.
Explanatory Variables
Prespecified demographic (sex, gender, and date of birth) and clinical variables (past medical history, current medications, date of diabetes diagnosis, diabetes complications, HbA1c, history of severe hypoglycemia, diabetic ketoacidosis, and other diabetes-related hospitalizations and insulin pump information) were obtained at the first study visit.
Outcomes
The primary study outcome was the percentage of CGM time in target range (glucose 70-180 mg/dL) with AAPS versus stand-alone pump therapy at the final week of the 4-week-study arms. Prespecified secondary outcomes were mean sensor glucose value with AAPS compared with stand-alone pump therapy, percentage of CGM time in hypoglycemia range (glucose <70 mg/dL), percentage of CGM time in clinically significant hypoglycemia range (glucose <54 mg/dL), percentage of CGM time in hyperglycemia range (glucose >180 mg/dL), percentage of CGM time in significant hyperglycemic range (glucose >270 mg/dL), number of symptomatic episodes of hypoglycemia (symptoms consistent with hypoglycemia confirmed by a finger-prick glucose level of less than 54 mg/dL), number of severe hypoglycemic episodes (any low glucose level requiring the assistance of another person to actively administer carbohydrate, glucagon, or take other corrective action), number of hospitalizations for diabetic ketoacidosis, the total daily dose of insulin, and patient-reported outcomes, including treatment satisfaction, diabetes distress, fear of hypoglycemia, and hypoglycemia awareness. These were assessed using the validated tools of the Diabetes Self-Management Questionnaire (DSMQ), 12 Problem Areas in Diabetes (PAID), 13 Hypoglycemia Fear Survey (HFS)-II short form, 14 and Gold score for hypoglycemia awareness. 15
Sample Size and Power
Based on previously published data, 16 the use of a day-and-night hybrid closed-loop insulin delivery system for four weeks was anticipated to improve the time in target range (70-180 mg/dL) by a mean of 10.5% (95% CI [7.6%-13.4%]) compared with standard pump therapy. Assuming an improvement in time in target range of 12% and a conventional deviation of 18%, 17 participants were required to provide 80% power at a 5% alpha level. Therefore, 20 participants were recruited, allowing for a dropout rate of 15%.
Statistical Methods
Categorical variables were summarized as percentages and continuous variables were reported as means with standard deviations or as medians with interquartile ranges (IQR) and tested for normality to determine appropriate statistical analysis (parametric or nonparametric). All outcomes, except for the number of symptomatic hypoglycemia episodes, were analyzed using a linear mixed model with random individual intercept. The number of symptomatic hypoglycemia episodes were analyzed using Poisson generalized linear mixed model. For the following outcomes, namely, the proportion of TIR, time in severe hypoglycemia, time in hypoglycemia, and time in hyperglycemia range variables, the outcome variables were log-transformed prior to statistical analysis. For all outcomes, the treatment effects were adjusted for the baseline variable (eg, baseline TIR when analyzing TIR), gender, age at diagnosis, and period effect. Type I error (α) was set at 5%, and P values <.05 indicated statistical significance. All analyses were performed using Project R Version 4.1.1.
Results
Participants
Twenty adults with type 1 diabetes, aged 45.8 ± 15.9 years, 60% women, participated in this study. The mean duration of type 1 diabetes was 23.9 ± 13.2 years and baseline HbA1c was 7.5% ± 0.6% (58 ± 7.1 mmol/mol). The mean body mass index (BMI) was 27.7 ± 4.9 kg/m2. At baseline, mean TIR (70-180 mg/dL) was 61.0% ± 12.7%, with percentage of time below range (glucose <70 mg/dL) 4.8% ± 4.9% and percentage of time above range (glucose >180 mg/dL) 34.1% ± 14.1%.
