Abstract
OBJECTIVE.
To define the extent of an outbreak of Achromobacter xylosoxidans bacteremia, determine the source of the outbreak, and implement control measures.
DESIGN.
An outbreak investigation, including environmental and infection control assessment, and evaluation of hypotheses using the binomial distribution and case control studies.
SETTING.
A 50-bed medical surgical unit in a hospital in Illinois during the period January 1-July 15, 2006.
INTERVENTIONS.
Discontinuation of use of opioid delivery via patient-controlled analgesia (PCA) until the source of the outbreak was identified and implementation of new protocols to ensure more rigorous observation of PCA pump cartridge manipulations.
RESULTS.
Calculations based on the binomial distribution indicated the probability that all 9 patients with A. xylosoxidans bacteremia were PCA pump users by chance alone was <.001. A subsequent case control study identified PCA pump use for administration of morphine as a risk factor for A. xylosoxidans bacteremia (odds ratio, undefined; P < .001). Having a PCA pump cartridge with morphine started by nurse C was significantly associated with becoming a case-patient (odds ratio, 46; 95% confidence interval, 4.0–525.0; P < .001).
CONCLUSIONS.
We hypothesize that actions related to diversion of morphine by nurse C were the likely cause of the outbreak. An aggressive pain control program involving the use of opioid medication warrants an equally aggressive policy to prevent diversion of medication by staff.
Achromobacter xylosoxidans (formerly known as Alcaligenes xylosoxidans) is a nonfermenting, motile gram-negative rod commonly found in water sources, such as well water,1 hos-pital faucets,2 swimming pools, and moist soil.3 A. xylosoxidans has been identified as a pathogen affecting cystic fibrosis patients4,5 and has also been described as a cause of infections at numerous sites in individuals without cystic fibrosis.3 Uncommonly, contamination of mouthwash, incubators, soaps, disinfectants, intravenous fluids, and devices by this microorganism has caused nosocomial infections and outbreak of infection, primarily involving bloodstream and wound infections.6-8 In early July 2006, hospital A notified the Illinois Department of Public Health that 8 cases of A. xylosoxidans bacteremia had been identified among inpatients from a 50-bed medical-surgical unit and that cultures of environmental samples obtained in the operating rooms had failed to identify a possible source. Our investigation of this outbreak was aimed at defining the outbreak’s size and scope, identifying risk factors, determining the source of the outbreak, and implementing infection control measures.
METHODS
Case Definition and Ascertainment
A case patient was defined as a patient with laboratory-confirmed A. xylosoxidans bacteremia who was admitted to hospital A from January 1, 2006, though July 15, 2006. Case patients were identified through review of microbiologic, medical, and infection control records.
Infection Control Assessment
We surveyed the hospital and reviewed procedures and practices in the pharmacy, operating rooms, recovery rooms, medication rooms, and medical-surgical unit. In addition, we also interviewed and observed the hospital staff in the operating room, pharmacy, and medical-surgical unit and reviewed medical and infection control records.
Laboratory Evaluation
Pulsed-field gel electrophoresis (PFGE) of available isolates was performed at Mayo Laboratories in Rochester, Minnesota, using methods for Salmonella species,9 with the exception that isolates were digested with restriction endonuclease Xba1. PFGE pattern comparisons were based on standard criteria.10
Case Control Studies
We conducted a case control study to identify specific staff and exposures associated with an increased risk of A. xylosoxidans bacteremia. Specifically, these included starting or changing patient-controlled analgesia (PCA) morphine cartridges and “wasting” PCA morphine cartridges. Wasting refers to discarding partially used PCA narcotic cartridges. Approximately 3 control subjects per case patient were randomly selected for a total of 24 control subjects. Criteria for control subjects were as follows: (1) patients admitted to the medical-surgical unit who used PCA pumps, (2) patients with no fever or bacteremia during hospitalization, and (3) patients admitted to the hospital during the outbreak period (January 1 through July 15, 2006).
