Abstract
Objective:
Real-time continuous glucose monitoring (CGM) is effective for diabetes management in cases of type 1 diabetes and adults with type 2 diabetes (T2D) but has not been assessed in adolescents and young adults (AYAs) with T2D. The objective of this pilot interventional study was to assess the feasibility and acceptability of real-time CGM use in AYAs with T2D.
Methods:
Adolescents and young adults (13-21 years old) with T2D for six months or more and hemoglobin A1c (A1c) greater than 7%, on any Food and Drug Administration–approved treatment regimen, were included. After a blinded run-in period, participants were given access to a real-time CGM system for 12 weeks. The use and acceptability of the real-time CGM were evaluated by sensor usage, surveys, and focus group qualitative data.
Results:
Participants’ (n = 9) median age was 19.1 (interquartile range [IQR] 16.8-20.5) years, 78% were female, 100% were people of color, and 67% were publicly insured. Baseline A1c was 11.9% (standard deviation ±2.8%), with median diabetes duration of 2.5 (IQR 1.4-6) years, and 67% were using insulin. Seven participants completed the study and demonstrated statistically significant improvement in diabetes-related quality of life, with the mean Pediatric Quality of Life inventory (PedsQL) diabetes score increasing from 70 to 75 after using CGM (P = .026). Focus group results supported survey results that CGM use among AYAs with T2D is feasible, can improve quality of life, and has the potential to modify behavior.
Conclusion:
Real-time CGM is feasible and acceptable for AYAs with T2D and may improve the quality of life of patients with diabetes. Larger randomized controlled trials are needed to assess the effects on glycemic control and healthy lifestyle changes.
Keywords: continuous glucose monitoring, lifestyle factors, pediatrics, sensors, type 2 diabetes, adolescents
Introduction
The incidence of type 2 diabetes (T2D) in adolescents and young adults (AYAs) is increasing at an alarming rate and continues to disproportionally affect AYAs of color. 1 Compared to adults with T2D, AYAs diagnosed with T2D show more rapid disease progression and have higher rates of complications and mortality, even exceeding the rates of AYAs with type 1 diabetes (T1D).2,3 Adolescents and young adults with T2D do not have access to the same therapeutic options, such as diabetes device technology, as do AYAs with T1D, even when using the same amount of insulin and/or additional medications. 4 Optimal glycemic control is necessary to prevent long-term complications of diabetes.5-7 Access to innovative and effective tools is needed to improve clinical care and reduce the risk of diabetes complications in AYAs with T2D.
Real-time continuous glucose monitoring (CGM) is rapidly replacing blood glucose (BG) monitoring by a glucose meter in patients with T1D and some adults with T2D. Multicenter randomized controlled trials and meta-analyses show that CGM use in both adults and children with T1D is associated with achievement and maintenance of target hemoglobin A1c (A1c) levels, reduction of severe hypoglycemia, and higher treatment satisfaction.8-13 The use of CGM in adults with T2D is also associated with improved outcomes, including more optimal A1c when used periodically, increase in physical activity, and reduction in caloric intake, weight, body mass index (BMI), and postprandial glucose levels.14-18 Recently a large retrospective cohort study showed that among adults who started real-time CGM, those with T2D on insulin had greater improvements in A1c than did patients with T1D, suggesting that adults with T2D can receive as much or more benefit from this technology. 19 Practice guidelines encourage regular use of CGM in both children and adults with T1D and in adults with T2D who are not achieving glucose targets or who are experiencing hypoglycemia.20-24
Despite evidence suggesting efficacy of CGM for patients with diabetes, to our knowledge, no studies have explored the use of real-time CGM in adolescents with T2D, and CGM is rarely prescribed in this population. 4 Payors frequently do not provide coverage of real-time CGM for adolescents with T2D, creating a barrier to access that contributes to lower technology use in this population. Prior work on CGM in AYAs with T2D has focused only on the clinician’s retrospective review of CGM data to provide medication recommendations, describe glycemic variability, or assist in diagnosis,4,25,26 while self-review by adolescents with T2D or their caregivers has yet to be described. Our objective was to assess the use and acceptability of real-time CGM in AYAs with T2D. Our hypothesis was that the use of real-time CGM in AYAs with T2D would be readily accepted and would provide opportunities to educate patients about glycemic excursions and the effect of their lifestyle choices on glucose levels, which would in turn promote behavior changes and improve clinical outcomes.