Primary and Secondary Outcomes
Time in range during the AAPS period was 76.6% ± 11.7%, compared with 58.0% ± 15.6% during the stand-alone pump therapy period, a mean difference of 18.6 (11.4-25.9)%, P < .001. Time below range (glucose <70 mg/dL) during the AAPS arm was 2.1% ± 1.7%, compared with 4.2% ± 3.8% during the stand-alone pump therapy arm, with a difference of −2.0 (−3.8 to −0.3)%, P = .191. Time above range (glucose >180 mg/dL) during the AAPS arm was 21.3% ± 11.5%, compared with 37.9% ± 14.3% during the stand-alone pump arm, a difference of −16.6 (−23.4 to −9.8)%, P < .001. The glycemia risk index (GRI) during the AAPS arm was 30% ± 18%, compared with 45% ± 17% during the stand-alone pump therapy arm, a difference of −15 (−34 to −4)%, P = .06 (Table 1).
Table 1.
Primary and Secondary Outcomes During Treatment Periods.
| AAPS period (N = 20) | Stand-alone pump therapy period (N = 20) | Mean diff (AAPS-YpsoPump) | P value a | |
|---|---|---|---|---|
| Primary outcomes | ||||
| % time glucose 70-180 mg/dL | 76.6 (11.7) | 58.0 (15.6) | 18.6 (11.4 to 25.9) | <.001 |
| % time glucose <70 mg/dL | 2.1 (1.7) | 4.2 (3.8) | −2.0(−3.8 to −0.3) | .191 |
| % time glucose >180 mg/dL | 21.3 (11.5) | 37.9 (14.3) | −16.6 (−23.4 to −9.8) | <.001 |
| Secondary outcomes | ||||
| HbA1c (%) | 7.1 (0.6) | 7.5 (0.6) | −0.4 (−0.7 to 0) | <.001 |
| HbA1c (mmol/mol) | 54.6 (6.2) | 58.5 (6.2) | −3.9 (−8.2 to 0.4) | <.001 |
| Mean glucose (mg/dL) | 149.4 (19.8) | 167.4 (23.9) | −19.8 (−32.4 to −5.6) | .011 |
| Insulin daily dose (units) | 40.5 (24.6) | 41.7 (24.1) | −1.2 (−3.2 to 0.8) | .276 |
| Number of symptomatic hypoglycemia events | 3.8 (2.2) | 1.4 (1.8) | 2.4 (1.3-3.5) | <.001 |
| Time in glucose < 54 mg/dL (%) | 0.3 (0.4) | 1.0 (1.9) | −0.7 (−1.6 to 0.1) | .663 |
| Time in glucose > 270 mg/dL (%) | 2.4 (0.4) | 9.3 (1.9) | −6.9 (−11 to −2.8) | .012 |
| Glycemia risk index | 30 (18) | 45 (17) | −15 (−34 to 4) | .06 |
| Problem areas in diabetes score | 13.5 (14.7) | 14.6 (12) | −1.1 (−9.8 to 7.7) | .739 |
| Hypoglycemia Fear Survey-II | 16.3 (14.9) | 14.3 (13.8) | 2.0 (−7.6 to 11.6) | .268 |
| Diabetes treatment satisfaction questionnaire combined | 5.5 (0.6) | 5.7 (0.6) | −0.2 (−0.6 to 0.2) | .168 |
Each outcome is reported using mean (SD).
Abbreviations: AAPS, AndroidAPS; APS, artificial pancreas systems.
For testing treatment effects (AAPS-stand-alone pump) adjusted for patient-specific baseline, gender, age at Type 1 diabetes diagnosis, and potential ordering effect. Endpoints were calculated with the use of data from blinded CGM from week 4 of the treatment period.
