Abstract
Several different strains of simian-human immunodeficiency virus (SHIV) that contain the envelope glycoproteins of either T-cell-line-adapted (TCLA) strains or primary isolates of human immunodeficiency virus type 1 (HIV-1) are now available. One of the advantages of these chimeric viruses is their application to studies of HIV-1-specific neutralizing antibodies in preclinical AIDS vaccine studies in nonhuman primates. In this regard, an important consideration is the spectrum of antigenic properties exhibited by the different envelope glycoproteins used for SHIV construction. The antigenic properties of six SHIV variants were characterized here in neutralization assays with recombinant soluble CD4 (rsCD4), monoclonal antibodies, and serum samples from SHIV-infected macaques and HIV-1-infected individuals. Neutralization of SHIV variants HXBc2, KU2, 89.6, and 89.6P by autologous and heterologous sera from SHIV-infected macaques was restricted to an extent that these viruses may be considered heterologous to one another in their major neutralization determinants. Little or no variation was seen in the neutralization determinants on SHIV variants 89.6P, 89.6PD, and SHIV-KB9. Neutralization of SHIV HXBc2 by sera from HXBc2-infected macaques could be blocked with autologous V3-loop peptide; this was less true in the case of SHIV 89.6 and sera from SHIV 89.6-infected macaques. The poorly immunogenic but highly conserved epitope for monoclonal antibody IgG1b12 was a target for neutralization on SHIV variants HXBc2, KU2, and 89.6 but not on 89.6P and KB9. The 2G12 epitope was a target for neutralization on all five SHIV variants. SHIV variants KU2, 89.6, 89.6P, 89.6PD, and KB9 exhibited antigenic properties characteristic of primary isolates by being relatively insensitive to neutralization in peripheral blood mononuclear cells with serum samples from HIV-1-infected individuals and 12-fold to 38-fold less sensitive to inhibition with recombinant soluble CD4 than TCLA strains of HIV-1. The utility of nonhuman primate models in AIDS vaccine development is strengthened by the availability of SHIV variants that are heterologous in their neutralization determinants and exhibit antigenic properties shared with primary isolates.
Multiple simian-human immunodeficiency virus (SHIV) variants have been constructed by replacing env, tat, and rev of molecularly cloned SIVmac239 with the corresponding genes of human immunodeficiency virus type 1 (HIV-1). These variants broaden the scope of studies to assess efficacy and correlates of immunity in preclinical stages of vaccine development. SHIV is particularly advantageous for studies of HIV-1 envelope subunit vaccines in nonhuman primates. The surface gp120 and transmembrane gp41 of HIV-1, both of which are present on SHIV, are major targets for neutralizing antibodies (8). These envelope glycoproteins exhibit extensive genetic variability (26) and most likely exist as a trimolecular complex of heterodimers in their native oligomeric form on the virus surface (10, 14, 32, 71, 74). Genetic and structural variability in gp120 and gp41 are potential obstacles for the development of a broadly effective HIV-1 vaccine and add complexity to the in vitro and in vivo assessment of neutralizing antibodies (40).
Optimal use of the SHIV model requires knowledge of the antigenic properties of the chimeric viruses. Assessments of the breadth of antibody efficacy, for example, may require multiple virus variants that are heterologous to one another in their neutralization determinants. It is also important to know whether the antigenicity of the SHIV envelope glycoproteins resembles T-cell-line-adapted (TCLA) variants or primary isolates of HIV-1. For example, as with other lentiviruses (2, 11, 37), primary isolates of HIV-1 are less sensitive to antibody-mediated neutralization in vitro than TCLA strains (45, 60, 73). Primary isolates are also less sensitive to inhibition by recombinant soluble CD4 (rsCD4) (12, 47). The sensitivity of HIV-1 to neutralization by antibody and rsCD4 is strongly influenced by the structure of the native oligomeric envelope glycoproteins. Specifically, some epitopes are exposed for efficient antibody binding on TCLA strains more so than on primary isolates (10, 46, 74). This is especially true for epitopes residing in the V3 cysteine-cysteine loop of gp120 (6, 65, 70). A major emphasis is placed on achieving primary isolate neutralization with candidate HIV-1 vaccines (8, 40, 46, 50).
Envelope glycoproteins of both TCLA strains and primary isolates of HIV-1 have been used for SHIV construction. Some SHIV variants replicate poorly and are relatively avirulent in macaques (5, 18, 21, 27, 30, 31, 33, 35, 54, 55, 64), whereas others replicate at high levels persistently and induce AIDS (18, 20, 22–24, 34, 53, 55, 64, 66). Assessing vaccine efficacy with nonpathogenic SHIV is limited to observations of sterilizing immunity (i.e., absence of infection) and perhaps a reduction in transient virus loads, whereas assessments made with pathogenic SHIV include protection from immunologic suppression and AIDS. The validity of the SHIV model for studies of antibody efficacy is supported by the observation that passively administered antibodies can achieve both levels of protection in macaques (16, 36, 40, 63).
Six SHIV variants were selected here for study. One SHIV contained the envelope glycoproteins of the HXBc2 molecular clone of the IIIB TCLA strain of HIV-1 (30, 31, 33). SHIV HXBc2 was later engineered to contain the envelope glycoproteins of a primary isolate, designated 89.6 (54). Both SHIV variants are relatively avirulent in macaques and were subsequently passaged multiple times in vivo to increase their virulence. A highly pathogenic variant of HXBc2 was termed SHIV KU2 (22), whereas highly pathogenic variants of 89.6 were designated either 89.6P (53) or 89.6PD (34, 66). The latter two SHIV variants were isolated from the cells and plasma of the same infected animal, respectively, and therefore are closely related. We also examined the KB9 molecular clone of SHIV 89.6P, which exhibits many of the pathogenic properties of 89.6P (24). SHIV variants 89.6 and KB9 differ by 12 amino acids throughout the gp120 and gp41 sequences (24). The pathogenicity of KB9 has been attributed to changes specific to the ectodomain of the envelope glycoproteins (25).
The major neutralization determinants on SHIV variants HXBc2, 89.6, and 89.6P were shown previously to be different for each virus (15, 42). Most striking was the fact that 89.6P and its molecular clone, KB9, no longer possessed at least some of the neutralization determinants found on parental SHIV 89.6 and had actually acquired new determinants not found on SHIV 89.6 (15, 42). One of the goals of the present study was to determine whether a similar change in neutralization determinants occurred when the nonpathogenic SHIV HXBc2 was passaged in macaques to obtain the pathogenic variant KU2. We also assessed multiple SHIV variants to determine whether the antigenic properties of their envelope glycoprotein resembled those of primary isolates.
MATERIALS AND METHODS
Viruses.
The derivation and biologic properties of SHIV variants HXBc2 (30), KU2 (22), 89.6 (54), 89.6P (53), 89.6PD (34, 66), and KB9 (24) are summarized in Table 1. Briefly, inoculation with either HXBc2 or 89.6 in macaques results in a relatively attenuated infection, whereas inoculation with either KU2, 89.6P, 89.6PD, or KB9 results in variable and sometimes rapid immune suppression and death from AIDS-like illness. The IIIB and MN strains of HIV-1 were obtained from Robert Gallo and have been described previously (17). HIV-1 strain SF-2 (29) was obtained from the NIH AIDS Research and Reference Reagent Program (Bethesda, Md.) as donated by Jay Levy. Cell-free stocks of SHIV variant HXBc2 and HIV-1 strains IIIB and MN were produced in H9 cells as described previously (43). Cell-free stocks of the remaining SHIV variants were produced in human peripheral blood mononuclear cells (PBMC) as described previously for primary HIV-1 isolates (41).
TABLE 1.
Description of SHIV variants used for study
| SHIV varianta | Derivation | Pathogenic in macaques |
|---|---|---|
| HXBc2 | TCLA strain of HIV-1 IIIB | No |
| KU2 | Macaque-passaged SHIV HXBc2 | Yes |
| 89.6 | Primary isolate that is dual tropic for T cells and macrophages | No |
| 89.6P | Macaque-passaged SHIV 89.6, isolated from cells | Yes |
| 89.6PD | Macaque-passaged SHIV 89.6, isolated from plasma | Yes |
| KB9 | Molecularly cloned SHIV 89.6P | Yes |
HXBc2, 89.6, and KB9 SHIV variants are molecularly cloned. KU2, 89.6P, and 89.6PD SHIV variants are minor quasispecies.