Research Design and Methods
Study Design and Participants
Participants were recruited via consecutive sampling of the electronic medical record from an academic pediatric diabetes center, offering patient-centered multidisciplinary care for children with diabetes in the greater San Francisco Bay Area, staffed by faculty who are board-certified in pediatric endocrinology, clinical fellows, nurse practitioners, behavioral health providers, certified diabetes care and education specialists, dieticians, and support staff. This single-arm interventional pilot study included AYAs aged 13 to 21 years with T2D for six months or more on a stable medication regimen, defined as no medication change in the month prior to enrollment and, if they were on exogenous insulin, without an increase in the total daily dose of insulin by more than 20% over the past month. Participants were English and Spanish speakers and had access to a personal electronic smart device compatible with the Dexcom G6 CGM system. Eligible AYAs had suboptimal glycemic control, defined as an A1c greater than 7% per the American Diabetes Association Standards of Medical Care. 27 Medication regimens including metformin, glucagon-like peptide-1 (GLP-1) receptor agonists, and basal and/or bolus insulin were permissible as these were Food and Drug Administration (FDA)-approved and the most commonly prescribed agents at the time the study was conducted. We offered participation to AYAs receiving all treatment regimens to recruit a participant sample representative of our clinical patient population. We excluded patients taking non–FDA-approved medications because being prescribed off-label medications may have indicated atypical T2D disease progression that required nonstandard treatment.
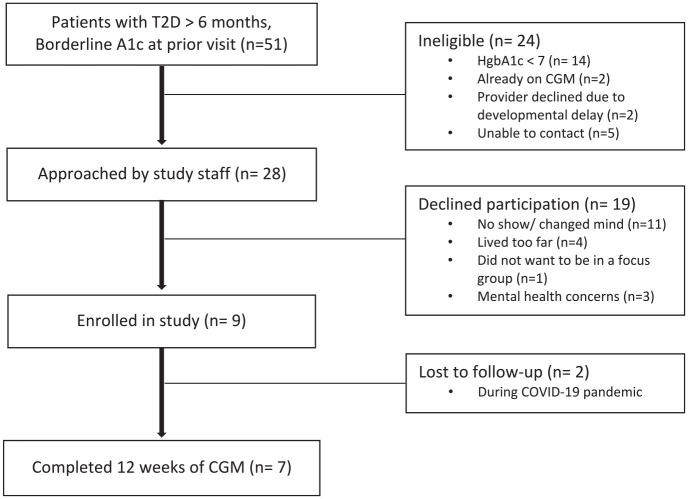
Participants were recruited from August 2019 through October 2020, with a pause in recruitment from March 2020 to July 2020 due to research restrictions related to the coronavirus disease 2019 (COVID-19) pandemic. A total of 51 patients with T2D for more than six months were screened for potential participation. After excluding ineligible patients due to the A1c being less than 7%, the current use of CGM, developmental delay, or inability to contact (n = 24), an additional 19 participants declined participation due to living too far from the clinic, mental health concerns, not wanting to participate in focus groups, or changing their mind (Figure 1). Participants provided written and verbal informed consent prior to study initiation. For those participants who were minors (younger than 18 years) at the time of enrollment, a caregiver (parent or legal guardian) provided informed consent, and the minor assented to participation. The protocol and procedures were approved by the Institutional Review Board at the University of California, San Francisco.
Figure 1.
Consolidating Standards of Reporting Trials (CONSORT) diagram.
CGM Intervention
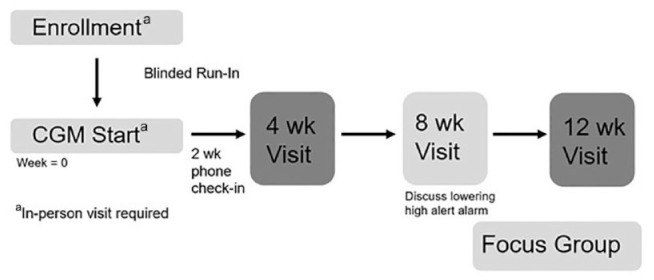
Each participant first completed a run-in period, in which they wore a blinded Dexcom G6 CGM device to ensure that they would tolerate wearing the sensor prior to the start of the study. This run-in period lasted 7 to 10 days, based on the length of wear of the CGM sensor. After the run-in period, participants were given access to a Dexcom G6 CGM system for 12 weeks (Figure 2).
Figure 2.
Study design. Abbreviation: CGM, continuous glucose monitoring.