After outbreak control measures were implemented, we performed an additional case control study to identify risk factors associated with acquiring A. xylosoxidans bacteremia by comparing case patients with bacteremia with 30 control subjects. Control subjects were randomly selected from a list of patients admitted to the hospital during the epidemic period (January 1 through July 15, 2006) who did not acquire gram-negative bacteremia. We assessed factors that included type of surgery, type of anesthesia, surgical prep regimen, surgical irrigation, method of pain control, pain control medications, type of intravenous fluid, and location of patient care. Exposure data were collected from the time of admission to the hospital until the collection of the culture that led to the diagnosis of A. xylosoxidans infection in the case patient. Data regarding exposure for control subjects were collected from the time of hospital admission to the median length of stay at which A. xylosoxidans infection was diagnosed in the case patients (2 days) or until discharge from the hospital if the patient was discharged after less than 2 days.
Statistical Analysis
Data regarding case patient admission, procedures, medications, and culture collection times were collected on standardized forms and analyzed with Epi Info software, version 3.32 (Centers for Disease Control and Prevention). Univariate analyses were performed for both case control studies in which odds ratios (ORs) were calculated and hypotheses tested by χ2 analysis. Odds ratios with 95% confidence intervals (CIs) that excluded 1.0 and P values <.05 were considered statistically significant. The binomial probability (single tailed) of all 9 cases of A. xylosoxidans that occurred among PCA users attributable to chance alone was calculated using an online statistical calculator.11 This analysis assumed that 8% of patients on the medical-surgical unit at hospital A used a PCA pump for administration of morphine and that A. xylosoxidans bacteremia occurred randomly among medical-surgical unit patients. Assumptions regarding PCA pump use were based on a review of electronic PCA records from hospital A’s pharmacy.
Ethics Statement
This work was performed as part of a public health outbreak investigation and was therefore not subject to institutional review board approval.
RESULTS
Infection Control Assessment
Review of infection control and laboratory records did not indicate any evidence of an increase in other noscomial infections, misuse of multidose cartridges, or improper intravenous line care. Additional information regarding assessment of PCA pump use and procedures is provided below.
Case Ascertainment and Description
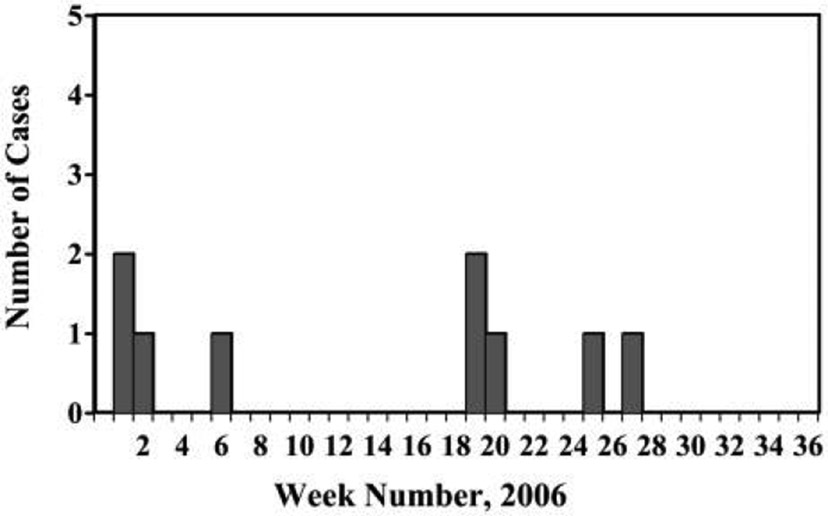
Based on inquiries to infection control practitioners and laboratories at local hospitals and the Centers for Disease Control and Prevention, the incidence of A. xylosoxidans infection had not increased elsewhere in the region, nor had it increased in the United States overall. One additional case of A. xylosoxidans bacteremia was identified via review of microbiology records. Of the 9 case patients identified, 6 were female and 3 were male, with an age range of 23–85 years (Figure 1). All 9 cases of bacteremia developed within 26–62 hours after hospitalization. Seven cases occurred in patients who underwent surgical procedures, 1 case occurred after endoscopic retrograde cholangiopancreatography (ERCP), and 1 case occurred in a patient who was admitted to the hospital for magnetic resonance imaging. All 9 case patients received morphine via PCA pump before developing bacteremia. No deaths or serious complications occurred.
