Abstract
Regulatory B cells (Bregs) that produce IL-35 and IL-10 (i35-Bregs) regulate central nervous system (CNS) autoimmune diseases including uveitis. In the mouse model of uveitis, i35-Breg cells suppress intraocular inflammation by inducing expansion of IL-10-producing B cells (B10), IL-10-producing T cells (Tregs), and IL-35-producing T cells (iTR35), suggesting that i35-Bregs orchestrate an immunesuppressive milieu that regulates immunity during autoimmune diseases. In this chapter, we discuss uveitis and therapeutic challenges that necessitate the development of cell-based therapies for the treatment of these potentially blinding diseases that cause 10% visual handicap. We then describe the methods we set up for ex vivo generation of i35-Breg cells employed in i35-Breg immunotherapy in uveitis and in other CNS autoimmune diseases.
Keywords: Regulatory B cells, i35-Breg, Uveitis, EAU, IL-35, IL-10, CNS autoimmune disease
1. Introduction
Uveitis is a general term that groups diverse intraocular inflammatory diseases such as birdshot retinochoroidopathy, Behcet’s disease, and ocular sarcoidosis, and it accounts for 10% of severe visual handicaps in the United States [1, 2]. Similarly to other central nervous system (CNS) tissues, the neuroretina is an immune privileged tissue comprised of intricate and highly vulnerable physiology shielded by the blood-ocular barrier [3]. The endothelial and parenchymal basement membranes, pericytes, and perivascular space are barrier-forming constituents that limit paracellular and transcellular diffusion, while allowing import of nutrients and export of toxic metabolites out of the neuroretina [3]. Ocular immune privilege is maintained by retinal microglia and resident ocular parenchymal cells that constitutively secrete immunosuppressive and anti-inflammatory cytokines which inactivate immunological effector cells [4, 5]. However, during uveitis, lymphocytes bearing receptors specific to retinal proteins (uveitogenic T cells) breach the blood-ocular-barrier, attack and destroy photoreceptor cells. Thus, extravasation of inflammatory cells into the neuroretina plays an important role in the development of uveitis, and these cells are prime therapeutic targets (see Fig. 1). However, therapeutic intervention in uveitis presents formidable challenges, due to the need of striking a balance between controlling pathogenic immune responses on one hand and preventing generalized immunosuppression that would undermine the patients’ vital immune surveillance functions and immunity.