We chose the Dexcom G6 CGM system because of accuracy and availability of real-time continuous data without prompting. At the CGM start visit, participants received training on how to use the Dexcom G6 CGM system and education on how to respond to various glucose ranges and alerts (see Supplemental Material). The low-alert alarm for the sensor was set at a BG level of 70 mg/dl, and the high-alert alarm was set at a BG level of 400 mg/dl. The high-alert alarm was initially set at this level to prevent alarm fatigue, with the intent that this would increase consistent wearing of the device. Participants had a clinic appointment with their usual diabetes clinician at four weeks and then at 12 weeks after the CGM start. Participants also received a phone call to check-in at two weeks after CGM start and had a telehealth visit with research staff at eight weeks (Figure 2). During the two-week check-in phone call, study staff asked participants if they had any questions about the CGM device and helped with technical problems associated with wearing the CGM device or alerts. At the eight-week telehealth visit, study staff asked participants if they noted patterns of high and low glucoses in relation to diet, exercise, or medications, and CGM data were reviewed. The option to reduce the high-alert alarm to a lower BG level was discussed. Documentation of this eight-week telehealth visit was forwarded to their diabetes clinician for glucose review and possible medication adjustments. Medications could be adjusted during visits with their providers based off CGM data. Participants received up to two, one-way text messages per week to encourage them to correlate their BG trends with their real-time eating and exercise habits.
Measurements
The use and acceptability of the real-time CGM were evaluated by sensor usage, preintervention and postintervention surveys, and focus group qualitative data. Data were extracted from the electronic medical record and Web-based data-visualization systems, Tidepool (Palo Alto, CA, USA), and Clarity (Dexcom, Inc., San Diego, CA, USA). Electronic surveys were completed at study visits on tablet computers or by remote link via REDcap (Vanderbilt, Nashville, TN, USA), 28 an electronic data-capture tool hosted at University of California, San Francisco.
Demographic and clinical data for participants included age, gender, insurance, primary language, weight, BMI, insulin dose, diabetes medication doses, self-reported medication adherence assessed by using participant questionnaires, and duration of diabetes as identified in the electronic medical records and baseline surveys. Continuous glucose monitoring glycemic metrics were obtained from Tidepool or Clarity over a two-week period at each study visit and included the mean glucose level, percent time above range (between 180 and 250 mg/dl and >250 mg/dl), percent time in range (70-180 mg/dl), and percent time below range (between 54 and 70 mg/dl and <54 mg/dl), along with standard deviation and coefficient of variation to account for changes in glucose variability. Glucose management indicator (GMI), an approximation of the A1c level based on the average glucose readings obtained from the CGM device, and A1c were collected if available, as markers of glycemic control.29,30
Validated pre-intervention and post-intervention surveys included physical activity questionnaires, 31 the Dietary Screener Questionnaire, 32 Problem Areas in Diabetes for teenagers (PAID-T) to assess diabetes distress, 33 and the Pediatric Quality of Life inventory (PedsQL) which is validated in T2D. 34 Surveys rating user experience, satisfaction in diabetes management, and the likelihood to continue CGM use or recommend the device to a friend were completed at the end of the study.
Focus Groups
At the end of the study, all participants (AYAs and their parents/caregivers) were invited to join one of two optional online focus groups via secure videoconferencing to discuss their experiences in the study and to add contextual detail and information to supplement survey findings. The purpose of the focus group was to further explore research questions about device acceptance, opportunities to educate patients, and ways to promote behavior change and improvement of clinical outcomes. Each group consisted of two to three participants, as smaller groups are recommended to better facilitate online conversation, and lasted 45 to 60 minutes. 35 Focus groups began with AYAs alone, and caregivers were invited to join for the last 15 minutes of the group. We offered the focus group in both English and Spanish, but only groups conducted in English were needed.
During the focus group, questions posed by two facilitators (H.C. and J.C.W.) addressed the participants’ overall reaction to wearing the device, experiences and challenges in learning how to use the CGM device, and their emotional, physical, and behavioral responses to being able to view their glucose levels and be alerted to high and low glucose levels. All focus group participants were asked if they would like to continue wearing the device, if they requested a prescription for CGM from their providers upon completion of the study, and if they would recommend CGM to other patients with T2D. At the end of the AYA focus groups, the caregivers were given the option to speak with the facilitators and provide their perspectives on using CGM.
Statistical Analysis
Statistical analysis was performed using Stata 16.0 (StataCorp, College Station, TX, USA). Comparisons of glycemic metrics in two-week periods between the blinded run-in period and the end of the study were compared, using two-sided unpaired t tests. Survey scores were also compared using two-sided unpaired t tests. A P value <.05 was considered statistically significant.
