Abstract
Background
Bacterial vaginosis is an imbalance of the normal vaginal flora with an overgrowth of anaerobic bacteria and a lack of the normal lactobacillary flora. Women may have symptoms of a characteristic vaginal discharge but are often asymptomatic. Bacterial vaginosis during pregnancy has been associated with poor perinatal outcomes and, in particular, preterm birth (PTB). Identification and treatment may reduce the risk of PTB and its consequences.
Objectives
To assess the effects of antibiotic treatment of bacterial vaginosis in pregnancy.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 May 2012), searched cited references from retrieved articles and reviewed abstracts, letters to the editor and editorials.
Selection criteria
Randomised trials comparing antibiotic treatment with placebo or no treatment, or comparing two or more antibiotic regimens in pregnant women with bacterial vaginosis or intermediate vaginal flora whether symptomatic or asymptomatic and detected through screening.
Data collection and analysis
Two review authors independently assessed trials for inclusion, trial quality and extracted data. We contacted study authors for additional information.
Main results
We included 21 trials of good quality, involving 7847 women diagnosed with bacterial vaginosis or intermediate vaginal flora.
Antibiotic therapy was shown to be effective at eradicating bacterial vaginosis during pregnancy (average risk ratio (RR) 0.42; 95% confidence interval (CI) 0.31 to 0.56; 10 trials, 4403 women; random‐effects, T² = 0.19, I² = 91%). Antibiotic treatment also reduced the risk of late miscarriage (RR 0.20; 95% CI 0.05 to 0.76; two trials, 1270 women, fixed‐effect, I² = 0%).
Treatment did not reduce the risk of PTB before 37 weeks (average RR 0.88; 95% CI 0.71 to 1.09; 13 trials, 6491 women; random‐effects, T² = 0.06, I² = 48%), or the risk of preterm prelabour rupture of membranes (RR 0.74; 95% CI 0.30 to 1.84; two trials, 493 women). It did increase the risk of side‐effects sufficient to stop or change treatment (RR 1.66; 95% CI 1.02 to 2.68; four trials, 2323 women, fixed‐effect, I² = 0%).
In this updated review, treatment before 20 weeks' gestation did not reduce the risk of PTB less than 37 weeks (average RR 0.85; 95% CI 0.62 to 1.17; five trials, 4088 women; random‐effects, T² = 0.06, I² = 49%).
In women with a previous PTB, treatment did not affect the risk of subsequent PTB (average RR 0.78; 95% CI 0.42 to 1.48; three trials, 421 women; random‐effects, T² = 0.19, I² = 72%).
In women with abnormal vaginal flora (intermediate flora or bacterial vaginosis), treatment may reduce the risk of PTB before 37 weeks (RR 0.53; 95% CI 0.34 to 0.84; two trials, 894 women).
One small trial of 156 women compared metronidazole and clindamycin, both oral and vaginal, with no significant differences seen for any of the pre‐specified primary outcomes. Statistically significant differences were seen for the outcomes of prolongation of gestational age (days) (mean difference (MD) 1.00; 95% CI 0.26 to 1.74) and birthweight (grams) (MD 75.18; 95% CI 25.37 to 124.99) however these represent relatively small differences in the clinical setting.
Oral antibiotics versus vaginal antibiotics did not reduce the risk of PTB (RR 1.09; 95% CI 0.78 to 1.52; two trials, 264 women). Oral antibiotics had some advantage over vaginal antibiotics (whether metronidazole or clindamycin) with respect to admission to neonatal unit (RR 0.63; 95% CI 0.42 to 0.92, one trial, 156 women), prolongation of gestational age (days) (MD 9.00; 95% CI 8.20 to 9.80; one trial, 156 women) and birthweight (grams) (MD 342.13; 95% CI 293.04 to 391.22; one trial, 156 women).
Different frequency of dosing of antibiotics was assessed in one small trial and showed no significant difference for any outcome assessed.
Authors' conclusions
Antibiotic treatment can eradicate bacterial vaginosis in pregnancy. The overall risk of PTB was not significantly reduced. This review provides little evidence that screening and treating all pregnant women with bacterial vaginosis will prevent PTB and its consequences. When screening criteria were broadened to include women with abnormal flora there was a 47% reduction in preterm birth, however this is limited to two included studies.
Keywords: Female; Humans; Pregnancy; Anti‐Bacterial Agents; Anti‐Bacterial Agents/therapeutic use; Fetal Membranes, Premature Rupture; Fetal Membranes, Premature Rupture/prevention & control; Pregnancy Complications, Infectious; Pregnancy Complications, Infectious/drug therapy; Premature Birth; Premature Birth/prevention & control; Randomized Controlled Trials as Topic; Vaginosis, Bacterial; Vaginosis, Bacterial/drug therapy
Plain language summary
Antibiotics for treating bacterial vaginosis in pregnancy
Bacteria are normally present in the birth canal and are useful in maintaining the health of the vagina. However, if the numbers of some of the bacteria increase, this is called bacterial vaginosis. For some women, there are no symptoms but for others it may cause an unpleasant discharge and may cause some babies to be born too early. These babies can suffer from problems related to their immaturity both in the weeks following birth such as breathing difficulty, infection and bleeding within the brain as well as problems when growing up such as poor growth, chronic lung disease and delayed development.
The review looked to see whether the use of antibiotics in women with bacterial vaginosis reduced the symptoms for women and reduced the incidence of babies being born too early. We identified 21 trials, involving 7847 women. We found that antibiotics given to pregnant women reduced this overgrowth of bacteria, but did not reduce the numbers of babies who were born too early. There were adverse effects sufficient to stop treatment or have the treatment changed when antibiotics were used and this needs further investigation. The effect of screening and treating women with abnormal flora needs to be studied in further trials and the effects of screening and treating proven vaginal infections is the subject of another Cochrane review.
Background
Description of the condition
Bacterial vaginosis is an imbalance of vaginal flora caused by a reduction of the normal lactobacillary bacteria, and a heavy overgrowth of mixed anaerobic flora including Gardnerella vaginalis, Mycoplasma hominis and Mobiluncus species. Why these organisms multiply, many of which are normally present in small numbers in the vagina, while the usually prevalent lactobacilli decrease, is not clear. The role of hydrogen peroxide‐producing lactobacilli appears to be important in preventing overgrowth of anaerobes in normal vaginal flora (Hillier 1993). Bacterial vaginosis does not appear to be sexually transmitted but may be associated with sexual activity.
Bacterial vaginosis is often asymptomatic but may result in a vaginal discharge which can be grey in colour with a characteristic 'fishy' odour. It is not associated with vaginal mucosal inflammation and rarely causes vulval itch.
The classical diagnosis of bacterial vaginosis is confirmed by fulfilling three out of four criteria (Amsel 1983). These are (i) a vaginal pH greater than 4.7, (ii) the presence of 'clue cells' on a Gram stain or wet mount of the vaginal discharge, (iii) the presence of a thin homogenous discharge and (iv) the release of a fishy odour when potassium hydroxide is added to a sample of the discharge. The use of these criteria for diagnosis, however, is complex and time consuming in some settings. Use of a Gram stain of a vaginal swab with semi‐quantification of the microbial flora has high sensitivity and specificity and is an accepted alternative method which has been used in many studies due to ease of standardisation, ability to be read at a later date and potential to be blinded (Nugent 1991). In the Nugent system, the numbers of different bacterial morphotypes in Gram stained smears are counted in high power fields using microscopy with a 1000x magnification. The points achieved from the number of different bacterial morphotypes are added together, with a total score of zero to three considered normal and a score of seven to 10 consistent with bacterial vaginosis. A score of four to six is classified as intermediate.
Natural history in pregnancy
Bacterial vaginosis is present in up to 20% of women during pregnancy (Lamont 1993). The majority of these cases will be asymptomatic. The natural history of bacterial vaginosis is such that it may spontaneously resolve without treatment although most women identified as having bacterial vaginosis in early pregnancy are likely to have persistent infection later in pregnancy (Hay 1994).
Association with adverse outcomes
There is now a substantial body of evidence associating bacterial vaginosis in pregnancy with poor perinatal outcome, in particular an increased risk of preterm birth (Hay 1994a; Hillier 1995; Kurki 1992; McGregor 1990), with potential neonatal sequelae due to prematurity. A recent meta‐analysis of adverse outcomes associated with bacterial vaginosis and including over 30,000 women from 32 studies (Leitich 2007) showed that bacterial vaginosis approximately doubled the risk of preterm delivery in asymptomatic patients (odds ratio (OR) 2.16, 95% confidence interval (CI) 1.56 to 3.00) as well as significantly increased the risks of late miscarriage (OR 6.32, 95% CI 3.65 to 10.94) and maternal infection (OR 2.53, 95% CI 1.26 to 5.08). There is also evidence associating intermediate flora with adverse pregnancy outcome (Donders 2009; Hay 1994a; Leitich 2007). Although intra‐amniotic infection has a clear causal relationship with preterm delivery mediated via pattern recognition receptors, chemokines or inflammatory cytokines (Lamont 2011b), the exact mechanism by which by which the organisms associated with bacterial vaginosis may effect the initiation of preterm labour remains unclear. It is also unclear why bacterial vaginosis is associated with preterm birth in some women but not in others with recent studies suggesting individual immune responses (Jones 2010; Lamont 2011a) or specific gene polymorphisms (Annells 2004; Annells 2005; Simhan 2003; Witkin 2003) are responsible for individual susceptibility. Whilst a number of other genital micro‐organisms such as Escherichia coli, Listeria monocytogenes and Viridans streptococci may be involved in chorioamnionitis, carriage of these organisms during early to mid‐pregnancy has not been associated with an increased risk of preterm labour. Although maternal carriage of group B Streptococcus increases the risk of neonatal sepsis due to this organism, there is conflicting evidence about whether carriage during pregnancy increases the risk of preterm birth. Infections during pregnancy for which there is good evidence of an increased risk of preterm birth and preterm prelabour rupture of the membranes, include asymptomatic bacteriuria, Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis and bacterial vaginosis. The opportunity therefore exists to reduce the preterm birth rate by treatment of these infections during pregnancy.
Description of the intervention
Antibiotics used to treat bacterial vaginosis cover anaerobic organisms. Different antibiotics have differing specificity for the organisms present. Commonly used antibiotics for bacterial vaginosis are metronidazole and clindamycin.
How the intervention might work
Antibiotic treatment aims to reduce the overgrowth of the abnormal anaerobic bacteria, restore the balance of the protective lactobacillary bacteria and prevent the development of an inflammatory response and the initiation of preterm labour.
Why it is important to do this review
Bacterial vaginosis is relatively common, even in populations of women at low risk of adverse events and it is amenable to treatment (Burtin 1995; Fischbach 1993; McDonald 1994). Identification during pregnancy and treatment may present a rare opportunity to reduce the preterm birth rate, and resulting risk of prematurity to the newborn. Such treatment may also reduce other adverse perinatal outcomes such as postpartum infection. Much evidence exists to support the association of bacterial vaginosis with preterm birth, however, the exact underlying mechanisms remain unclear. The results of randomised controlled trials of treatment are needed to provide more direct evidence of the role of bacterial vaginosis in preterm birth.
Objectives
To determine whether the use of antibiotics for bacterial vaginosis in pregnancy can: (a) improve maternal symptoms; (b) decrease incidence of adverse perinatal outcomes.
To determine if antibiotics are helpful and which antibiotic regimens are the most effective.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials that compare (i) one antibiotic regimen with placebo or no treatment or (ii) two or more alternative antibiotic regimens in pregnant women with bacterial vaginosis (however defined).
Types of participants
Women of any age, at any stage of pregnancy with a diagnosis of bacterial vaginosis regardless of method of diagnosis (detected because of symptoms or asymptomatic as part of a screening programme). Co‐infection with other sexually transmitted infections is not a reason to exclude a study from the review. Women classified as Nugent score four to six (intermediate flora) are included.
Types of interventions
Any antibiotic (any dosage regimen, any route of administration) compared with either placebo or no treatment. Any two antibiotic regimens compared.
Types of outcome measures
The outcome measures in this review are as follows.
Primary outcomes
Maternal symptoms
Failure to eradicate bacterial vaginosis on examination (failure to achieve 'microbiological cure').
Incidence of pregnancy loss up to 24 weeks' gestation (late miscarriage).
Neonatal outcomes
Clinical
Perinatal death including stillbirth after 24 weeks' gestation and neonatal death, up to 28 days after birth.
Neonatal sepsis (defined as definite symptoms or positive cultures from a sterile site ‐ positive culture of gastric aspirates alone will not be sufficient).
Birth less than 37 weeks' gestation.
Birth less than 34 weeks' gestation.
Birth less than 32 weeks' gestation.
Incidence of low birthweight (however defined).
Birthweight (not a prespecified outcome).
Prolongation of gestation age (not a prespecified outcome).
Maternal side‐effects
Side‐effects sufficient to stop or change treatment.
Other side‐effects not sufficient to stop or change treatment.
Secondary outcomes
Maternal symptoms
Clinical report by women of failure of symptoms to improve.
Incidence of preterm prelabour rupture of membranes.
Incidence of fever during labour or delivery.
Incidence of chorioamnionitis treated with antibiotics.
Incidence of postpartum fever.
Incidence of postpartum uterine infection.
Neonatal outcomes
Clinical
Severe neonatal morbidity (moderate to severe respiratory distress syndrome ‐ defined as any ventilatory support, intraventricular haemorrhage, necrotising enterocolitis, chronic lung disease).
Cerebral palsy at childhood follow‐up.
Moderate/severe visual impairment at childhood follow‐up.
Moderate/severe hearing impairment at childhood follow‐up.
Economic
Total duration of ventilatory support.
Admission to neonatal unit.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 May 2012).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We applied no language restrictions. We searched cited references from retrieved articles for additional studies and reviewed abstracts and letters to the editor to identify randomised controlled trials that have not been published. We also reviewed editorials, indicating expert opinion, to identify and ensure that no key studies were missed for inclusion in this review.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeAppendix 1.
For this update, we used the following methods when assessing the reports identified by the updated search.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies that were identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
A data extraction form was designed. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion. We entered the data into Review Manager software (RevMan 2011) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies are at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook. We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated” analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
Where applicable, we made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we have presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measure the same outcome, but used different methods.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if the T² was greater than zero and either the I² was greater than 30% or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Where 10 or more studies reported a particular outcome in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful we did not combine trials.
Where we used random‐effects analyses, the results were presented as the average treatment effect with its 95% confidence interval, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses.
We performed the following subgroup analyses.
Women with previous preterm birth.
Women with intermediate flora or bacterial vaginosis.
Treatment prior to 20 weeks' gestation.
In addition to the subgroups above, we also investigated the effect of different types of antibiotics, although this was not a subgroup pre‐specified in the protocol. We did not restrict subgroups of antibiotics to particular outcomes.
Subgroup analyses were restricted to the following outcomes for prespecified subgroups.
Failure of test of cure.
Perinatal death.
Preterm birth before 37, before 34 and before 32 weeks' gestation.
Incidence of low birthweight.
Neonatal sepsis.
Side‐effects.
Late miscarriage.
Admission to neonatal unit.
We assessed differences between subgroups by interaction tests available in RevMan 2011.
Sensitivity analysis
We undertook sensitivity analyses as part of investigation of heterogeneity in various analyses.
Results
Description of studies
Results of the search
This review is now comprised of 21 included studies, involving 13,209 women, of which 7847 were bacterial vaginosis or intermediate flora positive. Twenty studies are excluded and one study is ongoing (Subtil 2008), seeCharacteristics of ongoing studies.
Included studies
See table of Characteristics of included studies for further details of the 21 included studies.
Nine trials used oral metronidazole alone (Darwish 2007; McDonald 1997; Mitchell 2009; Moniri 2009; Morales 1994; NICHD MFMU 2000; NICHD MFMU 2001; Odendaal 2002; Shennan 2006), one used oral metronidazole plus erythromycin (Hauth 1995), one used oral clindamycin (Darwish 2007), one amoxicillin (Duff 1991), and one used vaginal metronidazole gel (Darwish 2007), whilst nine used intravaginal clindamycin (Vermeulen 1999; Darwish 2007; Giuffrida 2006; Guaschino 2003; Joesoef 1995; Kekki 2001; Kiss 2004; Lamont 2003; Larsson 2006). Sixteen trials performed microbiological follow‐up and 11 trials gave a second course of treatment (seven only if bacterial vaginosis was not eradicated). One trial (Porter 2001) compared different antibiotic regimens (once daily versus twice daily vaginal metronidazole).
Two trials (Lamont 2003; Ugwumadu 2003), which used intermediate vaginal flora (Nugent score four to six) as well as bacterial vaginosis as the basis for recruitment, have been included as a separate comparison (Analysis 7).
Excluded studies
For details of the 20 excluded studies, seeCharacteristics of excluded studies.
Risk of bias in included studies
Overall, the majority of included studies performed well in a 'Risk of bias' analysis, as detailed below. See Figure 1; Figure 2 for summaries of risk of bias in included studies.
1.
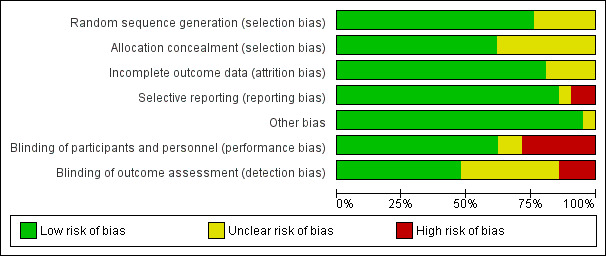
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
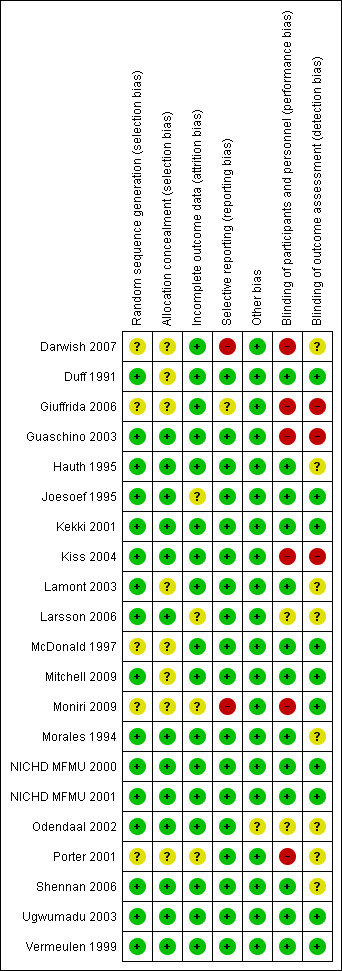
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Overall, the reporting of the method of random sequence generation was good, with all but five studies (Darwish 2007; Giuffrida 2006; McDonald 1997; Moniri 2009 ; Porter 2001) reporting adequate methods of random sequence generation. These studies did not report their method of random sequence generation, and therefore, the risk of selection bias was unclear.
Reporting of the method of allocation concealment was much less consistent. Thirteen studies reported an adequate method (Guaschino 2003; Hauth 1995; Joesoef 1995; Kekki 2001; Kiss 2004; Larsson 2006; Morales 1994; NICHD MFMU 2000; NICHD MFMU 2001; Odendaal 2002; Shennan 2006; Ugwumadu 2003; Vermeulen 1999). The remaining seven studies did not report their method of allocation concealment: (Darwish 2007; Duff 1991; Giuffrida 2006; Lamont 2003; McDonald 1997; Mitchell 2009; Porter 2001).
Blinding
When the intervention is a medication, as in the trials in this review, it is reasonable to expect trials to use a placebo control, which is identical in appearance to the active intervention, and for participants, clinicians and outcome assessors to be blinded to which treatment was given.
It should also be noted that although blinding was considered adequate for the majority of the included studies (for both participants and personnel and outcome assessment), for those studies that rescreened and therefore retreated some women there may be a potential risk of unblinding.
Blinding of participants and personnel
The majority of studies were adequately blinded (13/21).
Blinding methods were assessed as low risk in 13 studies: Duff 1991 (placebo group, triple‐blind); Hauth 1995 (placebo group, double‐blind); Joesoef 1995 (placebo group, double‐blind); Kekki 2001 (placebo group, triple‐blind); Lamont 2003 (placebo group, double‐blind); McDonald 1997 (placebo group, triple‐blind); Mitchell 2009 (placebo group, double‐blind); Morales 1994 (placebo group, double‐blind); NICHD MFMU 2000 (placebo group, double‐blind); NICHD MFMU 2001 (placebo group, double‐blind); Ugwumadu 2003 (placebo group, double‐blind); Vermeulen 1999 (placebo group, double‐blind);Shennan 2006 (placebo group, double blind).
Blinding methods were unclear (e.g. due to failure to report method) in the following studies: Larsson 2006 (complicated method of blinding; see 'Risk of bias' table for this trial); Odendaal 2002 (blinding not reported, placebo not identical).
Blinding methods were high risk in the following studies: Darwish 2007 (no placebo, different modes of administration so unable to blind participants/clinicians); Giuffrida 2006 (no placebo group, no reporting of blinding); Guaschino 2003 (no placebo group; no reporting of blinding); Kiss 2004 (no placebo group, no blinding); Porter 2001 (blinding not done, since comparison between two frequencies of administration and no placebo used) and Moniri 2009 (no placebo, no blinding).
Blinding of outcome assessment
Blinding of outcome assessment was reported and assessed as low risk in 10 studies (Duff 1991; Joesoef 1995; Kekki 2001; McDonald 1997; Mitchell 2009; NICHD MFMU 2000; NICHD MFMU 2001; Ugwumadu 2003; Vermeulen 1999;Moniri 2009), not reported and assessed as unclear in eight studies (Darwish 2007; Hauth 1995; Lamont 2003; Larsson 2006; Morales 1994; Odendaal 2002; Porter 2001; Shennan 2006) and high risk in three studies, where it was clear that blinding was not undertaken (Giuffrida 2006; Guaschino 2003; Kiss 2004).
Incomplete outcome data
Overall reporting of outcome data for all participants was done well, with 17/21 trials assessed as low risk: (Darwish 2007; Duff 1991; Giuffrida 2006; Guaschino 2003; Hauth 1995; Kekki 2001; Kiss 2004; Lamont 2003; McDonald 1997; Mitchell 2009; Morales 1994; NICHD MFMU 2000; NICHD MFMU 2001; Odendaal 2002; Shennan 2006; Ugwumadu 2003; Vermeulen 1999).
Three trials were assessed as being at unclear risk of attrition bias: Joesoef 1995 (please refer to 'Risk of bias' table for explanation); Larsson 2006 (please refer to 'Risk of bias' table for explanation); Porter 2001 (abstract only) and Moniri 2009 (please refer to 'Risk of bias' table for explanation) .
Selective reporting
Eighteen of the 21 trials were assessed as being at low risk of reporting bias: (Duff 1991; Guaschino 2003; Hauth 1995; Joesoef 1995; Kiss 2004; Kekki 2001; Lamont 2003; Larsson 2006; McDonald 1997; Mitchell 2009; Morales 1994; NICHD MFMU 2000; NICHD MFMU 2001; Odendaal 2002; Porter 2001; Shennan 2006; Ugwumadu 2003; Vermeulen 1999). The Giuffrida 2006 trial was judged to be at unclear risk due to not adequately labelling results tables (unclear as to whether mean values were being reported) and a small number of outcomes being reported. Darwish 2007 was judged to be at high risk of selective reporting bias due to inadequate statistical analysis and inadequate reporting of outcomes. Moniri 2009 was also assessed as high risk only reporting one of the three prespecified outcomes.