FIGURE 1.
Epidemic curve of Achromobacter xylosoxidans bacteremia from January 1 through July 15, 2006.
Binomial Distribution Method
Results demonstrated that the probability that all 9 patients with A. xylosoxidans bacteremia would be PCA pump users by chance alone was <.001. This result was considered sufficient to immediately proceed to a case control study aimed at identifying an increased risk of A. xylosoxidans bacteremia associated with specific staff manipulating case patients’ PCA pumps.
Case Control Study: Risk Factors for A. xylosoxidans Bacteremia
This case control study aimed to assess risk factors for A. xylosoxidans bacteremia after outbreak control measures had been implemented and to validate the hypothesis generated through use of the binomial probability model. In a univariate analysis, case and control patients were similar in terms of age, sex, and the proportion of patients undergoing surgery. Patients with A. xylosoxidans bacteremia were significantly more likely than control subjects to have morphine administered via a PCA pump and be admitted to the medical-surgical unit (Table 1).
TABLE 1.
Risk Factors for Achromobacter xylosoxidans Bacteremia
| Risk factor | No. (%) of case patients (n = 9) |
No. (%) of control subjects (n = 30) |
Odds ratio (95% CI) | P |
|---|---|---|---|---|
| Exposure | ||||
| D5 normal saline | 4 (44) | 13 (43) | 1.0 (0.2–4.7) | .6 |
| Lactated ringers | 6 (67) | 19 (63.3) | 1.2 (0.2–5.6) | .6 |
| Normal saline | 4 (44) | 6 (20) | 3.2 (0.7–15.7) | .1 |
| Non-PCA narcotics | 9 (100) | 24 (80) | Undefined | .2 |
| Demerol | 7 (78) | 15 (50) | 3.5 (0.6–19.7) | .1 |
| Dilaudid | 1 (11) | 1 (3.3) | 3.6 (0.2–64.6) | .4 |
| Fentanyl | 1 (11) | 1 (3.3) | 3.6 (0.2–64.6) | .4 |
| Hydrocodone | 0 (0) | 4 (13.3) | Undefined | .3 |
| Ketorlac | 0 (0) | 1 (3.3) | Undefined | .8 |
| Morphine | 4 (44) | 8 (26.7) | 2.2 (0.5–10.3) | .3 |
| PCA morphine | 9 (100) | 1 (3.3) | Undefined | <.001 |
| Location | .3 | |||
| Radiology | 0 (0) | 4 (13.3) | Undefined | .3 |
| ED | 3 (33) | 5 (16.7) | 2.5 (0.5–13.5) | .5 |
| OR | 9 (89) | 24 (80) | 2.0 (0.2–19.2) | .5 |
| Postoperation recovery | 9 (89) | 24 (80) | 2.0 (0.2–19.2) | <.001 |
| Medical-surgical unit | 9 (100) | 13 (43) | Undefined |
note. CI, confidence interval; ED, emergency department; OR, operating room; PCA, patient-controlled analgesia.
Case Control Study: Nurse Staffing
All cases occurred among patients who were admitted to the medical-surgical unit. During the outbreak period, 33 registered nurses (9 full-time and 24 part-time employees) and 2 licensed practical nurses (1 full-time and 1 part-time employee) were employed to work on the medical-surgical unit. The number of nurses on duty per shift fluctuated with the census, but the staffing ratio was consistent. The nurse-to-patient ratio was 1 : 3.5 (1 nurse per 3–4 patients).