Focus group data were analyzed by using a thematic content analysis. 36 Data were reviewed independently by the authors, an interdisciplinary team of medical researchers and a social scientist (C.P.), using a constant comparison analysis to create codes, categories of codes, and broader themes that express findings across focus groups, as well as to compare focus group findings across members of the research team. 37
Results
A total of nine participants with T2D, a median age of 19.1 (IQR 16.8-20.5) years, and a baseline mean A1c of 11.9% (±2.8%) were enrolled in the study. The majority of participants had public insurance, and one third primarily spoke Spanish at home (Table 1). The median duration of diabetes was 2.5 (1.4-6.0) years (Table 1). Forty-four percent of participants were using both basal-bolus insulin regimens given by multiple daily injections at baseline, and 22% used basal insulin only. At baseline, 33% of participants reported not regularly checking their BG at all, 22% reported checking their BG one to two times per week, and 44% reported checking their BG one to three times per day. Of the nine enrolled participants, two were lost to follow-up during the COVID-19 pandemic prior to the completion of any follow-up study visits after enrollment.
Table 1.
Participant Characteristics.
| n = 9 | |
|---|---|
| Median age (years) | 19.1 (16.8-20.5) |
| Female sex | 78% |
| Diabetes characteristics | |
| Median duration of diabetes (years) | 2.5 (1.4-6.0) |
| Mean baseline A1c (%) | 11.9 ± 2.8 |
| Mean baseline time above 180 mg/dL (%) | 84 ± 13 |
| Mean baseline time in range, 70-180 mg/dl (%) | 16 ± 14 |
| Mean baseline time below 70 mg/dL (%) | 0 |
| Using insulin by multiple daily injections (basal-bolus) | 44% |
| Using basal insulin only | 22% |
| Using metformin | 67% |
| Using liraglutide | 11% |
| Public insurance | 67% |
| Race and ethnicity | |
| Hispanic/Latinx | 44% |
| Non-Hispanic Black or African American | 22% |
| Asian | 11% |
| Non-Hispanic White or Caucasian | — |
| More than one race, non-Hispanic a | 22% |
| Spanish as primary language at home | 33% |
| Parent with at least some college education | 33% |
Data are given as median values (interquartile range), mean ± standard deviation values, or frequencies (%). Medication regimens were self-reported in baseline surveys.
Black and Asian, Black and Pacific Islander.
Survey Data
All seven participants reported using the CGM device at the end of the 12-week intervention. Participants demonstrated statistically significant improvement in the PedsQL diabetes score, increasing from 70 to 75 after using the CGM device (P = .026). There were no statistically significant changes in glycemic metrics before and after CGM use (see Supplemental Material), in physical activity scores, dietary surveys, or reported medication adherence. Due to the small sample size, no conclusions could be drawn between the frequency of BG meter use at baseline and changes in outcome measures. The final CGM data were available for five of seven participants; two participants were unable to upload their CGM data remotely, or their transmitters were lost. Of those with CGM data, CGM usage was 80% in a two-week period at study completion.
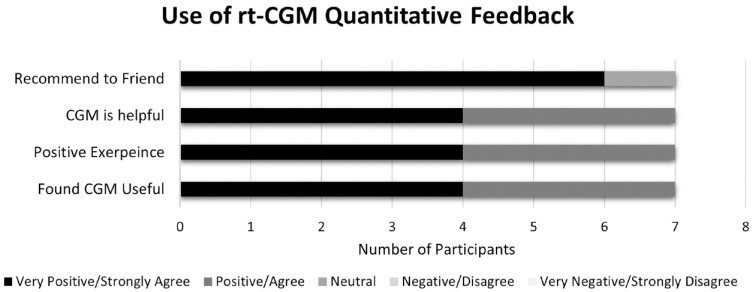
Of those who completed the satisfaction survey (n = 7), 100% had a positive experience with CGM, found it easy to use, found it useful, and desired to continue to use CGM in the future. Eighty-five percent were extremely likely to recommend real-time CGM to friends (Figure 3). Sixty-seven percent of participants self-reported eating fewer meals while using the CGM device.
Figure 3.
Quantitative feedback regarding the use of real-time continuous glucose monitoring (rt-CGM). Abbreviation: rt-CGM, real-time continuous glucose monitoring.
Focus Group Data
Five AYAs participated in the optional focus groups. While some CGM users experienced challenges with initial use, such as difficulty attaching the device and keeping it on while exercising or doing other activities like bathing, four of five focus group participants said they wore the CGM for “the majority of the time,” all wanted to continue using it, and all participants would recommend it to other patients with T2D. Using this device was “easier” than using a glucose meter throughout the day, a word all patients used to describe CGM, indicating improvement in quality of life. For example, one female participant said, “[I]t was relieving not to have to do finger stick[s]. . .I really liked that. That made [me] feel happier, not having to do that every single day, multiple times a day.”