Mean HbA1c was 7.1% ± 0.6% (54.6 ± 6.2 mmol/mol) after 4 weeks of AAPS compared with 7.5% ± 0.5% (58.5 ± 6.2 mmol/mol) after 4 weeks of stand-alone pump therapy, a mean adjusted difference of −0.4 (−0.7 to −0.0)%, −3.9 (−8.2 to −0.4) mmol/mol, P < .001. Mean glucose was 149.4 ± 19.8 mg/dL with AAPS, compared with 167.4 ± 23.4 mg/dL with stand-alone pump therapy, a mean adjusted difference of −19.8(−32.4 to −5.6) mg/dL P = .011. The total daily insulin dose did not vary between the AAPS and stand-alone pump therapy arms, 40.5 ± 24.6 units and 41.7 ± 24.1 units, respectively, P = .276. Time in significant hypoglycemia (glucose <54. mg/dL) was not increased during AAPS 0.3% ± 0.4% compared with stand-alone pump therapy 1.0% ± 1.9%, P = .663. Time in significant hyperglycemia (glucose >270 mg/dL) was less during AAPS 2.4% ± 0.4% than with stand-alone pump therapy 9.3% ± 1.9%, P = .012.
The number of symptomatic hypoglycemic events was more in the AAPS period compared with the stand-alone pump therapy, 3.8 ± 2.2 versus 1.4 ± 1.8 episodes/wk, respectively, P < .001. During the AAPS arm, 95% (IQR = 5.2) of time was spent in closed-loop mode. Figure 2 shows the 24-hour glucose profiles during AAPS and stand-alone pump therapy periods.
Figure 2.
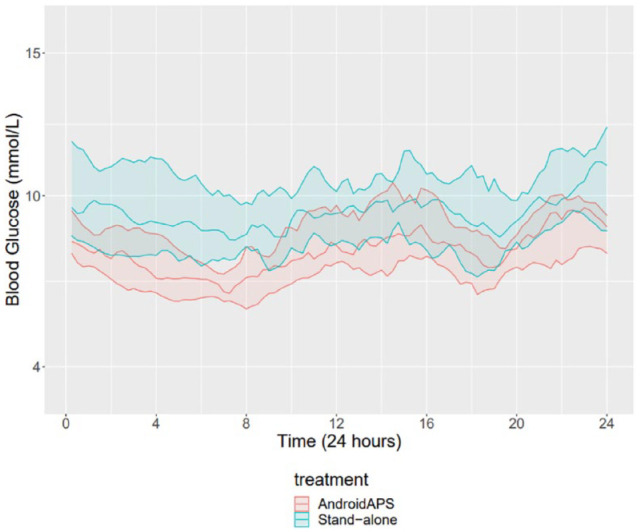
Median sensor glucose levels during AAPS and stand-alone pump therapy. The teal and red shaded areas indicate interquartile ranges.
Abbreviations: AAPS, AndroidAPS; APS, artificial pancreas systems.
There were no significant differences in psychological parameters between AAPS and stand-alone pump therapy arms. The mean PAID score was 13.5 ± 14.7 during the AAPS period compared with 14.6 ± 12.0 in the stand-alone pump therapy period, P = .739. The mean HFS II short form score was 16.3 ± 14.9 in the AAPS period compared with 14.3 ± 13.8 in the stand-alone pump therapy period, P = .739. The mean Diabetes Treatment Satisfaction Questionnaire (DTSQ) score was 5.5 ± 0.6 in the AAPS period compared with 5.7 ± 0.6 in the stand-alone pump therapy period, P = .168. According to the Gold score for hypoglycemia awareness, 17 patients had normal hypoglycemia awareness, three patients had undetermined hypoglycemia awareness, and no patients had impaired awareness of hypoglycemia. This did not change from baseline during the AAPS and stand-alone pump therapy periods.
Adverse Events
No serious adverse events or episodes of severe hypoglycemia were recorded during the study period. There were no episodes of diabetic ketoacidosis. Three medical adverse events were investigated and found to be unrelated to the intervention. These were as follows: Helicobacter pylori was detected during routine endoscopy before the start of the study, an episode of gastroenteritis, and hyperglycemia after the insulin pump was inadvertently not restarted following a cannula change. Three technical adverse events occurred during the AAPS period due to connectivity issues. Four technical adverse events occurred: connection lost after the study phone ran out of battery, pump not restarted after cannula change, Nightscout not receiving data after sensor loosened without participant realizing, and ratio change not updated. These did not result in any clinically significant events.