Among the nurses who worked on the medical-surgical unit, 11 had documented starting or changing a PCA pump morphine cartridge for at least 1 case patient, according to charting on PCA flow sheets that were included in patient medical records. The association of each of these staff members with the risk of becoming a case patient was calculated (Table 2). There was no significant association between A. xylosoxidans bacteremia and any of the 11 nurses wasting a patient’s PCA pump cartridge (data not shown). However, having a PCA pump cartridge started by one of these nurses (nurse C) was statistically significantly associated with becoming a case patient (OR, 46; 95% CI, 4.0–525.0; P < .001). Additional review of timekeeping records established that nurse C was the only nurse on the unit who worked during the period from hospital admission to before fever onset for all 9 case patients.
TABLE 2.
Association of Bacteremia with Having a Patient-Controlled Analgesia Pump Morphine Cartridge Started or Changed by Individual Staff
| Nurse | No. (%) of case patients (n = 9) |
No. (%) of control subjects (n = 24) |
Odds ratio (95% CI) | P |
|---|---|---|---|---|
| A | 1 (11) | 1 (4) | 2.9 (0.2–51.5) | .5 |
| B | 1 (11) | 1 (4) | 2.9 (0.2–51.5) | .5 |
| C | 6 (67) | 1 (4) | 46 (4.0–525.0) | <.001 |
| D | 2 (22) | 1 (4) | 6.6 (0.5–83.8) | .2 |
| E | 1 (11) | 0 (0) | Undefined | .3 |
| F | 2 (22) | 2 (8) | 3.1 (0.4–26.6) | .3 |
| G | 1 (11) | 0 (0) | Undefined | .3 |
| H | 1 (11) | 1 (4) | 2.9 (0.2–51.5) | .5 |
| I | 1 (11) | 0 (0) | Undefined | .3 |
| J | 1 (11) | 2 (8) | 1.4 (0.1–17.3) | .6 |
| K | 1 (11) | 1 (4) | 2.9 (0.2–51.5) | .5 |
NOTE. CI, confidence interval.
PCA Pump Use
The PCA pumps (Abbott LifeCare PCA PLUS II) used by the hospital at the time that the outbreak occurred are medication-dispensing units equipped with a pump attached to an intravenous line that is inserted into a vein in the patient’s hand or arm. By means of a push-button mechanism, the patient self-administers doses of a short-acting narcotic in response to their own pain needs. At hospital A, the standard narcotic for PCA pumps was morphine, which came in 30-mL glass cartridges. The morphine arrived to the medical-surgical unit from the pharmacy in tamper-resistant packages and was stocked in a medication-dispensing unit (Pyxis) that tracked access and dispensing for each nurse. When the PCA pump was initially set up or the cartridge was changed, 2 nurses were required to sign off that this was done properly.
We observed the passage of the medication being delivered to the hospital, being stored and dispensed from the pharmacy, and being set up and infused on the nursing floor. It appeared unlikely that the medication or packaging could have been tampered with before it was dispensed on the medical-surgical floor because of the tamper-resistant nature of the packaging. We demonstrated that it would be relatively easy, after the morphine cartridge was removed from the packaging and before it was attached to the PCA pump, to insert a needle through the cartridge’s rubber stopper and withdraw morphine. Morphine solution could then be replaced by injecting another fluid into the cartridge through its rubber stopper.
Case Patient Testing
PFGE analysis was performed on 5 isolates from the last 3 case patients (2 isolates from each of 2 cases and 1 isolate from a third case). Three different PFGE patterns were observed, with 1 isolate from each of the 3 case patients having a pattern that matched the pattern of an isolate from another case patient.
Outbreak Control Measures
PCA pump use was discontinued on day 2 of the outbreak investigation. We recommended observations of nurse C’s practices, drug testing of nurse C, and culturing of samples from nurse C’s hands and water sources on and near the medical surgical unit that might harbor A. xylosoxidans. Nurse C resigned from the hospital upon being informed by the hospital of her association with A. xylosoxidans bacteremia. Drug testing and cultures of samples from water sources and from nurse C’s hands were not performed because the results were not considered likely to lead to additional actions by the hospital.