Other potential sources of bias
All studies apart from Odendaal 2002 were assessed as being at low risk of other sources of bias. In the case of Odendaal 2002, in which primigravidae and multigravidae groups were analysed separately, the baseline characteristics were significantly different between groups in the multigravidae women, with significant differences present in age, percentage antenatal antibiotic use and percentage asymptomatic bacteriuria. It is possible but not necessary that these differences could significantly influence the outcomes of the study.
Effects of interventions
Twenty‐one trials involving 7847 women with bacterial vaginosis or intermediate flora were included.
This updated review has restructured the comparisons analysed to assess outcomes for the following groups: 1) antibiotic versus placebo/no treatment; 2) antibiotic versus another antibiotic; 3) antibiotics: different routes of administration; 4) antibiotic versus another treatment; 5) antibiotics: different frequency/dose of administration.
The following subgroup analyses were also undertaken: 1) whether the women had experienced a previous preterm birth; 2) whether the women had intermediate vaginal flora (including bacterial vaginosis); and 3) treatment before 20 weeks' gestation.
Separate comparisons
1. Any antibiotic versus placebo/no treatment
Primary outcomes
In terms of preterm births before 37 weeks, there is overall no significant advantage for antibiotics when compared to placebo/no treatment, from 13 trials with 6491 participants (average risk ratio (RR) 0.88; 95% confidence interval (CI) 0.71 to 1.09, T² = 0.06, I² = 48% (Analysis 1.5)). However, there is substantial heterogeneity in this result (I² of 48%). The one trial comparing two oral antibiotics (metronidazole + erythromycin) against placebo (Hauth 1995) shows a significant benefit (RR 0.64; 95% CI 0.47 to 0.88; one trial 258 women). The test for subgroup differences to investigate heterogeneity present for preterm births before 37 weeks was not significant (P = 0.39, I² = 5.1%, (Analysis 1.5).
1.5. Analysis.
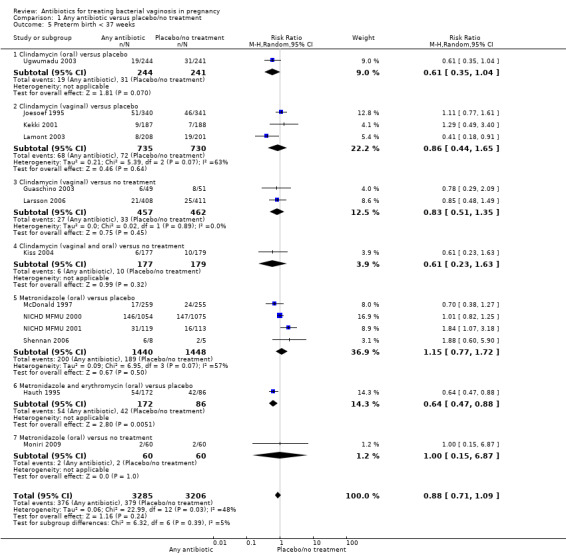
Comparison 1 Any antibiotic versus placebo/no treatment, Outcome 5 Preterm birth < 37 weeks.
Similarly, there is no overall significant advantage for antibiotics when compared to placebo/no treatment for preterm births before 34 weeks (RR 1.16; 95% CI 0.52 to 2.59; three trials, 515 women (Analysis 1.6). There is also no indication of benefit from antibiotics when compared to placebo/no treatment for preterm births before 32 weeks (Analysis 1.7) or for low birthweight (RR 0.99; 95% CI 0.82 to 1.20; seven trials, 4040 women (Analysis 1.8)).
1.6. Analysis.
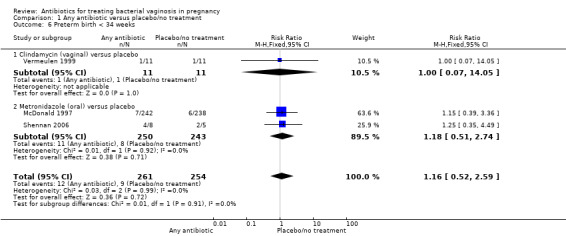
Comparison 1 Any antibiotic versus placebo/no treatment, Outcome 6 Preterm birth < 34 weeks.
1.7. Analysis.
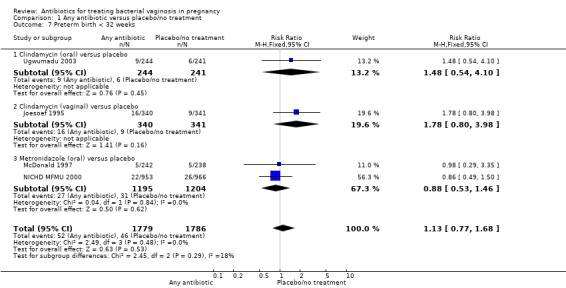
Comparison 1 Any antibiotic versus placebo/no treatment, Outcome 7 Preterm birth < 32 weeks.
1.8. Analysis.
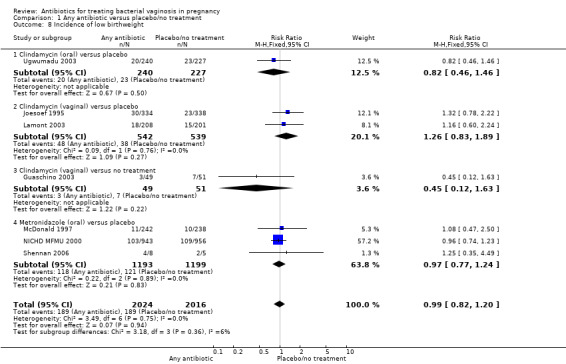
Comparison 1 Any antibiotic versus placebo/no treatment, Outcome 8 Incidence of low birthweight.
There was a significant benefit in reduction of late miscarriage for antibiotics compared with placebo/no treatment with very low heterogeneity for this result (RR 0.20; 95% CI 0.05 to 0.76; two trials,1270 women (Analysis 1.14)).
1.14. Analysis.
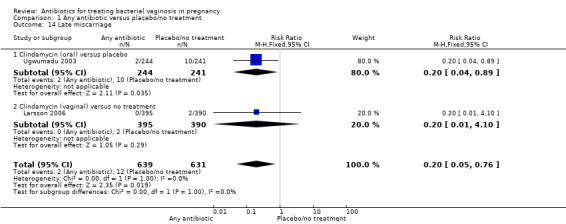
Comparison 1 Any antibiotic versus placebo/no treatment, Outcome 14 Late miscarriage.
Overall, 10 trials with 4403 women indicated that antibiotics are generally beneficial, when compared to placebo/no treatment, with respect to failure of test of cure (average RR 0.42; 95% CI 0.31 to 0.56; T² = 0.19, I² = 91% (Analysis 1.1)). The very high level of heterogeneity in this analysis (I² = 91%) could be due to the differences in treatment effects between different antibiotics. The test for subgroup differences is significant (P < 0.00001, I² = 92.2%, (Analysis 1.1), and does appear to indicate this.
1.1. Analysis.
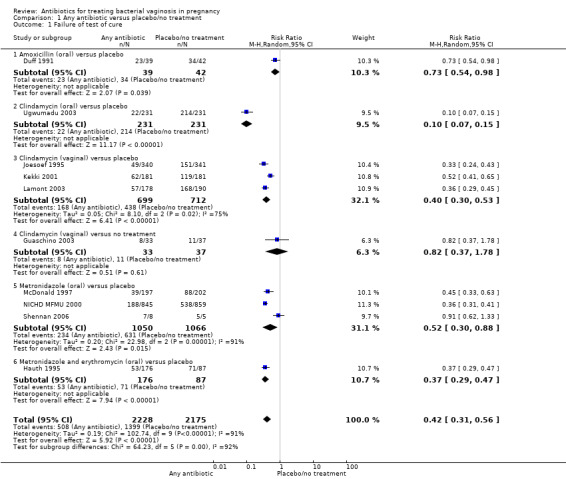
Comparison 1 Any antibiotic versus placebo/no treatment, Outcome 1 Failure of test of cure.
There were significantly higher rates of side‐effects sufficient to stop or change treatment in the antibiotics groups overall (RR 1.66; 95% CI 1.02 to 2.68; four trials, 2235 women (Analysis 1.10)).
1.10. Analysis.
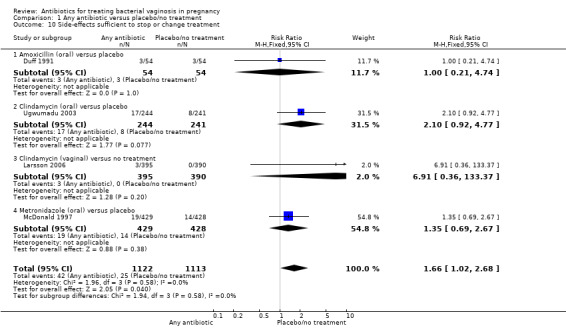
Comparison 1 Any antibiotic versus placebo/no treatment, Outcome 10 Side‐effects sufficient to stop or change treatment.
Secondary outcomes
There was no statistically significant advantage for antibiotics when compared to placebo/no treatment, with respect to postpartum infection (average RR 0.91; 95% CI 0.26 to 3.21; two trials, 618 participants; T² = 0.47, I² = 41% (Analysis 1.2)) or in terms of perinatal death from four trials with 3195 participants (RR 0.71; 95%; CI 0.36 to 1.39 (Analysis 1.3)).
1.2. Analysis.
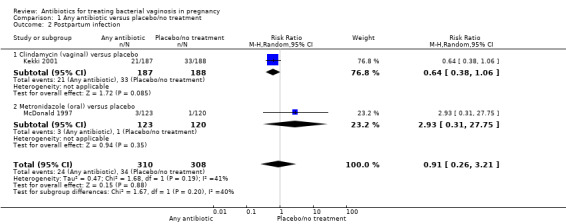
Comparison 1 Any antibiotic versus placebo/no treatment, Outcome 2 Postpartum infection.
1.3. Analysis.
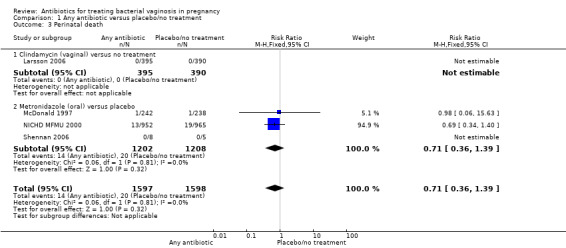
Comparison 1 Any antibiotic versus placebo/no treatment, Outcome 3 Perinatal death.
Similarly, there was no statistically significant advantage for antibiotics when compared to placebo/no treatment, with respect to the incidence of preterm prelabour rupture of membranes from two trials (McDonald 1997; Shennan 2006) with 493 participants (RR 0.74; 95% CI 0.30 to 1.84 (Analysis 1.4)). Please see Table 1 for information regarding reporting of preterm prelabour rupture of membranes in the included studies.
1.4. Analysis.
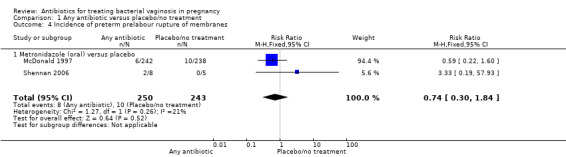
Comparison 1 Any antibiotic versus placebo/no treatment, Outcome 4 Incidence of preterm prelabour rupture of membranes.
1. Reporting of rupture of membranes in included trials.
| Trial | Was PPROM reported? | What outcome was reported regarding ROM? |
| Darwish 2007 | no | PROM |
| Duff 1991 | no | PROM |
| Giuffrida 2006 | no | PROM |
| Guaschino 2003 | no | PROM |
| Hauth 1995 | no | DNR |
| Joesoef 1995 | no | DNR |
| Kekki 2001 | no | DNR |
| Kiss 2004 | no | DNR |
| Lamont 2003 | no | DNR |
| Larsson 2006 | no | DNR |
| McDonald 1997 | yes | PPROM |
| Mitchell 2009 | no | DNR |
| Morales 1994 | no | PROM |
| NICHD MFMU 2000 | no | preterm SROM |
| NICHD MFMU 2001 | no | preterm membrane rupture |
| Odendaal 2002 | no | DNR |
| Porter 2001 | no | SROM |
| Shennan 2006 | yes | PPROM |
| Ugwumadu 2003 | no | DNR |
| Vermeulen 1999 | no | DNR |
DNR = did not report PROM = prelabour rupture of membranes PPROM = preterm prelabour rupture of membranes SROM = spontaneous rupture of membranes
There was no significant difference between antibiotics and placebo/no treatment in terms of: neonatal sepsis (RR 1.40; 95% CI 0.45 to 4.41; three trials, 2345 women (Analysis 1.9)); side‐effects not sufficient to stop or change treatment (RR 1.27; 95% CI 0.76 to 2.13; three trials,1340 women (Analysis 1.11)); severe neonatal morbidity (RR 0.97; 95% CI 0.54 to 1.75; three trials, 2715 women (Analysis 1.12)); admission to neonatal unit (average RR 1.02; 95% CI 0.69 to 1.50; two trials, 2383 women; T² = 0.04, I² = 45% (Analysis 1.13)); and moderate/severe visual impairment at childhood follow‐up (RR 0.33; 95% CI 0.01 to 8.05; one trial, 785 women (Analysis 1.15)).
1.9. Analysis.
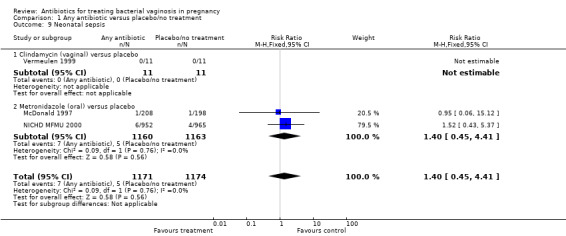
Comparison 1 Any antibiotic versus placebo/no treatment, Outcome 9 Neonatal sepsis.
1.11. Analysis.
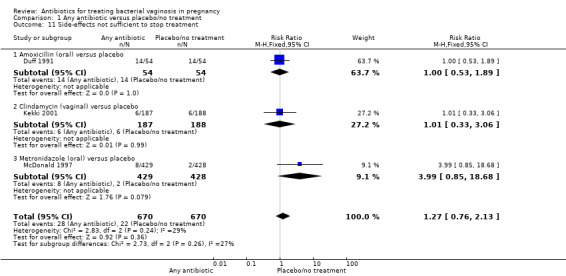
Comparison 1 Any antibiotic versus placebo/no treatment, Outcome 11 Side‐effects not sufficient to stop treatment.
1.12. Analysis.
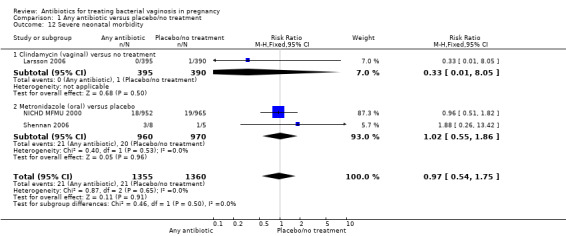
Comparison 1 Any antibiotic versus placebo/no treatment, Outcome 12 Severe neonatal morbidity.
1.13. Analysis.
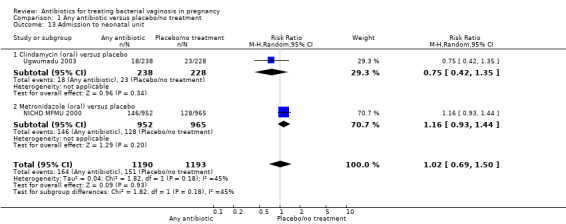
Comparison 1 Any antibiotic versus placebo/no treatment, Outcome 13 Admission to neonatal unit.
1.15. Analysis.
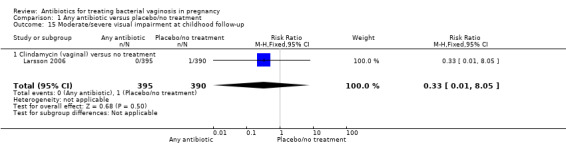
Comparison 1 Any antibiotic versus placebo/no treatment, Outcome 15 Moderate/severe visual impairment at childhood follow‐up.
2. Antibiotic versus another antibiotic
There was no significant difference between metronidazole and clindamycin in relation to the incidence of premature rupture of membranes (RR 1.10; 95% CI 0.83 to 1.46 (Analysis 2.1)), preterm birth before 37 weeks (RR 0.89; 95% CI 0.63 to 1.26 (Analysis 2.2)), or admission to neonatal unit (RR 0.97; 95% CI 0.67 to 1.40 (Analysis 2.3)). Prolongation of gestational age (days) (mean difference (MD) 1.00; 95% CI 0.26 to 1.74 (Analysis 2.4)) and birthweight (grams) (MD 75.18; 95% CI 25.37 to 124.99 (Analysis 2.5)) were significantly higher with metronidazole when compared with clindamycin. However, these data are from a single trial (Darwish 2007) with 156 participants and although statistically significant represent less significance clinically.
2.1. Analysis.
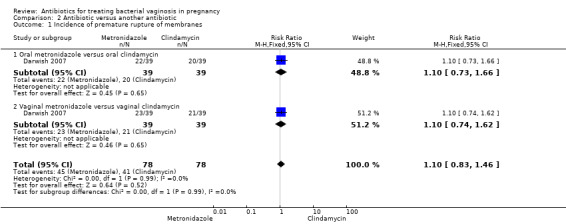
Comparison 2 Antibiotic versus another antibiotic, Outcome 1 Incidence of premature rupture of membranes.
2.2. Analysis.
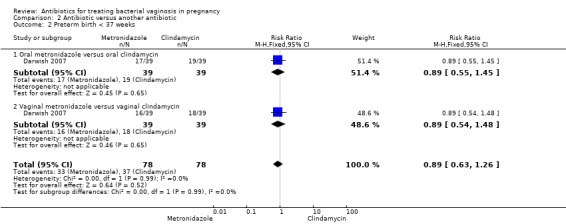
Comparison 2 Antibiotic versus another antibiotic, Outcome 2 Preterm birth < 37 weeks.
2.3. Analysis.

Comparison 2 Antibiotic versus another antibiotic, Outcome 3 Admission to neonatal unit.
2.4. Analysis.
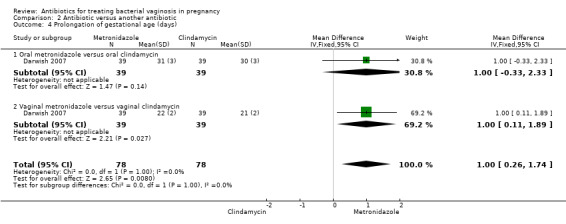
Comparison 2 Antibiotic versus another antibiotic, Outcome 4 Prolongation of gestational age (days).
2.5. Analysis.
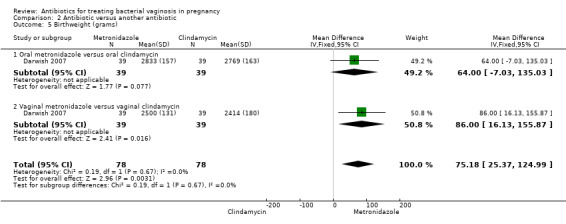
Comparison 2 Antibiotic versus another antibiotic, Outcome 5 Birthweight (grams).
3. Antibiotics: different routes of administration
There was no significant difference between oral and vaginal antibiotics in relation to the eradication of bacterial vaginosis on examination (RR 0.96; 95% CI 0.67 to 1.39; one trial, 108 women (Analysis 3.1)), preterm birth before 37 weeks (RR 1.09; 95% CI 0.78 to 1.52; two trials, 264 women (Analysis 3.2)), incidence of low birthweight (RR 1.93; 95% CI 0.51 to 7.31; one trial, 108 women (Analysis 3.3), absence of abnormal clinical signs (RR 1.09; 95% CI 0.78 to 1.53; one trial, 99 women (Analysis 3.4)), or incidence of premature rupture of membranes (RR 0.95; 95% CI 0.72 to 1.27; one trial, 156 women (Analysis 3.5)).
3.1. Analysis.
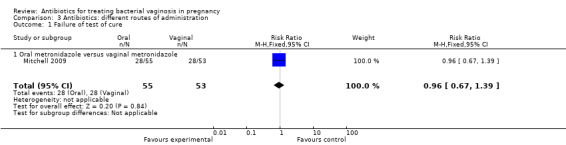
Comparison 3 Antibiotics: different routes of administration, Outcome 1 Failure of test of cure.
3.2. Analysis.
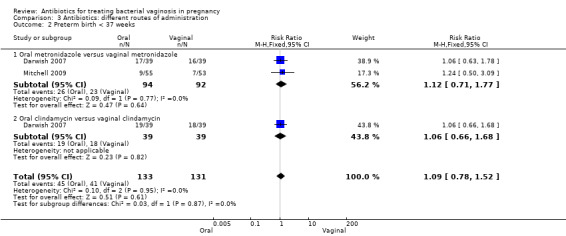
Comparison 3 Antibiotics: different routes of administration, Outcome 2 Preterm birth < 37 weeks.
3.3. Analysis.
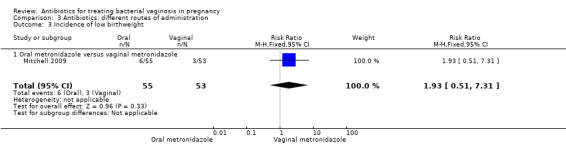
Comparison 3 Antibiotics: different routes of administration, Outcome 3 Incidence of low birthweight.
3.4. Analysis.
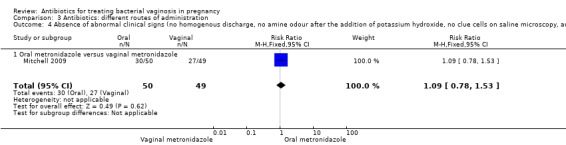
Comparison 3 Antibiotics: different routes of administration, Outcome 4 Absence of abnormal clinical signs (no homogenous discharge, no amine odour after the addition of potassium hydroxide, no clue cells on saline microscopy, and PH<4.5).
3.5. Analysis.
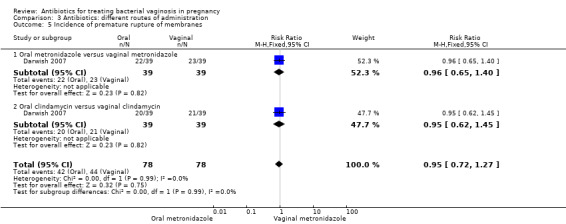
Comparison 3 Antibiotics: different routes of administration, Outcome 5 Incidence of premature rupture of membranes.
However, there was an advantage for oral antibiotics over vaginal antibiotics (whether metronidazole or clindamycin) with respect to admission to neonatal unit (RR 0.63; 95% CI 0.42 to 0.92; two trials with 156 women (Analysis 3.6), prolongation of gestational age (MD 9.00; 95% CI 8.20 to 9.80; one trial, 156 women (Analysis 3.7)) and birthweight (grams) (MD 342.13; 95% CI 293.04 to 391.22; one trial, 156 women (Analysis 3.8)).