Fig. 1.
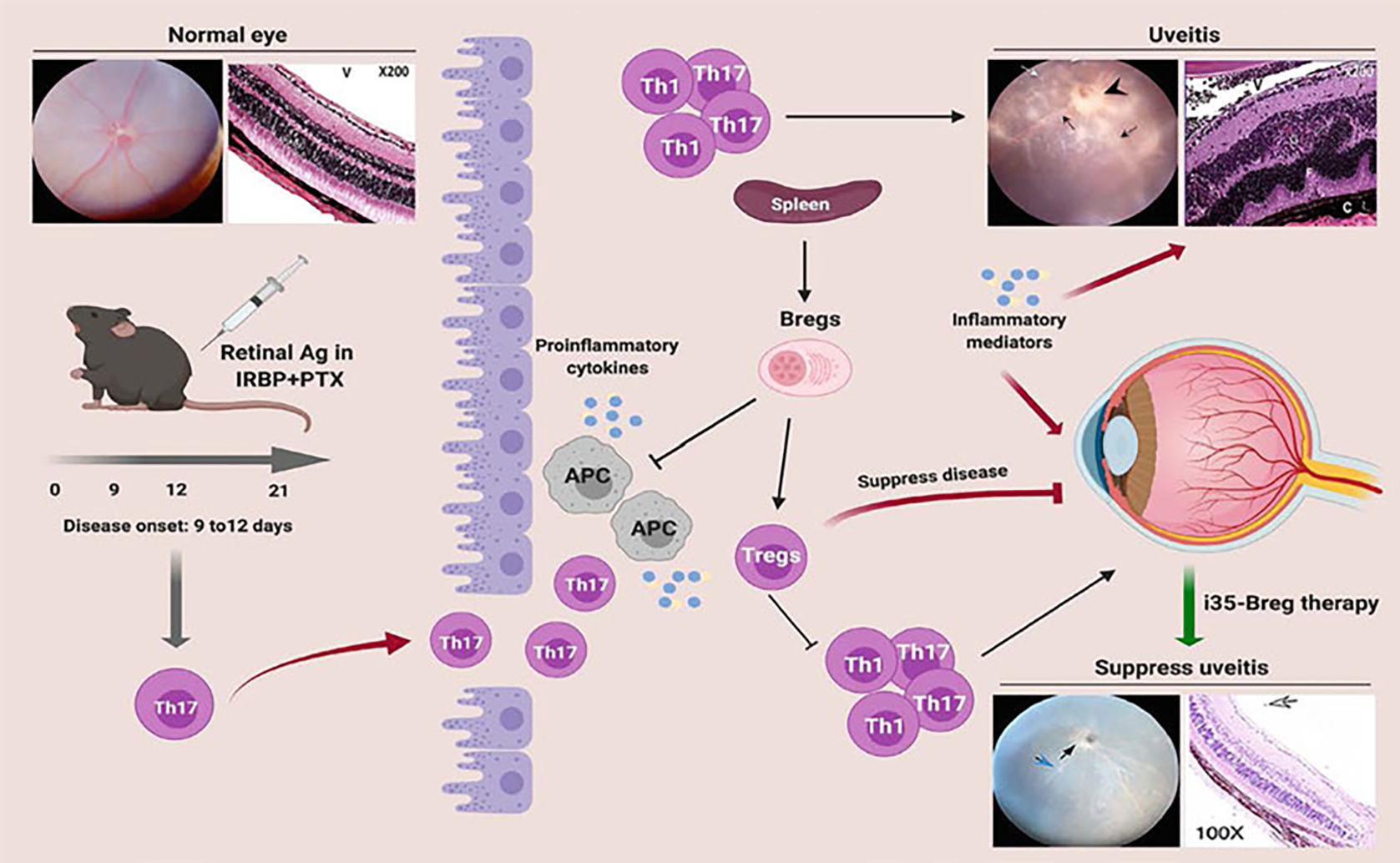
Schematic representation of early events associated with loss of immune privilege of the eye, Th17/Th1-mediated pathology in the neuroretina and efficacy of Regulatory B-cell (Breg) immunotherapy in suppressing uveitis. Retinal fold (F); Choroiditis (C); Granuloma (G); Vitreous (V)
Although steroids constitute an effective therapy for uveitis, serious adverse effects preclude their prolonged use. Immune modulation therapies that target inflammatory cells, cytokines, and cytokine receptors have been used in uveitis, and these include anti-IL-2R, anti-TNF-α, Etanercept (Enbrel®), and Infliximab (Remicade®) therapies. However, these biologics are mostly used as steroid-sparing agents that allow reduction of therapeutic dose of steroids. Although some of these biologics are used in uveitis, the therapeutic efficacy is a matter of some debate. Thus, there is considerable impetus to develop more effective biologics and cell-based therapies for uveitis. Experimental autoimmune uveitis (EAU) shares essential immunopathogenic features of human uveitis, and this animal model provides a useful framework for evaluating therapies purported to suppress and ameliorate uveitis.
The cytokines belonging to the interleukin-12 (IL-12) family have profound effects on lymphocyte developmental decisions and have emerged as important biologics that regulate immunity [6]. The family is comprised of pro-inflammatory members (IL-12, IL-23, IL-39) implicated in several autoimmune diseases while two members (IL-27, IL-35) suppress CNS inflammation [7–10] and contribute to mechanisms that ameliorate uveitis in mice [11–13]. Regulatory B cells (Bregs) that secrete IL-35 (i35-Bregs) have recently been shown to suppress uveitis and encephalomyelitis [8, 14]. These studies suggest that the i35-Breg can produce both IL-10 and IL-35 in a time-dependent manner, although production of either cytokine is mutually exclusive [8, 14]. At early time points in culture, Bregs predominantly produce IL-10, but, at later time points, the majority of these cells produce IL-35. Thus, IL-10-producing Bregs and IL-35-producing Bregs are not distinct lineages but represent transitory states of the i35-Breg lineage. In this chapter, we describe the methods to generate and enrich Breg cells that exclusively produce IL-35 and the efficacy of autologous i35-Breg therapy in uveitis.