Cells.
H9 (17), MT-2 (19), and CEMx174 (59) are human CD4+ lymphoblastoid cell lines that are highly permissive to infection with TCLA strains of HIV-1 and many SHIV strains. These cells were maintained in growth medium consisting of RPMI 1640 supplemented with 12% heat-inactivated fetal bovine serum and 50 μg of gentamicin/ml. Human PBMC were prepared from buffy coats of healthy, HIV-1-negative individuals obtained through the Laboratory Services of the American Red Cross Carolina Region in Charlotte, N.C. The PBMC were isolated by centrifugation over lymphocyte separation medium (Organon-Teknika/Akzo, Durham, N.C.). Cells at the interface were washed twice in growth medium containing 20% heat-inactivated fetal bovine serum and resuspended at a density of 2.5 × 107 cells/ml and frozen in 1-ml portions in liquid nitrogen with the aid of a Gordonier controlled-rate cryostat. Prior to their use in neutralization assays and to grow virus stocks, aliquots of PBMC were thawed in a room-temperature water bath and incubated for 1 day at 37°C in 5% CO2–95% humidified air in growth medium supplemented with phytohemagglutinin-P (5 μg/ml) and 4% human interleukin-2 (IL-2). All human PBMC were prescreened for an ability to support the replication of syncytium-inducing (SI) and non-SI (NSI) primary isolates of HIV-1 to confirm the expression of appropriate coreceptors for the virus, including CCR5. PBMC from rhesus macaques (Macacca mulata) were similarly isolated from heparinized peripheral blood.
Serum samples and other immunologic reagents.
Serum samples were obtained from SHIV-infected macaques housed in primate facilities maintained in accordance with guidelines set forth in Guide for the Care and Use of Laboratory Animals (49). Additional serum samples were obtained from patients who had been infected with HIV-1 for at least 2 years and were attended by physicians at the Duke University Medical Center. Due to either adverse reactions or personal preference, these patients were not being treated with antiretroviral drugs. All serum samples were heat inactivated for 1 h at 37°C prior to use. Human monoclonal antibodies IgG1b12 and 2G12 have been described previously (9, 57, 69); the latter antibody was obtained from the NIH AIDS Research and Reference Reagent Program as contributed by Hermann Katinger. Human rsCD4 was a generous gift from Progenics Pharmaceuticals, Inc. (Tarrytown, N.Y.).
Peptides.
Peptides corresponding to the V3-loop of HIV-1 strains IIIB and 89.6 were synthesized and purified by the Laboratory of Molecular Structure at the National Institute of Allergy and Infectious Diseases, National Institutes of Health. The IIIB V3-loop peptide consisted of the amino acid sequence CTRPNNNTRKSIRIQRGPGRAFVTIGKIGNMRQA; the 89.6 V3-loop peptide had the amino acid sequence CTRPNNNTRRRLSIGPGRAFYARRNIIGDIRQA.
Neutralizing antibody assays.
Two assays were used to assess virus neutralization by antibody and rsCD4. One assay was performed in either MT-2 or CEMx174 cells and used a reduction in virus-induced cell killing as measured by neutral red uptake by viable cells as described previously (43). HIV-1 strains IIIB and MN and all SHIV variants used in these studies were highly infectious and cytopathic in the MT-2 cells. Assays with SF2 were sometimes performed in CEMx174 cells because of a greater cytopathic effect relative to that of MT-2 cells with this virus. Briefly, 50 μl of cell-free virus containing 500 50% tissue culture infectious doses (TCID50) were added to multiple dilutions of test serum, monoclonal antibodies, and rsCD4 in 100 μl of growth medium in triplicate in 96-well culture plates. The mixtures were incubated at 37°C for 1 h followed by the addition of either MT-2 or CEMx174 cells (5 × 104 cells in 100 μl) to each well. Infection led to extensive syncytium formation and virus-induced cell killing in approximately 4 to 6 days in the absence of antibodies. Neutralization was measured by staining viable cells with Finter’s neutral red in poly-l-lysine-coated plates (43). Percent protection was determined by calculating the difference in absorption (A540) between test wells (cells plus serum sample plus virus) and virus control wells (cells plus virus), dividing this result by the difference in absorption between cell control wells (cells only) and virus control wells, and multiplying by 100. Neutralization was measured at a time when virus-induced cell killing in virus control wells was greater than 70% but less than 100%. Neutralization titers are given as the reciprocal dilution required to protect 50% of cells from virus-induced killing.
For assays in which V3-loop peptides were tested for their ability to block neutralizing antibodies, serum samples were first diluted twofold in growth medium and then incubated for 1 h at 37°C in the presence and absence of peptide (50 μg/ml). The titer of neutralizing antibodies was then determined in MT-2 cells as described above.
Assays were also performed in either human or macaque PBMC by using a reduction in SHIV p27 Gag antigen synthesis as a measurement of neutralization as described previously (42). Briefly, diluted serum samples or monoclonal antibodies were incubated with virus (500 TCID50) in a total of 50 μl in triplicate for 1 h at 37°C in 96-well U-bottom culture plates. Six wells containing virus only (no test sample) were included as controls. Following a 1-h incubation at 37°C, PBMC (4 × 105 cells in 150 μl of IL-2 growth medium) were added to each well and the mixtures were incubated for 24 h at 37°C. The cells were then washed three times with 200 μl of growth medium to remove the virus inoculum and antibodies. Washed cells were resuspended in 200 μl of IL-2 growth medium and incubated in fresh 96-well U-bottom plates until p27 production reached peak concentrations. In some experiments, 25 μl was removed after the final resuspension and mixed with 225 μl of 0.5% Triton X-100 spiked with a known amount of p27 and incubated at 4°C overnight; the p27 content of the mixture was then measured to test for interference by residual anti-p27 antibody in the antigen detection enzyme-linked immunosorbent assay (ELISA). Culture supernatants (25 μl) were collected on a daily basis thereafter and mixed with 225 μl of 0.5% Triton X-100 for the later quantification of p27 produced by infection. Viral p27 was quantified with an antigen ELISA as described by the supplier (Organon-Teknika/Akzo). The 25-μl volume of culture fluid removed each day was replaced with 25 μl of fresh IL-2-containing growth medium. Measurements of p27 for the detection of neutralization were made on a harvest prior to the time when p27 production in virus control wells had reached peak concentrations, which is when optimum sensitivity is achieved in this assay (75).
ELISA.
Nunc (Roskilde, Denmark) Immuno plates (MaxiSorb F96) were coated with V3-loop peptides by adding 100 μl of a solution containing peptide (1 μg/ml) in carbonate buffer (15 mM Na2CO3, 35 mM NaHCO3 [pH 9.8]) overnight at 4°C. The solution was aspirated, and the wells were washed once with 100 μl of phosphate-buffered saline (PBS; 0.14 M NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.7 mM NaH2PO4 [pH 7.4]) and then filled with 100 μl of blocking buffer (95% [vol/vol] PBS, 5% [wt/vol] bovine serum albumin, 5% [vol/vol] growth medium) and incubated for 2 h at 37°C. The plates were washed four times with PBS containing 0.05% [vol/vol] Tween 20. Serum samples were diluted 1:50 in borate buffer (0.1 M boric acid, 47 mM sodium borate, 75 mM NaCl, 0.05% [vol/vol] Tween 20) containing 2.5% fetal bovine serum, added to the wells (each in duplicate), and incubated for 2 h at 37°C. The wells were washed four times with PBS-Tween 20 and received 100 μl of alkaline phosphatase-conjugated, goat anti-monkey immunoglobulin G (IgG) (whole molecule; Sigma Chemical Co., St. Louis, Mo.) and incubated for 2 h at 37°C. The wells were washed four times with PBS-Tween 20 and incubated with 100 μl of p-nitrophenylphosphate disodium hexahydrate (Sigma 104 phosphatase substrate) in diethanolamine buffer (0.9 M diethanolamine, 7 mM MgCl2 [pH 9.8] with concentrated HCl). Following color development, the absorbance was read at 405 nm.
RESULTS
Neutralization of SHIV and TCLA strains of HIV-1 with sera from SHIV-infected macaques.