3.6. Analysis.
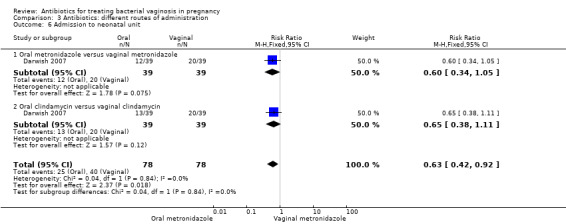
Comparison 3 Antibiotics: different routes of administration, Outcome 6 Admission to neonatal unit.
3.7. Analysis.
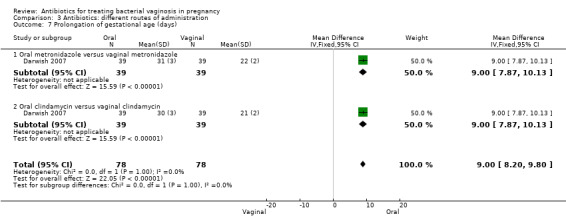
Comparison 3 Antibiotics: different routes of administration, Outcome 7 Prolongation of gestational age (days).
3.8. Analysis.
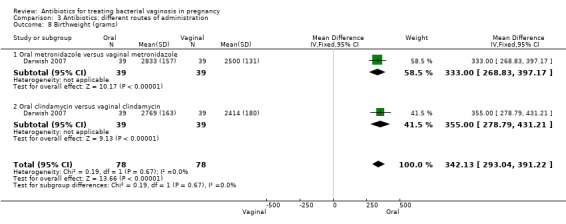
Comparison 3 Antibiotics: different routes of administration, Outcome 8 Birthweight (grams).
4. Antibiotic versus another treatment
Only three trials compared antibiotic treatments with non‐antibiotic treatments; one of these trials (Giuffrida 2006) compared vaginal clindamycin with vaginal peroxen (hydrogen peroxide 0.5%), and the other two trials (Morales 1994; Odendaal 2002) compared oral metronidazole with oral vitamin C. Vitamin C was classified as a placebo by both of these trials, although vitamin C has potential anti‐infective properties (Hemilä 2007) so this group was separately analysed in this review.
Treatment with oral metronidazole was significantly more likely to result in eradication of bacterial vaginosis on microbiological examination (decreased failure of test of cure) compared with oral vitamin C (average RR 0.25; 95% CI 0.08 to 0.83; two trials, 335 women; T² = 0.64, I² = 86%). The Giuffrida 2006 trial also reported failure of test of cure, but no events of failure of test of cure were reported for this study, so no RR was calculable (Analysis 4.1). Giuffrida 2006 reported no significant difference in side‐effects (not sufficient to stop or change treatment), RR 1.75; 95% CI 0.57 to 5.36, 60 women, (Analysis 4.2).
4.1. Analysis.
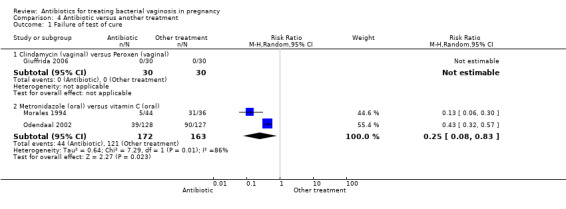
Comparison 4 Antibiotic versus another treatment, Outcome 1 Failure of test of cure.
4.2. Analysis.
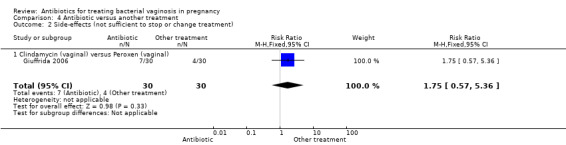
Comparison 4 Antibiotic versus another treatment, Outcome 2 Side‐effects (not sufficient to stop or change treatment).
Morales 1994 only reported the incidence of low birthweight, with lower numbers of low birthweight infants in the group treated with oral metronidazole versus the group treated with vitamin C, although this was only just significant (RR 0.41; 95% CI 0.17 to 0.98, one trial, 80 women (Analysis 4.6)).
4.6. Analysis.
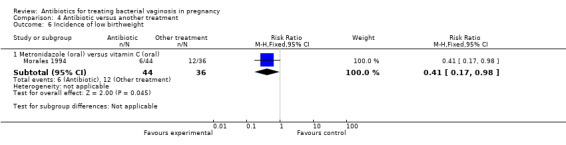
Comparison 4 Antibiotic versus another treatment, Outcome 6 Incidence of low birthweight.
Odendaal 2002 only reported rates of perinatal death, although the result did not show any significant difference (RR 2.61; 95% CI 0.71 to 9.62; one trial, 269 women (Analysis 4.3)). Similarly, the results for preterm birth before 37 weeks or 34 weeks overall were not significantly different (Analysis 4.4; Analysis 4.5).
4.3. Analysis.
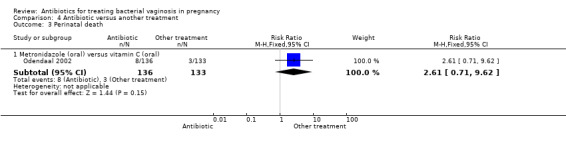
Comparison 4 Antibiotic versus another treatment, Outcome 3 Perinatal death.
4.4. Analysis.

Comparison 4 Antibiotic versus another treatment, Outcome 4 Preterm birth < 37 weeks.
4.5. Analysis.
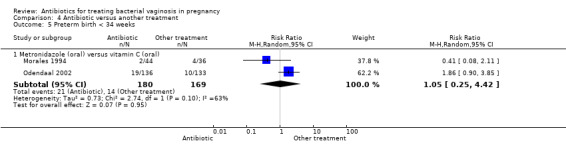
Comparison 4 Antibiotic versus another treatment, Outcome 5 Preterm birth < 34 weeks.
Significant heterogeneity was seen for the analyses that included the Morales 1994 and Odendaal 2002 data. This likely relates to the differing study settings, timing of treatment and exclusion criteria for the trials.
5. Antibiotics: different frequency/dose of administration
Results from a single trial (Porter 2001, n = 94) comparing outcomes of use of vaginal metronidazole gel in once daily versus twice daily dosing regimens, indicated that there was no significant benefit for a double dose over a single dose with respect to postpartum uterine infection (RR 3.27; 95% CI 0.35 to 30.28; one trial, 94 women (Analysis 5.1)), preterm delivery before 37 weeks (RR 0.41; 95% CI 0.12 to 1.44; one trial, 94 women (Analysis 5.2)) or incidence of low birthweight (RR 1.19; 95% CI 0.58 to 2.42; one trial, 94 women (Analysis 5.3)).
5.1. Analysis.
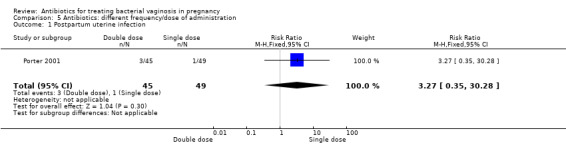
Comparison 5 Antibiotics: different frequency/dose of administration, Outcome 1 Postpartum uterine infection.
5.2. Analysis.
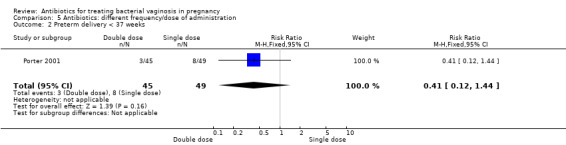
Comparison 5 Antibiotics: different frequency/dose of administration, Outcome 2 Preterm delivery < 37 weeks.
5.3. Analysis.
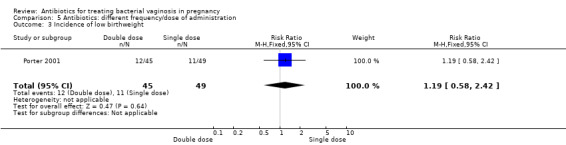
Comparison 5 Antibiotics: different frequency/dose of administration, Outcome 3 Incidence of low birthweight.
Subgroup analyses
Sugroup analyses have been performed only on studies from the main comparison of any antibiotic versus placebo/no treatment. There were insufficient studies included in the remaining comparisons (Analysis 2; Analysis 3; Analysis 4; Analysis 5) to shed any light on differences between subgroups.
6. Previous preterm birth versus no previous preterm birth
The use of antibiotics was beneficial in terms of failure of test of cure in comparison with placebo/no treatment for the subgroup of women with no previous preterm birth (no previous preterm birth: average RR 0.39; 95% CI 0.28 to 0.53; eight trials, 4127 women; random‐effects, T² = 0.18, I² = 91%), however no difference was observed for women with a history of previous preterm birth: average RR 0.57; 95% CI 0.22 to 1.50; two trials; 276 women; random‐effects, T² = 0.45, I² = 94% (Analysis 6.1)). Substantial heterogeneity was apparent in both subgroups (I² = 94%; I² = 91%) and in the overall analysis (I² = 91%). There was no evidence of any subgroup differences (P = 0.45; I² = 0%, Analysis 6.1).
6.1. Analysis.
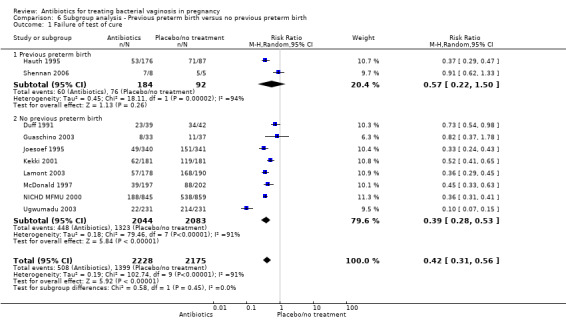
Comparison 6 Subgroup analysis ‐ Previous preterm birth versus no previous preterm birth, Outcome 1 Failure of test of cure.
For all other subgroup analyses, there were no evidence of an effect for any of the outcomes examined (perinatal death; preterm delivery less than 37 weeks; preterm delivery less than 34 weeks; low birthweight; neonatal sepsis) and no evidence of a difference between subgroups (Analysis 6.2; Analysis 6.3; Analysis 6.4; Analysis 6.5; Analysis 6.6).
6.2. Analysis.
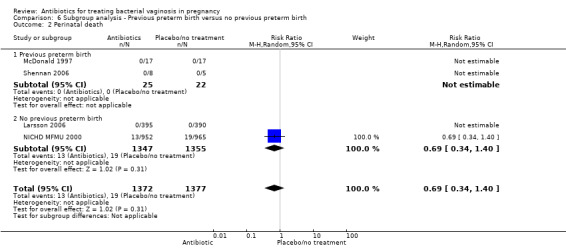
Comparison 6 Subgroup analysis ‐ Previous preterm birth versus no previous preterm birth, Outcome 2 Perinatal death.
6.3. Analysis.
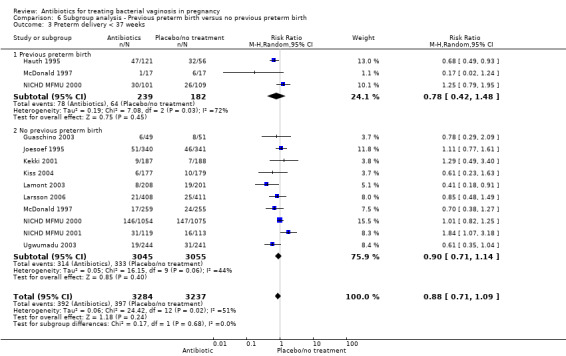
Comparison 6 Subgroup analysis ‐ Previous preterm birth versus no previous preterm birth, Outcome 3 Preterm delivery < 37 weeks.
6.4. Analysis.
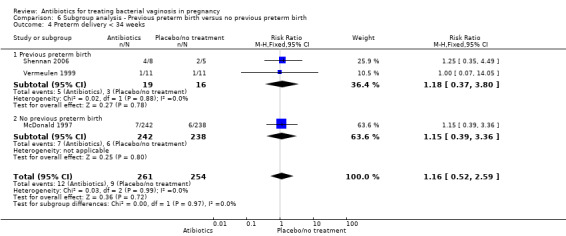
Comparison 6 Subgroup analysis ‐ Previous preterm birth versus no previous preterm birth, Outcome 4 Preterm delivery < 34 weeks.
6.5. Analysis.
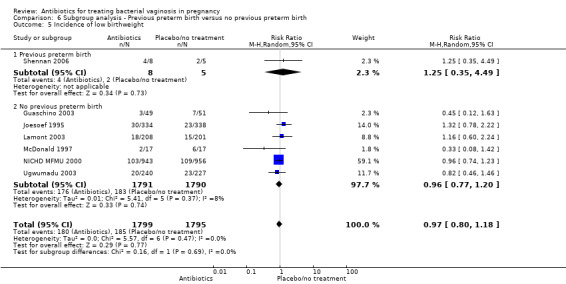
Comparison 6 Subgroup analysis ‐ Previous preterm birth versus no previous preterm birth, Outcome 5 Incidence of low birthweight.
6.6. Analysis.
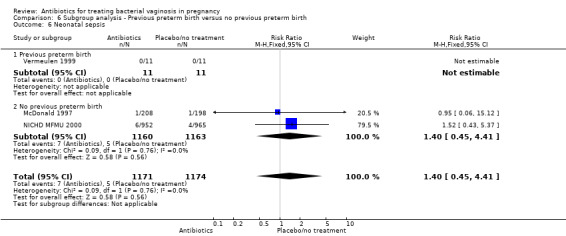
Comparison 6 Subgroup analysis ‐ Previous preterm birth versus no previous preterm birth, Outcome 6 Neonatal sepsis.
7. Women with intermediate flora or bacterial vaginosis (Nugent score four to 10) versus women with no intermediate flora or bacterial vaginosis
Only two trials reported outcomes for the subgroup of patients diagnosed with either intermediate flora or bacterial vaginosis (Nugent score four to 10).These trials were Ugwumadu 2003, with 485 participants, and Lamont 2003, with 409 participants.
In the subgroup of women with intermediate flora or bacterial vaginosis, the use of antibiotics was associated with a significant reduction in preterm birth less than 37 weeks (average RR 0.53; 95% CI 0.34 to 0.84; two trials 894 women; random‐effects T² = 0.00, I² = 0% (Analysis 7.3). There was no significant difference in preterm birth less than 37 weeks in the subgroup of studies with no intermediate flora or bacterial vaginosis (average RR 0.93; 95% CI 0.75 to 1.16; 10 trials 5584 women; random‐effects T² = 0.04, I² = 42% (Analysis 7.3). There was evidence for a difference in subgroups (P = 0.03; I² = 79.2%, Analysis 7.3); however, this should be interpreted with caution because there were only two studies in the intermediate flora or bacterial vaginosis subgroup.
7.3. Analysis.
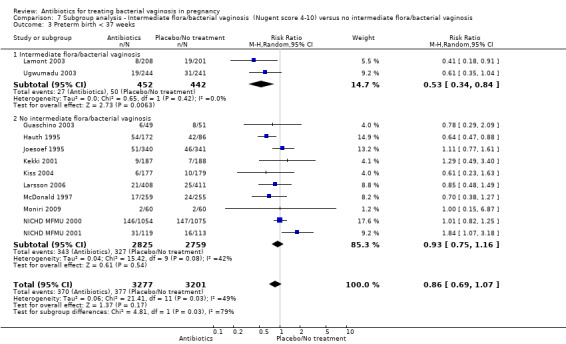
Comparison 7 Subgroup analysis ‐ Intermediate flora/bacterial vaginosis (Nugent score 4‐10) versus no intermediate flora/bacterial vaginosis, Outcome 3 Preterm birth < 37 weeks.
There were no significant differences between antibiotic and placebo/no treatment groups for any of the outcomes examined for any of the subgroups (failure of test of cure; perinatal death; preterm birth less than 32 weeks; incidence of low birthweight; side‐effects not sufficient to stop treatment; late miscarriage; admission to neonatal unit) and no evidence of a difference between subgroups for any of these outcomes (Analysis 7.1; Analysis 7.2; Analysis 7.4; Analysis 7.5; Analysis 7.6; Analysis 7.7; Analysis 7.8; Analysis 7.9).
7.1. Analysis.
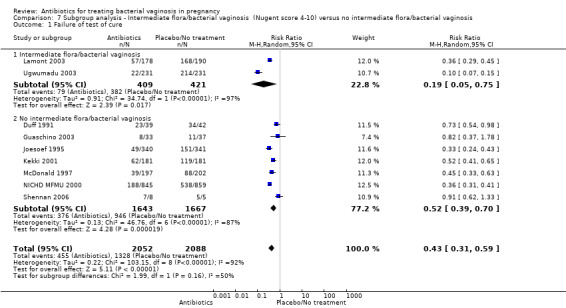
Comparison 7 Subgroup analysis ‐ Intermediate flora/bacterial vaginosis (Nugent score 4‐10) versus no intermediate flora/bacterial vaginosis, Outcome 1 Failure of test of cure.
7.2. Analysis.
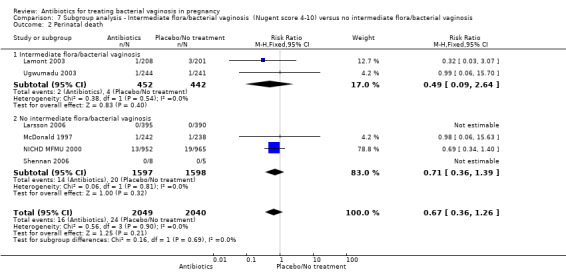
Comparison 7 Subgroup analysis ‐ Intermediate flora/bacterial vaginosis (Nugent score 4‐10) versus no intermediate flora/bacterial vaginosis, Outcome 2 Perinatal death.
7.4. Analysis.
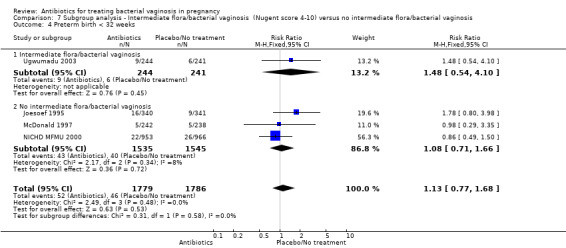
Comparison 7 Subgroup analysis ‐ Intermediate flora/bacterial vaginosis (Nugent score 4‐10) versus no intermediate flora/bacterial vaginosis, Outcome 4 Preterm birth < 32 weeks.
7.5. Analysis.
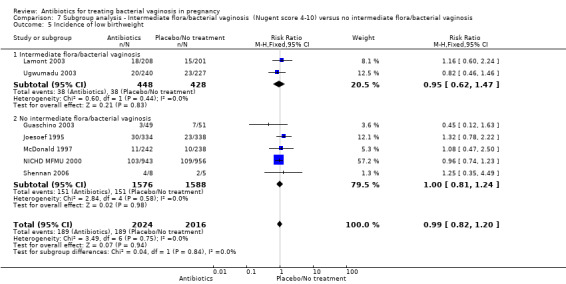
Comparison 7 Subgroup analysis ‐ Intermediate flora/bacterial vaginosis (Nugent score 4‐10) versus no intermediate flora/bacterial vaginosis, Outcome 5 Incidence of low birthweight.
7.6. Analysis.
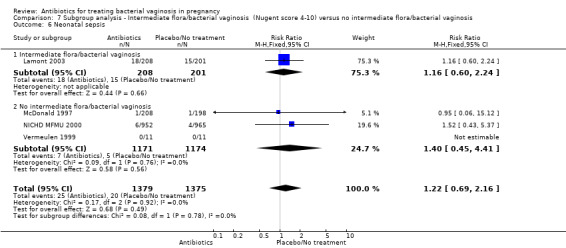
Comparison 7 Subgroup analysis ‐ Intermediate flora/bacterial vaginosis (Nugent score 4‐10) versus no intermediate flora/bacterial vaginosis, Outcome 6 Neonatal sepsis.
7.7. Analysis.
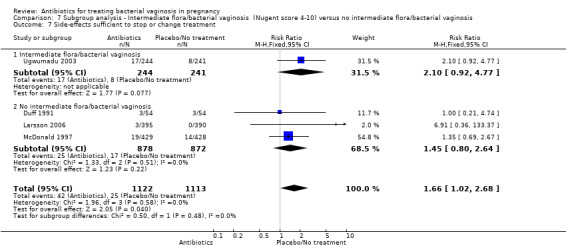
Comparison 7 Subgroup analysis ‐ Intermediate flora/bacterial vaginosis (Nugent score 4‐10) versus no intermediate flora/bacterial vaginosis, Outcome 7 Side‐effects sufficient to stop or change treatment.
7.8. Analysis.
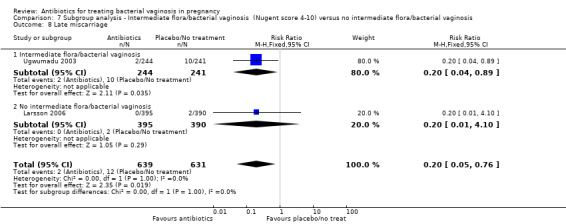
Comparison 7 Subgroup analysis ‐ Intermediate flora/bacterial vaginosis (Nugent score 4‐10) versus no intermediate flora/bacterial vaginosis, Outcome 8 Late miscarriage.
7.9. Analysis.
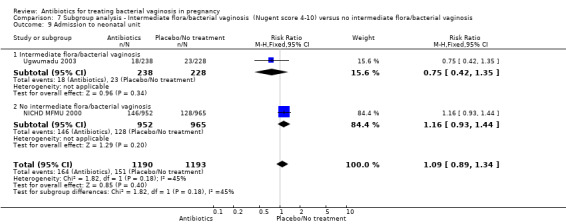
Comparison 7 Subgroup analysis ‐ Intermediate flora/bacterial vaginosis (Nugent score 4‐10) versus no intermediate flora/bacterial vaginosis, Outcome 9 Admission to neonatal unit.
8. Treatment before 20 weeks' gestation versus treatment after 20 weeks' gestation
In both subgroups of women, the use of antibiotics were beneficial in terms of failure of test of cure in comparison with placebo/no treatment (treatment before 20 weeks': average RR 0.40; 95% CI 0.32 to 0.51; three trials, 2434 women; random‐effects, T² = 0.03, I² = 76%; and treatment after 20 weeks': average RR 0.38; 95% CI 0.19 to 0.78; five trials; 1693 women; random‐effects, T² = 0.62, I² = 95% (Analysis 8.1). However, substantial heterogeneity was apparent in both subgroups (I² = 76%; I² = 95%) and in the overall analysis (I² = 91%). There was no evidence of any subgroup differences (P = 0.90; I² = 0%, Analysis 8.1).
8.1. Analysis.
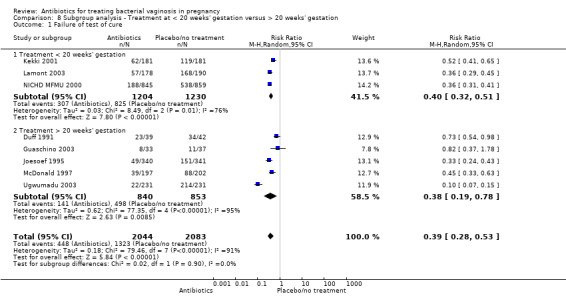
Comparison 8 Subgroup analysis ‐ Treatment at < 20 weeks' gestation versus > 20 weeks' gestation, Outcome 1 Failure of test of cure.