All focus group participants also said CGM use made them more aware of their glucose levels and encouraged them to modify their behaviors, including selecting healthier foods, exercising, and taking medications. One female patient said, “[W]hen I saw my numbers. . .I was like, I need to eat healthier. I want to see my numbers change.” Another male patient said when he connected the CGM to his phone, he noticed his glucose numbers more because he was already “constantly” on his phone and that “everything was [on] a graph” which helped him understand his body’s response to meals and what happened when he took medication. He explained, “[W]ith my metformin and insulin it would show the decrease [on] the graph, visually. I’m a visual learner so it helped me a lot.” Another female participant said that if she received an alert that her glucose was high, she would “go for a walk or something” and “the numbers [would] level out.”
Two participants noted that prior to the study, they had no knowledge of the existence of CGM and were thankful for having access through the study. The legacy of mistrust of research and lack of opportunities for AYAs with T2D to participate in research was also noted. One parent stated, “I’m always skeptical or I have my doubts whenever there’s something that they want to test on my kids [. . .] I don’t want my kids to be guinea pigs [. . .] We are thankful and appreciative we got the opportunity.”
Through this process, we noted the salience of the following themes: feasibility, quality of life, and behavior modifications. For example, when AYAs mentioned the amount of time they wore the CGM device during the study (for most, the majority of the time), we noted this as evidence of the feasibility for AYAs with T2D to consistently use a CGM device, if prescribed. Through this analysis, we found evidence supporting our hypotheses that devices would be readily accepted, devices provide opportunities to educate AYAs with T2D, and CGM use by AYAs with T2D has the potential to support behavior changes and improve clinical outcomes.
Discussion
This pilot feasibility study evaluated three months of real-time CGM use in AYAs with T2D, providing the first analysis of real-time CGM use in this population. Our results suggest that for those AYAs with T2D who are interested, real-time CGM is both feasible and acceptable. Participants who completed the intervention noted a high degree of satisfaction and demonstrated sustained use of the devices. Notably, half of the participants were enrolled during the COVID-19 pandemic, which is known to have negatively impacted mental health and diabetes self-management.38-40 Even with the challenge of the pandemic, participants in our study reported modest improvements in their quality of life during three months of using real-time CGM. This result was supported by both validated measures and focus group data. Our results showing the positive impact of CGM on patient-oriented outcomes parallel findings in other populations, including improved quality of life in children with T1D 41 and higher treatment satisfaction in adults with T2D. 18
Although we did not observe changes in self-reported exercise, eating patterns, or medication adherence, the participants in the focus group interviews did express a desire to change their eating and exercise patterns based on their visualized glucose patterns. Quantitative outcome measures for exercise and eating patterns may have been affected by the COVID-19 pandemic, which generally has led to reduced exercise and healthy eating patterns in AYAs.42-44 Future studies of real-time CGM use in AYAs with T2D in the postpandemic era could be designed to prompt participants to notice BG patterns in response to food and exercise.
The ability to access CGM data remotely has the potential to improve diabetes management for AYAs with T2D. Patients with T2D have high rates of missed clinic appointments. 45 Telehealth interventions have improved glycemic control for patients with T2D 46 and can lead to higher rates of visit attendance.47,48 Adolescents and young adults, including those from lower-income households, generally have access to smartphones compatible with video platforms used for telehealth visits. 49 Conducting telehealth visits with remotely acquired CGM data can increase the interaction with the clinic team and enhance the quality of visits for AYAs with T2D. In our study, some participants had difficulty uploading CGM data remotely. In clinical practice, adequate education and support must be provided to AYAs and families regarding viewing, collecting, and sharing CGM data. With this support, CGM data could enable diabetes teams to provide more meaningful support to AYAs with T2D, even when they are unable to physically come to the clinic. 50
Historically, it has been challenging to recruit and retain AYAs with T2D in clinical studies.51,52 Indeed, in this study, two of the nine enrolled were lost to follow-up. Some of this difficulty was likely due to public health restrictions during the COVID-19 pandemic, but concerns about new technology and uncertainty about research might have also played a role. There is an overrepresentation of adolescents with T2D who come from lower-income households, have public insurance, have parents with lower levels of education, and are from historically marginalized racial and ethnic groups.50-52 Recruitment barriers in this population include a lack of awareness of research and hesitance to participate due to past medical trauma. Systemic racism and implicit bias in clinicians and researchers toward these populations may also play a role. 53 Despite these challenges, we successfully recruited a racially and ethnically diverse cohort of participants who were majority publicly insured, reflective of the population living with T2D. The positive response to the technology and willingness to continue to use it shows that researchers and clinicians need to be aware of bias in designing studies and offering treatment options such that all patients have equitable access and opportunity.