2. Materials
2.1. General Instrumentation
Tissue culture hood.
Incubator at 37 °C with 5% CO2 atmosphere.
0.2 μm syringe filters with 1 mL syringes.
Hamilton 1 mL glass syringes.
16-Gauge blunt-end needles.
Alcohol swab and gauze.
27-Gauge needles and 1 mL syringes.
40- and 70-μm pore-size nylon cell strainers.
Refrigerated centrifuge and microcentrifuge for 96-well plates and conical tubes.
Plastic serological pipettes.
15 mL and 50 mL polypropylene conical tubes.
5 mL polystyrene round-bottom flow tubes.
1.5–1.7 mL microcentrifuge tubes.
Photomicroscope.
Electroretinography console.
Ganzfeld dome.
Dissecting microscope.
Cell counting chamber or cell viability analyzer such as Vi-Cell XR cell viability analyzer (Beckman Coulter).
Cell sorter, flow cytometer, and software for analyzing flow cytometry data.
2.2. Tissue Dissection and Cell Culture Reagents
Incomplete culture medium: RPMI-1640.
Complete culture medium: 1% L-glutamine, 10% fetal bovine serum (FBS), 1% HEPES, 1% sodium pyruvate, 1% MEM nonessential amino acids, 1% penicillin/streptomycin, and 0.1% mercaptoethanol in RPMI-1640.
Red blood cell lysing buffer: 150 mM NH4Cl, 10 mM KHCO3, and 0.1 mM Na2EDTA in distilled water.
Mouse restrainer with tail access.
Sterile scissors and forceps.
Sterile 100 mm Petri dishes.
Phosphate-buffered saline (PBS): 8 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4, and 0.24 g KH2PO4 in 1 liter distilled water. Keep sterile.
Trypan blue solution.
6-Well tissue culture plates.
Collagenase.
DNase.
2.3. Reagents for the Study of i35-Breg Cells
Fluorescence-activated cell sorting (FACS) buffer: 2% fetal calf serum (FCS), 2 mM EDTA, 0.1% sodium azide in PBS.
Cell sort collection buffer: 1 mL FBS in 15 mL conical tubes.
Magnetic-activated cell sorting (MACS) buffer: 0.5% bovine serum albumin (BSA) and 2 mM EDTA in PBS, pH 7.2.
Equipment for immunomagnetic separation (e.g., columns and magnets).
CD45R/B220 MicroBeads.
Cell stimulation reagents: LPS (Escherichia coli 0111: B4) and anti-mouse CD40 (clone FGK4.5/FGK45).
Phorbol 12-myristate 13-acetate (PMA).
Ionomycin.
Brefeldin A.
4′,6-Diamidino-2-phenylindole (DAPI) viability dye for dead cell exclusion.
PE rat anti-mouse CD138 antibody, clone 281–2.
APC anti-human/mouse IL-12/IL-35 p35 antibody, clone 27537.
PerCP anti-mouse EBI3 antibody, clone 355022.
Dye for the discrimination of viable from nonviable cells in multicolor flow cytometric applications. Reconstitute the dye according to manufacturer’s instructions.
Fixation and permeabilization solutions for intracellular staining.
2.4. Mice and Immunization Reagents
6- to 8-week-old C57BL/6 J for CD45.2+ recipient mice.
6- to 8-week-old B6.SJL-Ptprca Pepcb/BoyJ (CD45.1+) for donor mice.
Interphotoreceptor retinoid-binding protein peptide (IRBP651–670).