For all other subgroup analyses, there were no evidence of an effect for any of the outcomes examined (preterm delivery less than 37 weeks; low birthweight; side‐effects not sufficient to stop treatment; late miscarriage) and no evidence of a difference between subgroups (Analysis 8.2; Analysis 8.3; Analysis 8.4; Analysis 8.5).
8.2. Analysis.
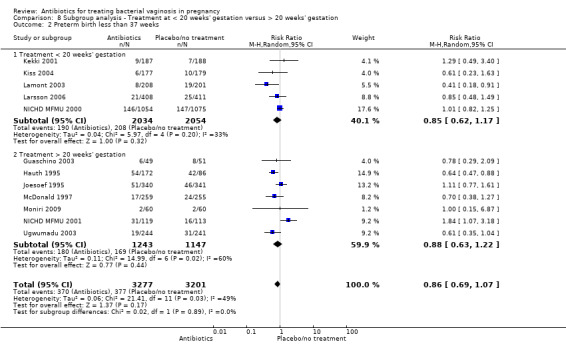
Comparison 8 Subgroup analysis ‐ Treatment at < 20 weeks' gestation versus > 20 weeks' gestation, Outcome 2 Preterm birth less than 37 weeks.
8.3. Analysis.
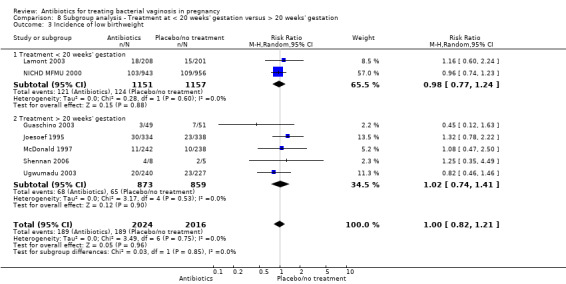
Comparison 8 Subgroup analysis ‐ Treatment at < 20 weeks' gestation versus > 20 weeks' gestation, Outcome 3 Incidence of low birthweight.
8.4. Analysis.

Comparison 8 Subgroup analysis ‐ Treatment at < 20 weeks' gestation versus > 20 weeks' gestation, Outcome 4 Side‐effects not sufficient to stop treatment.
8.5. Analysis.
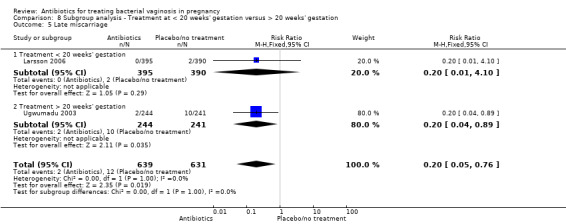
Comparison 8 Subgroup analysis ‐ Treatment at < 20 weeks' gestation versus > 20 weeks' gestation, Outcome 5 Late miscarriage.
Assessment of reporting biases
Only two outcomes were reported in 10 or more studies, so publication bias was assessed for these outcomes using funnel plots. Funnel plots were constructed for Analysis 1.1, (failure of test of cure; antibiotic versus placebo/no treatment) Figure 3 and for Analysis 1.5 (preterm birth before 37 weeks; antibiotic versus placebo/no treatment) Figure 4.
3.
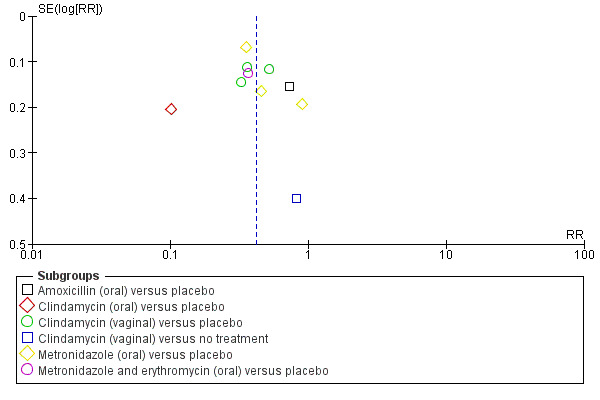
Funnel plot of comparison: 1 Any antibiotic versus placebo/no treatment, outcome: 1.1 Failure of test of cure.
4.
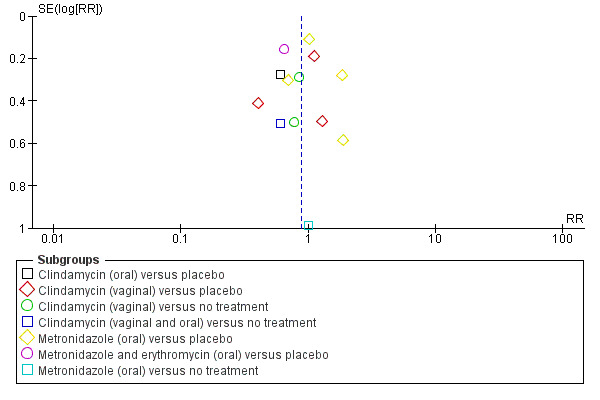
Funnel plot of comparison: 1 Any antibiotic versus placebo/no treatment, outcome: 1.5 Preterm birth < 37 weeks.
On visual inspection of the funnel plot for failure of test of cure, (seeFigure 3) there appears to be a risk of reporting bias for this outcome, as indicated by an asymmetrical plot.
The funnel plot for preterm birth before 37 weeks, (seeFigure 4) should be interpreted with caution, given that the studies that reported the outcome of preterm birth were of similar sample size (similar standard errors of intervention effect estimates) (see Cochrane Handbook for Systematic Reviews of Interventions section 10.4.3.1). On visual inspection of the funnel plot, there appears to be a low risk of reporting bias for this outcome.
Discussion
Summary of main results
Twenty‐one trials involving 13,209 women were included (of which 7847 women were bacterial vaginosis or intermediate flora positive). Many of the prespecified outcomes for this review were not assessed by the included trials. The available evidence from these trials suggests that antibiotic treatment given to women with bacterial vaginosis in pregnancy is highly effective at eradicating bacterial vaginosis infection (risk ratio (RR) 0.42; 95% confidence interval (CI) 0.31 to 0.56; 10 trials of 4403 women). There was significant heterogeneity between trials for many of the comparisons; therefore, random‐effects analyses were used where necessary.
There was no statistically significant decrease in the risk of preterm birth (PTB) at less than 37 weeks' gestation for any antibiotic treatment versus no treatment or placebo (average RR 0.88; 95% CI 0.71 to 1.09; 13 trials, 6491 women). There was also no evidence of an effect on birth before 34 weeks (RR 1.16; 95% CI 0.52 to 2.59; three trials of 515 women), nor for an effect on birth before 32 weeks (RR 1.13; 95% CI 0.77 to 1.68; four trials of 3565 women). The effect of treatment on the incidence of low birthweight suggests no difference (RR 0.99; 95% CI 0.82 to 1.2; seven trials of 4040 women). Antibiotics were not associated with a decrease in the risk of preterm prelabour rupture of membranes (RR 0.74; 95% CI 0.30 to 1.84; two trials of 493 women).
In this updated review, treatment before 20 weeks' gestation did not reduce the risk of PTB less than 37 weeks and in women with a previous PTB, again, there was no reduction in the risk of subsequent PTB. However, in women with abnormal vaginal flora (intermediate flora or bacterial vaginosis), the evidence from two trials suggests that treatment may reduce the risk of PTB before 37 weeks.
Very few perinatal deaths were reported and only one trial (NICHD MFMU 2000 unpublished) reported substantive measures of neonatal morbidity or economic outcomes such as health service utilisation. There was, however, a substantial reduction in late miscarriage with the use of antibiotics (RR 0.20; 95% CI 0.05 to 0.76; two trials with 1270 women), but also an increase in side‐effects sufficient to stop treatment (RR 1.66; 95% CI 1.02 to 2.68; four trials, 2235 women).
Agreements and disagreements with other studies or reviews
There is now a substantial body of evidence that associates bacterial vaginosis in pregnancy with a poor perinatal outcome, in particular an increased risk of preterm birth (Donders 2009; Hay 1994a; Hillier 1995; Kurki 1992; Lamont 2011a; Leitich 2007; McGregor 1990). This strong association between bacterial vaginosis and preterm birth has led many researchers and clinicians to believe that bacterial vaginosis may be the cause of preterm birth in these women.
The results of trials that treat bacterial vaginosis in pregnancy, however, are not encouraging. This updated review now includes six additional trials, one from this update (Moniri 2009) and five from the previous update (Darwish 2007; Giuffrida 2006; Larsson 2006; Mitchell 2009; Shennan 2006) and continues to show no significant reduction in preterm birth from 13 trials involving 6491 women. The review has been restructured to make it clearer to see the differences between trials in terms of antibiotic used, frequency and route and whether compared with placebo or another treatment. Subgroup analyses have also been re‐done for the subgroups of women with previous preterm birth, intermediate flora or bacterial vaginosis or treatment prior to 20 weeks' gestation. The evidence does not now suggest any differential effect between subgroups of women with a previous/no previous preterm birth or treatment before 20 weeks's gestation/after 20 weeks' gestation for the outcomes examined. There is some suggestion that in women identified as having abnormal vaginal flora (intermediate flora or bacterial vaginosis), treatment may reduce the risk of PTB before 37 weeks; however, this result comes from only two studies of 894 women.
Interestingly, there is a benefit from antibiotics in terms of an 80% reduction in late miscarriage. This result is from only two trials but does involve 1270 women. Both studies used clindamycin. This finding may be consistent with the increasing evidence that for treatment to be effective it needs to be started early.
In this update however, for the subgroup of women treated before 20 weeks' gestation, there was no evidence of an effect for any of the outcomes examined (preterm delivery less than 37 weeks; low birthweight; side‐effects not sufficient to stop treatment; late miscarriage). As there have been no head‐to‐head comparisons of early versus late treatment, trials in this area are warranted. The only trial large enough to stratify their results by early or later treatment failed to show any difference in effect when comparing earlier versus later treatment, although it could be argued that even in the early group, treatment was not started early enough. A recent meta‐analysis of clindamycin treatment of bacterial vaginosis prior to 22 weeks' gestation included five of the studies assessed in this review and demonstrated a significantly reduced risk of preterm birth and late miscarriage (Lamont 2011b). Their results remained significant when sensitivity analysis was performed including two further studies.
The two trials of women with abnormal vaginal flora, i.e. intermediate flora or bacterial vaginosis (Lamont 2003; Ugwumadu 2003), showed significantly decreased rates of preterm birth less than 37 weeks' gestation with treatment, which the authors postulate may be due to the earlier gestation of treatment in both these studies (13 to 20 weeks for Lamont 2003 and 12 to 22 weeks (mean 15.6) (for Ugwumadu 2003). When analysed as a separate category, antibiotic treatment of abnormal flora resulted in a significant decrease in preterm birth. However, their inclusion in meta‐analysis of all the trials has not made a substantial difference to the overall picture.
Additional information on neonatal sepsis from the large NICHD MFMU trial is included in the analysis but provides no evidence of a reduction in neonatal sepsis.
Significant heterogeneity was found in several analyses; in the 'failure of test of cure' analysis this is probably due to differences in the timing of the test of cure and the method for determining test of cure. Also, trials in this review have used several different methods of diagnosing bacterial vaginosis or abnormal genital flora (Amsel or clinical criteria, Gram stain criteria, and abnormal Nugent score four to 10).
Limitations of the trials
The trial protocols differ in a number of ways such as the method for diagnosing bacterial vaginosis, timing of screening, timing of treatment, and the period between screening and treatment. Most trials have tested treatment in the second trimester; some as late as 28 weeks' gestation. This may be too late to prevent the consequences of any ascending infection and may be one of the main reasons for the observed lack of a statistically significant effect on the preterm birth rates. The efficacy of antibiotic treatment in long‐term eradication of bacterial vaginosis is at best 80%. The subgroups of women in whom bacterial vaginosis was successfully eradicated, and those with recurring bacterial vaginosis, need to be identified and studied more closely in future trials.
Most trials have concentrated on the timing of birth and have made the assumption that the later in gestation a baby is born, the greater are its chances of disability‐free survival. This may not be the case, however. Neonatal well being and measures of maternal postpartum morbidity were each reported by two trials. However, the majority of outcomes we considered important for this review were not mentioned.
Since the first publication of the earlier Cochrane review in 1998 (Brocklehurst 1998), the number of women in this meta‐analysis has more than trebled, largely due to the inclusion of the NICHD MFMU 2000 and NICHD MFMU 2001 studies with 2132 women. This fourth review has increased the trial numbers by 1294. There remains no clear evidence that screening and treating all women with bacterial vaginosis in the antenatal period will have a major impact on the consequences of preterm birth, however the area of screening and treating abnormal flora deserves further investigation.
Authors' conclusions
Implications for practice.
The evidence to date does not suggest any benefit in screening and treating all pregnant women for asymptomatic bacterial vaginosis to prevent preterm birth. The lack of a significant effect despite large numbers of women in the included trials may be due to many differences within the trials regarding diagnosis, timing of treatment and antibiotic choice. In considering the implications for clinical practice it should be remembered, however, that many of the trials excluded women with symptomatic bacterial vaginosis due to treatment of their symptoms with antibiotics. These women, especially those with recurrent or persistent bacterial vaginosis, may be at highest risk of associated adverse outcomes. Unfortunately, from studies to date we know almost nothing about the impact of these interventions on the health of the baby.
At the present time, there seems little justification for initiating a policy of screening for asymptomatic bacterial vaginosis in pregnancy. Any impact may be dependent upon early detection and treatment.
Implications for research.
The consequences of preterm birth to the individuals concerned and the health services are of major importance. Any intervention with the potential to decrease the risk of mortality and morbidity associated with neonatal immaturity, therefore, needs prompt and appropriate evaluation so that any benefits may be maximised. The focus of current research is to identify those subgroups of pregnant women who are at highest risk for adverse sequelae of bacterial vaginosis. These subgroups include women with recurrent or persistent bacterial vaginosis. Individual susceptibility to preterm birth may also be increased by the presence of specific gene polymorphisms, producing a heightened inflammatory response to vaginal or intrauterine infection. In addition, recent findings suggest future studies may need to focus on earlier detection and treatment of bacterial vaginosis in the first trimester of pregnancy, or even preconception.
What then remains to be demonstrated is that a policy for screening and treatment for asymptomatic bacterial vaginosis in pregnancy can reduce substantive measures of morbidity associated with neonatal immaturity, and that this results in cost savings to families and the health services. Large trials are needed which can determine the effect of a screening programme on neonatal mortality and major measures of morbidity such as intracranial damage and chronic lung disease. For example, in the NICHD MFMU trial, to reduce the incidence of neonatal morbidity by 25% (1.9% to 1.4%) with 90% power, significant at the 5% level, would require recruitment of at least 28,000 women.
Identification of specific subgroups who may benefit most from treatment may be improved further by an individual patient data analysis of the trials included in this review. If the detection and treatment of bacterial vaginosis can be shown to improve neonatal outcome, further trials will be necessary to determine the most effective antibiotic regimen.
Feedback
Klebanoff, October 2005
Summary
I have a couple of minor technical corrections. First, the NICHD trial (NICHD MFMU 2000) randomised women from 16 to 24 weeks, not from 18 to 24 weeks. In fact, these women were randomised not much later than those in Ugwumadu 2003.
Second, women with bacterial vaginosis plus Trichomonas were not eligible for the NICHD study included in this review. However, they were randomised into a parallel NICHD Trichomonas study .(1] In that study, we presented results separately for women who had Trichomonas only and women who had Trichomonas plus bacterial vaginosis. Since many other bacterial vaginosis trials did not screen for Trichomonas, and therefore probably randomised some women who had both, there is no reason to exclude such women recruited to our second study from your review.
Finally, our original draft of the paper for NICHD MFMU 2000 included data on neonatal mortality and morbidity. This table was removed at the request of the NEJM Editor. If you wish, I can investigate whether we can provide you with this additional data.
Reference (1] Klebanoff MA, Carey JC, Hauth JC, Hillier SL, Nugent RP, Thom EA, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487‐93.
(Summary of comments from Mark Klebanoff, October 2005)
Reply
Thank you very much for your comments. We have addressed each of your points in this update as follows: (1) the information about NICHD MFMU 2000 is now correct, women were enrolled between 16 to 24 weeks; (2) we have included the published data from the NICHD Trichomonas study (NICHD MFMU 2001); (3) data from NICHD MFMU 2000 on neonatal mortality and morbidity supplied by the authors have been included.
(Summary of response from Helen McDonald, November 2006)
Contributors
Mark Klebanoff
What's new
| Date | Event | Description |
|---|---|---|
| 31 May 2012 | New search has been performed | Search updated 31 May 2012. |
| 31 May 2012 | New citation required and conclusions have changed | Six new trials incorporated into the review (Darwish 2007; Giuffrida 2006; Larsson 2006; Mitchell 2009; Moniri 2009; Shennan 2006). Subgroup analyses re‐done. For this update there is no evidence for a difference between subgroups of women with previous preterm birth/no previous preterm birth or in women treated prior to 20 weeks' gestation/treated after 20 weeks' gestation for preterm pre‐rupture of membranes or in preterm birth before 37 weeks' gestation. |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 4, 1998
| Date | Event | Description |
|---|---|---|
| 21 June 2011 | New citation required but conclusions have not changed | New authors helped to prepare this review update. |
| 18 November 2010 | Amended | Search updated. Eleven new reports added to Studies awaiting classification. |
| 21 June 2010 | New search has been performed | Search updated ‐ no new trials identified. Eleven trial reports identified in the earlier search have been incorporated into the review. Four new studies have been included (Darwish 2007; Giuffrida 2006; Larsson 2006; Mitchell 2009) and four new studies have been excluded (Kurtzman 2008; Mitchell 2009a; Schoeman 2005; Ugwumadu 2006). One new study is ongoing (Subtil 2008) and additional reports were identified for McDonald 1997 and Shennan 2006. One trial, (Shennan 2006) previously excluded is now an included study due to unpublished relevant outcome data. This review is now comprised of 20 included studies, 20 excluded studies and one ongoing study. |
| 29 April 2008 | Amended | Corrected data input error for Morales 1994 for comparison 08.04. |
| 29 April 2008 | Amended | Converted to new review format. |
| 13 November 2006 | Feedback has been incorporated | Response to feedback from Mark Klebanoff added. |
| 25 September 2006 | New search has been performed | (1) Search updated May 2006. (2) Addition of co‐author Dr Adrienne Gordon. (3) Addition of extra neonatal data in NICHD MFMU 2000 study. (4) Addition of three new studies (NICHD MFMU 2001 with parallel data to NICHD MFMU 2000; Lamont 2003; Kiss 2004). (5) Analysis of clindamycin trials. (6) Analysis of abnormal vaginal flora trials (recruited on the basis of Nugent score 4‐10). (7) Analysis of treatment at less than 20 weeks' gestation. |
| 25 September 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Professor Mary Hannah, Women's College Hospital, University of Toronto, Ontario, Canada: co‐author on the first review.
Dr Rasiah Vigneswaran, deceased: co‐author to 2002.
Helen McDonald, was the contact author from 2003 to 2006, and was responsible for performing searches; personal communications with researchers; extracted and entered data; revised text and responded to comments, etc, for the second and third reviews.
Jacqueline Parsons: co‐author on the 2005 review.
Professor Caroline Crowther, Discipline of Obstetrics and Gynaecology, The University of Adelaide, for editorial advice.
Thanks to Elena Intra for translating the Giuffrida 2006 article from Italian into English.
As part of the pre‐publication editorial process, this updated review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Leanne Jones for her contributions to the revisions made to the final version of the 2012 update in response to editorial feedback.
Appendices
Appendix 1. Methods used to assess trials included in previous versions of this review
We selected all potential trials for eligibility according to the criteria specified in the protocol. Each of the review authors independently abstracted the information necessary for the review from the report and, where necessary, we sought additional information from the authors.
We assessed all trials for methodological quality using the standard Cochrane criteria. As there are a sufficient number of trials in the review, we stratified the trials by quality to explore the robustness of the findings. We calculated summary Peto odds ratios when appropriate (i.e. there was no evidence of significant heterogeneity) using the Cochrane Review Manager software (RevMan 2003).
Stratified analysis
As there are sufficient trials in the review, the comparisons are stratified to explore the effect of the intervention on the outcomes by the following factors:
oral versus vaginal antibiotics;
women with a previous preterm birth;
women with intermediate flora/bacterial vaginosis;
clindamycin versus placebo treatment;
treatment before 20 weeks' gestation.
It was not possible to stratify results into symptomatic versus asymptomatic bacterial vaginosis because in most trials, women with symptoms were treated with antibiotics and were therefore excluded.