The pharmacy implemented new protocols to ensure observation of all PCA pump cartridge handling by a second staff member and transfer of responsibility for wasting of residual medication to the pharmacy. As of March 2010, no additional cases of A. xylosoxidans bacteremia had occurred at hospital A (hospital A, personal communication). The Illinois Department of Professional Regulation was informed of the association of nurse C with cases of A. xylosoxidans bacteremia. Because direct evidence that nurse C was diverting narcotics was lacking, disciplinary action did not take place.
DISCUSSION
Although direct evidence of nurse C causing cases of bacteremia is lacking, the results of this investigation indicate that practices of nurse C were likely responsible for this outbreak. Nurse C may have substituted contaminated water for morphine or used a contaminated needle and syringe to extract morphine from cartridges; morphine cartridges that had been manipulated were then used for PCA pump infusions for affected patients. Contamination of artificial fingernails and skin with gram-negative organisms is well described, and contamination of nurse C’s fingers or fingernails was considered as a possible cause of this outbreak; however, this explanation of contamination is considered unlikely in this situation, because nurse C was not reported to wear artificial nails and had no recognizable skin disorder that might predispose to gram-negative colonization (eg, psoriasis),12 and because contaminated hands or nails should have resulted in infections other than bacteremia. In addition, the identification of heterogeneous PFGE patterns is consistent with an environmental source.
According to the Substance Abuse and Mental Health Services Administration National Household Survey on Drug Abuse, 3.6% of nurses and 4.0% of physicians admit to the use of illicit drugs,13 and substance abuse is reportedly the most common reason for disciplinary action against nurses and physicians by state licensing boards.14,15
Adverse events, including outbreaks, that are related to employee diversion of drugs are infrequently recognized and even more infrequently reported. A review of medical literature since 1990 identified only 2 outbreaks in hospital settings that were clearly related to narcotic tampering; each of these outbreaks were also caused by gram-negative bacteria (Serratia marcescens and Pseudomonas picketti).16,17
Employees were not drug tested during the Pseudomonas outbreak “because of the sensitive issue of drug testing in the workplace.”17 The employee implicated in the Serratia outbreak was tested for narcotics via hair sampling, which led to a positive result. Drug testing of hospital employees is controversial18 and infrequently addressed by national organizations. In 1992, the American Hospital Association recommended preemployment testing of applicants and for-cause testing of employees but did not recommend random or no-cause testing.19 A 1995 statement from the American Medical Association opposed random drug testing of hospital employees, including physicians.20 Clear-cut hospital policies on drug testing after hiring, including policies on the use of epidemiologic evidence to support for-cause drug testing, could lead to improved identification of the source of certain adverse events in hospitals, including outbreaks associated with contaminated infusion fluids. In addition, regardless of a hospital’s drug testing policy, an aggressive pain control program, such as that in hospital A, warrants the use of multifaceted strategies, including controlled access, audits, and investigation to ensure that drug diversion is prevented, given the potential risk for diversion.
ACKNOWLEDGMENTS
We thank A. Srinivasan for helpful advice and S. Hudson for assistance with manuscript preparation.
Footnotes
Financial support. This outbreak investigation was supported by an appointment to the Centers for Disease Control and Prevention Public Health Prevention Service Fellowship (to J.Y.).