This study has limitations. As a pilot study, the sample size was small, and it was not powered to detect statistically significant changes in glycemic metrics. Because the objective was to assess feasibility of using CGM, the high-glucose alert on the CGM devices was initially set to 400 mg/dl to prevent alarm fatigue. Setting the high-glucose alert to a lower glucose value might have prompted greater change in dietary and exercise behaviors. Participants had contact with the research team at regular intervals, which was needed, given the large volume of CGM data presented to participants. 24 This frequent contact, including text message reminders, could have influenced quality-of-life measures and improved patient satisfaction. Selection bias might have influenced the focus group results and may not represent the opinions of the total cohort. Future studies with comparisons to standard-of-care control groups are needed to address these issues.
CGM use in AYAs with T2D warrants further assessment in larger randomized controlled trials over longer periods of time. These studies could show the potential benefits of CGM in AYAs with T2D, potentially reducing long-term complications and costs to the healthcare system. Current payor restrictions for CGM use in children with T2D may ignore the burden that BG monitoring places on individuals. 54 Finally, our study participants report a desire to see more research involving AYAs with T2D. These future studies of CGM and other diabetes technologies should include evaluation of effectiveness, design, and adaptation to the patient population, and tailored education supporting the continued use of the technology for AYAs living with T2D. 55
Conclusion
Real-time CGM is feasible and acceptable for AYAs with T2D. Modest improvements were seen in quality of life, and focus group participants expressed a desire to change their diet and exercise in response to glucose trends. Larger randomized controlled trials are needed to assess real-time CGM effects on glycemic outcomes and lifestyle modifications.
Supplemental Material
Supplemental material, sj-docx-1-dst-10.1177_19322968221139873 for Real-Time Continuous Glucose Monitoring in Adolescents and Young Adults With Type 2 Diabetes Can Improve Quality of Life by Hannah Chesser, Shylaja Srinivasan, Cassidy Puckett, Stephen E. Gitelman and Jenise C. Wong in Journal of Diabetes Science and Technology
Acknowledgments
The authors would like to thank Laura A. Dapkus, NCPT, for coordinating this research study.
Footnotes
Abbreviations: A1c, hemoglobin A1c; ADA, American Diabetes Association; AYAs, adolescents and young adults; BG, blood glucose; BMI, body mass index; CGM, continuous glucose monitoring; CONSORT, Consolidating Standards of Reporting Trials; COVID-19, coronovirus disease 2019; GLP-1, glucagon like peptide-1, GMI, glucose management indicator; PedsQL, Pediatric Quality of Life Inventory; PAID-T, Problem Areas in Diabetes for teenagers; T1D, type 1 diabetes; T2D, type 2 diabetes.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (award nos: T32DK007161 for H.C. and K23DK120932-02 for S.S.), and a University of California San Francisco Research Evaluation and Allocation Committee grant from the Brooks Fund for J.C.W. Additional funding was from a philanthropic gift, donor anonymous, to the Pediatric Diabetes Program at University of California, San Francisco.