Data and analyses
Comparison 1. Any antibiotic versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of test of cure | 10 | 4403 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.31, 0.56] |
| 1.1 Amoxicillin (oral) versus placebo | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.54, 0.98] |
| 1.2 Clindamycin (oral) versus placebo | 1 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.07, 0.15] |
| 1.3 Clindamycin (vaginal) versus placebo | 3 | 1411 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.30, 0.53] |
| 1.4 Clindamycin (vaginal) versus no treatment | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.37, 1.78] |
| 1.5 Metronidazole (oral) versus placebo | 3 | 2116 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.30, 0.88] |
| 1.6 Metronidazole and erythromycin (oral) versus placebo | 1 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.29, 0.47] |
| 2 Postpartum infection | 2 | 618 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.26, 3.21] |
| 2.1 Clindamycin (vaginal) versus placebo | 1 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.38, 1.06] |
| 2.2 Metronidazole (oral) versus placebo | 1 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 2.93 [0.31, 27.75] |
| 3 Perinatal death | 4 | 3195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.36, 1.39] |
| 3.1 Clindamycin (vaginal) versus no treatment | 1 | 785 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Metronidazole (oral) versus placebo | 3 | 2410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.36, 1.39] |
| 4 Incidence of preterm prelabour rupture of membranes | 2 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.30, 1.84] |
| 4.1 Metronidazole (oral) versus placebo | 2 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.30, 1.84] |
| 5 Preterm birth < 37 weeks | 13 | 6491 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.71, 1.09] |
| 5.1 Clindamycin (oral) versus placebo | 1 | 485 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.35, 1.04] |
| 5.2 Clindamycin (vaginal) versus placebo | 3 | 1465 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.44, 1.65] |
| 5.3 Clindamycin (vaginal) versus no treatment | 2 | 919 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.51, 1.35] |
| 5.4 Clindamycin (vaginal and oral) versus no treatment | 1 | 356 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.23, 1.63] |
| 5.5 Metronidazole (oral) versus placebo | 4 | 2888 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.77, 1.72] |
| 5.6 Metronidazole and erythromycin (oral) versus placebo | 1 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.47, 0.88] |
| 5.7 Metronidazole (oral) versus no treatment | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.87] |
| 6 Preterm birth < 34 weeks | 3 | 515 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.52, 2.59] |
| 6.1 Clindamycin (vaginal) versus placebo | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.05] |
| 6.2 Metronidazole (oral) versus placebo | 2 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.51, 2.74] |
| 7 Preterm birth < 32 weeks | 4 | 3565 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.77, 1.68] |
| 7.1 Clindamycin (oral) versus placebo | 1 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.54, 4.10] |
| 7.2 Clindamycin (vaginal) versus placebo | 1 | 681 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.80, 3.98] |
| 7.3 Metronidazole (oral) versus placebo | 2 | 2399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.53, 1.46] |
| 8 Incidence of low birthweight | 7 | 4040 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.82, 1.20] |
| 8.1 Clindamycin (oral) versus placebo | 1 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.46, 1.46] |
| 8.2 Clindamycin (vaginal) versus placebo | 2 | 1081 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.83, 1.89] |
| 8.3 Clindamycin (vaginal) versus no treatment | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.12, 1.63] |
| 8.4 Metronidazole (oral) versus placebo | 3 | 2392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.24] |
| 9 Neonatal sepsis | 3 | 2345 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.45, 4.41] |
| 9.1 Clindamycin (vaginal) versus placebo | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Metronidazole (oral) versus placebo | 2 | 2323 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.45, 4.41] |
| 10 Side‐effects sufficient to stop or change treatment | 4 | 2235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.02, 2.68] |
| 10.1 Amoxicillin (oral) versus placebo | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.74] |
| 10.2 Clindamycin (oral) versus placebo | 1 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.92, 4.77] |
| 10.3 Clindamycin (vaginal) versus no treatment | 1 | 785 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.91 [0.36, 133.37] |
| 10.4 Metronidazole (oral) versus placebo | 1 | 857 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.69, 2.67] |
| 11 Side‐effects not sufficient to stop treatment | 3 | 1340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.76, 2.13] |
| 11.1 Amoxicillin (oral) versus placebo | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.53, 1.89] |
| 11.2 Clindamycin (vaginal) versus placebo | 1 | 375 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.33, 3.06] |
| 11.3 Metronidazole (oral) versus placebo | 1 | 857 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.99 [0.85, 18.68] |
| 12 Severe neonatal morbidity | 3 | 2715 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.54, 1.75] |
| 12.1 Clindamycin (vaginal) versus no treatment | 1 | 785 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.05] |
| 12.2 Metronidazole (oral) versus placebo | 2 | 1930 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.55, 1.86] |
| 13 Admission to neonatal unit | 2 | 2383 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.69, 1.50] |
| 13.1 Clindamycin (oral) versus placebo | 1 | 466 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.42, 1.35] |
| 13.2 Metronidazole (oral) versus placebo | 1 | 1917 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.93, 1.44] |
| 14 Late miscarriage | 2 | 1270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.05, 0.76] |
| 14.1 Clindamycin (oral) versus placebo | 1 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.89] |
| 14.2 Clindamycin (vaginal) versus no treatment | 1 | 785 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.10] |
| 15 Moderate/severe visual impairment at childhood follow‐up | 1 | 785 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.05] |
| 15.1 Clindamycin (vaginal) versus no treatment | 1 | 785 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.05] |
Comparison 2. Antibiotic versus another antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of premature rupture of membranes | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.83, 1.46] |
| 1.1 Oral metronidazole versus oral clindamycin | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.73, 1.66] |
| 1.2 Vaginal metronidazole versus vaginal clindamycin | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.74, 1.62] |
| 2 Preterm birth < 37 weeks | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.63, 1.26] |
| 2.1 Oral metronidazole versus oral clindamycin | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.55, 1.45] |
| 2.2 Vaginal metronidazole versus vaginal clindamycin | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.54, 1.48] |
| 3 Admission to neonatal unit | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.67, 1.40] |
| 3.1 Oral metronidazole versus oral clindamycin | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.48, 1.76] |
| 3.2 Vaginal metronidazole versus vaginal clindamycin | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.65, 1.54] |
| 4 Prolongation of gestational age (days) | 1 | 156 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [0.26, 1.74] |
| 4.1 Oral metronidazole versus oral clindamycin | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.33, 2.33] |
| 4.2 Vaginal metronidazole versus vaginal clindamycin | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [0.11, 1.89] |
| 5 Birthweight (grams) | 1 | 156 | Mean Difference (IV, Fixed, 95% CI) | 75.18 [25.37, 124.99] |
| 5.1 Oral metronidazole versus oral clindamycin | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 64.0 [‐7.03, 135.03] |
| 5.2 Vaginal metronidazole versus vaginal clindamycin | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 86.0 [16.13, 155.87] |
Comparison 3. Antibiotics: different routes of administration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of test of cure | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.67, 1.39] |
| 1.1 Oral metronidazole versus vaginal metronidazole | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.67, 1.39] |
| 2 Preterm birth < 37 weeks | 2 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.78, 1.52] |
| 2.1 Oral metronidazole versus vaginal metronidazole | 2 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.71, 1.77] |
| 2.2 Oral clindamycin versus vaginal clindamycin | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.66, 1.68] |
| 3 Incidence of low birthweight | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.51, 7.31] |
| 3.1 Oral metronidazole versus vaginal metronidazole | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.51, 7.31] |
| 4 Absence of abnormal clinical signs (no homogenous discharge, no amine odour after the addition of potassium hydroxide, no clue cells on saline microscopy, and PH<4.5) | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.78, 1.53] |
| 4.1 Oral metronidazole versus vaginal metronidazole | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.78, 1.53] |
| 5 Incidence of premature rupture of membranes | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.72, 1.27] |
| 5.1 Oral metronidazole versus vaginal metronidazole | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.65, 1.40] |
| 5.2 Oral clindamycin versus vaginal clindamycin | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.62, 1.45] |
| 6 Admission to neonatal unit | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.42, 0.92] |
| 6.1 Oral metronidazole versus vaginal metronidazole | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.34, 1.05] |
| 6.2 Oral clindamycin versus vaginal clindamycin | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.38, 1.11] |
| 7 Prolongation of gestational age (days) | 1 | 156 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [8.20, 9.80] |
| 7.1 Oral metronidazole versus vaginal metronidazole | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [7.87, 10.13] |
| 7.2 Oral clindamycin versus vaginal clindamycin | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [7.87, 10.13] |
| 8 Birthweight (grams) | 1 | 156 | Mean Difference (IV, Fixed, 95% CI) | 342.13 [293.04, 391.22] |
| 8.1 Oral metronidazole versus vaginal metronidazole | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 333.0 [268.83, 397.17] |
| 8.2 Oral clindamycin versus vaginal clindamycin | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 355.0 [278.79, 431.21] |
Comparison 4. Antibiotic versus another treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of test of cure | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Clindamycin (vaginal) versus Peroxen (vaginal) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Metronidazole (oral) versus vitamin C (oral) | 2 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.08, 0.83] |
| 2 Side‐effects (not sufficient to stop or change treatment) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.57, 5.36] |
| 2.1 Clindamycin (vaginal) versus Peroxen (vaginal) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.57, 5.36] |
| 3 Perinatal death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Metronidazole (oral) versus vitamin C (oral) | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.61 [0.71, 9.62] |
| 4 Preterm birth < 37 weeks | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Metronidazole (oral) versus vitamin C (oral) | 2 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.22, 3.30] |
| 5 Preterm birth < 34 weeks | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Metronidazole (oral) versus vitamin C (oral) | 2 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.25, 4.42] |
| 6 Incidence of low birthweight | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Metronidazole (oral) versus vitamin C (oral) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.17, 0.98] |
Comparison 5. Antibiotics: different frequency/dose of administration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postpartum uterine infection | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [0.35, 30.28] |
| 2 Preterm delivery < 37 weeks | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.12, 1.44] |
| 3 Incidence of low birthweight | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.58, 2.42] |
Comparison 6. Subgroup analysis ‐ Previous preterm birth versus no previous preterm birth.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of test of cure | 10 | 4403 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.31, 0.56] |
| 1.1 Previous preterm birth | 2 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.22, 1.50] |
| 1.2 No previous preterm birth | 8 | 4127 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.28, 0.53] |
| 2 Perinatal death | 4 | 2749 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.34, 1.40] |
| 2.1 Previous preterm birth | 2 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 No previous preterm birth | 2 | 2702 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.34, 1.40] |
| 3 Preterm delivery < 37 weeks | 11 | 6521 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.71, 1.09] |
| 3.1 Previous preterm birth | 3 | 421 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.42, 1.48] |
| 3.2 No previous preterm birth | 10 | 6100 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.71, 1.14] |
| 4 Preterm delivery < 34 weeks | 3 | 515 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.52, 2.59] |
| 4.1 Previous preterm birth | 2 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.37, 3.80] |
| 4.2 No previous preterm birth | 1 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.39, 3.36] |
| 5 Incidence of low birthweight | 7 | 3594 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.18] |
| 5.1 Previous preterm birth | 1 | 13 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.35, 4.49] |
| 5.2 No previous preterm birth | 6 | 3581 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.77, 1.20] |
| 6 Neonatal sepsis | 3 | 2345 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.45, 4.41] |
| 6.1 Previous preterm birth | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 No previous preterm birth | 2 | 2323 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.45, 4.41] |
Comparison 7. Subgroup analysis ‐ Intermediate flora/bacterial vaginosis (Nugent score 4‐10) versus no intermediate flora/bacterial vaginosis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of test of cure | 9 | 4140 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.31, 0.59] |
| 1.1 Intermediate flora/bacterial vaginosis | 2 | 830 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.05, 0.75] |
| 1.2 No intermediate flora/bacterial vaginosis | 7 | 3310 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.39, 0.70] |
| 2 Perinatal death | 6 | 4089 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.36, 1.26] |
| 2.1 Intermediate flora/bacterial vaginosis | 2 | 894 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.64] |
| 2.2 No intermediate flora/bacterial vaginosis | 4 | 3195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.36, 1.39] |
| 3 Preterm birth < 37 weeks | 12 | 6478 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.69, 1.07] |
| 3.1 Intermediate flora/bacterial vaginosis | 2 | 894 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.34, 0.84] |
| 3.2 No intermediate flora/bacterial vaginosis | 10 | 5584 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.16] |
| 4 Preterm birth < 32 weeks | 4 | 3565 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.77, 1.68] |
| 4.1 Intermediate flora/bacterial vaginosis | 1 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.54, 4.10] |
| 4.2 No intermediate flora/bacterial vaginosis | 3 | 3080 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.71, 1.66] |
| 5 Incidence of low birthweight | 7 | 4040 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.82, 1.20] |
| 5.1 Intermediate flora/bacterial vaginosis | 2 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.62, 1.47] |
| 5.2 No intermediate flora/bacterial vaginosis | 5 | 3164 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.81, 1.24] |
| 6 Neonatal sepsis | 4 | 2754 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.69, 2.16] |
| 6.1 Intermediate flora/bacterial vaginosis | 1 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.60, 2.24] |
| 6.2 No intermediate flora/bacterial vaginosis | 3 | 2345 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.45, 4.41] |
| 7 Side‐effects sufficient to stop or change treatment | 4 | 2235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.02, 2.68] |
| 7.1 Intermediate flora/bacterial vaginosis | 1 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.92, 4.77] |
| 7.2 No intermediate flora/bacterial vaginosis | 3 | 1750 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.80, 2.64] |
| 8 Late miscarriage | 2 | 1270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.05, 0.76] |
| 8.1 Intermediate flora/bacterial vaginosis | 1 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.89] |
| 8.2 No intermediate flora/bacterial vaginosis | 1 | 785 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.10] |
| 9 Admission to neonatal unit | 2 | 2383 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.89, 1.34] |
| 9.1 Intermediate flora/bacterial vaginosis | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.42, 1.35] |
| 9.2 No intermediate flora/bacterial vaginosis | 1 | 1917 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.93, 1.44] |
Comparison 8. Subgroup analysis ‐ Treatment at < 20 weeks' gestation versus > 20 weeks' gestation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of test of cure | 8 | 4127 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.28, 0.53] |
| 1.1 Treatment < 20 weeks' gestation | 3 | 2434 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.32, 0.51] |
| 1.2 Treatment > 20 weeks' gestation | 5 | 1693 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.19, 0.78] |
| 2 Preterm birth less than 37 weeks | 12 | 6478 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.69, 1.07] |
| 2.1 Treatment < 20 weeks' gestation | 5 | 4088 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.62, 1.17] |
| 2.2 Treatment > 20 weeks' gestation | 7 | 2390 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.22] |
| 3 Incidence of low birthweight | 7 | 4040 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.82, 1.21] |
| 3.1 Treatment < 20 weeks' gestation | 2 | 2308 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.77, 1.24] |
| 3.2 Treatment > 20 weeks' gestation | 5 | 1732 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.74, 1.41] |
| 4 Side‐effects not sufficient to stop treatment | 4 | 2045 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.92, 2.50] |
| 4.1 Treatment < 20 weeks' gestation | 1 | 785 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.91 [0.36, 133.37] |
| 4.2 Treatment > 20 weeks' gestation | 3 | 1260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.84, 2.34] |
| 5 Late miscarriage | 2 | 1270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.05, 0.76] |
| 5.1 Treatment < 20 weeks' gestation | 1 | 785 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.10] |
| 5.2 Treatment > 20 weeks' gestation | 1 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.89] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Darwish 2007.
| Methods | Longitudinal prospective comparative study with random assignment to 4 groups. | |
| Participants |
Setting: antenatal outpatient clinic of the Department of Obstetrics and Gynaecology, Faculty of Medicine, Assiut University, Assiut, Egypt. Inclusion criteria: (a) clinical picture of TPL or at high risk of PROM in the third trimester. (b) positive for BV using Amsels criteria. Exclusion criteria: definite preterm labour, definite PROM, other causes explaining TPL or PROM, e.g. multiple pregnancy, uterine anomalies, polyhydramnios, fibroid uterus, fetal anomalies, patients receiving medical treatment (for diabetes, pre‐eclampsia or other condition) patients in active phase of labour, incompetent os and other gynaecologic or obstetric problems. Gestational age at trial entry: third trimester (no further info). Total number of participants: 156. |
|
| Interventions | (a) Oral MET (250 mg 3x/day for 7 days) n = 39 vs (b) Clindamycin vaginal cream (0.2%, once daily at night for 7 days) OR n = 39 vs (c) oral clindamycin (300 mg 2x/day for 7 days) OR n = 39 vs (d) MET vaginal suppositories (500 mg once daily at night for 7 days) n = 39. |
|
| Outcomes | (a) Changes of Amsel’s criteria after treatment. (b) Effect on BW of each treatment modality. (c) Percentage of neonates who required neonatal intensive care admission. (d) Days until delivery after therapy. (e) Maternal tolerability and adverse effects. (f) PROM. |
|
| Notes | Unclear from methods whether this study is actually an RCT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It was reported that no participants were lost to follow‐up after randomisation. No CONSORT diagram is presented. The analysis was intention‐to‐treat since all participants were followed up and all remained in their allocated treatment groups (no drop‐outs/withdrawals were reported). |
| Selective reporting (reporting bias) | High risk | Changes in Amsels criteria were presented as a figure with percentages for each criteria. Numerical values were not available for cure rates for each intervention group. Statistical significance for comparisons were not comprehensively reported. Only selected comparisons were reported. |
| Other bias | Low risk | No evidence of other bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo, different modes of administration so unable to blind participants/clinicians. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessment blinding not reported. |
Duff 1991.
| Methods | RCT. | |
| Participants |
Setting: University of Washington Medical Center and Madigan Army Center. Inclusion criteria: BV diagnosed on Gram stain (Nugents criteria). Exclusion criteria: penicillin allergy, antimicrobial use within 2 weeks of enrolment, anticipated movement away from area, inability to speak English, diabetes, cervical cerclage, multiple pregnancy, hypertension on treatment, pregnancy‐induced hypertension, fetal anomalies. Gestational age at trial entry: women were screened for BV at 15‐25 weeks' gestation. Total number of participants: 108. |
|
| Interventions | (a) Amoxycillin 500 mg x 3/day for 14 days or n = 54 vs (b) Matching placebo n = 54. |
|
| Outcomes | "Test‐of‐cure" 2 weeks after treatment complete, at 34‐36 weeks and at admission in labour; preterm delivery; low BW; PROM; neonatal sepsis; maternal infection. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Biased‐coin technique, which balanced the groups after every 6 participants. "Adaptive randomization plan using a biased‐can technique which balanced the groups after every 6 enrollees." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1702 women were screened for BV. 357 screened positive. 45 women declined participation. 45 did not keep enrolment clinic appointments. 149 women were excluded. 118 women, 54 in each treatment group were enrolled in the treatment trial. It appears that no participants were excluded after randomisation (only before). The paper indicates that intention‐to‐treat analysis occurred. 15 patients in the intervention group and 12 in the placebo group did not return for their follow‐up appointments. Loss to follow‐up ‐ 7/54 (13%) in amoxicillin group, 9/54 (17%) in placebo group. |
| Selective reporting (reporting bias) | Low risk | Outcomes mentioned in the methods section were all reported on in the results section. |
| Other bias | Low risk | There were no significant differences between the 2 groups with respect to selected demographic variables such as maternal age, race, level of education, marital status, and mean family income. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The physician, study nurse and participant were blinded. Placebo capsules were identical to intervention capsules. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The physician, study nurse and participant were blinded. Placebo capsules were identical to intervention capsules. |
Giuffrida 2006.
| Methods | RCT. | |
| Participants |
Setting: Casa di Cura Lucina (private clinic), Catania, Italy. Inclusion criteria: (a) Positive for BV. (b) Asymptomatic of vaginal infection. (c) LMP from 3/6/2005 ‐ 30/11/2005. Exclusion criteria: (a) Multiple pregnancy. (b) Cervical cerclage. (c) Diabetes. (d) Screened positive for a sexually transmitted infection. (e) Patients treated with systemic or local AV therapy < 2 weeks before recruiting. (f) Previous premature delivery, previous PROM. Gestational age at trial entry: 13‐16 weeks. Total number of participants: 150. |
|
| Interventions | (a) Initial treatment: Clindamycin intravaginal ovules 100 mg for 3 days, n = 30 vs (b) Hydrogen peroxide (0.5%) vaginal cream for 5 days, n = 30. All participants were retested via vaginal smear for BV 20 days after treatment and if positive received a second treatment course of the same AB as first randomised to but a longer duration. Those that retested negative received no further treatment. Repeat treatment: clindamycin intravaginal ovules 100 mg for 7 days or Hydrogen peroxide (0.5 %) vaginal cream for 10 days. |
|
| Outcomes | (a) PROM. (b) Gestational age at labour in weeks. (c) BW in grams. (d) Maternal side‐effects: vaginal burning, dysuria, vulvovaginal pruritis,vaginal discharge. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of participants to follow‐up at each data collection point. No exclusion of participants after randomisation. It appears that no withdrawals occurred post‐randomisation. Adverse events are reported, although it appears these patients did not withdraw. |
| Selective reporting (reporting bias) | Unclear risk | Maternal age, weight, height, parity and gestational age were reported for comparison, although it was not clear whether this was a mean, median or mode value, nor were P values for significance provided in the table. The small number of outcomes reported was not optimal. More maternal, neonatal and economic outcomes would have been useful. |
| Other bias | Low risk | It appears that the study ran to its expected conclusion. The translation did not provide further information regarding this analysis. No differential diagnosis was likely. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding did not occur. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding did not occur. |
Guaschino 2003.
| Methods | Randomised trial (no placebo, but no treatment group as control). | |
| Participants |
Setting: outpatient obstetric services of participating centres (Clinica Ostetrica e Ginecologica di Trieste, Torino e Milano). Inclusion criteria: women between 14 and 25 weeks of gestation with asymptomatic BV. Exclusion criteria: multifetal gestation, symptoms of vaginal or urinary tract infection, AV therapy in the previous 15 days or contraindications to the use of clindamycin. Gestational age at trial entry: 14‐25 weeks. Total number of participants: 100. |
|
| Interventions | (a) Intravaginal clindamycin 2% cream once daily for 7 days, n = 49 vs (b) no treatment n = 51 (no placebo, just no treatment). |
|
| Outcomes | Preterm delivery < 37 weeks. Low BW, PROM. | |
| Notes | 10.7% lost to follow‐up, 6 in each group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation lists stratified for centre. |
| Allocation concealment (selection bias) | Low risk | Women were randomised by phoning the randomisation centre (third party). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1890 women were screened. BV was diagnosed in 112 cases. 55 women were assigned to intervention and 57 to no treatment. No participants were excluded after randomisation. The analysis was not intention‐to‐treat. The 12 lost to follow‐up were not included in the analysis. Of the 112 women randomised, 12 were lost to follow‐up. 6 in each group. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported in the reported in the results section. |
| Other bias | Low risk | The study was not stopped early. There were no significant differences in baseline characteristics between the 2 groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no placebo; the control group was a “no treatment” group, so participants were not blinded. Blinding of clinicians and outcome assessors also appears not to have occurred. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not occur. |
Hauth 1995.
| Methods | RCT. | |
| Participants |
Setting: abstract only Inclusion criteria: pregnant women at 22‐24 weeks' gestation with history of previous preterm delivery or who weighed < 50 kg in current pregnancy. All women were screened for BV (diagnosed by Amsels criteria). Exclusion criteria: allergies to MET or erythromycin, uncertain gestational age, multiple pregnancy, prior vaginal bleeding, medical complications, any AV use in the previous 4 weeks, co‐infection with gonorrhoea, trichomonas or vaginal candida. Gestational age at trial entry: 22‐24 weeks' gestation. Total number of participants: 624. |
|
| Interventions | (a) MET 250 mg x 3/day for 7 days plus erythromycin base 333 mg x 3/day for 14 days
vs (b) matching placebo. Treatment repeated if BV still present at "test‐of‐cure". Rx mean 27.6 weeks. |
|
| Outcomes | "Test‐of‐cure" 2 to 4 weeks after treatment; preterm delivery before 37 weeks. | |
| Notes | All women who were enrolled in the trial were treated with ABs/placebo at trial entry regardless of whether they had BV ‐ this formed a post‐randomisation stratification. BV positive women ‐ 176 AB vs 87 placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation scheme in a ratio of 2:1. |
| Allocation concealment (selection bias) | Low risk | Investigational drug service (third party) generated the randomisation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 624 women randomised to treatment (433) or placebo (191). 8 were lost after randomisation. No post‐randomisation exclusion occurred. The analysis was not intention‐to‐treat. Women who were lost to follow‐up examination were not included in analysis. 8 women were lost to follow‐up. 7 were lost from the treatment group (from 433 to 426) and 1 was lost from the control group (191 to 190). Loss to follow‐up for the whole trial cohort ‐ 4/176 (2%) in AV group, 1/87 (1%) in placebo group. |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were reported in the results section. |
| Other bias | Low risk | The study was not stopped early. It was reported that there were no significant baseline differences between the treatment group and the placebo group. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as double blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
Joesoef 1995.