Complete Freund’s adjuvant (CFA): Incomplete Freund’s (IFA) supplemented with heat-killed Mycobacterium tuberculosis (strain H37Ra) at a final concentration of 2.5 mg/mL.
1% mouse normal serum: restore the lyophilized normal mouse serum with sterile water in order to obtain a 1% solution. Filter through a 0.2-μm filter.
Bordetella pertussis toxin (PTX). Lyophilized powder in 50 μg vials.
2.5. Reagents for the Assessment of Uveitis
Ketamine.
Xylazine.
Midrin P: 0.5% tropicamide, 0.5% phenylephrine hydrochloride eye drops.
4% glutaraldehyde.
4% formaldehyde.
Alcohol.
Methacrylate.
Photomicroscope.
Gonioscopic prism solution.
3. Methods
3.1. Induction of EAU
EAU is a T-cell-mediated autoimmune disease that serves as a model of human intraocular inflammatory disease [11, 14]. EAU is induced in susceptible species by active immunization with ocular-specific proteins (or peptides derived from them) and is transferable to naive syngeneic animals by injection of in vitro-activated CD4+ MHC class II-restricted T cell lines specific to retinal antigens. The mouse EAU model is commonly induced by immunization with IRBP651–670, a 140-kDa retinal glycoprotein that functions in the transport of retinoids between the neuroretina and retinal pigment epithelium. For immunization, the IRBP651–670 is emulsified in CFA (see Note 1).
Prepare CFA by pestle crushing in a porcelain mortar 100 mg of heat-killed Mycobacterium tuberculosis to a fine powder, and mix with 40 mL of IFA. Store indefinitely at 4 °C.
Dissolve the IRBP651–670 peptide in PBS to a final concentration of 10 mg/mL (see Note 2).
Make an emulsion of the IRBP651–670 peptide in CFA (1:1; v/v) by vortexing for 10 min at high speed, and sonicate 1 time with 30 sec bursts. After mixing peptide and CFA, ensure that the amount of emulsion is sufficient, bearing in mind that 300 μg peptide in 0.2 mL emulsion must be provided to each mouse. Emulsion for two additional mice should be prepared.
Spin-down the emulsion for 5 min at 300 × g to remove bubbles from the emulsion, and vortex briefly to mix. Well-made emulsion should appear as a very sticky cream.
Draw emulsion into a 1-mL Hamilton glass syringe using 16-Gauge blunt-end needles. Make sure that no air bubble is present in the syringe. Replace the 16-Gauge needle with a 23-Gauge needle to proceed with immunization.
Prepare a 10-μg/mL PTX stock solution by adding the filtered 1% mouse serum solution to the PTX vial (see Note 3).
Use 6- to 8-weeks-old female C57BL/6 J mice (see Note 4). Inject 100 μL of the IRBP651–670 emulsion subcutaneously into the back of the mouse, and 50 μL to each thigh. After IRBP651–670, immediately inject intraperitoneally 1 μg/mouse (100 μL) of PTX stock solution (see Note 5). Return the animal to the cage for daily evaluation of EAU development.
3.2. Assessment of Uveitis and Pathological Score
Initial signs of uveitis in C57BL/6 J mice are observed by day 13 post-immunization (p.i.), with full-blown uveitis occurring between day 16 and day 22 p.i.. Disease progression is monitored by fundoscopy, histology, optical coherence tomography (OCT), and electroretinography (ERG).
Fundoscopy: This is a noninvasive method routinely used to examine the retina of a dilated eye pupil under a binocular microscope. EAU severity is evaluated at various time points during the course of the disease. Briefly, following administration of systemic anesthesia (intraperitoneal injection of 1.4 mg/mouse ketamine and of 0.12 mg/mouse xylazine), the pupil is dilated by topical administration of 1% tropicamide ophthalmic solution. At least six images (two posterior central retinal view, four peripheral retinal views) are taken from each eye by positioning the endoscope and viewing from superior, inferior, lateral, and medial fields, and each individual lesion is identified, mapped, and recorded (see Note 6).