Discussion
This randomized crossover study of 20 adults with type 1 diabetes comparing an AAPS system with stand-alone insulin pump therapy demonstrates the efficacy and safety of AAPS. Time in range was improved by 18.6% from baseline for AAPS compared with stand-alone insulin pump therapy; this equates to an additional 4 hours, 27 minutes of TIR per day. Time below range and GRI were not increased, and a significant reduction in time above range was observed. There were no severe adverse events and no changes in patient-reported outcomes. This is one of the first clinical trials designed to assess this system in a controlled randomized prospective study, consistent with the findings of other studies looking at this automated insulin delivery system. The achieved parameters with AAPS during this study were within the recommendations of the American Diabetes Association (ADA), 17 TIR >70%, TBR <4%, and time below 54 mg/dL <1%.
The improvement in TIR observed here is comparable to previously reported literature using DIY APSs18 -22 and greater than the initial studies of some commercial systems. 23 The CREATE (Community deRivEd AutomaTEd insulin delivery) trial was the first published study evaluating AAPS in children and adults with type 1 diabetes and reported an increase of 14% time in target with AAPS compared with sensor-augmented insulin pump at 24 weeks. 24 The TIR reported in this study was consistent with that reported in the CREATE trial (74.5% ± 11.9%). 24 A cross-sectional analysis of OpenAPS reported an increase in the percentage of time in target range from 58% to 81%. 25 Our results are also consistent with analyses of TIR for commercially available hybrid closed-loop systems, using the Medtronic MiniMed 780G hybrid closed-loop system (76.2% ± 9.1%) 26 and Tandem t:slim X2 with Control-IQ (73.5 [IQR 64.4-81.6]%).
The relatively short duration of the study may have precluded observation of the full extent of the HbA1c lowering effect of AAPS. Nonetheless, an HbA1c improvement of this magnitude is clinically significant and would be expected to result in fewer complications. We would expect to see an even bigger change in HbA1c if this study were conducted over a longer period. Despite this, the decrease in HbA1c observed with AAPS compared with stand-alone pump therapy here was comparable to the findings of other studies conducted in both adults,22,27 older children, and adolescents18,28 with type 1 diabetes. The CREATE study reported a similar degree of HbA1c lowering (0.45%, 5.9 mmol/mol) to our study, despite being conducted over a more extended period of 24 weeks. A systematic review 29 reported an improvement in HbA1c by up to 0.85% with the use of DIY APS systems although no prospective studies were included in this review.
Similar to our findings, other studies also report low incidences of adverse events such as ketoacidosis and severe hypoglycemia, supporting the favorable safety profile of DIY hybrid closed-loop therapy.22,24,28 The rate of technical problems was very low, as evidenced by the high percentage of time in closed-loop mode during the AAPS arm of the study. However, the relatively short duration of the study may have limited observation of rarer adverse events, such as ketoacidosis.
Some studies have reported improvements in diabetes-related quality of life and reduced fear of hypoglycemia10,27,30 but others reported increased challenges with using DIY APS systems. 31 However, unlike our study, validated tools or questionnaires were not used to measure outcomes. We did not identify any differences in patient-reported outcomes between AAPS and stand-alone pump therapy. However, this may be due to the relatively short duration of this study.