| Methods | RCT. | |
| Participants |
Setting: 3 maternity clinics in Jakarta and in 4 maternity clinics in Surabaya, Indonesia. The clinics were public clinics serving mostly low‐income families. Inclusion criteria: Gram stain score > 6 and pH of vaginal fluid > 4.5. Exclusion criteria: allergy to clindamycin, medical condition associated with preterm delivery (hypertension, multiple pregnancy, diabetes etc), previous tocolytic treatment, previous steroid treatment, AVs in 2 weeks before trial entry, age < 15 years, uterine or fetal abnormalities or incompetent Cx. Gestational age at trial entry: 14‐26 weeks. Total number of participants: 681. |
|
| Interventions | (a) Clindamycin cream 2% ‐ 5 g intravaginally at bedtime for 7 days, n =340 vs (b) Matching placebo. 43% enrolled @ less than 20 weeks, n = 341. |
|
| Outcomes | "Test‐of‐cure" 2 weeks after completion of treatment and again after 34 weeks; preterm delivery < 37 and < 32 weeks; low BW (< 2500 g). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator selected permuted blocks of size 6. Stratified by centre. |
| Allocation concealment (selection bias) | Low risk | Allocation was administered centrally (multi‐centre trial). Sealed envelopes containing information regarding patient allocations were not opened. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 745 women were enrolled, 681 were followed‐up through delivery. No exclusion of participants after randomisation was reported. The patients that were lost to follow‐up were not included in the analysis. It is unclear whether the analysis was intention‐to‐treat. 64 patients were lost to follow‐up, although the stage of loss and which groups were not reported. |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias occurred. |
| Other bias | Low risk | The study was not stopped early. It was reported that baseline characteristics were similar, and this appears to be correct, from inspection of the table of baseline characteristics provided, although no statistical test was undertaken to identify statistically significant differences. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A placebo was used. Neither the women nor the investigator knew which cream the women received (active or placebo). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A placebo was used. Neither the women nor the investigator knew which cream the women received (active or placebo). |
Kekki 2001.
| Methods | RCT. | |
| Participants |
Setting: the study centres were the Departments of Obstetrics and Gynecology, University of Helsinki and University of Oulu (17 antenatal clinics), Health Centers of the City Health Department of Helsinki (7 antenatal clinics), and the County of Vihti (4 antenatal clinics), Finland. Inclusion criteria: pregnant women with BV (screened at 10‐17 weeks, using Spiegel's criteria). Exclusion criteria: multiple pregnancy, history of PTB, induced/spontaneous abortion, move to another city. Gestational age at trial entry: screened at 10‐17 weeks and randomised at 12‐19 weeks. Total number of participants: 375. |
|
| Interventions | (a) 2% vaginal clindamycin cream (single course) for 7 days, n = 187 vs (b) matching placebo for 7 days, n = 188. |
|
| Outcomes | "Test‐of‐cure" 1 week after treatment; spontaneous preterm delivery < 37 weeks' gestation; peripartum infectious morbidity (postpartum endometritis, postpartum sepsis, caesarian section wound infection, episiotomy infection necessitating AV treatment). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was used within each centre. |
| Allocation concealment (selection bias) | Low risk | Treatment group allocations were kept sealed in opaque envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5432 were screened. 565 tested positive for BV. 190 were excluded for various reasons. 375 were randomised to intervention (187) or placebo (188). No post‐randomisation exclusion occurred. The analysis was intention‐to‐treat. There were no drop‐outs/withdrawals, although 13 patients did not attend the first follow‐up visit. No dropouts, but 35 attended only 1 follow‐up visit. 21 (6%) given additional topical treatment for symptomatic BV. Intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes described in the methods section were reported in the results section. |
| Other bias | Low risk | The study was not stopped early. At baseline, the intervention and control groups were similar in terms of age and parity. No other baseline characteristics were presented, and the baseline characteristics were presented in the same table as study outcomes, which is not optimal. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The placebo used effectively blinded participants. Clinician blinding appeared also to have occurred. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Gram stains were blinded as to intervention/control. |
Kiss 2004.
| Methods | RCT (no placebo, control is no treatment). | |
| Participants |
Setting: Vienna Inclusion criteria: pregnant women at 15‐19 weeks' gestation all screened for BV (Nugents criteria) Exclusion criteria: subjective complaints (contractions, vaginal bleeding, or symptoms suggestive of vaginal infection); multiple pregnancy. Gestational age at trial entry: enrolled between 15 + 0 (15 weeks plus 0 days) and 19 + 6 weeks (19 weeks plus 6 days) of gestation. Total number of participants: 4155 women screened but included in the review are 356 women with BV. |
|
| Interventions | (a) 2% vaginal clindamycin cream for 6 days, given 7‐10 days after diagnosis. (12‐19 weeks). Retreated if still present @ follow‐up, n = 2058 vs (b) no treatment for control group, n = 2097. |
|
| Outcomes | PTB < 37 weeks, intrauterine death, miscarriage, BW < 2500, < 2000 ,< 1500 and < 1000 g. | |
| Notes | Not intention‐to‐treat. 274 excluded from analysis post randomisation leaving 177 AB vs 179 placebo. Lost to follow‐up 8 in BV group and 13 in controls. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Random assignment was done by a central laboratory. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4429 women were randomised. 274 were not included in the analysis (140 were lost to follow‐up; 68 were erroneously included as they did not fulfil all inclusion criteria and 66 had multiple pregnancies). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias. |
| Other bias | Low risk | The study was not stopped early. No baseline differences in parity, or history of preterm delivery or in screening test results appeared to be present between the 2 main groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This trial was not blinded. The intervention group women and their clinicians received their screening test results and treated accordingly whereas the control group participants did not have their screening test results revealed. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This trial was not blinded. |
Lamont 2003.
| Methods | RCT. | |
| Participants |
Setting: Northwick Park Hospital, Jessop Hospital, Sheffield and Bolton General Hospital. Inclusion criteria: asymptomatic pregnant women 13‐20 weeks with BV or intermediate flora by Nugent's criteria. Exclusion criteria: women with sensitivity to clindamycin; history of AV‐related colitis; inflammatory bowel disease or frequent periodic diarrhoea. Gestational age at trial entry: between 13 and 20 weeks' gestation. Total number of participants: 409. |
|
| Interventions | (a) 5 g of 2% clindamycin intravaginal cream (+ 100 mg) for 3 nights, n = 199 vs (b) matched placebo, n = 205. In addition 7 extra days if vaginal swab still positive (BV/intermediate flora) at visit 2. |
|
| Outcomes | PTB < 37 weeks; low BW, very low BW, stillborn. | |
| Notes | Intent‐to‐treat analysis. 30 did not return for visit 2 in clindamycin group, and 11 in the placebo group, leaving 208 AV vs 201 placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised block procedure with a block size of 10, using a computer‐generated random code list. Computerised block randomisation (block size 10). |
| Allocation concealment (selection bias) | Unclear risk | It is unclear how allocation concealment was achieved. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 404 women were studied in an intention‐to‐treat analysis comprising 199 in the CVC group and 205 in the placebo group. No post‐randomisation exclusion of participants occurred. Analysis was intention‐to‐treat. Follow‐up and drop‐out details were provided in the text. |
| Selective reporting (reporting bias) | Low risk | There is no evidence of selective reporting bias. |
| Other bias | Low risk | The study was not stopped early. No statistically significant differences in baseline characteristics were reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active and placebo creams were packaged identically. Participants and clinicians were blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
Larsson 2006.
| Methods | RCT. | |
| Participants |
Setting: South East Health Care region of Sweden. Inclusion criteria: (a) 18 or older. (b) Swedish speaking. (c) No AV treatment in early pregnancy. (d) No symptomatic vaginal infection. (e) Nugent score of 6 and above (in this trial 6 ‐ 10 considered to have BV secondary to a change to a microscope with a larger image area). Exclusion criteria: (a) Therapeutic termination of pregnancy. (b) Early spontaneous miscarriage or missed miscarriage. (c) Post‐inclusion need for cervical cerclage. (d) Post‐inclusion treatment with either MET or clindamycin (outside the study). (e) Multiple pregnancy. Gestational age at trial entry: 10‐14 weeks. Total number of participants: 819. |
|
| Interventions | (a) 7 days of clindamycin vaginal cream, n = 408 vs (b) no treatment, n = 411. |
|
| Outcomes | Late miscarriage and spontaneous preterm delivery before 37 weeks. Birthweight < 2500 g. Length of NICU admission. 4‐year follow‐up. Maternal side‐effects sufficient to stop treatment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was carried out by computer generation in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by the Department of Clinical Microbiology at the University Hospital in Linköping (third party). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A total of 819 women with a Nugent score of 6+ were considered to have BV. Of those 819 women, 408 participants were randomised to treatment, and 411 participants were randomised to no treatment. Of those who were randomised to the treatment group (408), 55 declined treatment. These 55 women were included in the analysis according to intention‐to‐treat. All patients allocated to the no treatment group did not receive treatment. It was then reported that 19 multiple pregnancies, 12 iatrogenic or induced pre‐term deliveries and 3 women treated outside the study with MET or clindamycin were excluded from the study (giving a post‐randomisation exclusion total of 34). It was not specified which group and in what proportion each exclusion came from. However, further reported figures (395 for intervention group, 390 for control group) allowed it to be deduced that 13 of the 34 excluded participants came from the intervention group, whilst 21 of the 34 excluded participants came from the control group. Thus, the totals in each group came to 395 in the intervention group and 390 in the control group. The 34 excluded patients as mentioned above were not included in the analysis. Also, 3 patients withdrew from treatment due to minor adverse events, and appear to have been included in the intervention group analysis. Overall, reporting of post‐randomisation exclusion/withdrawal was unclear. |
| Selective reporting (reporting bias) | Low risk | The outcome measures detailed in the methods section correspond to the results section. |
| Other bias | Low risk | The study was not stopped early. No baseline imbalances appear to be present, although the reported baseline characteristics are not sufficiently comprehensive. No plausible differential diagnosis is present. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The participants were un‐blinded to their intervention; those allocated to the intervention group were told the result of their vaginal smear and treated, whereas those who were in the control group were untreated and uninformed regarding their BV status. It is reported that clinicians caring for the BV‐positive group randomised to non‐treatment were not told of the diagnosis. It would be unclear to the clinicians whether the patient was positive for BV or negative for BV, but it would be clear that they were not treating the patient for BV, hence it is possible that this process un‐blinded the clinicians. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not reported whether the outcome assessor was blinded. |
McDonald 1997.
| Methods | RCT. | |
| Participants |
Setting: 4 South Australian perinatal centres serving a large metropolitan area. Inclusion criteria: BV or Gardnerella vaginalis. Exclusion criteria: multiple pregnancy, age < 17 years, in‐vitro fertilisation, allergy to MET, symptomatic BV requiring treatment, ruptured membranes, cervical cerclage, diabetes, placenta previa, AV treatment for vaginitis within 2 weeks of trial entry, inability to attend before 28 weeks, language difficulties. Gestational age at trial entry: 16‐26 weeks. Total number of participants: 879. |
|
| Interventions | (a) MET 400 mg x 2/day for 2 days at 24 weeks' gestation, n = 439 vs (b) matching placebo, n = 440. If repeat swabs remained positive at 28 weeks' gestation a further course of treatment was given. |
|
| Outcomes | PTB < 37 weeks; preterm premature rupture of the membranes; stratified by previous history of preterm delivery. | |
| Notes | The women included in this review were the subset of women with BV, (56% of total trial cohort). Women with a heavy growth of Gardnerella but no BV have not been included. Loss to follow‐up ‐ 10/439 (2%) MET group, 12/440 (3%) placebo group. Leaving BV positive randomised to 242 AV vs 238 placebo. Additional information supplied by investigator. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed on an intention‐to‐treat basis regardless of compliance with the protocol. 879 women were randomised. 439 were allocated to the intervention and 440 were allocated to the placebo group. All randomised women were included except 22 women who were lost to follow‐up (12 placebo and 10 intervention). |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting bias. |
| Other bias | Low risk | The study was not stopped early. The demographic characteristics of the women in the 2 groups were similar (except for a small number of teenage deliveries). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo tablets were manufactured to resemble MET in size, texture and colour. Patients, clinicians and outcome assessors were blinded to the allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See row above. |
Mitchell 2009.
| Methods | Randomised double blind placebo‐controlled trial. | |
| Participants |
Setting: Public Health Clinics in Seattle, Washington from May 200 ‐Sept 2004. Inclusion criteria: pregnant women positive for BV via gram stain. Exclusion criteria: (a) > 20 weeks' gestation. (b) < 16 years of age. (c) Used AVs within 7 days prior to their screening visit. (d) Had a history of PTB (< 37 weeks). (e) Had a multiple gestation pregnancy. (f) Had major medical complications, such as chronic hypertension or pre‐existing diabetes. (g) Recent alcohol dependence. (h) Allergy to MET. Gestational age at trial entry: < 20 weeks. Total number of participants: 126. |
|
| Interventions | (a) Oral MET (250 mg 3x/day for 7 days) plus intravaginal placebo, n = 63 vs (b) intravaginal MET (5 g of 0.75% gel 2x/day for 5 days) plus oral placebo, n = 63. Placebos indistinguishable form active therapy. |
|
| Outcomes | Follow‐up was undertaken at 4 and 8 weeks after completion of treatment and at delivery.Outcomes reported are for the 4‐week visit. (a) Microbiological cure‐ Normal vaginal gram stain (score of 0‐3). (b) Clinical cure‐ Absence of all 4 clinical signs (no homogenous discharge, no amine odour after the addition of potassium hydroxide, no clue cells on saline microscopy, and pH < 4.5). (c) Therapeutic cure ‐ gram stain 0‐3 plus no symptoms. (d) Treatment failure ‐ persistent BV by gram stain or any symptoms. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random number tables with a 1: 1 ratio between study groups. |
| Allocation concealment (selection bias) | Unclear risk | The Investigational Drug Service at the University of Washington performed the randomisation and provided the treatment assignments in opaque, sealed envelopes. According to the Cochrane Handbook, http://www.cochrane‐handbook.org/, methods using envelopes are more susceptible to manipulation than other approaches (Schulz 1995). If investigators use envelopes, they should develop and monitor the allocation process to preserve concealment. In addition to use of sequentially numbered, opaque, sealed envelopes, they should ensure that the envelopes are opened sequentially, and only after the envelope has been irreversibly assigned to the participant. No mention was made of numbering and sequential opening in the article, thus the adequacy of allocation concealment was unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusion of participants after randomisation occurred. The analysis was not intention‐to‐treat. The data can not be re‐included. There were 10 patients lost to follow‐up in the vaginal MTZ group and 8 lost to follow‐up from the oral MTZ group. Details of why these participants were not followed‐up were not included. |
| Selective reporting (reporting bias) | Low risk | The outcome measures detailed in the methods section correspond to the results section. |
| Other bias | Low risk | No evidence of other bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither the participants nor the study personnel assessing treatment effect were aware of which active agent had been assigned. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
Moniri 2009.
| Methods | Randomised controlled trial. | |
| Participants |
Setting: Shabih Khani maternity hospital, Kashan, Iran. Inclusion criteria: women suffering from bacterial vaginosis (diagnosed based on clinical and laboratory findings). Exclusion criteria: not stated. Gestational age at trial entry: 20‐34 weeks. Total number of participants: 120. |
|
| Interventions | (a) MET 500 mg BID for 7 days, n = 60 vs (b) No treatment, n = 60. The no treatment group were untreated and uninformed of their BV status. All women studied had similar healthcare management throughout pregnancy. |
|
| Outcomes | (a) Delivery prior to 37 completed gestational weeks. (b) Delivery method. (c) Infection. (d) Fever. |
|
| Notes | Study only reports on the PTB outcome. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Appears to be no loss to follow‐up according to the results presented but not documented. |
| Selective reporting (reporting bias) | High risk | No reporting of 3 outcomes described in the abstract (delivery method; infection; fever). |
| Other bias | Low risk | None apparent, baseline characteristics similar between groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants were un‐blinded to their intervention; those allocated to the intervention group were told the result of their vaginal smear and treated, whereas those who were in the control group were untreated and uninformed regarding their BV status. It is reported that clinicians caring for the BV‐positive group randomised to non‐treatment were not told of the diagnosis. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Appears to be blinding with respect to outcome assessment: “Double‐blind follow‐up of the patients at the whole stages of parturition and after delivery with respect to the delivery methods, infection and fever was done by a practitioner besides the main researcher”. |
Morales 1994.
| Methods | Randomised trial (control was vitamin C). | |
| Participants |
Setting: high‐risk obstetric clinic (no further info). Inclusion criteria: pregnant women with a previous preterm delivery who were screened for BV at 13‐20 weeks (Amsels criteria). Exclusion criteria: trichomonas infection, medical complications, cocaine use, previous preterm delivery due to intrauterine infection or incompetent Cx, AB use during 2 weeks prior to trial entry, lethal fetal abnormality, 2nd trimester bleeding, asymptomatic bacteriuria on initial screen. Gestational age at trial entry: between 13 and 20 weeks. Total number of participants: 80. |
|
| Interventions | (a) MET 250 mg x 3/day for 7 days, n = 44 vs (b) matching vitamin C placebo, n = 36. |
|
| Outcomes | Admission for preterm labour; PTB (< 34 and < 37 weeks); low BW (< 2500 g); preterm rupture of membranes. | |
| Notes | Not intention‐to‐treat analysis ‐ women were excluded from the analysis if they failed to complete the assigned treatment ‐ 6/94 women in total (6%). Loss to follow‐up ‐ 5/94 in total (5%). Leaving 44 AB vs 36 placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Low risk | Pharmacy department (third party). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 94 were enrolled in the study. 14 patients were excluded from the analysis. 3 were excluded post randomisation due to requiring AB therapy for other conditions. The analysis was not intention‐to‐treat. 14 in total were lost from the study: 5 lost to follow‐up. 6 failed to complete assigned treatment; 3 (mentioned above) were excluded post‐randomisation due to requiring additional AB therapy for other conditions. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias. |
| Other bias | Low risk | No statistically significant differences at baseline were present and the study was not stopped early. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Control tablet was identical to intervention. Double‐blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
NICHD MFMU 2000.
| Methods | RCT. | |
| Participants |
Setting: multi‐centre. Inclusion criteria: pregnant women at 16‐23 + 6 weeks with asymptomatic BV (not TV+) (screened at 8‐22 + 6 weeks') gestation. Exclusion criteria: multifetal gestation; allergy to MET; current abuse of ethanol; AV treatment within previous 14 days; intention‐to‐receive antenatal care or deliver at location where no follow‐up possible; planned AV treatment before delivery; current/planned Cx cerclage; preterm labour before screening; current/planned tocolytic treatment; fetal death/known life‐threatening anomaly; medical illnesses requiring long‐term/intermittent drug treatment: if received any AVs between screening and study treatment, if time between screening and randomisation exceeded 8 weeks, or if tests for syphilis or chlamydia were positive. Gestational age at trial entry: 16‐ 23 weeks. Total number of participants: 1953 women. |
|
| Interventions | (a) 8 x 250 mg dose oral MET or plus repeat dose in 48 hrs (@ 16‐23 + 6 weeks' gestation). Second treatment at 24‐30 weeks' gestation, n = 966 vs (b) matching lactose placebo, n = 987. |
|
| Outcomes | "Test‐of‐cure" at least 14 days after initial visit and before second treatment; gestational age at delivery; BW; preterm SROM; clinical intra‐amniotic infection; postpartum endometritis; neonatal sepsis; use of tocolytic drugs; visits and admissions to hospital; preterm labour. | |
| Notes | Low recruitment response ‐ only 29% BV+ women were enrolled. 10% did not return for follow‐up visit, leaving 953 AV vs 966 placebo. Unpublished data on neonatal morbidity and admission to a neonatal unit were supplied by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, with stratification according to clinical centre. |
| Allocation concealment (selection bias) | Low risk | Central allocation, in correspondence with the author ‐ "Each woman was assigned a package of 4 bottles ‐ one for each treatment. The bottles were pre‐packaged at a central (remote) site, numbered to match allocation sequence, and shipped to each center. When a woman was randomized she was given the next sequentially numbered package. The package was stored, and her re‐treatment came from the same package." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on the week of gestation at delivery were missing for 34 women (1.7%), 13 in the MET group and 21 in the placebo group. Outcome data were available for 1919 of the 1953 women (98.3%). |
| Selective reporting (reporting bias) | Low risk | Outcomes were adequately reported. |
| Other bias | Low risk | Low risk of other bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In correspondence with author "Similarly, I think the primary outcome was free of blinding of outcome assessment. All women were required to have firm gestational dating (LMP and sonogram) before they could be randomized, and the estimate could not be changed after randomization. Therefore the only piece of information needed to assess the primary outcome was date of delivery, which is an objective data point." |
NICHD MFMU 2001.
| Methods | RCT. | |
| Participants |
Setting: multi‐centre (15 participating sites). Inclusion criteria: pregnant women at 16‐23 + 6 weeks with positive culture for TV plus asymptomatic BV (screened at 8‐22 + 6 weeks' gestation). Exclusion criteria: multifetal gestation; allergy to MET; current abuse of ethanol; AV treatment within previous 14 days; intention to receive antenatal care or deliver at location where no follow‐up possible; planned AV treatment before delivery; current/planned Cx cerclage; preterm labour before screening; current/planned tocolytic treatment; fetal death/known life‐threatening anomaly; medical illnesses requiring long‐term/intermittent drug treatment: if received any AVs between screening and study treatment, if time between screening and randomisation exceeded 8 weeks, or if tests for syphilis or chlamydia were positive. Gestational age at trial entry: 16‐23 weeks. Total number of participants: 617. |
|
| Interventions | (a) 8 x 250 mg dose oral MET plus repeat dose in 48 hrs n = 320 Second treatment at 24‐30 weeks' gestation vs (b) placebo with same regimen n = 297. |
|
| Outcomes | Preterm delivery; BW; ABs prescribed after randomisation; hospital admissions for preterm labour or PPROM; tocolysis; preterm rupture of membranes; clinical intra‐amniotic infection; postpartum endometritis; suspected or confirmed neonatal sepsis. | |
| Notes | Parallel study to NICHD MFMU 2000 assessing Met vs placebo for those with positive trichomonas. Subgroup that had BV plus trichomonas analysed. 119 AB vs 113 placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence with stratification by clinical centre. Computer‐generated randomisation, with stratification according to clinical centre, based on Trichomonas positive result. |
| Allocation concealment (selection bias) | Low risk | Central allocation, in correspondence with the author ‐ "Each woman was assigned a package of 4 bottles ‐ one for each treatment. The bottles were pre‐packaged at a central (remote) site, numbered to match allocation sequence, and shipped to each center. When a woman was randomized she was given the next sequentially numbered package. The package was stored, and her re‐treatment came from the same package." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were available for 604 out of 617 women who underwent randomisation (97.9 %). |
| Selective reporting (reporting bias) | Low risk | Outcomes were adequately reported. |
| Other bias | Low risk | Low risk of other bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In correspondence with author "Similarly, I think the primary outcome was free of blinding of outcome assessment. All women were required to have firm gestational dating (LMP and sonogram) before they could be randomized, and the estimate could not be changed after randomization. Therefore the only piece of information needed to assess the primary outcome was date of delivery, which is an objective data point." |
Odendaal 2002.