One of our first goals was to assess the relatedness of six SHIV variants by using sera from macaques infected with either SHIV variant HXBc2, KU2, 89.6, or 89.6PD. Neutralization of HXBc2, 89.6, and 89.6P was shown previously to be distinct, and therefore these viruses were considered heterologous in their neutralization determinants (15, 42). We confirmed and extended those observations here by including SHIV variants KU2, 89.6PD, and KB9 in cross-sectional assessments. We also included HIV-1 strains MN and SF2 as representative TCLA viruses. The data are shown in Table 2. For comparison, this table contains titers reported previously for serum samples from long-term SHIV 89.6-infected animals, 123-93 and 504-92, when assayed with SHIV variants HXBc2, 89.6, and 89.6P and HIV-1 strains MN and SF2 (42).
TABLE 2.
Neutralizing antibodies generated by SHIV infection
| SHIV variant and animal no. | Wk of infection | Neutralizing antibody titer toa:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| SHIV
|
HIV-1
|
||||||||
| HXBc2 | KU2 | 89.6 | 89.6P | 89.6PD | KB9 | MN | SF2 | ||
| HXBc2 | |||||||||
| 18001 | 27 | 469 | <20 | <20 | <20 | <20 | <60 | <60 | 85 |
| 18024 | 27 | 968 | <20 | <20 | <20 | <20 | <60 | 77 | 154 |
| 18062 | 27 | 793 | <20 | <20 | <20 | <20 | <60 | <60 | 99 |
| L28 | 146 | 5,340 | 112 | 145 | <20 | <20 | <20 | 487 | 7,321 |
| H123 | 156 | 1,622 | <20 | 193 | <20 | <20 | <20 | 1,526 | 2,918 |
| T06 | 156 | 799 | 80 | 63 | <20 | <20 | <20 | 254 | 1,641 |
| KU2 | |||||||||
| UV3 | 14 | 258 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| 14984 | 14 | 236 | <20 | <20 | <20 | <20 | <20 | <20 | 33 |
| E2V | 14 | 3,818 | 553 | <20 | <20 | <20 | <20 | 47 | 221 |
| T429 | 28 | 2,146 | 1,612 | 48 | 20 | 22 | <20 | 320 | 523 |
| T443 | 28 | 1,293 | 159 | <20 | <20 | <20 | <20 | 83 | 112 |
| V540 | 28 | 1,053 | 4,338 | 29 | <20 | <20 | <20 | <20 | 101 |
| T225 | 28 | 3,313 | 884 | 27 | <20 | <20 | <20 | 90 | 171 |
| T155 | 28 | 2,241 | 51 | 49 | <20 | <20 | <20 | 100 | 305 |
| 89.6 | |||||||||
| 259-94 | 16 | <20 | <20 | 951 | <20 | <20 | <20 | <20 | <20 |
| 305-94 | 16 | <20 | <20 | 195 | <20 | <20 | <20 | 42 | <20 |
| 123-93 | 124 | 40 | <20 | 2,484 | 30 | 67 | 55 | 659 | 388 |
| 504-92 | 124 | <10 | <20 | 3,539 | 13 | 27 | <20 | 124 | 48 |
| 89.6PD | |||||||||
| R94056 | 31 | <20 | <20 | <20 | 2,528 | 2,188 | 2,434 | 101 | <20 |
| R92014 | 53 | <20 | <20 | 357 | 547 | 824 | 763 | 594 | 300 |
| R94085 | 53 | <20 | <20 | 630 | 840 | 1,168 | 962 | 1,282 | 733 |
SHIV HXBc2 and HIV-1 strains MN and SF2 were grown in H9 cells. All other viruses were grown in human PBMC. Titers of neutralizing antibodies were measured in either MT-2 cells (all viruses except SF2) or CEMx174 cells (SF2) and are given as the reciprocal serum dilution at which 50% of cells were protected from virus-induced killing.
As can be seen in Table 2, each SHIV was highly sensitive to neutralization by autologous serum samples. Sera from SHIV-infected animals also contained variable and sometimes potent neutralizing activity against HIV-1 strains MN and SF2. Notably, however, sera from animals infected with either SHIV variant HXBc2 or 89.6 had little or no neutralizing activity against heterologous SHIV. This was also true for sera from animals infected with either SHIV variant KU2 or 89.6PD, with two exceptions: (i) serum from KU2-infected animals often neutralized HXBc2 better than KU2, and (ii) serum from some 89.6PD-infected animals had nearly equivalent neutralization potencies against SHIV variants 89.6, 89.6P, 89.6PD, and KB9. It was interesting that sera from 89.6PD-infected animals could neutralize 89.6 so well when sera from 89.6-infected animals had little neutralizing activity against SHIV variants 89.6P, 89.6PD, and KB9. This unidirectional cross-neutralizing activity might be explained by either an enhanced immunogenicity of the 89.6PD envelope glycoproteins or, alternatively, by higher levels of persistent SHIV 89.6PD replication relative to SHIV 89.6 replication in macaques.
Serum samples from SHIV 89.6PD-infected animals that cross-neutralized SHIV 89.6 were obtained after 53 weeks of infection. In contrast, serum from a third animal (R94056) infected with 89.6PD for only 31 weeks failed to neutralize 89.6 despite having potent neutralizing activity against SHIV variants 89.6P, 89.6PD, and KB9. This was also true for serum samples obtained after 11 weeks of infection with 89.6PD in an earlier study (42). Due to the heterologous nature of the neutralization determinants on these two viruses, antibodies that neutralize 89.6 are not generated until high titers of 89.6PD-specific neutralizing antibodies are detected in 89.6PD-infected macaques. This is consistent with the observation that potent neutralization of SHIV 89.6 was detected after only 16 weeks of infection with SHIV 89.6 (Table 2).
Neutralization of SHIV with sera from HIV-1-infected individuals.
We next examined the neutralization sensitivity of each SHIV variant in cell lines and in human PBMC with serum samples from HIV-1-infected individuals. Results of assays performed in cell lines are shown in Table 3. All serum samples contained high titers of neutralizing antibodies against the TCLA strains IIIB, MN, and SF2. SHIV variant KU2 was clearly the least sensitive to neutralization by these serum samples, where positive neutralization was low or undetectable with nine of nine samples tested. By comparison, SHIV variants 89.6, 89.6P, 89.6PD, and KB9 were more sensitive to neutralization in these assays, although the overall sensitivity was moderate in comparison to that of MN and SF2 and more closely resembled that of IIIB. Of note, 89.6P, 89.6PD, and KB9 had approximately equal levels of neutralization sensitivity, again indicating that the neutralization determinants of these three SHIV variants are remarkably similar. Interestingly, SHIV HXBc2 was somewhat less sensitive to neutralization than HIV-1 strain IIIB. It is possible in this case that the HXBc2 molecular clone of IIIB differs antigenically from the dominant quasispecies present in the uncloned IIIB stock.
TABLE 3.
Neutralization sensitivity of SHIV when assayed in MT-2 cells with sera from HIV-1-infected individuals
| Serum | Neutralizing antibody titer toa:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| SHIV
|
HIV-1
|
||||||||
| HXBc2 | KU2 | 89.6 | 89.6P | 89.6PD | KB9 | IIIB | MN | SF2 | |
| T00953 | 169 | 34 | 120 | 125 | 86 | 72 | 97 | 102 | 287 |
| W97464 | 58 | <20 | 97 | 25 | 29 | 31 | 124 | 647 | 731 |
| P46471 | 215 | <20 | 200 | 51 | 40 | 45 | 960 | 1,102 | 1,301 |
| T88580 | 71 | 21 | 305 | 86 | 192 | 69 | 136 | 5,171 | 6,408 |
| X00250 | <20 | <20 | 33 | 22 | 25 | 41 | 25 | 166 | 5,889 |
| F22934 | <20 | <20 | 82 | 53 | 55 | 88 | 36 | 427 | 775 |
| M00333 | 113 | 69 | 84 | 110 | 83 | 202 | 332 | 2,446 | 1,524 |
| X39840 | 74 | <20 | 249 | 100 | 94 | 85 | 172 | 1,688 | 2,077 |
| V91008 | 139 | <20 | 186 | 32 | <20 | 21 | 312 | 3,689 | 194 |
SHIV HXBc2 and HIV-1 strains IIIB, MN, and SF2 were grown in H9 cells. All other viruses were grown in human PBMC. Titers of neutralizing antibodies were measured in either MT-2 cells (all viruses except SF2) or CEMx174 cells (SF2) and are given as the reciprocal serum dilution at which 50% of cells were protected from virus-induced killing.