Histology: This technique is generally used to assess and grade pathological lesions and involves enucleation of the eye. Eyes are fixed in 4% glutaraldehyde for 30 min and transferred to a 4% formaldehyde solution. Specimens are then dehydrated through graded alcohol series, embedded in methacrylate, and 4 μm serial transverse sections are cut and stained with hematoxylin and eosin. Photographs of representative sections are taken on a photomicroscope.
Optical coherence tomography: This noninvasive procedure allows visualization of internal microstructures of various eye structures in living animals. It allows the spatial localization and assessment of the extent of accumulation of inflammatory cells in vitreous and optic nerve head. Before OCT imaging is performed, each animal is anesthetized, and the pupils dilated. The anesthetized mouse is immobilized using adjustable holder that rotates easily allowing for horizontal or vertical scanning. Each scan is performed at least twice, with realignment each time. The dimension of the scan (in depth and transverse extent) is adjusted until the optimal signal intensity and contrast are achieved.
Electroretinogram: This technique measures changes in electrical potentials in response to light stimulation of the retina and is a well-established tool for identifying gross physiologic changes indicative of defects in cone and/or rod signaling functions and visual function defects. Before ERG recordings, mice are dark-adapted overnight, and experiments performed under dim red illumination. Mice are anesthetized with a single intraperitoneal injection of 1.4 mg/mouse ketamine and 0.12 mg/mouse xylazine and pupils dilated with Midrin P drops. ERGs are recorded using an electroretinography console that generates and controls the light stimulus. Dark-adapted ERG is recorded with a single flash delivered in a Ganzfeld dome with intensity of −4 to 1 log cd·s/m2 delivered in 6 steps. Light-adapted ERG was obtained with a 20 cd/m2 background, and light stimuli started at 0.3 to 100 cd·s/m2 in six steps. Gonioscopic prism solution is used to provide good electrical contact and maintain corneal moisture. A reference electrode (gold wire) is placed in the mouth, and a ground electrode (subcutaneous stainless-steel needle) is positioned at the base of the tail. Signals are differentially amplified and digitized. Amplitudes of the major ERG components (a- and b-wave) are measured by automated methods.
3.3. Isolation of Regulatory or Inflammatory Cells from the Neuroretina
To characterize inflammatory cells that cross the blood–retina barrier during EAU, mice should be euthanized and perfused with PBS. Enucleated eyes are put in a Petri dish containing incomplete culture medium for immediate isolation of the retina under a dissecting microscope.
Cut the eye along the limbus of the eye, and then carefully remove lens and cornea.
Peel off the retina and remove the attached optic nerve.
Digest the freshly isolated retina with 1 mg/mL collagenase in incomplete culture medium containing 10 μg/mL DNase for 1 h at 37 °C. Cells are pipetted intermittently every 30 min. Digestion is quenched with 5–10 folds volume of 10% FBS in complete culture medium.
Wash the cells twice in complete culture medium and count cells using the trypan blue dye exclusion method and either a counting chamber or a cell viability analyzer.
3.4. Isolation of Splenocytes
Isolate cells from the spleen of unimmunized or EAU-bearing mice and sort primary B cells based on the positive expression of the CD19 or B220 markers.
Euthanize B6 CD45.1+ mice via CO2 asphyxiation and dissect the spleen from animals.
Place the spleen in a sterile Petri dish with 10–15 mL of complete culture medium.
Under a sterile tissue culture hood, gently rub the spleen between the frosted end of a glass slide or plunger (butt) of a disposable 1 mL syringe, to gradually break the tissue apart into a suspension. Repeat the mechanic disruption procedure until no large pieces of tissue remain in the buffer.
Use 10 mL plastic serological pipettes to recover the splenocyte suspension from the dish into a 50-mL conical tube. Pass the cell suspension several times through a 70-μm pore-size nylon cell strainer or filter. Make sure the cell suspension contains no visible fragments.
Centrifuge the cells in 50 mL conical tubes for 10 min at 300 × g, 4 °C.
Discard supernatant and resuspend cell pellet in 2 mL of red blood cell lysis buffer for 5 min.