Strengths of this prospective trial include the randomized crossover design, in an at-home setting, with consistently high use of the hybrid closed-loop AAPS system, validated quality of life scales, and extensive safety and efficacy monitoring procedures. This trial had several limitations. The conventional therapy arm of the study did not use real-time CGM, therefore it is possible that some of the improvements in time, in range achieved in the AAPS arm, may partially be attributable to the use of real-time CGM. Despite relatively broad eligibility criteria, the trial population may not represent the general population of type 1 diabetes patients. This single-center study recruited patients attending outpatient services at a specialist diabetes service. The study contained a relatively small number of participants who were well educated and highly motivated. As this trial comprised participants with relatively tight glycemic control at enrolment, it is not clear how well these findings would translate to other populations with diverse ethnic and sociodemographic characteristics or with poorer glycemic control. However, evidence suggests that AAPS is of benefit irrespective of baseline glycemic control. 27 This study did not use advanced oref1 algorithm features, such as super micro boluses, which may have provided even greater glycemic benefits. Dexcom G5 sensors were used in this trial, which required calibration and had shorter wear time than the Dexcom G6 sensors. This may have limited the percentage of time in closed loop, and further studies using G6 sensors in association with AAPS are of potential importance. The CGM data were obtained over a period of one week, since the protocol for this study was designed, an international consensus statement has been published, recommending the use of 14 days of CGM data. 32
Conclusions
This randomized crossover trial demonstrated that automated insulin delivery with the use of an open-source AAPS hybrid closed-loop system led to significant improvements in time, in range and reduced time above range with no increase in TBR, severe hypoglycemia, adverse events, or quality of life compared with stand-alone pump therapy. Therefore, we conclude that open source AAPS presents an acceptable safety and efficacy profile.
Footnotes
Abbreviations: AAPS, Android Artificial Pancreas System; ADA, American Diabetes Association; ANZCTR, Australian New Zealand clinical trial registry; BMI, body mass index; CGM, continuous glucose monitor; DIY APS, do it yourself artificial pancreas system; DSMQ, diabetes self-management questionnaire; DTSQ, diabetes treatment satisfaction questionnaire; eGFR, estimated glomerular filtration rate; GRI, glycemia risk index; HbA1c, glycated hemoglobin; HFS, Hypoglycemia Fear Survey; HREC, Human Research Ethics Committee; IQR, interquartile range; MPC, model predictive control; Oref1, OpenAPS reference design 1; PAID, problem areas in diabetes; RCT, randomized control trial; TBR, time below range; TIR, time in range.
Author Contributions and Guarantor Statement: AS and NC were responsible for the concept and design of the study. AS and NC supervised the study. NN, DB, AS, YE, DHL, and NC contributed to data acquisition, analysis, or interpretation. The manuscript was drafted by NN, DB, AS, YE, DHL and NC. DHL carried out the statistical analysis. All authors revised the manuscript critically and approved the final version. AS and NC had full access to the data and take responsibility for the integrity of the data and the accuracy of the analysis.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was financially supported with an unrestricted grant by Ypsomed Selfcare Solutions. Ypsomed Selfcare Solutions did not have any involvement or input in the study design, data collection, statistical analyses, or completion of any aspect of this trial.
ORCID iDs: Natalie Nanayakkara  https://orcid.org/0000-0001-9271-9917
https://orcid.org/0000-0001-9271-9917
Yasser Elghattis  https://orcid.org/0000-0001-8351-4784
https://orcid.org/0000-0001-8351-4784
References
- 1. Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55(12):3155-3162. Accessed January 24, 2023. https://pubmed.ncbi.nlm.nih.gov/22965294/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369(3):224-232. https://pubmed.ncbi.nlm.nih.gov/23789889/. Accessed January 24, 2023. [DOI] [PubMed] [Google Scholar]
- 3. Battelino T, Nimri R, Dovc K, Phillip M, Bratina N. Prevention of hypoglycemia with predictive low glucose insulin suspension in children with type 1 diabetes: a randomized controlled trial. Diabetes Care. 2017;40(6):764-770. http://care.diabetesjournals.org/lookup/suppl/. Accessed January 24, 2023. [DOI] [PubMed] [Google Scholar]
- 4. Kesavadev Seshadhri Srinivasan Banshi Saboo Meera Krishna Gopika Krishnan JB. The do-it-yourself artificial pancreas: a comprehensive review. Diabetes Ther. 2020;11(6):1217-1235. doi: 10.6084/m9.figshare.12145824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. OpenAPS.org. OpenAPS Outcomes. https://openaps.org/outcomes/. Accessed January 24, 2023.