We next examined the sensitivity of five SHIV variants in neutralization assays performed with human PBMC with sera from seven of the HIV-1-infected individuals mentioned above. As shown in Table 4, SHIV variants KU2, 89.6, 89.6P, and 89.6PD were relatively insensitive to neutralization in these assays. Specifically, each variant was less sensitive than SHIV HXBc2. Three of the serum samples (T00953, W97464, and P46471) had been assessed previously with six primary HIV-1 isolates (7). Serum samples that were most potent against those primary isolates were more likely to neutralize SHIV variants KU2, 89.6, 89.6P, and 89.6PD. These latter SHIV variants were, overall, moderately more sensitive to neutralization than the primary HIV-1 isolates, perhaps because they consisted of less-complex quasispecies of genetic variants. Also, neutralization of 89.6, 89.6P, and 89.6PD was less potent in human PBMC than in MT-2 cells (Table 3). This discordant outcome is unlikely to be due to the different end points in the two assays, since a 50% reduction in cell viability in the MT-2 assay corresponds consistently to >80% reductions in p27 synthesis (unpublished data). Differential neutralization in these two cell types has also been observed with the HIV-1 89.6 primary isolate, where the phenomenon was unrelated to coreceptor usage (39).
TABLE 4.
Neutralization sensitivity of SHIV when assayed in human PBMC with sera from HIV-1-infected individuals
| Serum | Neutralizing antibody titer to SHIV varianta:
|
||||
|---|---|---|---|---|---|
| HXBc2 | KU2 | 89.6 | 89.6P | 89.6PD | |
| T00953 | 423 | 44 | 64 | 67 | 40 |
| W97464 | 14 | 5 | 7 | <3 | <3 |
| P46471 | 234 | 29 | 59 | 27 | 4 |
| T88580 | 70 | <3 | 20 | 4 | 7 |
| X00250 | <3 | <3 | <3 | <3 | <3 |
| F22934 | <3 | <3 | 11 | <3 | <3 |
| M00333 | 83 | 6 | 5 | 6 | 4 |
SHIV HXBc2 was grown in H9 cells. All other viruses were grown in human PBMC. Titers of neutralizing antibodies were measured in human PBMC and are given as the reciprocal serum dilution at which SHIV p27 antigen production was reduced by 80%.
Sensitivity to inhibition by rsCD4.
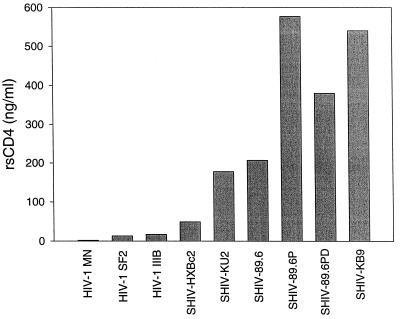
Six SHIV variants were compared to TCLA strains IIIB, MN, and SF2 in their sensitivity to inhibition with rsCD4. As shown in Fig. 1, several SHIV variants were much less sensitive to inhibition than TCLA strains in the MT-2 assay. Specifically, the 50% inhibitory doses for SHIV variants 89.6, 89.6P, 89.6PD, KB9, and KU2 were 14-, 38-, 25-, 36- and 12-fold higher, respectively, than the average dose required for 50% inhibition of HIV-1 strains SF2 and IIIB and were even higher when compared to that of MN. Of note, SHIV HXBc2 was approximately threefold less sensitive to inhibition than uncloned HIV-1 IIIB, a result consistent with the differential sensitivity to antibody-mediated neutralization exhibited by these two viruses in the MT-2 assay (Table 3). It should also be noted that variant KU2, which was the most resistant SHIV in antibody-mediated neutralization assays, was not the most resistant to inhibition by rsCD4. Nonetheless, it was at least 12-fold less sensitive to inhibition with rsCD4 than the TCLA strains were. The ability of rsCD4 inhibition to predict minor differences in neutralization sensitivity between HIV-1 IIIB and SHIV HXBc2 but not between KU2 and other SHIV variants might be explained by the fact that the neutralization epitope(s) on HIV-1 IIIB and SHIV HXBc2 are similar, whereas those on less-related viruses would be more divergent.
FIG. 1.
Inhibition of HIV-1 and SHIV infection with rsCD4. Viruses were incubated with various concentrations of rsCD4 ranging from 0.002 to 5 μg/ml and then examined for infectivity in MT-2 cells as described for antibody-mediated neutralization in Materials and Methods. The height of each bar corresponds to the dose of rsCD4 that was required to provide 50% protection from virus-induced cell killing.
V3-loop-specific neutralization of SHIV variants HXBc2 and 89.6.
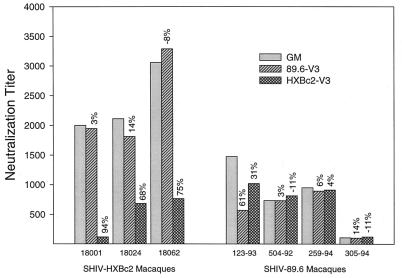
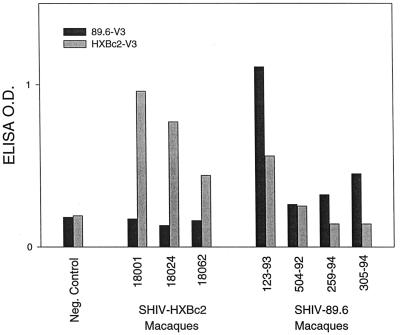
Serum samples from SHIV-infected macaques were assessed for V3-loop-specific neutralizing antibodies. This was done by testing whether the addition of V3-loop peptides to serum samples reduced the neutralization titer against the autologous SHIV. The results are shown in Fig. 2. Autologous neutralization titers of sera from three of three SHIV HXBc2-infected macaques were reduced 68- to 94-fold by the addition of HXBc2 V3-loop peptide, in comparison to when no peptide was added. As a relevant negative control, addition of 89.6 V3-loop peptide had no significant effect on the neutralization titer of these serum samples. These outcomes roughly corresponded to positive ELISA reactivity to an HXBc2 V3-loop peptide and negative reactivity to an 89.6 V3-loop peptide, respectively (Fig. 3).
FIG. 2.
Ability of V3-loop peptides to absorb neutralizing antibodies in serum samples from SHIV-infected macaques. Serum samples from macaques infected with either SHIV variant HXBc2 or 89.6 were incubated in the presence and absence of V3-loop peptides (50 μg/ml) and then examined for neutralizing antibody titer to the corresponding homologous SHIV in MT-2 cells as described in Materials and Methods. The height of each bar corresponds to the reciprocal serum dilution at which 50% of cells were protected from virus-induced killing effects. Values above each bar are the percent reductions in neutralization titer relative to that of the corresponding serum sample that was incubated with an equal volume of growth medium (GM) in place of V3-loop peptide. Serum samples from animals 18001, 18024, and 18062 were obtained after 27 weeks of infection with HXBc2. Serum samples from animals 123-93 and 504-92 were obtained after 124 weeks of infection, and serum samples 259-94 and 305-94 were obtained after 16 weeks of infection with 89.6.
FIG. 3.
V3-loop reactivity measured by peptide ELISA with serum samples from SHIV-infected macaques. Serum samples were assessed at a 1:50 dilution for antibodies reactive with IIIB and 89.6 V3-loop peptides in an ELISA as described in Materials and Methods. Serum samples are the same as those described in the legend to Fig. 2. A negative control serum sample was obtained from a healthy, noninfected rhesus macaque. O.D., optical density.
A different result was obtained with serum samples from animals infected with SHIV 89.6. In this case, only one of four animals had neutralizing antibodies that could be blocked by the addition of an 89.6 V3-loop peptide (animal 123-93). This same animal also had the strongest reactivity to the 89.6 V3-loop peptide by ELISA (Fig. 3). A moderate degree of SHIV 89.6 neutralizing activity could also be blocked by the addition of an HXBc2 V3-loop peptide, a result in agreement with the reactivity of this serum sample with the HXBc2 V3-loop peptide by ELISA. Serum samples from three remaining SHIV 89.6-infected animals showed no evidence of having V3-loop-specific antibodies as detected by neutralization-blocking assays or peptide ELISA.
Neutralization with IgG1b12 and 2G12.