| Methods | RCT. | |
| Participants |
Setting: tertiary academic hospital. Inclusion criteria: 2 groups of women with BV (Spiegel's criteria): primigravidae at first antenatal visit, between 15 and 26 weeks' gestation; women with a previous preterm labour/midtrimester miscarriage. Exclusion criteria: multiple pregnancy; known cervical incompetence. Gestational age at trial entry: 15‐26 weeks. Total number of participants: 277 women. |
|
| Interventions | (a) Oral MET 400 mg twice daily for 2 days and if still BV positive after 4 weeks, repeat treatment course, n = 141 vs (b) oral placebo containing 100 mg vitamin C at matching times. n = 136. |
|
| Outcomes | "Test‐of‐cure" 4 weeks after; preterm delivery < 37, < 34, < 28 weeks' gestation; BW; intrauterine death; neonatal death; perinatal death; 5‐minute Apgar score. | |
| Notes | Women with a history of taking ABs within the previous 2 weeks had enrolment postponed for 2 weeks. Lost to follow‐up participants not separated into treatment/placebo. Intention‐to‐treat analysis of 128 AB vs 127 placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers were used to determine the sequence of vitamin C or MET tablets, which were kept in duplicated numbered sealed envelopes. Selection was done by picking the next envelope from a box. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment appears to have been achieved. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 177 women were randomised (150 primigravidae and 127 multigravidae). Of the primigravidae women, 67 were randomised to intervention and 83 to vitamin C. Of the multigravidae, 74 were randomised to intervention and 53 to vitamin C. A total of 141 women received the intervention, whilst only 128 returned for the second visit. A total of 136 women received vitamin C, whilst only 127 returned for the second visit. No women were excluded after randomisation. The analysis was not intention‐to‐treat. A total of 141 women received the intervention, whilst only 128 returned for the second visit. A total of 136 women received vitamin C, whilst only 127 returned for the second visit. |
| Selective reporting (reporting bias) | Low risk | The results were well reported and there was no evidence of selective reporting bias. |
| Other bias | Unclear risk | The study was not stopped early. Describe any baseline imbalance: The primigravidae and multigravidae groups were analysed separately. Thus, their baseline characteristics were also analysed separately. The baseline characteristics for the primigravidae were not significantly different between groups. However, the baseline characteristics were significantly different between groups in the multigravidae women, with significant differences present in age, % antenatal AV use and % asymptomatic bacteriuria. It is possible but not necessary that these differences could significantly influence the outcomes of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “Vitamin C was used for a placebo since the manufacturers could not supply a placebo identical to metronidazole”. Blinding was not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | See above. |
Porter 2001.
| Methods | Randomisation method unknown. | |
| Participants |
Setting: abstract only. Inclusion criteria: pregnant women with BV at 12 to 28 weeks' gestation (3 out of 4 Amsel criteria confirmed by Nugent's and Spiegel's criteria). Exclusion criteria: abstract only. Gestational age at trial entry: 12‐28 weeks. Total number of participants: 186. |
|
| Interventions | (a) Once daily vaginal MET gel (0.75%) for 5 days, n = 104 vs (b) twice daily vaginal MET gel (0.75%) for 5 days (no placebo), n = 82. Repeat treatment if positive at follow‐up. |
|
| Outcomes | "Test‐of‐cure" at unknown time after treatment; gestation at delivery; BW; 1‐min and 5‐min Apgar scores; caesarean section rate; spontaneous rupture of membranes; intra‐amniotic infection; endometritis; bladder infection. | |
| Notes | Study not yet completed. 186 out of 194 delivered at his point. No further publication of data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported in abstract. |
| Allocation concealment (selection bias) | Unclear risk | Not reported in abstract. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only abstract available, therefore none of the below information is available. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting bias. |
| Other bias | Low risk | The study was not stopped early. With the exception of a slightly older gestational age at enrolment in women treated with a single dose per day, there were no differences in the study populations at baseline. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comparison made between 2 frequencies of administration of MET vaginal gel without the use of a placebo therefore participants and personnel aware of the frequency of use. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was unclear. |
Shennan 2006.
| Methods | RCT. | |
| Participants |
Setting: 14 UK Hospitals. Inclusion Criteria: singleton pregnancy with history of previous PTB or PPROM before 37 weeks of gestation, previous late miscarriage (16 + 0–23 + 6 weeks of gestation), uterine anatomical abnormality, cervical surgery prior to the index pregnancy or current cervical cerclage were enrolled in the larger study. All women screened for BV using Nugents criteria. For this review we have included those women who screened positive for BV at trial entry. Exclusion Criteria: women who were unable or unwilling to give informed consent or who had been prescribed MET within a 4‐week period prior to recruitment. were excluded. Sexual intercourse within 48 hrs of fetal fibronectin testing was also an exclusion criterion. Gestational age at trial entry: pregnant women between 23 + 0 and 24 + 6 weeks of gestation. Total no. of participants: for this review have only included those who screened positive for BV at PREMET trial entry n = 13. |
|
| Interventions | MET 400 mg tds for 7 days or identical placebo. | |
| Outcomes | Delivery at < 30 weeks , < 34 weeks and < 37 weeks' gestation, PPROM, changes in BV status, onset of labour (spontaneous, induced or augmented SROM, prelabour CS), mode of delivery, gender, mean BW, low BW (< 2.5 kg), 1‐ and 5‐minute Apgar scores, SCN/NNU admission, severe neonatal morbidity (oxygen at 28 days, cerebral US abnormality, active resuscitation, IPPV > 12 hrs), mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation lists prepared by trial statistician. |
| Allocation concealment (selection bias) | Low risk | Randomisation lists sent to a commercial packaging company where the medication was prepackaged and labelled. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100 women with positive fetal fibronectin test were randomised. (47 to placebo and 53 to MET). Of these included in the review are those who screened positive for BV = 5 in placebo and 8 in MET. 1 lost to follow‐up in overall study but analysis by intention‐to‐treat. No loss to follow‐up of the women who screened positive for BV. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Tablets encased in identical capsules and sealed in opaque containers. When trial drug prescribed pharmacist dispensed the next number from the list at each of the trial centres. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
Ugwumadu 2003.
| Methods | RCT. | |
| Participants |
Setting: St George’s Hospital, London, UK, and St Helier Hospital, Surrey, UK. Inclusion criteria: pregnant women (12‐22 weeks) with asymptomatic intermediate flora (Nugent score 4‐6) or BV (Nugent 7‐10). Exclusion criteria: multiple pregnancy; needed or had cervical cerclage; history of cone biopsy; uterine, cervical, or fetal anomaly; disorders such as diabetes, renal disease, collagen disease, lupus, antiphospholipid syndrome, or essential hypertension; known allergy to clindamycin; or were younger than 16 years of age. Women who reported a fishy smelling vaginal discharge, either voluntarily or on direct questioning, received treatment and further genitourinary screening for sexually transmitted pathogens, but were excluded from randomisation. Gestational age at trial entry: 12‐22 weeks. Total number of participants: 494. |
|
| Interventions | (a) Oral clindamycin 300 mg, n = 244 vs (B) placebo twice daily for 5 days, n = 241. |
|
| Outcomes | Spontaneous PTB (> or = 24 to < 37 weeks and late miscarriage (> or = 13 weeks but < 24 weeks). Death in utero. "Test of cure" at 14 days post AB or placebo, NICU admission, BW < 2500, BW < 1500. | |
| Notes | Intention‐to‐treat analysis. 9 women lost to follow‐up, leaving 244 AB vs 241 placebo. PTB stratified by Nugent score 1‐10, previous PTB, and race. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer program was used to randomly assign the numbers 1‐500 to clindamycin or placebo treatment. |
| Allocation concealment (selection bias) | Low risk | A trial pharmacist used this randomised list to package bottles of 5‐day courses of either clindamycin (intervention) or placebo. The trial pharmacist retained the code for the group allocation within a sealed envelope until all study data had been obtained and analysed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 494 were randomly allocated. 294 were randomised to the intervention group whilst 245 were randomised to the placebo. Of those in the intervention group (249), 4 were lost to follow‐up and 1 was lost to elective termination. Of those in the placebo group (245), 1 was lost to follow‐up and 3 were lost to elective termination. 25 women (5%) had side‐effects and discontinued therapy; 8 took placebo and 17 clindamycin (intervention). 4 women (2 intervention, 2 placebo) were treated with AVs for STIs. 12 women received AV treatment for other indications before onset of labour. All were included in the analysis. The analysis was intention‐to‐treat, although, the patients that were lost to follow‐up (5 from the intervention group and 4 in the placebo group). |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias was evident. |
| Other bias | Low risk | The study was not stopped early. No statistical test was undertaken to determine the differences between intervention and placebo groups, although a baseline characteristics table was provided in the text, complete with standard deviations and visual inspection of the figures showed no differences that are likely to be significant. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The intervention and placebo capsules were identical. Neither the participants nor the investigators knew the contents of any of the pre‐packed bottles. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See row above. |
Vermeulen 1999.
| Methods | RCT. | |
| Participants |
Setting: 12 city hospitals in The Netherlands. Inclusion criteria: pregnant women with a history of spontaneous PTB in preceding pregnancy. Exclusion criteria: multiple pregnancy, major fetal congenital anomalies, previous PTB associated with hypertension or pre‐eclampsia, placental disorders, congenital uterine anomalies, maternal diseases or allergy to clindamycin. Gestational age at trial entry: 26 weeks. Total number of participants: 168 women. |
|
| Interventions | (a) 2% clindamycin vaginal cream for 7 days at 26 weeks and again at 32 weeks or n = 83 vs (b) matching placebo cream daily for 7 days at 26 weeks and again at 32 weeks n = 85. |
|
| Outcomes | Spontaneous preterm delivery < 37 weeks; admission for threatened preterm labour; neonatal infectious morbidity; infectious morbidity associated with sepsis; pneumonia. | |
| Notes | Treated all high‐risk women with and without BV. Low sample size: needed 566 but enrolled 168. Only 11 BV positive women in AB group vs 11 placebo. Intention‐to‐treat analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed in blocks of 4 and was stratified by centre and by BV. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was achieved by a research co‐ordinator allocating medication or placebo using a pre‐determined randomisation list. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 168 women were randomised. 83 women were randomised to the intervention group whilst 85 were randomised to the placebo group. Analysis was undertaken in both intention‐to‐treat and complete trial of medication groups. 8 women in the placebo group and 4 in the clindamycin group had not taken any medication at all. In 5 women in the placebo group and 9 in the clindamycin group, follow‐up was not complete because of not applying medication at the scheduled times or being lost to follow‐up. Thus, a total of 13 women were not included in the placebo group and a total of 13 women were not included in the intervention group for the analysis “complete trial of medication”. However, all of the above mentioned women were included in the intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported in the results and there is no evidence of selective reporting bias. |
| Other bias | Low risk | The study was not stopped early. No statistical test was undertaken to check for baseline imbalances, although mean/n values and SDs were provided for baseline characteristics. There may have been a significant difference between the numbers of primiparous (10 more in the placebo group than the clindamycin group). No other noteworthy differences were apparent on visual inspection. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The placebo was identical to the intervention. Care providers and patients were blinded regarding the presence of BV and to the intervention allocation. The randomisation code was left unbroken until the last patient had delivered. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind. |
AB: antibiotic BV: bacterial vaginosis BW: birthweight CS: caesarean section CVC: Clindamycin vaginal cream Cx: cervix hr: hour LMP: last menstrual period MET: metronidazole min: minutes NICU: neonatal intensive care unit PPROM: preterm premature rupture of membranes PROM: premature rupture of membranes PTB: preterm birth RCT: randomised controlled trial SROM = spontaneous rupture of membranes tds: three times daily TPL: threatened preterm labour TV: Trichomonas vaginalis vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andrews 2005 | Women not pregnant at randomisation and treatment plus women not specifically screened for bacterial vaginosis. |
| Goldenberg 2006 | Studied 3120 HIV+ and 600 HIV‐ women. No pregnancy outcomes for bacterial vaginosis positive women. |
| Hawkinson 1966 | Study conducted before diagnostic criteria for bacterial vaginosis were established. |
| Hitti 2002 | No pregnancy outcome data. |
| Holst 1990 | Not a randomised trial. Not an evaluation of an antibiotic regimen. |
| Klebanoff 2004 | Study of regression of asymptomatic BV. No pregnancy outcome data. |
| Kurtzman 2008 | Abstract for the diagnostic test aspect of fetal fibronectin in the PREMET (Shennan 2006) study. No BV data. |
| Leitich 2003 | Meta‐analysis of existing studies. |
| McGregor 1994 | This was a 2‐ phase observational trial (phase 1 ‐ examination for BV and micro‐organisms: phase 2 ‐ treatment for infected women) and is not a randomised placebo‐controlled trial. |
| Mitchell 2009a | Nested case‐control sub‐study of the larger Mitchell 2009 study which is already included in review. The smaller study focused on bacterial concentrations in oral vs vaginal metronidazole groups. |
| Neri 1993 | Intervention agent yogurt. Did not fulfil entry criteria for review. |
| Paternoster 2004 | Intervention not antibiotic. |
| Rosnes 2002 | No evaluation of pregnancy outcome. |
| Schoeman 2005 | Substudy of the predictive value of BV on PTD but within the Steyn 2003 study of women randomised to vitamin C or placebo and not an antibiotic regimen. |
| Steyn 2003 | Not an evaluation of an antibiotic regimen. |
| Thiagarajan 1998 | No evaluation of pregnancy outcome. |
| Ugwumadu 1999 | No usable data available, trial report in abstract form only. |
| Ugwumadu 2006 | Substudy of the included Ugwumadu 2003 study assessing histological chorioamnionitis in clindamycin vs placebo groups. Placental inflammation reported by areas but areas not mutually exclusive therefore cannot from the published data report overall histological chorioamnionitis incidence in each group. Author contacted for these data ‐ response awaited. |
| Yudin 2002 | No evaluation of pregnancy outcome. |
| Yudin 2003 | No evaluation of pregnancy outcome. |
BV: bacterial vaginosis vs: versus
Characteristics of ongoing studies [ordered by study ID]
Subtil 2008.
| Trial name or title | Prevention of Very Preterm Delivery by Testing for and Treatment of Bacterial Vaginosis (PREMEVA). |
| Methods | Double blind placebo‐controlled RCT. |
| Participants | Pregnant women > 18 years of age and diagnosed with BV before 13 weeks will be divided into low‐ and high‐risk groups. They will be defined as at low risk when they have no history of spontaneous preterm delivery or late abortion. Women with such histories will be defined as at high risk. Inclusion criteria:
Exclusion criteria:
|
| Interventions | Low‐risk patients will be asked to participate in a trial with 3 equal parallel groups, comparing 2 regimes of clindamycin (1 or 3 4‐day treatments of clindamycin 300 mgx2/d) and placebo. High‐risk patients will be asked to participate in a trial with 2 parallel groups to assess the usefulness of repeating antibiotic treatment monthly by comparing the administration of 1 4‐day treatment of clindamycin (300 mgx2/d) to 3 4‐day treatments, 1 month apart. |
| Outcomes | Primary outcome measures: premature delivery (16 to 32 weeks of gestation) (time frame: at delivery) (designated as safety issue: yes). Secondary outcome measures: preterm labour, PPROM, spontaneous preterm labor, PROM, abruptio placentae, chorioamnionitis, fever > 38°C during labour, postpartum fever (> 38°), postpartum wound infections, perinatal death, NICU transfer, bacterial neonatal colonisation (time frame: at delivery) (designated as safety issue: yes). |
| Starting date | April 2006. |
| Contact information | Contact: Damien SUBTIL, PHD 33 +3 20 44 66 26 d‐subtil@chru‐lille.fr |
| Notes | Estimated study completion December 2011. |
BV: bacterial vaginosis NICU: neonatal intensive care unit PROM: premature rupture of membranes PPROM: preterm premature rupture of membranes RCT: randomised controlled trial
Differences between protocol and review
Two outcomes, prolongation of gestational age and birthweight are outcomes which were included and analysed within this review. They were not prespecified outcomes in the protocol but were included in this update as they represent the continuous versions of the prespecifed categorical variables of preterm birth and low birthweight.
In addition to the subgroups specified in the protocol, we also investigated the effect of different types of antibiotics. We did not restrict investigation of different subgroups of antibiotics to particular outcomes.
Contributions of authors
Peter Brocklehurst, as contact author for the first review, was primarily responsible for writing the text, responding to comments, etc. As co‐author for the second, third and fourth reviews, he reviewed studies and contributed to the revised text.
Adrienne Gordon, co‐author for the third and contact author for the fourth review, reviewed studies, extracted data, performed double entry of data, restructured tables, assessed risk of bias, contacted trial authors and contributed to the revised text.
Emer Heatley, co‐author for the fourth review reviewed studies, extracted data, assessed risk of bias, initiated review restructuring, added additional tables and references, and contributed to the revised text.
Stephen Milan, co‐author for the fourth review reviewed studies, extracted data, assessed risk of bias, performed review restructuring, and contributed to the revised text.
Sources of support
Internal sources
Women's and Children's Hospital, North Adelaide, Australia.
-
Cochrane Pregnancy and Childbirth Group, Institute of Translational Medicine, University of Liverpool, UK.
Provision of research associate Steve Milan to assist with review update.
External sources
-
National Institute for Health Research, UK.
NIHR Programme of centrally‐managed pregnancy and childbirth systematic reviews of priority to the NHS and users of the NHS:10/4001/02
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Darwish 2007 {published data only}
- Darwish A, Elnshar EM, Hamadeh SM, Makarem MH. Treatment options for bacterial vaginosis in patients at high risk of preterm labor and premature rupture of membranes. Journal of Obstetrics and Gynaecology Research 2007;33(6):781‐7. [DOI] [PubMed] [Google Scholar]
Duff 1991 {published data only}
- Duff P, Lee ML, Hillier SL, Herd LM, Krohn MA, Eschenbach DA. Amoxicillin treatment of bacterial vaginosis during pregnancy. Obstetrics & Gynecology 1991;77:431‐5. [PubMed] [Google Scholar]
Giuffrida 2006 {published data only}
- Giuffrida G, Mangiacasale A. Bacterial vaginosis in pregnancy: treatment with peroxen vs vaginal clindamycin [Vaginosi batteriche in gravidanza: trattamento con peroxen vs clindamicina intravaginale.]. Giornale Italiano di Ostetricia e Ginecologia 2006;28(12):539‐43. [Google Scholar]
Guaschino 2003 {published data only}
- Guaschino S, Grimaldi E, Seta F, Mangino F. Bacterial vaginosis and preterm delivery. Acta Obstetricia et Gynecologica Scandinavica Supplement 1997;76(167):35. [Google Scholar]
- Guaschino S, Ricci E, Franchi M, Frate GD, Tibaldi C, Santo DD, et al. Treatment of asymptomatic bacterial vaginosis to prevent pre‐term delivery: a randomised trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2003;110:149‐52. [DOI] [PubMed] [Google Scholar]
Hauth 1995 {published data only}
- Hauth J, Goldenberg R, Andrews W, Copper R, Schmid T. Efficacy of metronidazole plus erythromycin to decrease bacterial vaginosis and other markers of altered vaginal flora [abstract]. American Journal of Obstetrics and Gynecology 1993;168:421. [Google Scholar]
- Hauth J, Goldenberg R, Andrews W, Dubard M, Copper R. Mid trimester treatment with metronidazole plus erythromycin reduces preterm delivery only in women with bacterial vaginosis. American Journal of Obstetrics and Gynecology 1995;172:253. [Google Scholar]
- Hauth J, Goldenberg R, Andrews W, Dubard M, Copper R. Reduced incidence of preterm delivery with metronidazole and erythromycin in women with bacterial vaginosis. Obstetrical & Gynecological Survey 1996;51(6):329‐30. [DOI] [PubMed] [Google Scholar]
- Hauth JC, Goldenberg RL, Andrews WW, Copper RL, DuBard MB, Schmid T. The effect of mid‐trimester metronidazole plus erythromycin on bacterial vaginosis and other markers of altered vaginal flora [abstract]. American Journal of Obstetrics and Gynecology 1995;172:303. [Google Scholar]
- Hauth JC, Goldenberg RL, Andrews WW, DuBard MB, Copper RL. Reduced incidence of preterm delivery with metronidazole and erythromycin in women with bacterial vaginosis. New England Journal of Medicine 1995;333:1732‐6. [DOI] [PubMed] [Google Scholar]
Joesoef 1995 {published data only}
- Joesoef MR, Hillier SL, Wiknjosastro G, Sumampouw H, Linnan M, Norojono W, et al. Intravaginal clindamycin treatment for bacterial vaginosis: effects on preterm delivery and low birth weight. American Journal of Obstetrics and Gynecology 1995;173:1527‐31. [DOI] [PubMed] [Google Scholar]
Kekki 2001 {published data only}
- Kekki M, Kurki T, Kurkinen‐Raty M, Pelkonen J, Paavonen J. Recurrent bacterial vaginosis in pregnancy predisposes to infectious morbidity: a double‐blind, placebo‐controlled multicenter intervention trial with vaginal clindamycin. International Journal of Gynecology & Obstetrics 1999;67(Suppl 2):S42. [Google Scholar]
- Kekki M, Kurki T, Paavonen J, Rutanen E‐M. Insulin‐like growth factor binding protein‐1 in cervix as a marker of infectious complications in pregnant women with bacterial vaginosis. Lancet 1999;353:1494. [DOI] [PubMed] [Google Scholar]
- Kekki M, Kurki T, Pelkonen J, Kurkinen‐Raty M, Cacciatore B, Paavonen J. Bacterial vaginosis in pregnancy is associated with infectious morbidity ‐ a randomized placebo‐controlled intervention trial with vaginal clindamycin. Acta Obstetricia et Gynecologica Scandinavica Supplement 1996;76(167):28. [Google Scholar]
- Kekki M, Kurki T, Pelkonen J, Kurkinen‐Raty M, Cacciatore B, Paavonen J. Vaginal clindamycin in preventing preterm birth and peripartal infections in asymptomatic women with bacterial vaginosis: a randomized, controlled trial. Obstetrics & Gynecology 2001;97(5 Pt 1):643‐8. [DOI] [PubMed] [Google Scholar]
- Kekki M, Kurki T, Pelkonen J, Kurkinen‐Raty M, Cacciatore B, Paavonen J. Vaginal clindamycin is ineffective in preventing preterm birth and peripartum infections in a low risk population with bacterial vaginosis: a double‐blind placebo‐controlled multicenter trial. XVI FIGO World Congress of Obstetrics and Gynecology (Book 3); 2000 September 3‐8; Washington DC, USA:18. 2000.
- Kekki M, Kurki T, Polkonen J, Cacciatore B, Paavonen J. Bacterial vaginosis in pregnancy is associated with infectious morbidity ‐ a randomized placebo‐controlled trial with vaginal clindamycin. Acta Obstetricia et Gynecologica Scandinavica 1996;75:87. [Google Scholar]
- Kurkinen‐Raty M, Vuopala S, Koskela M, Kekki M, Kurki T, Paavonen J, et al. A randomised controlled trial of vaginal clindamycin for early pregnancy bacterial vaginosis. BJOG: an international journal of obstetrics and gynaecology 2000;107:1427‐32. [DOI] [PubMed] [Google Scholar]
Kiss 2004 {published data only}
- Kiss H, Petricevic L, Husslein P. Prospective randomised controlled trial of an infection screening programme to reduce the rate of preterm delivery. BMJ 2004;329:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamont 2003 {published data only}
- Lamont R, Adinkra P, Mason M. The use of clindamycin intravaginal cream in the treatment of abnormal genital tract flora‐ safety and efficacy. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3‐6; Cape Town, South Africa. 1999:82.