- 6. Lewis DM, Swain RS, Donner TW. Improvements in A1c and time-in-range in DIY closed-loop (OpenAPS) users. Diabetes. 2018;67(suppl 1):352-OR. https://diabetesjournals.org/diabetes/article/67/Supplement_1/352-OR/57141/Improvements-in-A1c-and-Time-in-Range-in-DIY. Accessed January 24, 2023. [Google Scholar]
- 7. Wilmot EG, Langeland L, Mclay A, Taylor N, Idris IR. 1067-P: open source artificial pancreas system (APS) vs. combination insulin pump with flash glucose monitoring in adults with type 1 diabetes: an observational study. Diabetes. 2019;68:1067. https://diabetesjournals.org/diabetes/article/68/Supplement_1/1067-P/58808. Accessed January 24, 2023. [Google Scholar]
- 8. Hng TM. Do-it-yourself (DIY) closed loop systems: perspectives of an endocrinologist. J Diabetes Sci Technol. 2019;14(6):1104-1106. doi: 10.1177/1932296819890855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murray JA, Clayton MF, Litchman ML. Healthcare provider knowledge and perceptions of FDA-approved and do-it-yourself automated insulin delivery. 2019;14(6):1017-1021. doi: 10.1177/1932296819895567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hng TM, Burren D. Appearance of Do-It-Yourself closed-loop systems to manage type 1 diabetes. Intern Med J. 2018;48(11):1400-1404. doi: 10.1111/imj.14105. [DOI] [PubMed] [Google Scholar]
- 11. OpenAPS.org. OpenAPS reference design. https://openaps.org/reference-design/. Accessed January 24, 2023.
- 12. Schmitt A, Gahr A, Hermanns N, Kulzer B, Huber J, Haak T. The Diabetes Self-Management Questionnaire (DSMQ): development and evaluation of an instrument to assess diabetes self-care activities associated with glycaemic control. Health Qual Life Outcomes. 2013;11(1):1-14. doi: 10.1186/1477-7525-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Welch GW, Jacobson AM, Polonsky WH. The Problem Areas in Diabetes Scale An evaluation of its clinical utility. Diabetes Care. 1997;20 (5):760-766. http://diabetesjournals.org/care/article-pdf/20/5/760/584254/20-5-760.pdf. Accessed January 24, 2023. [DOI] [PubMed] [Google Scholar]
- 14. Grabman J, Vajda Bailey K, Schmidt K, et al. Short report: educational and psychological aspects an empirically derived short form of the hypoglycaemia fear survey II. Diabetic Medicine. 2017;34(4):500-504. doi: 10.1111/dme.13162. [DOI] [PubMed] [Google Scholar]
- 15. Gold AE, Macleod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care. 1994;17(7):697-703. https://diabetesjournals.org/care/article/17/7/697/18251/Frequency-of-Severe-Hypoglycemia-in-Patients-With. Accessed January 24, 2023. [DOI] [PubMed] [Google Scholar]
- 16. Bally L, Thabit H, Kojzar H, et al. Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: an open-label, randomised, crossover study. Lancet Diabetes Endocrinol. 2017;5:(4):261-270. https://pubmed.ncbi.nlm.nih.gov/28094136/. Accessed January 24, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Committee ADAPP. 6. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S83–S96. https://diabetesjournals.org/care/article/45/Supplement_1/S83/138927/6-Glycemic-Targets-Standards-of-Medical-Care-in. Accessed January 24, 2023. [DOI] [PubMed] [Google Scholar]
- 18. Choi SB, Hong ES, Noh YH. Open artificial pancreas system reduced hypoglycemia and improved glycemic control in patients with type 1 diabetes. Diabetes. 2018;67(suppl 1):964-P. https://diabetesjournals.org/diabetes/article/67/Supplement_1/964-P/54870/Open-Artificial-Pancreas-System-Reduced. Accessed January 24, 2023. [Google Scholar]