Wash the pellet once in PBS by centrifuging 10 min at 300 × g, 4 °C.
Resuspend cells in 10 mL complete culture medium and determine the cell number.
3.5. Regulatory B-Cell Enrichment and Isolation
Bregs that produce IL-10 and/or IL-35 (i35-Breg) suppress CNS autoimmune diseases and are enriched in CD138+ B cells. Although i35-Bregs can produce both IL-10 and IL-35, these cells are developmentally and functionally distinct from the CD5hiCD1dhi B10 subset that exclusively produce IL-10. Here we describe the method we use to generate and enrich i35-Bregs that exclusively produce IL-35 from CD138+ cells (see Fig. 2a and Note 7).
Fig. 2.

Schematic and diagrammatic representations of procedures for (a) ex vivo generation of IL-35-producing Regulatory B cells (i35-Bregs) and (b) autologous transfer of the CD45.2+ Breg cells to CD45.1+ mice as treatment for experimental autoimmune uveitis (EAU)
3.5.1. Induction and Isolation of CD138+ Cells
Isolate splenocytes from healthy or EAU-bearing C57BL/6 J mice as described in Subheading 3.4.
Centrifuge the isolated spleen cell suspension at 300 × g for 10 min and resuspend the cell pellet in MACS buffer. Determine the cell number.
Isolate CD45R/B220+ cells by immunomagnetic separation, using the specific microbeads and columns according to manufacturer’s instructions (see Note 8).
Wash the obtained cells once more in 10 mL PBS by centrifuging at 10 min 300 × g, 4 °C.
Aspirate supernatant completely and resuspend the cell pellet in fresh complete culture medium.
Induce plasma cell differentiation by resuspending isolated CD45R/B220+ cells in complete culture medium containing 25 μg/mL LPS and incubate for 96 h in a 6-well plate, at a final concentration of 1 × 106 cells/mL. Alternatively, isolated CD45R/B220+ cells from healthy and EAU-bearing mice can be stimulated in complete culture medium containing 5 μg/mL anti-CD40 antibody in IRBP-coated (20 μg/mL) 96-well plates.
At the end of the 96 h incubation, harvest the stimulated cells and wash twice with cold FACS buffer.
Resuspend 1 × 108 cells in 5 mL of FACS buffer and add PE rat anti-mouse CD138 antibody for the detection of CD138+ cells. Incubate cells for a minimum of 20 min on 4 °C.
Wash the cells twice in 10 mL FACS buffer by centrifuging at 10 min 300 × g, 4 °C.
Resuspend cells in 5 mL FACS buffer and remove clumped cells by passing the cell suspension through 40-μm pore-size nylon cell strainers.
Before proceeding with cell sorting, transfer cells to 5 mL polystyrene FACS tubes and add the DAPI viability dye to samples at the final concentration of 0.05 μg/mL.
Run samples and collect sorted CD138+ cells in 15 mL conical tubes in cell sort collection buffer.
Wash sorted cells twice by centrifuging 10 min 300 × g, 4 °C. Resuspend cell pellet in PBS and count the number of cells (see Note 9).
3.5.2. Detection of IL-35-Producing CD138+ Cells by Intracellular Cytokine Staining
Determine the percentage of IL-35-producing B cells among sorted CD138+ cells by intracellular cytokine staining using monoclonal Abs specific to p35 or Ebi3.
Plate sorted CD138+ cells at 1 × 106 cells/mL and add 50 ng/mL PMA, 500 ng/mL ionomycin, and 5 μg/mL Brefeldin A in complete culture medium. Incubate for 5 h.
After 5 h incubation, wash cells twice with cold PBS and stain the cells with a dye specific for the discrimination of viable from nonviable cells in multicolor flow cytometric applications for 20 min at 4 °C.
Wash the cells twice in FACS buffer by centrifuging at 10 min at 300 × g, 4 °C and add 200 μL/well intracellular fixation buffer for 30 min on 4 °C.
Permeabilize cells by washing twice with permeabilization buffer and centrifuge 10 min at 300 × g, 4 °C.