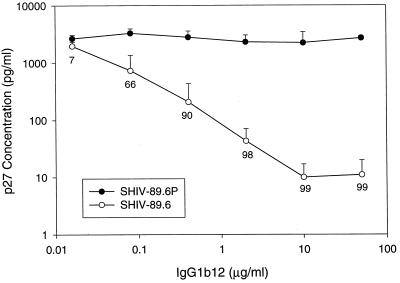
Three human monoclonal antibodies have been identified that recognize conserved epitopes in either the CD4 binding domain of gp120 (IgG1b12) (8, 57), the base of the V3/V4 region of gp120 (2G12) (69) or the membrane-proximal ectodomain of gp41 (2F5) (48, 52), and are capable of neutralizing TCLA strains and primary isolates broadly and potently (13, 68). Two of these monoclonal antibodies, IgG1b12 and 2G12, were tested for their ability to neutralize SHIV. As shown in Table 5, 2G12 was capable of neutralizing SHIV variants HXBc2, KU2, 89.6, 89.6P, and KB9 in MT-2 cells. Also, IgG1b12 was capable of neutralizing SHIV variants HXBc2, KU2, and 89.6, but not 89.6P and KB9 in MT-2 cells. To determine whether the inability of IgG1b12 to neutralize these latter two SHIV variants was a property of the virus or the cells used for assay, additional tests were performed in either rhesus or human PBMC. As shown in Fig. 4, SHIV 89.6 remained sensitive whereas 89.6P was highly resistant to IgG1b12 in rhesus PBMC. In addition, KB9 resisted neutralization by IgG1b12 at 18 μg/ml in human PBMC (data not shown). These results indicate that the resistance of 89.6P and KB9 to neutralization by IgG1b12 is independent of the cells used for assay. In support of this conclusion, Karlsson et al. obtained similar results when viruses bearing the 89.6 and KB9 envelope glycoproteins were assessed with IgG1b12 in CEMx174 cells (25).
TABLE 5.
Sensitivity to neutralization with human monoclonal antibodies IgG1b12 and 2G12
| Monoclonal antibody | Minimum concn (μg/ml) for neutralization of SHIV varianta:
|
||||
|---|---|---|---|---|---|
| HXBc2 | KU2 | 89.6 | 89.6P | KB9 | |
| IgG1b12 | 0.22 | 1.75 | 0.12 | >50 | >18 |
| 2G12 | 1.93 | 2.25 | 2.28 | 1.14 | 1.52 |
Neutralization was assessed in MT-2 cells. Values are the concentrations of antibody that provided 50% protection from virus-induced cell killing.
FIG. 4.
Neutralization of SHIV variants 89.6 and 89.6P by IgG1b12 in rhesus PBMC. Virus stocks were grown in human PBMC and assayed in rhesus macaque PBMC. Virus (500 TCID50) was incubated for 1 h at 37°C with various concentrations of IgG1b12 in 96-well U-bottom plates. Rhesus PBMC (stimulated with phytohemagglutinin-P) in IL-2 growth medium were added (500,000 cells/well), and the plates were incubated overnight. The medium was changed completely twice to remove the virus inoculum. Viral p27 was quantified at a time when virus production in the absence of IgG1b12 was in a linear phase of increase and had yet to peak (2,133 ± 438 pg/ml for SHIV variant 89.6 and 2,319 ± 571 pg/ml for SHIV variant 89.6P). Error bars are used to show the standard deviation for average p27 values in triplicate wells. Values below the data points on the 89.6 curve represent percent reductions in p27 synthesis relative to infection in the absence of IgG1b12.
DISCUSSION
Several SHIV variants were shown here to represent a cross-section of heterologous neutralization determinants and to resemble primary isolates in terms of the antigenicity of their envelope glycoproteins. These variants should prove useful when assessing breadth and potency of antibody efficacy in preclinical HIV-1 vaccine studies in macaques. Specifically, results obtained with serum samples from SHIV-infected macaques indicated that SHIV variants HXBc2, KU2, 89.6, and 89.6P (including 89.6PD and KB9) are heterologous to one another in their major neutralization determinants. In addition, KU2, 89.6, 89.6P, 89.6PD, and KB9 exhibit antigenic properties characteristic of primary isolates. Specifically, these SHIV variants were less sensitive to rsCD4 and neutralizing sera than TCLA strains of HIV-1. As another feature of primary isolates, neutralizing antibodies to SHIV 89.6 generated by SHIV 89.6 infection were not easily blocked by an 89.6 V3-loop peptide. This latter finding is in agreement with an earlier study where 89.6 neutralization was affected mostly by changes in V2 and less commonly by changes in V3 (15).
The antigenic properties of SHIV variants 89.6P, 89.6PD, and KB9 were nearly indistinguishable in assays with rsCD4 and sera from SHIV-infected animals, suggesting that these viruses are homologous in their neutralization determinants. In this regard, the fact that 89.6P and 89.6PD were isolated from cells and plasma of the same animal, respectively, and that KB9 is a molecular clone of a minor 89.6P quasispecies seems to have had little impact on the determinants of neutralization as detected by a variety of serologic reagents in vitro.
Our results also demonstrate that multiple passages in macaques can have a profound influence on the neutralization determinants of SHIV. One of the more striking examples of this was found with the nonpathogenic SHIV HXBc2 and its animal-passaged pathogenic variant, KU2. In particular, serum samples from HXBc2-infected animals had little or no neutralizing activity against KU2 despite having potent neutralizing activity against HXBc2. This outcome is unlikely to be explained by a lack of shared epitopes between HXBc2 and KU2, since infection with KU2 gave rise to antibodies that neutralized HXBc2. A more likely explanation is that the neutralization epitopes on KU2 were poorly exposed for efficient antibody binding relative to their exposure on HXBc2. This would explain why sera from some KU2-infected animals neutralized HXBc2 more potently than KU2. The envelope glycoproteins of KU2 are likely to have adopted a structure resembling primary isolate envelope glycoproteins as a consequence of in vivo passage. Results of crystallographic and serologic studies suggest that some neutralization epitopes on primary isolates are masked by the V1/V2 loops (74) and the positioning of N-glycans (1, 56, 61, 62) on the native gp120 molecule. Other epitopes might be occluded by subunit-subunit interactions in the quartenary structure of the native oligomeric complex. It has been proposed that poorly exposed epitopes on primary isolates are recognized by B cells in the context of monomers and debris of envelope glycoproteins present during infection to generate antibodies to epitopes exposed in a similar fashion on TCLA strains (50, 51). Our results with SHIV variants HXBc2 and KU2 are consistent with this hypothesis.
At least one neutralization epitope on KU2 must have been adequately exposed to permit potent neutralization of this SHIV by sera from some KU2-infected animals. This result is consistent with the antigenic properties of primary isolates. For example, some epitopes on primary isolates are potent targets for autologous neutralizing antibodies generated by infection; such epitopes are relatively strain specific, and it may take many months for the antibodies to be generated (44, 51, 72). The fact that sera from KU2-infected animals had little or no neutralizing activity against SHIV variants 89.6, 89.6P, 89.6PD, and KB9 is consistent with the notion that the neutralization epitope(s) detected on KU2 is relatively strain specific.
Similar to our observations with SHIV KU2, multiple animal passage had a profound effect on the neutralization determinants of SHIV 89.6 when deriving 89.6P and 89.6PD. In contrast to KU2 infection, however, serum from one 89.6PD-infected animal failed to neutralize parental 89.6 despite the serum’s ability to neutralize 89.6PD potently. Such strain-specific neutralization by serum from 89.6PD-infected animals has been observed previously and seems to be associated with earlier stages of infection (42). This outcome is consistent with the SHIV 89.6 envelope glycoproteins originating from a primary isolate and having at least some of its neutralization epitopes masked. An unusual feature of both viruses, however, was their increased sensitivity to neutralization in MT-2 cells relative to human PBMC, which was also observed for 89.6P. Although unusual for primary isolates, this property has also been observed with primary HIV-1 89.6 (39). The exact nature of this phenomenon is uncertain and did not appear to be related to coreceptor usage in the case of HIV-1 89.6 (39). Along these same lines, the titers of autologous neutralizing antibodies generated by infection with SHIV variants 89.6 and 89.6PD were higher than would be expected for a primary isolate when measured in MT-2 cells. Titers were much lower, however, when measured previously in PBMC (42), where they more closely approximated the titers expected for primary isolate neutralization (44, 51, 72). These observations suggest that SHIV 89.6 and its derivatives are somewhat atypical for primary isolates in terms of their high sensitivity to neutralization in MT-2 cells, although they resemble primary isolates in other important features.