- 19. Burnside MJ, Lewis DM, Crocket H, et al. 286-OR: the CREATE trial: randomized clinical trial comparing open-source automated insulin delivery with sensor augmented pump therapy in type 1 diabetes. Diabetes. 2022;71(suppl 1):286-OR. https://diabetesjournals.org/diabetes/article/71/Supplement_1/286-OR/146634/286-OR-The-CREATE-Trial-Randomized-Clinical-Trial. Accessed January 24, 2023. [Google Scholar]
- 20. Ware J, Allen JM, Boughton CK, et al. Randomized trial of closed-loop control in very young children with type 1 diabetes. N Engl J Med. 2022;386(3):209-219. doi: 10.1056/NEJMoa2111673. [DOI] [PubMed] [Google Scholar]
- 21. Lewis D, Leibrand S. Real-world use of open source artificial pancreas systems. J Diabetes Sci Technol. 2016;10(6):1411. doi: 10.1177/1932296816665635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gawrecki A, Zozulinska-Ziolkiewicz D, Michalak MA, et al. Safety and glycemic outcomes of do-it-yourself AndroidAPS hybrid closed-loop system in adults with type 1 diabetes. PLoS ONE. 2021;16(4):e0248965. doi: 10.1371/journal.pone.0248965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316(13):1407-1408. https://jamanetwork.com/journals/jama/fullarticle/2552454. Accessed January 24, 2023. [DOI] [PubMed] [Google Scholar]
- 24. Burnside MJ, Lewis DM, Crocket HR, et al. Open-source automated insulin delivery in type 1 diabetes. N Engl J Med. 2022;387(10):869-881. doi: 10.1056/NEJMoa2203913. [DOI] [PubMed] [Google Scholar]
- 25. Lewis D, Leibrand S. OpenAPS Community Real-world use of open source artificial pancreas systems. J Diabetes Sci Technol. 2016;10(6):1411–1411. doi: 10.1177/1932296816665635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Silva J, da Lepore G, Battelino T, et al. Real-world performance of the MiniMedTM 780G system: first report of outcomes from 4120 users. Diabetes Technol Ther. 2022;24(2):113-119. doi: 10.1089/dia.2021.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu Z, Luo S, Zheng X, et al. Use of a do-it-yourself artificial pancreas system is associated with better glucose management and higher quality of life among adults with type 1 diabetes. Ther Adv Endocrinol Metab. 2020;11:2042018820950146. http://www.ncbi.nlm.nih.gov/pubmed/32922721. Accessed January 24, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet. 2018;392(10155):1321-1329. https://www.sciencedirect.com/science/article/pii/S0140673618319470. Accessed January 24, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Asarani NAM, Reynolds AN, Elbalshy M, et al. Efficacy, safety, and user experience of DIY or open-source artificial pancreas systems: a systematic review. Acta Diabetol. 2021;58(5):539-547. https://link.springer.com/article/10.1007/s00592-020-01623-4. Accessed January 24, 2023. [DOI] [PubMed] [Google Scholar]
- 30. Kesavadev J, Saboo B, Kar P, Sethi J. DIY artificial pancreas: a narrative of the first patient and the physicians’ experiences from India. Diabetes Metab Syndr. 2021;15(2):615-620. https://www.sciencedirect.com/science/article/abs/pii/S1871402121000631. Accessed January 24, 2023. [DOI] [PubMed] [Google Scholar]
- 31. Barnard KD, Hood KK, Weissberg-Benchell J, Aldred C, Oliver N, Laffel L. Psychosocial assessment of artificial pancreas (AP): commentary and review of existing measures and their applicability in AP research. Diabetes Technol Ther. 2015;17(4):295-300. doi: 10.1089/dia.2014.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Battelino T, Alexander CM, Amiel SA, et al. Continuous glucose monitoring and metrics for clinical trials: an international consensus statement. Lancet Diabetes Endocrinol. 2022;11:42-57. [DOI] [PubMed] [Google Scholar]