Add the anti-mouse p35 and Ebi3 antibodies diluted according to manufacturer’s instructions in 100 μL permeabilization buffer. Incubate for 1 h at 4 °C.
Wash the cells twice and resuspend in 200 μL FACS buffer. Transfer cells to 5 mL polystyrene FACS tubes and acquire data on a flow cytometer. Analyze data using a software specific for the analysis of flow cytometry data, e.g., FlowJo. A representative intracellular staining analysis of i35-Breg cells is shown in Fig.3.
Fig. 3.

Gating strategy for the analysis of IL-35-producing CD138+ B cells. Live cells from LPS-stimulated B220/CD45R cells were stained with an anti-CD138 antibody and sorted by FACS. CD138+ or CD138− cells were then subjected to intracellular cytokine staining analysis. The representative flow cytometry plot shows the percentage of IL-35-expressing CD138+ B cells (p35+ and Ebi3+). Bar chart shows the mean percentage of IL-35 expressing CD138+ or CD138− cells from 10 independent i35-Breg preparations. ****p < 0.0001
3.6. Adoptive transfer of IL-35-Producing B Cells in Recipient Uveitis Mice
To establish if i35-Bregs can directly suppress uveitis, it is necessary to induce EAU in C57BL/6 J mice (CD45.2+), purify i35-Breg cells from the spleen of the donor CD45.2+ mouse, and examine whether adoptive transfer of the i35-Breg cells would suppress EAU in a CD45.1+-recipient congenic mouse. B6 CD45.1 (recipient) and B6 CD45.2 (donor) mice should be age-matched (see Note 10). The i35-Bregs are transferred to naïve CD45.1+ mice (1 × 107 cells/mouse), and EAU is induced 24 h after cell transfer. Efficacy of the i35-Bregs treatment is evaluated starting from 10 days after adoptive transfer (see Fig. 2b).
The day before EAU induction, prepare i35-Bregs in fresh complete medium (see Subheading 3.5.1) and inject 107 cells in a < 200 μL volume per mouse by tail vein injection. For this procedure, the mouse should be placed under a heat lamp to promote peripheral vasodilation. Then the mouse should be mechanically restrained using a mouse restrainer with a tail access chamber. Finally, the tail should be swabbed with alcohol gauze and placed horizontally for cell injection through a 27-Gauge needle.
On day 0, induce EAU by immunization with IRBP651–670 in CFA emulsion (see Subheading 3.1).
From day 10, start monitoring mice (see Note 11).
After 14 days post-immunization, examine the eyes using a microscope with coaxial illumination to assess disease severity.
After 21 days post-immunization, use one of the eyes of every mouse for histological analysis. Use the other remaining eye to isolate the retina cells and to evaluate the inflammatory cell changes in the i35-Bregs treatment group using FACS analysis.
4. Notes
The emulsion of the IRBP651–670 peptide in CFA must be mixed well and prepared just before using it as the suspended Mycobacterium tuberculosis easily settles at the bottom of the bottle.
If you purchase a large quantity of the IRBP651–670 peptide (50 mg/vial), do not try to dissolve it all at once since it does not dissolve well. This makes it difficult to ascertain the true concentration of the peptide. Using the recommended dose is crucial to obtain reproducible results in the induction of EAU.
The PTX vial contains 50 μg of the powdered toxin. It is expedient to add 5 mL of 1% sterile normal mouse serum to the vial to make the 10 μg/mL PTX stock solution.
The C57BL/6 mouse strain must not be used in EAU studies since it presents the RD8 mutation in the Crb1 gene. The RD8 mutation is a single nucleotide deletion in the Crb1 gene, which results in a form of retinal degeneration having a distinct clinical appearance. The C57BL/6 J strain does not have the RD8 mutation and can therefore be employed [15].
To induce severe EAU in most susceptible mouse species, concurrent injection of Bordetella pertussis toxin is required.
To avoid a subjective bias in scoring Fundoscopy examinations, evaluation of the fundus photographs is conducted without knowledge of the mouse identity by a masked observer.