Finally, the broadly cross-neutralizing human monoclonal antibodies 2G12 and IgG1b12 were used to examine the presence of conserved neutralization epitopes on SHIV. Our assessments showed that SHIV variants HXBc2, KU2, 89.6, 89.6P, and KB9 were sensitive to neutralization by 2G12, whereas only SHIV variants HXBc2, KU2, and 89.6 were sensitive to neutralization by IgG1b12. Thus, as reported previously (25), we confirm that the IgG1b12 determinant present on SHIV 89.6 was lost upon in vivo passage of this virus when deriving 89.6P and KB9. The 89.6 and KB9 envelope glycoproteins differ by 12 amino acids (24). Three of these changes in gp120, including I186V, E187K, and N190S (this latter mutation resulted in the acquisition of a potential site of N-glycan addition in KB9) are located in a region identified as important for in vitro escape from IgG1b12 in the case of the JR-FL strain as reported previously (38).
The differential neutralization of nonpathogenic SHIV 89.6 relative to pathogenic variants 89.6P and KB9 with IgG1b12 suggest that resistance to this monoclonal antibody is somehow related to in vivo virulence. In support of this notion, nonpathogenic SHIV HXBc2 was approximately 10 times more sensitive to IgG1b12 than its pathogenic counterpart, KU2. The determinants of KB9 pathogenicity have been shown to reside in the ectodomain of the envelope glycoproteins (25), which is where the IgG1b12 epitope resides (8). It is doubtful that virulence was related to escape from neutralizing antibodies equivalent to IgG1b12, since sera from SHIV-infected animals did not exhibit IgG1b12-like potency. Thus, the amino acid changes affecting IgG1b12 resistance and SHIV virulence are similar in location but would appear to arise independently. These SHIV variants will be useful when assessing the efficacy of IgG1b12 and 2G12 in passive immunization experiments. They also will be useful in testing the efficacy of vaccines that are capable of generating antibodies equivalent to IgG1b12 and 2G12. Unfortunately, the poor immunogenicity of the epitopes recognized by IgG1b12 and 2G12 has so far precluded their successful addition as a component of current HIV-1 vaccine candidates.
In summary, the utility of nonhuman primate models for HIV-1 vaccine development is strengthened by the availability of heterologous SHIV challenge stocks, some of which exhibit antigenic properties of primary isolates. It may be argued that one remaining obstacle to overcome when optimizing this model for studies of neutralizing antibodies is to develop a similar repertoire of SHIV variants that exhibit an NSI biologic phenotype and utilize CCR5 as their sole coreceptor. CCR5 and CXCR4 chemokine receptors are the major coreceptors used for HIV-1 entry (3, 58). Strains that use CXCR4 alone (X4 viruses) or in addition to CCR5 (X4R5 viruses) have an SI phenotype in MT-2 cells, whereas those that use CCR5 exclusively (R5 viruses) have an NSI phenotype in MT-2 cells (4). All of the SHIV variants described here are either X4 or X4R5 viruses. Given that most strains of HIV-1 isolated soon after infection are R5 viruses (3, 4, 58), one might question the relevance of using X4 or X4R5 viruses to assess the efficacy of antibodies generated by candidate HIV-1 vaccines. As a counterargument, however, antibody-mediated neutralization of HIV-1 has not been shown to be influenced by the differential use of CXCR4 and CCR5 coreceptors (28, 39, 67). In fact, no evidence exists to support the notion that neutralizing antibodies broadly effective against X4 or X4R5 viruses will not be equally effective against R5 viruses. We believe that the repertoire of SHIV variants described here are appropriate for studies of antibody efficacy in macaques as they relate to HIV-1 vaccine development.
ACKNOWLEDGMENTS
We thank Yichen Lu for SHIV 89.6PD, Opendra Narayan for SHIV KU2, Paul Maddon for rsCD4, and James Blanchard for serum samples from a subset of KU2-infected macaques.
This work was supported by grants from the NIH, including AI-85343 (D.C.M. and N.L.L.), AI65303 (M.S.W.), AI36643 (C.D.P.), AI33292 (D.R.B.), and AI40377 (P.W.H.I.P.).
REFERENCES
- 1.Back N K T, Smit L, de Jong J-J, Keulen W, Schutten M, Goudsmit J, Tersmette M. An N-glycan within the human immunodeficiency virus type 1 gp120 V3 loop affects virus neutralization. Virology. 1994;199:431–438. doi: 10.1006/viro.1994.1141. [DOI] [PubMed] [Google Scholar]
- 2.Baldinotti F, Matteucci D, Mazzetti P, Gianelli L, Bandecchi P, Tozzini F, Bendinelli M. Serum neutralization of feline immunodeficiency virus is markedly dependent on passage history of the virus and host system. J Virol. 1994;68:4572–4579. doi: 10.1128/jvi.68.7.4572-4579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger E A. HIV entry and tropism: the chemokine receptor connection. AIDS. 1997;11(Suppl. A):S3–S16. [PubMed] [Google Scholar]
- 4.Berger E A, Doms R W, Fenyö E M, Korber B T M, Littman D R, Moore J P, Sattentau Q J, Schuitemaker H, Sodroski J, Weiss R A. HIV-1 phenotypes classified by coreceptor usage. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 5.Bogers W M J M, Dubbes R, Haaft P T, Niphuis H, Cheng-Mayer C, Stahl-Hennig C, Hunsmann G, Kuwata T, Hayami M, Jones S, Ranjbar S, Almond N, Stott J, Rosenwirth B, Heeney J L. Comparison of in vitro and in vivo infectivity of different clade B HIV-1 envelope chimeric simian/human immunodeficiency viruses in Macaca mulatta. Virology. 1997;236:110–117. doi: 10.1006/viro.1997.8744. [DOI] [PubMed] [Google Scholar]
- 6.Bou-Habib D C, Roderiquez G, Oravecz T, Berman P W, Lusso P, Norcross M A. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J Virol. 1994;68:6006–6013. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradney A P, Scheer S, Crawford J M, Buchbinder S P, Montefiori D C. Neutralization escape in human immunodeficiency virus type 1-infected long-term nonprogressors. J Infect Dis. 1999;179:1264–1267. doi: 10.1086/314711. [DOI] [PubMed] [Google Scholar]
- 8.Burton D R, Montefiori D C. The antibody response in HIV-1 infection. AIDS. 1997;11(Suppl. A):S87–S98. [PubMed] [Google Scholar]
- 9.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W H I, Sawyer L S W, Hendry R M, Dunlop N, Nara P L, Lamacchia M, Garratty E, Stiehm E R, Bryson Y J, Cao Y, Moore J P, Ho D D, Barbas C F., III Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 10.Chan D C, Kim P S. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 11.Cook R F, Berger S L, Rushlow K E, McMannus J M, Cook S J, Harrold S, Raabe M L, Montelaro R C, Issel C J. Enhanced sensitivity to neutralizing antibodies in a variant of equine infectious anemia virus is linked to amino acid substitutions in the surface unit envelope glycoprotein. J Virol. 1995;69:1493–1499. doi: 10.1128/jvi.69.3.1493-1499.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daar E S, Li X L, Moudgil T, Ho D D. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency type 1 isolates. Proc Natl Acad Sci USA. 1990;87:6574–6578. doi: 10.1073/pnas.87.17.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Souza P M, Livnat D, Bradac J A, Bridges S H the AIDS Clinical Trials Group Antibody Selection Working Group, and Collaborating Investigators. Evaluation of monoclonal antibodies to human immunodeficiency virus type 1 primary isolates by neutralization assays: performance criteria for selecting candidate antibodies for clinical trials. J Infect Dis. 1997;175:1056–1062. doi: 10.1086/516443. [DOI] [PubMed] [Google Scholar]