ORCID iDs: Hannah Chesser  https://orcid.org/0000-0002-0724-4310
https://orcid.org/0000-0002-0724-4310
Jenise C. Wong  https://orcid.org/0000-0003-0573-6650
https://orcid.org/0000-0003-0573-6650
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Lawrence JM, Divers J, Isom S, et al. Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001-2017. JAMA. 2021;326(8):717-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Viner R, White B, Christie D. Type 2 diabetes in adolescents: a severe phenotype posing major clinical challenges and public health burden. The Lancet. 2017;389(10085):2252-2260. [DOI] [PubMed] [Google Scholar]
- 3. Nadeau KJ, Anderson BJ, Berg EG, et al. Youth-onset type 2 diabetes consensus report: current status, challenges, and priorities. Diabetes Care. 2016;39(9):1635-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan CL. Use of continuous glucose monitoring in youth-onset type 2 diabetes. Curr Diab Rep. 2017;17(9):66. [DOI] [PubMed] [Google Scholar]
- 5. Nathan DM, Cleary PA, Backlund JYC, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group; Nathan DM, Zinman B, Cleary PA, et al. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983-2005). Arch Intern Med. 2009;169(14):1307-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. TODAY Study Group; Bjornstad P, Drews KL, et al. Long-term complications in youth-onset type 2 diabetes. N Engl J Med. 2021;385(5):416-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group; Beck RW, Hirsch IB, Laffel L, et al. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32(8):1378-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33(1):17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34(4):795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Langendam M, Luijf YM, Hooft L, Devries JH, Mudde AH, Scholten RJPM. Continuous glucose monitoring systems for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2012;1:CD008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012;157(5):336-347. [DOI] [PubMed] [Google Scholar]
- 14. Vigersky R, Shrivastav M. Role of continuous glucose monitoring for type 2 in diabetes management and research. J Diabetes Complications. 2017;31(1):280-287. [DOI] [PubMed] [Google Scholar]
- 15. Park C, Le QA. The effectiveness of continuous glucose monitoring in patients with type 2 diabetes: a systematic review of literature and meta-analysis. Diabetes Technol Ther. 2018;20(9):613-621. [DOI] [PubMed] [Google Scholar]
- 16. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82(1):73-79. [DOI] [PubMed] [Google Scholar]
- 17. Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections. Ann Intern Med. 2017;167(6):365-374. [DOI] [PubMed] [Google Scholar]
- 19. Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA. 2021;325(22):2273-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klonoff DC, Buckingham B, Christiansen JS, et al. Continuous glucose monitoring: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(10):2968-2979. [DOI] [PubMed] [Google Scholar]
- 21. Fonseca VA, Grunberger G, Anhalt H, et al. Continuous glucose monitoring: a consensus conference of the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr Pract. 2016;22(8):1008-1021. [DOI] [PubMed] [Google Scholar]
- 22. Peters AL, Ahmann AJ, Battelino T, et al. Diabetes technology-continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(11):3922-3937. [DOI] [PubMed] [Google Scholar]
- 23. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. American Diabetes Association. 7. Diabetes technology: standards of medical care in diabetes—2021. Diabetes Care. 2020;44(suppl 1):S85-99. [DOI] [PubMed] [Google Scholar]
- 25. Boland EA, Tamborlane WV. Continuous glucose monitoring in youth with type 2 diabetes: overcoming barriers to successful treatment. Diabetes Technol Ther. 2000;2(suppl 1):S53-S59. [DOI] [PubMed] [Google Scholar]
- 26. Chan CL, Pyle L, Newnes L, Nadeau KJ, Zeitler PS, Kelsey MM. Continuous glucose monitoring and its relationship to hemoglobin A1c and oral glucose tolerance testing in obese and prediabetic youth. J Clin Endocrinol Metab. 2015;100(3):902-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Association AD. 13. Children and adolescents: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S180-S199. [DOI] [PubMed] [Google Scholar]
- 28. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bergenstal RM, Beck RW, Close KL, et al. Glucose management indicator (GMI): a new term for estimating A1c from continuous glucose monitoring. Diabetes Care. 2018;41(11):2275-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kowalski KC, Crocker PRE, Kowalski NP. Convergent validity of the physical activity questionnaire for adolescents. Pediatr Exerc Sci. 1997;9(4):342-352. [Google Scholar]
- 32. Thompson FE, Midthune D, Kahle L, Dodd KW. Development and evaluation of the National Cancer Institute’s dietary screener questionnaire scoring algorithms. J Nutr. 2017;147(6):1226-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shapiro JB, Vesco AT, Weil LEG, Evans MA, Hood KK, Weissberg-Benchell J. Psychometric properties of the problem areas in diabetes: teen and parent of teen versions. J Pediatr Psychol. 2018;43(5):561-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Varni JW, Delamater AM, Hood KK, et al. Pediatric Quality of Life Inventory (PedsQL) 3.2 Diabetes Module for youth with Type 2 diabetes: reliability and validity. Diabet Med. 2019;36(4):465-472. [DOI] [PubMed] [Google Scholar]