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article. All authors submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and the conflicts that the editors consider relevant to this article are disclosed here.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Spear JB, Fuhrer J, Kirby BD. Achromobacter xylosoxidans (Al-calagines xylosoxidans subsp. xylosoxidans) bacteremia associated with a well-water source: case report and review of the literature. J Clin Microbiol 1988;26:598–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J, Chen M, Lin YE, et al. Association between contaminated faucets and colonization or infection by nonfermenting gram-negative bacteria in intensive care units in Taiwan. J Clin Microbiol 2009;47(10):3226–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberg JP, Burd EM. Other gram-negative and gram-variable bacilli. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 7th ed. Philadelphia: Churchill Livingstone, 2010. [Google Scholar]
- 4.De Baets F, Schelstraete P, Van Daele S, Haerynck F, Vaneechoutte M. Achromobacter xylosoxidans in cystic fibrosis: prevalence and clinical relevance. J Cyst Fibros 2007;6(1):75–78. [DOI] [PubMed] [Google Scholar]
- 5.Kanellopoulou M, Pournaras S, Iglezos H, Skarmoutsou N, Papafrangas E, Maniatis AN. Persistent colonization of nine cystic fibrosis patients with an Achromobacter (Alcaligenes) xylosoxidans clone. Eur J Clin Microbiol Infect Dis 2004;23(4):336–339. [DOI] [PubMed] [Google Scholar]
- 6.Tena D, Carranza R, Barberá JR, Valdezate S, Garrancho JM, Arranz M, Sáez-Nieto JA. Outbreak of long-term intravascular catheter-related bacteremia due to Achromobacter xylosoxidans subspecies xylosoxidans in a hemodialysis unit. Eur J Clin Microbiol Infect Dis 2005;24(11):727–732. [DOI] [PubMed] [Google Scholar]
- 7.Kim MJ, Bancroft E, Lehnkering E, Donlan R, Mascola L. Alcaligenes xylosoxidans bloodstream infections in outpatient oncology office. Emerg Infect Dis 2008;14(7):1046–1052. [Google Scholar]
- 8.Vu-Thien H, Darbord JC, Moissenet D, Dulot C, Dufourcq JB, Marsol P, Garbarg-Chenon A. Investigation of an outbreak of wound infections due to Alcaligenes xylosoxidans transmitted by chlorhexidine in a burn unit. Eur J Clin Microbiol Infect Dis 1998;17:724–726. [DOI] [PubMed] [Google Scholar]
- 9.Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, Barrett TJ. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157: H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis 2006;3(1):59–67. [DOI] [PubMed] [Google Scholar]
- 10.Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 1995;33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binomial calculator. http://www.stat.tamu.edu/ῶvest/applets/binomialdemo.html. Accessed April 29, 2011.
- 12.Larson EL, Cimiotti JP, Haas J, et al. Gram-negative bacilli associated with catheter-associated and non-catheter-associated bloodstream infections and hand carriage by healthcare workers in neonatal intensive care units. Pediatr Crit Care Med 2005;6:457–461. [DOI] [PubMed] [Google Scholar]
- 13.Substance Abuse and Mental Health Services Administration, Office of Applied Studies (OAS). Preliminary Results from the 1996 National Household Survey on Drug Abuse. Rockville, MD: OAS series #H-13, DHHS Publication No. (SMA) 97–3149; 1997. [Google Scholar]
- 14.Public Health Citizens Research Group. Questionable doctors. http://www.citizen.org/hrg/qdsite/map.htm. Accessed April 30, 2011.
- 15.Sullivan E, Decker P. Effective Leadership and Management in Nursing. Upper Saddle River, NJ: Prentice Hall, 2001. [Google Scholar]
- 16.Ostrowsky BE, Whitener C, Bredenberg HK, et al. Serratia marcescens bacteremia traced to an infused narcotic. N Engl J Med 2002;346:1529–1537. [DOI] [PubMed] [Google Scholar]
- 17.Maki DG, Klein BS, McCormick RD, et al. Nosocomial Pseudomonas picketti bacteremias traced to narcotic tampering. JAMA 1991;265:981–986. [PubMed] [Google Scholar]
- 18.McGrath T. Addicted docs put patients in peril: random drug screening urged for health care workers. MSNBC. http://www.msnbc.msn.com/id/37396390/ns/health-addictions//. Updated June 24, 2010. Accessed April 30, 2011. [Google Scholar]
- 19.American Hospital Association. Substance Abuse Policies for Health Care Institutions. Chicago: Management Advisory Human Resources, American Hospital Association, 1992. [Google Scholar]
- 20.American Medical Association (AMA) Council on Scientific Affairs. Issues in Employee Drug Testing. Chicago: AMA Policy 95.984, CSA Report A, I-90; 1990. [Google Scholar]