- 14.Earl P L, Doms R W, Moss B. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci USA. 1990;87:648–652. doi: 10.1073/pnas.87.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etemad-Moghadam B, Karlsson G B, Halloran M, Sun Y, Schenten D, Fernandes M, Letvin N L, Sodroski J. Characterization of simian-human immunodeficiency virus envelope glycoprotein epitopes recognized by neutralizing antibodies from infected macaques. J Virol. 1998;72:8437–8445. doi: 10.1128/jvi.72.10.8437-8445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foresman L, Jia F, Li Z, Wang C, Stephens E B, Sahni M, Narayan O, Joag S V. Neutralizing antibodies administered before, but not after, virulent SHIV prevent infection in macaques. AIDS Res Hum Retroviruses. 1998;14:1035–1043. doi: 10.1089/aid.1998.14.1035. [DOI] [PubMed] [Google Scholar]
- 17.Gallo R C, Salahuddin S Z, Popovic M, Shearer G M, Kaplan M, Haynes B F, Palker T J, Redfield R, Oleske J, Safai B, White G, Foster P, Markham P D. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 18.Haaft P T, Verstrepen B, Überla K, Rosenwirth B, Heeney J. A pathogenic threshold of virus load defined in simian immunodeficiency virus- or simian-human immunodeficiency virus-infected macaques. J Virol. 1998;72:10281–10285. doi: 10.1128/jvi.72.12.10281-10285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harada S, Koyanagi Y, Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985;229:563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- 20.Harouse J M, Tan R C H, Gettie A, Dailey P, Marx P A, Luciw P A, Cheng-Mayer C. Mucosal transmission of pathogenic CXCR4-utilizing SHIV-SF33A variants in rhesus macaques. Virology. 1998;248:95–107. doi: 10.1006/viro.1998.9236. [DOI] [PubMed] [Google Scholar]
- 21.Igarashi T, Shibata R, Hasebe F, Ami Y, Shinohara K, Komatsu T, Stahl-Henning C, Petry H, Hunsmann G, Kuwata T, Jin M, Adachi A, Kurimura T, Okada M, Miura T, Hayami M. Persistent infection with SIVmac chimeric virus having tat, rev, vpu, env and nef of HIV type 1 in macaque monkeys. AIDS Res Hum Retroviruses. 1994;10:1021–1029. doi: 10.1089/aid.1994.10.1021. [DOI] [PubMed] [Google Scholar]
- 22.Joag S V, Li Z, Foresman L, Pinson D M, Raghavan R, Zhuge W, Adany I, Wang C, Jia F, Sheffer D, Ranchalis J, Watson A, Narayan O. Characterization of the pathogenic KU-SHIV model of acquired immunodeficiency syndrome in macaques. AIDS Res Hum Retroviruses. 1997;13:635–645. doi: 10.1089/aid.1997.13.635. [DOI] [PubMed] [Google Scholar]
- 23.Joag S V, Li Z, Foresman L, Stephens E B, Zhao L J, Adany I, Pinson D M, McClure H M, Narayan O. Chimeric simian human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J Virol. 1996;70:3189–3197. doi: 10.1128/jvi.70.5.3189-3197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlsson G B, Halloran M, Li J, Park I-W, Gomila R, Reimann K A, Axthelm M K, Iliff S A, Letvin N L, Sodroski J. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J Virol. 1997;71:4218–4225. doi: 10.1128/jvi.71.6.4218-4225.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karlsson G B, Halloran M, Schenten D, Lee J, Racz P, Tenner-Racz K, Manola J, Gelman R, Etemad-Moghadam B, Desjardins E, Wyatt R, Gerard N P, Marcon L, Margolin D, Fanton J, Axthelm M K, Letvin N L, Sodroski J. The envelope glycoprotein ectodomains determine the efficacy of CD4+ T lymphocyte depletion in simian-human immunodeficiency virus-infected macaques. J Exp Med. 1998;188:1159–1171. doi: 10.1084/jem.188.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korber B T M, Allen E E, Farmer A D, Myers G L. Heterogeneity of HIV-1 and HIV-2. AIDS. 1995;9:S5–S18. [PubMed] [Google Scholar]
- 27.Kuwata T, Igarashi T, Ido E, Jin M, Mizuno A, Chen J, Hayami M. Construction of human immunodeficiency virus 1/simian immunodeficiency virus strain mac chimeric viruses having vpr and/or nef of different parental origins and their in vitro and in vivo replication. J Gen Virol. 1995;76:2181–2191. doi: 10.1099/0022-1317-76-9-2181. [DOI] [PubMed] [Google Scholar]
- 28.LaCasse R A, Follis K E, Moudgil T, Trahey M, Binley J M, Planelles V, Zolla-Pazner S, Nunberg J H. Coreceptor utilization by human immunodeficiency virus type 1 is not a primary determinant of neutralization sensitivity. J Virol. 1998;72:2491–2495. doi: 10.1128/jvi.72.3.2491-2495.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy J A, Hoffman A D, Kramer S M, Landis J A, Shimabukuro J M, Oshiro L S. Isolation of lymphocytopathic retrovirus from San Francisco patients with AIDS. Science. 1984;225:840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- 30.Li J T, Halloran M, Lord C I, Watson A, Ranchalis J, Fung M, Letvin N L, Sodroski J G. Persistent infection of macaques with simian-human immunodeficiency viruses. J Virol. 1995;69:7061–7071. doi: 10.1128/jvi.69.11.7061-7067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Lord C I, Haseltine W, Letvin N L, Sodroski J. Infection of cynomolgus monkeys with a chimeric HIV-1/SIVmac virus that expresses the HIV-1 envelope glycoproteins. J AIDS. 1993;5:639–646. [PubMed] [Google Scholar]
- 32.Lu M, Blacklow S C, Kim P S. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat Struct Biol. 1995;2:1075–1082. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- 33.Lu Y, Salvato M S, Pauza C D, Li J, Sodroski J, Manson K, Wyand M, Letvin N, Jenkins S, Touzjian N, Chutkowski C, Kushner N, LeFaile M, Payne L G, Roberts B. Utility of SHIV for testing HIV-1 vaccine candidates in macaques. J Acquir Immun Defic Syndr Hum Retrovirol. 1996;12:99–106. doi: 10.1097/00042560-199606010-00001. [DOI] [PubMed] [Google Scholar]
- 34.Lu Y C, Pauza C D, Lu X S, Montefiori D C, Miller C J. Rhesus macaques that become systemically infected with pathogenic SHIV 89.6-PD after intravenous, rectal, or vaginal inoculation and fail to make an antiviral antibody response rapidly develop AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:6–18. doi: 10.1097/00042560-199809010-00002. [DOI] [PubMed] [Google Scholar]
- 35.Luciw P A, Pratt-Lowe E, Shaw K E S, Levy J A, Cheng-Mayer C. Persistent infection of rhesus macaques with T-cell line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV) Proc Natl Acad Sci USA. 1995;92:7490–7494. doi: 10.1073/pnas.92.16.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mascola J R, Lewis M G, Stiegler G, Harris D, VanCott T C, Hayes D, Louder M K, Brown C R, Sapan C V, Frankel S S, Lu Y, Robb M L, Katinger H, Birx D L. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Means R E, Greenough T, Desrosiers R C. Neutralization sensitivity of cell culture-passaged simian immunodeficiency virus. J Virol. 1997;71:7895–7902. doi: 10.1128/jvi.71.10.7895-7902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mo H, Stamatatos L, Ip J E, Barbas C F, Parren P W H I, Burton D R, Moore J P, Ho D D. Human immunodeficiency virus type 1 mutants that escape neutralization by human monoclonal antibody IgG1b12. J Virol. 1997;71:6869–6874. doi: 10.1128/jvi.71.9.6869-6874.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montefiori D C, Collman R G, Fouts T R, Zhou J Y, Bilska M, Hoxie J A, Moore J P, Bolognesi D P. Evidence that antibody-mediated neutralization of human immunodeficiency virus type 1 is independent of coreceptor usage. J Virol. 1998;72:1886–1893. doi: 10.1128/jvi.72.3.1886-1893.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montefiori D C, Evans T G. Toward an HIV-1 vaccine that generates potent, broadly cross-reactive neutralizing antibodies. AIDS Res Hum Retroviruses. 1999;15:689–698. doi: 10.1089/088922299310773. [DOI] [PubMed] [Google Scholar]
- 41.Montefiori D C, Pantaleo G, Fink L M, Zhou J T, Zhou J Y, Bilska M, Miralles G D, Fauci A S. Neutralizing and infection-enhancing antibody responses to human immunodeficiency virus type 1 in long-term nonprogressors. J Infect Dis. 1996;173:60–67. doi: 10.1093/infdis/173.1.60. [DOI] [PubMed] [Google Scholar]