- Lamont R, Morgan D, Sheehan M, Duncan S, Mandal D. The outcome of pregnancy following the use of clindamycin intravaginal cream for the treatment of abnormal colonization: a prospective, randomised, double‐blind, placebo‐controlled multicenter study. International Journal of Gynecology & Obstetrics 1999;67(Suppl 2):S42. [Google Scholar]
- Lamont RF, Duncan SL, Mandal D, Bassett P. Intravaginal clindamycin to reduce preterm birth in women with abnormal genital tract flora. Obstetrics & Gynecology 2003;101(3):516‐22. [DOI] [PubMed] [Google Scholar]
- Lamont RF, Jones BM, Mandal D, Hay PE, Sheehan M. The efficacy of vaginal clindamycin for the treatment of abnormal genital tract flora in pregnancy. Infectious Diseases in Obstetrics and Gynecology 2003;11(4):181‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RF, Sheehan M, Morgan DJ, Mandal D, Duncan SLB. Safety and efficacy of clindamycin intravaginal cream for the treatment of bacterial vaginosis in pregnancy: a randomized, double‐blind placebo‐controlled multicenter study. International Journal of Gynecology & Obstetrics 1999;67(Suppl 2):S45. [Google Scholar]
- Mason MR, Lamont RF, Adinkra PE. Pregnancy outcome following treatment of abnormal genital tract flora with a three day course of 2% clindamycin. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3‐6; Cape Town, South Africa. 1999:82‐3.
- Rosenstein I, Morgan D, Sheehan M, Lamont R, Taylor‐Robinson D. Effect of topical clindamycin on bacterial vaginosis and outcome of pregnancy. International Journal of Gynecology & Obstetrics 1999;67(Suppl 2):S43. [Google Scholar]
- Rosenstein IJ, Morgan DJ, Lamont RF, Sheehan M, Dore CJ, Hay PE, et al. Effect of intravaginal clindamycin cream on pregnancy outcome and on abnormal vaginal microbial flora of pregnant women. Infectious Diseases in Obstetrics and Gynecology 2000;8:158‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor‐Robinson D, Morgan DJ, Sheehan M, Rosenstein IJ, Lamont RF. Relation between Gram‐stain and clinical criteria for diagnosing bacterial vaginosis with special reference to Gram grade II evaluation. International Journal of STD and AIDS 2003;14:6‐10. [DOI] [PubMed] [Google Scholar]
Larsson 2006 {published data only}
- Larsson PG, Fahraeus L, Carlsson B, Jakobsson T, Forsum U, the premature study group of the southeast health care region of Sweden. Late miscarriage and preterm birth after treatment with clindamycin: a randomised consent design study according to Zelen. BJOG: an international journal of obstetrics and gynaecology 2006;113(6):629‐37. [DOI] [PubMed] [Google Scholar]
McDonald 1997 {published and unpublished data}
- McDonald HM, O'Loughlin JA, Vigneswaran R, Jolley PT, Harvey JA, Bof A, et al. Impact of metronidazole therapy on preterm birth in women with bacterial vaginosis flora (Gardnerella vaginalis): a randomised, placebo controlled trial. British Journal of Obstetrics and Gynaecology 1997;104:1391‐7. [DOI] [PubMed] [Google Scholar]
- McDonald HM, O'Loughlin JA, Vigneswaran R, Jolley PT, McDonald PJ. Bacterial vaginosis in pregnancy and efficacy of short‐course oral metronidazole treatment: a randomised controlled trial. International Journal of Gynecology & Obstetrics 1995;49:225. [PubMed] [Google Scholar]
- McDonald HM, O'Loughlin JA, Vigneswaran R, Jolley PT, McDonald PJ. Bacterial vaginosis in pregnancy and efficacy of short‐course oral metronidazole treatment: a randomized controlled trial. Obstetrics & Gynecology 1994;84:343‐8. [PubMed] [Google Scholar]
- McDonald HM, O'Loughlin JA, Vigneswaran R, McDonald PJ, Jolley PT, Pharm B, et al. Metronidazole treatment of bacterial vaginosis in pregnancy, and preterm birth: a randomized, placebo‐controlled trial. Infectious Diseases in Obstetrics and Gynecology 1996;4:49. [PubMed] [Google Scholar]
- Vigneswaran R, O'Loughlin J, McDonald H, McDonald P, Jolley P, Harvey J, et al. Metronidazole treatment of bacterial vaginosis in pregnancy, and effect on preterm birth. Prenatal and Neonatal Medicine 1996;1(Suppl 1):160. [Google Scholar]
Mitchell 2009 {published data only}
- Mitchell C, Balkus J, Agnew K, Lawler R, Hitti J. Changes in the vaginal microenvironment with metronidazole treatment for bacterial vaginosis in early pregnancy. Journal of Women's Health 2009;18(11):1817‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moniri 2009 {published data only}
- Moniri R, Behrashi M. Effects of metronidazole therapy on preterm labor in women with bacterial vaginosis. Acta Medica Iranica 2009;47(3):181‐4. [Google Scholar]
Morales 1994 {published data only}
- Morales WJ, Schorr S, Albritton J. Effect of metronidazole in patients with preterm birth in preceeding pregnancy and bacterial vaginosis: a placebo‐contolled, double‐blind study. American Journal of Obstetrics and Gynecology 1994;171:345‐9. [DOI] [PubMed] [Google Scholar]
- Morales WJ, Schorr SJ, Albritton J. Effect of metronidazole in patients with history of preterm birth and bacterial vaginosis: a placebo control double blind study [abstract]. American Journal of Obstetrics and Gynecology 1993;168:377. [DOI] [PubMed] [Google Scholar]
NICHD MFMU 2000 {published data only}
- Carey J, Klebanoff M, Hauth J, Hillier S, Thom E, Ernest J, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. New England Journal of Medicine 2000;342:534‐40. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Klebanoff M, Carey JC, MacPherson C. Metronidazole treatment of women with a positive fetal fibronectin test result. American Journal of Obstetrics and Gynecology 2001;185:485‐6. [DOI] [PubMed] [Google Scholar]
- Hauth J. Response of the three components of a vaginal Gram stain score to metronidazole treatment and in relation to preterm birth. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S56. [Google Scholar]
- Hauth J, Cliver S, Hodgkins P, Andrews W, Schwebke J, Hook E, et al. Mid‐trimester metronidazole and azithromycin did not prevent preterm birth in women at increased risk: a double blind trial. American Journal of Obstetrics and Gynecology 2001;185(6):S86. [Google Scholar]
- Klebanoff M, Carey J. Metronidazole did not prevent preterm birth in asymptomatic women with bacterial vaginosis. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):2. [Google Scholar]
- Sheffield J. The effect of bacterial vaginosis treatment on the acquisition of other symptomatic sexually transmitted diseases during pregnancy [abstract]. American Journal of Obstetrics and Gynecology 2001;184(1 Pt 2):S83. [Google Scholar]
NICHD MFMU 2001 {published data only}
- Klebanoff M, Carey C, Hauth JC, Hillier SL, Nugent RP, National Institute of Child Health and Human Development Network of Maternal‐Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic trichomonas vaginalis infection. New England Journal of Medicine 2001;345:487‐93. [DOI] [PubMed] [Google Scholar]
Odendaal 2002 {published data only}
- Odendaal H, Popov I, Schoeman J, Smith M, Grove D. Preterm labour ‐ is bacterial vaginosis involved?. South African Medical Journal 2002;92(3):231‐4. [PubMed] [Google Scholar]
Porter 2001 {published data only}
- Porter K, Rambo D, Jazayeri A, Jazayeri M, Prien S. Prospective randomized trial of once versus twice a day metronidazole‐vaginal in obstetrical population identified with bacterial vaginosis. American Journal of Obstetrics and Gynecology 2001;184(1 Pt 2):S166. [Google Scholar]
Shennan 2006 {published data only}
- Shennan A, Crawshaw S, Briley A, Hawken J, Seed P, Jones G, et al. A randomised controlled trial of metronidazole for the prevention of preterm birth in women positive for cervicovaginal fetal fibronectin: the PREMET study. BJOG: an international journal of obstetrics and gynaecology 2006;113(1):65‐74. [DOI] [PubMed] [Google Scholar]
- Thompson C. Taken before their time. Nursing Times 2002;98(3):29. [Google Scholar]
Ugwumadu 2003 {published data only}
- Ugwumadu A, Manyonda I, Reid F, Hay P. Effect of early oral clindamycin on late miscarriage and preterm delivery in asymptomatic women with abnormal vaginal flora and bacterial vaginosis: a randomised controlled trial. Lancet 2003;361:983‐8. [DOI] [PubMed] [Google Scholar]
- Ugwumadu A, Manyonda I, Reid F, Hay P. Effect of early oral clindamycin on late miscarriage and preterm delivery in asymptomatic women with abnormal vaginal flora and bacterial vaginosis: a randomised controlled trial. Obstetrical & Gynecological Survey 2003;58(9):566‐7. [DOI] [PubMed] [Google Scholar]
- Ugwumadu A, Manyonda I, Reid F, Hay P. Oral clindamycin for asymptomatic abnormal vaginal flora early in the second trimester of pregnancy reduces late miscarriage and spontaneous preterm birth [abstract]. BJOG: an international journal of obstetrics and gynaecology 2003;110:447. [Google Scholar]
- Ugwumadu A, Reid F, Hay P, Manyonda I. Natural history of bacterial vaginosis and intermediate flora in pregnancy and effect of oral clindamycin. Obstetrics & Gynecology 2004;104(1):114‐9. [DOI] [PubMed] [Google Scholar]
Vermeulen 1999 {published data only}
- Mason MR, Adinkra PE, Lamont RF. Prophylactic administration of clindamycin 2% vaginal cream to reduce the incidence of spontaneous preterm birth in women with an increased recurrence risk: a randomised placebo‐controlled double ‐blind trial [letter]. BJOG: an international journal of obstetrics and gynaecology 2000;107(2):295‐6. [DOI] [PubMed] [Google Scholar]
- Vermeulen GM, Bruinse HW. Prophylactic administration of clindamycin 2% vaginal cream to reduce the incidence of spontaneous preterm birth in women with an increased recurrence risk: a randomised placebo‐controlled double‐blind trial. British Journal of Obstetrics and Gynaecology 1999;106:652‐7. [DOI] [PubMed] [Google Scholar]
- Vermeulen GM, Zwet AA, Bruinse HW. Changes in the vaginal flora after two percent clindamycin vaginal cream in women at high risk of spontaneous preterm birth. BJOG: an international journal of obstetrics and gynaecology 2001;108:697‐700. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Andrews 2005 {published data only}
- Andrews WW, Goldenberg RL, Hauth JC, Cliver SP, Copper R, Conner M. Interconceptional antibiotics to prevent spontaneous preterm birth: a randomized clinical trial. American Journal of Obstetrics and Gynecology 2006;194:617‐23. [DOI] [PubMed] [Google Scholar]
Goldenberg 2006 {published data only}
- Goldenberg RL, Mwatha A, Read J, Adeniyi‐Jones S, Sinkala M, Msmanga G, et al. The HPTN 024 study: the efficacy of antibiotics to prevent chorioamnionitis and preterm birth. American Journal of Obstetrics and Gynecology 2006;194(3):650‐61. [DOI] [PubMed] [Google Scholar]
Hawkinson 1966 {published data only}
- Hawkinson J, Schulman H. Prematurity associated with cervicitis and vaginitis during pregnancy. American Journal of Obstetrics and Gynecology 1966;94:898‐902. [DOI] [PubMed] [Google Scholar]
Hitti 2002 {published data only}
- Hitti J, Kullberg J, Lee Z, Culhane J, Lawler R, Hogan V, et al. Vaginal inflammation and secretory leukocyte protease inhibitor among women with bacterial vaginosis in early pregnancy: response to antibiotic treatment. Infectious Diseases in Obstetrics and Gynecology 2002;10(Suppl 1):S19. [Google Scholar]
Holst 1990 {published data only}
- Holst E, Brandberg A. Treatment of bacterial vaginosis in pregnancy with a lactate gel. Scandinavian Journal of Infectious Diseases 1990;22:625‐6. [DOI] [PubMed] [Google Scholar]
Klebanoff 2004 {published data only}
- Klebanoff M, Hauth J, MacPherson CA, Carey J, Heine R, Wapner R, et al. Time course of the regression of asymptomatic bacterial vaginosis in pregnancy with and without treatment. American Journal of Obstetrics and Gynecology 2004;190:363‐70. [DOI] [PubMed] [Google Scholar]
Kurtzman 2008 {published data only}
- Kurtzman J, Chandiramani M, Briley A, Poston L, Shennan A. Quantitative fetal fibronectin screening at 24 weeks substantially discriminates the risk of recurrent preterm delivery in asymptomatic patients with prior preterm birth. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S10. [DOI] [PubMed] [Google Scholar]
Leitich 2003 {published data only}
- Leitich H, Bodner‐Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta‐analysis. American Journal of Obstetrics and Gynecology 2003;189(1):139‐47. [DOI] [PubMed] [Google Scholar]
McGregor 1994 {published data only}
- McGregor JA, French JI, Jones W, Milligan K, McKinney PJ, Patterson E, et al. Bacterial vaginosis is associated with prematurity and vaginal fluid mucinase and sialidase: results of a controlled trial of topical clindamycin cream. American Journal of Obstetrics and Gynecology 1994;170:1048‐60. [DOI] [PubMed] [Google Scholar]
Mitchell 2009a {published data only}
- Mitchell CM, Hitti JE, Agnew KJ, Fredricks DN. Comparison of oral and vaginal metronidazole for treatment of bacterial vaginosis in pregnancy: impact on fastidious bacteria. BMC Infectious Diseases 2009;9:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neri 1993 {published data only}
- Neri A, Sabah G, Samra Z. Bacterial vaginosis in pregnancy treated with yoghurt. Acta Obstetricia et Gynecologica Scandinavica 1993;72:17‐9. [DOI] [PubMed] [Google Scholar]
Paternoster 2004 {published data only}
- Paternoster DM, Tudor L, Milani M, Maggino T, Ambrosini A. Efficacy of an acidic vaginal gel on vaginal pH and interleukin‐6 levels in low‐risk pregnant women: a double‐blind, randomized placebo‐controlled trial. Journal of Maternal‐Fetal & Neonatal Medicine 2004;15(3):198‐201. [DOI] [PubMed] [Google Scholar]
Rosnes 2002 {published data only}
- Rosnes J, NICHD MFMU Network. Does vaginal ph or gram stain score alter the likelihood of successful metronidazole treatment of bacterial vaginosis (bv) or trichomonas vaginalis (tv) during pregnancy [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S228. [Google Scholar]
Schoeman 2005 {published data only}
- Schoeman J, Steyn PS, Odendaal HJ, Grove D. Bacterial vaginosis diagnosed at the first antenatal visit better predicts preterm labour than diagnosis later in pregnancy. Journal of Obstetrics and Gynaecology 2005;25(8):751‐3. [DOI] [PubMed] [Google Scholar]
Steyn 2003 {published data only}
- Steyn PS, Odendaal HJ, Schoeman J, Stander C, Fanie N, Grove D. A randomised, double‐blind placebo‐controlled trial of ascorbic acid supplementation for the prevention of preterm labour. Journal of Obstetrics and Gynaecology 2003;23(2):150‐5. [DOI] [PubMed] [Google Scholar]
Thiagarajan 1998 {published data only}
- Thiagarajan M. Evaluation of the use of yogurt in treating bacterial vaginosis in pregnancy [abstract]. Journal of Clinical Epidemiology 1998;51 Suppl 1:22S. [Google Scholar]
Ugwumadu 1999 {published data only}
- Ugwumadu A, Manyonda I, Hay PE. The natural history of bacterial vaginosis in pregnancy: results from a randomized, placebo‐controlled and double‐blind trial of systemic clindamycin at 12‐18 weeks gestation. International Journal of Gynecology and Obstetrics 1999;67(Suppl 2):S43. [Google Scholar]
Ugwumadu 2006 {published data only}
- Ugwumadu A, Reid F, Hay P, Manyonda I, Jeffrey I. Oral clindamycin and histologic chorioamnionitis in women with abnormal vaginal flora. Obstetrics & Gynecology 2006;107(4):863‐8. [DOI] [PubMed] [Google Scholar]
Yudin 2002 {published data only}
- Yudin MH, Landers DV, Hillier SL. Cytokine profiles in response to bacterial vaginosis in pregnancy before and after treatment with oral or vaginal metronidazole. Infectious Diseases in Obstetrics and Gynecology 2002;10(Suppl 1):S18. [DOI] [PubMed] [Google Scholar]
Yudin 2003 {published data only}
- Yudin MH, Landers DV, Meyn L, Hillier SL. Clinical and cervical cytokine response to treatment with oral or vaginal metronidazole for bacterial vaginosis during pregnancy: a randomized trial. Obstetrics & Gynecology 2003;102(3):527‐34. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Subtil 2008 {published data only}
- Subtil D. Prevention of very preterm delivery by testing for and treatment of bacterial vaginosis. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 9 April 2008).
Additional references
Amsel 1983
- Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. American Journal of Medicine 1983;74:14‐22. [DOI] [PubMed] [Google Scholar]
Annells 2004
- Annells MF, Hart PH, Mullighan CG, Heatley SL, Robinson JS, Bardy P, et al. Interleukins‐1, ‐4, ‐6, ‐10, tumour necrosis factor, transforming growth factor‐B, FAS, and mannose‐binding protein C gene polymorphisms in Australian women: Risk of preterm birth. American Journal of Obstetrics and Gynecology 2004;191:2056‐67. [DOI] [PubMed] [Google Scholar]
Annells 2005
- Annells MF, Hart PH, Mullighan CG, Heatley SL, Robinson JS, McDonald HM. Polymorphisms in immunoregulatory genes and the risk of histologic chorioamnionitis in Caucasoid women: a case control study. BMC Pregnancy and Childbirth 2005;5(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burtin 1995
- Burtin P, Taddio A, Ariburnu O, Einarson TR, Koren G. Safety of metronidazole in pregnancy: a meta‐analysis. American Journal of Obstetrics and Gynecology 1995;172:525‐9. [DOI] [PubMed] [Google Scholar]
Donders 2009
- Donders GG, Calsteren K, Bellen G, Reybrouck R, Bosch T, Riphagen I, et al. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG 2009;116:1315‐24. [DOI] [PubMed] [Google Scholar]
Fischbach 1993
- Fischbach F, Petersen EE, Weissenbacher ER, Martius J, Hosmann J, Mayer H. Efficacy of clindamycin vaginal cream versus oral metronidazole in the treatment of bacterial vaginosis. Obstetrics & Gynecology 1993;82:405‐10. [PubMed] [Google Scholar]
Hay 1994
- Hay PE, Morgan DJ, Ison CA, Bhide SA, Romney M, McKenzie P, et al. A longitudinal study of bacterial vaginosis during pregnancy. British Journal of Obstetrics and Gynaecology 1994;101:1048‐53. [DOI] [PubMed] [Google Scholar]
Hay 1994a
- Hay PE, Lamont RF, Taylor‐Robinson D, Morgan DJ, Ison C, Pearson J. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. BMJ 1994;308:295‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hemilä 2007
- Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005532.pub2] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated September 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hillier 1993
- Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ, Eschenbach DA. The normal vaginal flora, H2O2‐producing lactobacilli and bacterial vaginosis in pregnant women. Clinical Infectious Diseases 1993;16(Suppl 4):S273‐S281. [DOI] [PubMed] [Google Scholar]
Hillier 1995
- Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, et al. Association between bacterial vaginosis and preterm delivery of a low‐birth‐weight infant. New England Journal of Medicine 1995;333:1737‐42. [DOI] [PubMed] [Google Scholar]
Jones 2010
- Jones NM, Holzman C, Friderici KH, Jernigan K, Chung H, Wirth J, et al. Interplay of cytokine polymorphisms and bacterial vaginosis in the etiology of preterm delivery. Journal of Reproductive Immunology 2010;87(1‐2):82‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurki 1992
- Kurki T, Sivonen A, Renkonen O‐V, Savia E, Ylikorkala O. Bacterial vaginosis in early pregnancy and pregnancy outcome. Obstetrics & Gynecology 1992;80:173‐7. [PubMed] [Google Scholar]
Lamont 1993
- Lamont RF, Fisk NM. The role of infection in the pathogenesis of preterm labour. In: Studd JWW editor(s). Progress in Obstetrics and Gynaecology. Vol. 10, London: Churchill Livingstone, 1993:135‐58. [Google Scholar]
Lamont 2011a
- Lamont RF, Sobel JD, Akins RA, Hassan SS, Chaiworapongsa T, Kusanovic JP, et al. The vaginal microbiome: new information about genital tract flora using molecular based techniques. BJOG 2011;118(5):533‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamont 2011b
- Lamont RF, Nhan‐Chang CL, Sobel JD, Workowski K, Conde‐Agudelo A, Romero R. Treatment of abnormal vaginal flora in early pregnancy with clindamycin for the prevention of spontaneous preterm birth: a systematic review and metaanalysis. American Journal of Obstetrics and Gynecology 2011;205(3):177‐90. [DOI: 10.1016/j.ajog.2011.03.047] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leitich 2007
- Leitich H, Kiss H. Asymptomatic bacterial vaginosis and intermediate flora as risk factors for adverse pregnancy outcome. Best Practice & Research Clinical Obstetrics and Gynaecology 2007;21(3):375‐90. [DOI: 10.1016/j.bpobgyn.2006.12.005] [DOI] [PubMed] [Google Scholar]
McDonald 1994
- McDonald HM, O'Loughlin JA, Vigneswaran R, Jolley PT, McDonald PJ. Bacterial vaginosis in pregnancy and efficacy of short course oral metronidazole treatment: a randomized controlled trial. Obstetrics & Gynecology 1994;84:343‐8. [PubMed] [Google Scholar]
McGregor 1990
- McGregor JA, French JI, Richter R, Franco‐Buff A, Johnson A, Hillier S, et al. Antenatal microbiological maternal risk factors associated with prematurity. American Journal of Obstetrics and Gynecology 1990;163:1465‐73. [DOI] [PubMed] [Google Scholar]
Nugent 1991
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. Journal of Clinical Microbiology 1991;29:297‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2003 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Simhan 2003
- Simhan HN, Krohn MA, Roberts JM, Zeevi A, Caritis SN. Interleukin‐6 promoter ‐174 polymorphism and spontaneous preterm birth. American Journal of Obstetrics and Gynecology 2003;189(4):915‐8. [DOI] [PubMed] [Google Scholar]
Witkin 2003
- Witkin SS, Vardhana S, Yih M, Doh K, Bongiovanni AM, Gerber S. Polymorphism in intron 2 of the fetal interleukin‐1 receptor antagonist genotype influences midtrimester amniotic fluid concentrations of interleukin‐1 beta and interleukin‐1 receptor antagonist and pregnancy outcome. American Journal of Obstetrics and Gynecology 2003;189(5):1413‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Brocklehurst 1998
- Brocklehurst P, Hannah M, McDonald H. Interventions for treating bacterial vaginosis in pregnancy. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI] [PubMed] [Google Scholar]
McDonald 2005
- McDonald H, Brocklehurst P, Parsons J. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD000262.pub2] [DOI] [PubMed] [Google Scholar]
McDonald 2007
- McDonald HM, Brocklehurst P, Gordon A. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2007, issue 1. [DOI: 10.1002/14651858.CD000262.pub3; CD000262] [DOI] [PMC free article] [PubMed]