- 35. Barbour RS, Morgan DL. A New Era in Focus Group Research: Challenges, Innovation and Practice. London, England: Palgrave Macmillan; 2017. http://link.springer.com/10.1057/978-1-137-58614-8. Accessed November 5, 2022. [Google Scholar]
- 36. Heary CM, Hennessy E. The use of focus group interviews in pediatric healthcare research. J Pediatr Psychol. 2002;27(1):47-57. [DOI] [PubMed] [Google Scholar]
- 37. Onwuegbuzie AJ, Dickinson WB, Leech NL, Zoran AG. A qualitative framework for collecting and analyzing data in focus group research. Int J Qual Methods. 2009;8(3):1-21. [Google Scholar]
- 38. Alshareef R, Al Zahrani A, Alzahrani A, Ghandoura L. Impact of the COVID-19 lockdown on diabetes patients in Jeddah, Saudi Arabia. Diabetes Metab Syndr. 2020;14(5):1583-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alessi J, de Oliveira GB, Franco DW, et al. Mental health in the era of COVID-19: prevalence of psychiatric disorders in a cohort of patients with type 1 and type 2 diabetes during the social distancing. Diabetol Metab Syndr. 2020;12:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nearchou F, Flinn C, Niland R, Subramaniam SS, Hennessy E. Exploring the impact of COVID-19 on mental health outcomes in children and adolescents: a systematic review. Int J Environ Res Public Health. 2020;17(22):8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pintus D, Ng SM. Freestyle libre flash glucose monitoring improves patient quality of life measures in children with Type 1 diabetes mellitus (T1DM) with appropriate provision of education and support by healthcare professionals. Diabetes Metab Syndr. 2019;13(5):2923-2926. [DOI] [PubMed] [Google Scholar]
- 42. Burkart S, Parker H, Weaver RG, et al. Impact of the COVID-19 pandemic on elementary schoolers’ physical activity, sleep, screen time and diet: a quasi-experimental interrupted time series study. Pediatr Obes. 2022;17(1):e12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Flaudias V, Iceta S, Zerhouni O, et al. COVID-19 pandemic lockdown and problematic eating behaviors in a student population. J Behav Addict. 2020;9(3):826-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ammar A, Brach M, Trabelsi K, et al. Effects of COVID-19 home confinement on eating behaviour and physical activity: results of the ECLB-COVID19 international online survey. Nutrients. 2020;12(6):E1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alvarado MM, Kum HC, Gonzalez Coronado K, Foster MJ, Ortega P, Lawley MA. Barriers to remote health interventions for type 2 diabetes: a systematic review and proposed classification scheme. J Med Internet Res. 2017;19(2):e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Heitkemper EM, Mamykina L, Travers J, Smaldone A. Do health information technology self-management interventions improve glycemic control in medically underserved adults with diabetes? a systematic review and meta-analysis. J Am Med Inform Assoc. 2017;24(5):1024-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Haynes SC, Marcin JP, Dayal P, Tancredi DJ, Crossen S. Impact of telemedicine on visit attendance for paediatric patients receiving endocrinology specialty care. J Telemed Telecare. 2020;0(0). doi: 10.1177/1357633X20972911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haynes SC, Kompala T, Neinstein A, Rosenthal J, Crossen S. Disparities in telemedicine use for subspecialty diabetes care during COVID-19 shelter-in-place orders. J Diabetes Sci Technol. 2021;15(5):986-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Common Sense Media. 2019. The Common Sense census: media use by tweens and teens. https://www.commonsensemedia.org/sites/default/files/research/report/2019-census-8-to-18-key-findings-updated.pdf. Published 2019. Accessed November 5, 2022.
- 50. Thornton PL, Kumanyika SK, Gregg EW, et al. New research directions on disparities in obesity and type 2 diabetes. Ann N Y Acad Sci. 2020;1461(1):5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. American Diabetes Association. Health Equity Now [Internet]. Date unknown. https://www.diabetes.org/healthequitynow. Accessed November 5, 2022.
- 52. McGavock J, Wicklow B, Dart AB. Type 2 diabetes in youth is a disease of poverty. The Lancet. 2017;390(10105):1829. [DOI] [PubMed] [Google Scholar]
- 53. Dhaliwal R, Pereira RI, Diaz-Thomas AM, Powe CE, Yanes Cardozo LL, Joseph JJ. Eradicating racism: an endocrine society policy perspective. J Clin Endocrinol Metab. 2022;107(5):1205-1215. [DOI] [PubMed] [Google Scholar]
- 54. Anderson JE, Gavin JR, Kruger DF. Current eligibility requirements for CGM coverage are harmful, costly, and unjustified. Diabetes Technol Ther. 2020;22(3):169-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Azhar A, Gillani SW, Mohiuddin G, Majeed RA. A systematic review on clinical implication of continuous glucose monitoring in diabetes management. J Pharm Bioallied Sci. 2020;12(2):102-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dst-10.1177_19322968221139873 for Real-Time Continuous Glucose Monitoring in Adolescents and Young Adults With Type 2 Diabetes Can Improve Quality of Life by Hannah Chesser, Shylaja Srinivasan, Cassidy Puckett, Stephen E. Gitelman and Jenise C. Wong in Journal of Diabetes Science and Technology