The use of p35 and/or Ebi3 reporter mouse strain would facilitate ex vivo production of i35-Breg cells. However, a IL-35 (p35/Ebi3) reporter mouse is not commercially available.
It is important to work fast and keep the cells cold (do not work at room temperature). This prevents capping of antibodies on the cell surface and non-specific cell labeling.
When working with normal mice, we generally obtain 70 × 106 cells from mouse spleen processing, then 25 × 106 CD45R/B220+ cells after immunomagnetic separation, and we end up with 0.5 × 106 viable regulatory plasmacytes per mouse after FACS sorting. In EAU-bearing mice, the number of cells varies depending on disease severity.
CD45.1 and CD45.2 mice are widely used in adoptive transfer studies to track the donor and recipient cells. B cells expressing either CD45.1+ or CD45.2+ can be distinguished by FACS using mouse antibodies that recognize either CD45 haplotypes. We use these mice to track donor and recipient Breg cells in mouse tissues during EAU.
Depending on the experimental goal, the end point of the EAU study can vary and the mouse might be euthanized from 15 to 21 days after immunization.
References
- 1.Nussenblatt RB (1990) The natural history of uveitis. Int Ophthalmol 14(5–6):303–308 [DOI] [PubMed] [Google Scholar]
- 2.Nussenblatt RB (1991) Proctor lecture. Experimental autoimmune uveitis: mechanisms of disease and clinical therapeutic indications. Invest Ophthalmol Vis Sci 32(13):3131–3141 [PubMed] [Google Scholar]
- 3.Wraith DC, Nicholson LB (2012) The adaptive immune system in diseases of the central nervous system. J Clin Invest 122(4):1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Streilein JW (2003) Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol 3(11):879–889 [DOI] [PubMed] [Google Scholar]
- 5.Caspi RR (2010) A look at autoimmunity and inflammation in the eye. J Clin Invest 120 (9):3073–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vignali DA, Kuchroo VK (2012) IL-12 family cytokines: immunological playmakers. Nat Immunol 13(8):722–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A (2007) Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol 179(5):3268–3275 [DOI] [PubMed] [Google Scholar]
- 8.Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C, Li R, Jouneau L, Boudinot P, Wilantri S, Sakwa I, Miyazaki Y, Leech MD, McPherson RC, Wirtz S, Neurath M, Hoehlig K, Meinl E, Grutzkau A, Grun JR, Horn K, Kuhl AA, Dorner T, Bar-Or A, Kaufmann SH, Anderton SM, Fillatreau S (2014) IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507 (7492):366–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zundler S, Neurath MF (2015) Interleukin-12: functional activities and implications for disease. Cytokine Growth Factor Rev 26 (5):559–568 [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Wei Y, Xiao H, Liu X, Zhang Y, Han G, Chen G, Hou C, Ma N, Shen B, Li Y, Egwuagu CE, Wang R (2016) A novel IL-23p19/Ebi3 (IL-39) cytokine mediates inflammation in lupus-like mice. Eur J Immunol 46(6):1343–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE (2007) T(H)17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med 13 (6):711–718 [DOI] [PubMed] [Google Scholar]
- 12.Choi JK, Dambuza IM, He C, Yu CR, Uche AN, Mattapallil MJ, Caspi RR, Egwuagu CE (2017) IL-12p35 inhibits Neuroinflammation and ameliorates autoimmune encephalomyelitis. Front Immunol 8:1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dambuza IM, He C, Choi JK, Yu CR, Wang R, Mattapallil MJ, Wingfield PT, Caspi RR, Egwuagu CE (2017) IL-12p35 induces expansion of IL-10 and IL-35-expressing regulatory B cells and ameliorates autoimmune disease. Nat Commun 8(1):719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, Wingfield PT, Kim SH, Egwuagu CE (2014) Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med 20(6):633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattapallil MJ, Wawrousek EF, Chan CC, Zhao H, Roychoudhury J, Ferguson TA, Caspi RR (2012) The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci 53 (6):2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]