- 42.Montefiori D C, Reimann K A, Wyand M S, Manson K, Lewis M G, Collman R G, Sodroski J G, Bolognesi D P, Letvin N L. Neutralizing antibodies in sera from macaques infected with chimeric simian-human immunodeficiency virus containing the envelope glycoproteins of either a laboratory-adapted variant or a primary isolate of human immunodeficiency virus type 1. J Virol. 1998;72:3427–3431. doi: 10.1128/jvi.72.4.3427-3431.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montefiori D C, Robinson W E, Jr, Schuffman S S, Mitchell W M. Evaluation of antiviral drugs and neutralizing antibodies to human immunodeficiency virus by a rapid and sensitive microtiter infection assay. J Clin Microbiol. 1988;26:231–235. doi: 10.1128/jcm.26.2.231-235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moog C, Fleury H J A, Pellegrin I, Kirn A, Aubertin A M. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J Virol. 1997;71:3734–3741. doi: 10.1128/jvi.71.5.3734-3741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore J P, Cao Y, Qing L, Sattentau Q J, Pyati J, Koduri R, Robinson J, Barbas C F, Burton D R, Ho D D. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J Virol. 1995;69:101–109. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore J P, Ho D D. HIV-1 neutralization: the consequences of viral adaptation to growth on transformed T cells. AIDS. 1995;9(Suppl. A):S117–S136. [PubMed] [Google Scholar]
- 47.Moore J P, McKeating J A, Huang Y X, Ashkenazi A, Ho D D. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J Virol. 1992;66:235–243. doi: 10.1128/jvi.66.1.235-243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muster T, Guinea R, Trkola A, Purtscher M, Klima A, Steindl F, Palese P, Katinger H. Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. J Virol. 1994;68:4031–4034. doi: 10.1128/jvi.68.6.4031-4034.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.National Institutes of Health. Guide for the care and use of laboratory animals. Washington, D.C.: National Research Council, National Academic Press; 1985. [Google Scholar]
- 50.Parren P W H I, Burton D R, Sattentau Q J. HIV-1 antibody—debris or virion? Nat Med. 1997;3:366–367. doi: 10.1038/nm0497-366d. [DOI] [PubMed] [Google Scholar]
- 51.Pilgrim A K, Pantaleo G, Cohen O J, Fink L M, Zhou J Y, Zhou J T, Bolognesi D P, Fauci A S, Montefiori D C. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term nonprogressive infection. J Infect Dis. 1997;176:924–932. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 52.Purtschur M, Trkola A, Grassauer A, Schultz P M, Klima A, Dopper S, Gruber G, Buchacher A, Muster T, Katinger H. Restricted antigenic variability of the epitope recognized by the neutralizing gp41 antibody 2F5. AIDS. 1996;10:587–593. doi: 10.1097/00002030-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Reimann K A, Li J T, Veazey R, Halloran M, Park I-W, Karlsson G B, Sodroski J, Letvin N L. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reimann K A, Li J T, Voss G, Lekutis C, Tenner-Racz K, Racz P, Lin W, Montefiori D C, Lee-Parritz D E, Lu Y C, Collman R G, Sodroski J, Letvin N L. An env gene derived from a primary HIV-1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J Virol. 1996;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reimann K A, Watson A, Dailey P J, Lin W, Lord C I, Steenbeke T D, Parker R A, Axthelm M K, Karlsson G B. Viral burden and disease progression in rhesus monkeys infected with chimeric simian human immunodeficiency viruses. Virology. 1999;256:15–21. doi: 10.1006/viro.1999.9632. [DOI] [PubMed] [Google Scholar]
- 56.Reitter J N, Means R E, Desrosiers R C. A role for carbohydrates in immune evasion in AIDS. Nat Med. 1998;4:679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 57.Roben P, Moore J P, Thali M, Sodroski J, Barbas III C F, Burton D R. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J Virol. 1994;68:4821–4828. doi: 10.1128/jvi.68.8.4821-4828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rucker J, Doms R W. Chemokine receptors as HIV coreceptors: implications and interactions. AIDS Res Hum Retroviruses. 1998;14(Suppl. 3):S241–S246. [PubMed] [Google Scholar]
- 59.Salter R D, Howell D N, Cresswell P. Gene regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics. 1985;21:235–246. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- 60.Sawyer L S W, Wrin M T, Crawford-Miksza L, Potts B, Wu Y, Weber P A, Alfonso R D, Hanson C V. Neutralization sensitivity of human immunodeficiency virus type 1 is determined in part by the cell in which the virus is propagated. J Virol. 1994;68:1342–1349. doi: 10.1128/jvi.68.3.1342-1349.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schønning K, Jansson B, Olofsson S, Hansen J-E S. Rapid selection for an N-linked oligosaccharide by monoclonal antibodies directed against the V3 loop of human immunodeficiency virus type 1. J Gen Virol. 1996;77:753–758. doi: 10.1099/0022-1317-77-4-753. [DOI] [PubMed] [Google Scholar]
- 62.Schønning K, Jansson B, Olofsson S, Neilsen J O, Hansen J-E S. Resistance to V3-directed neutralization caused by an N-linked oligosaccharide depends on the quaternary structure of the HIV-1 envelope oligomer. Virology. 1996;218:134–140. doi: 10.1006/viro.1996.0173. [DOI] [PubMed] [Google Scholar]
- 63.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho M W, Martin M A. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 64.Shibata R, Maldarelli F, Siemon C, Matano T, Parta M, Miller G, Fredrickson T, Martin M A. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J Infect Dis. 1997;176:362–373. doi: 10.1086/514053. [DOI] [PubMed] [Google Scholar]
- 65.Spenlehauer C, Saragosti S, Fleury H J A, Kirn A, Aubertin A-M, Moog C. Study of the V3 loop as a target epitope for antibodies involved in the neutralization of primary isolates versus T-cell-line-adapted strains of human immunodeficiency virus type 1. J Virol. 1998;72:9855–9864. doi: 10.1128/jvi.72.12.9855-9864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steger K K, Dykhuizen M, Mitchen J L, Hinds P W, Preuninger B L, Wallace M, Thomson J, Montefiori D C, Lu Y, Pauza C D. CD4+-T-cell and CD20+-B-cell changes predict rapid disease progression after simian-human immunodeficiency virus infection in macaques. J Virol. 1998;72:1600–1605. doi: 10.1128/jvi.72.2.1600-1605.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trkola A, Ketas T, KewalRamani V N, Endorf F, Binley J M, Katinger H, Robinson J, Littman D R, Moore J P. Neutralization sensitivity of human immunodeficiency virus type 1 primary isolates to antibodies and CD4-based reagents is independent of their coreceptor usage. J Virol. 1998;72:1876–1885. doi: 10.1128/jvi.72.3.1876-1885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trkola A, Pomales A B, Yuan H, Korber B, Maddon P J, Allaway G P, Katinger H, Barbas III C F, Burton D R, Ho D D, Moore J P. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasa K, Sodroski J, Moore J P, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vancott T C, Polonis V R, Loomis L D, Michael N L, Nara P L, Birx D L. Differential role of V3-specific antibodies in neutralization assays involving primary and laboratory-adapted isolates of HIV type 1. AIDS Res Hum Retroviruses. 1995;11:1379–1390. doi: 10.1089/aid.1995.11.1379. [DOI] [PubMed] [Google Scholar]
- 71.Weissenhorn W, Wharton S A, Calder L J, Earl P L, Moss B, Alirandis E, Skehel J J, Wiley D C. The ectodomain of HIV-1 env subunit gp41 forms a soluble, alpha-helical, rod-like oligomer in the ansence of gp120 and the N-terminal fusion peptide. EMBO J. 1996;15:1507–1514. [PMC free article] [PubMed] [Google Scholar]
- 72.Wrin T, Crawford L, Sawyer L, Weber P, Sheppard H W, Hanson C V. Neutralizing antibody responses to autologous and heterologous isolates of human immunodeficiency virus. J Acquir Immune Defic Syndr. 1994;7:211–219. [PubMed] [Google Scholar]
- 73.Wrin T, Loh T P, Vennari J C, Schuitemaker H, Nunberg J H. Adaptation to persistent growth in the H9 cell line renders a primary isolate of human immunodeficiency virus type 1 sensitive to neutralization by vaccine sera. J Virol. 1995;69:39–48. doi: 10.1128/jvi.69.1.39-48.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 75.Zhou J Y, Montefiori D C. Antibody-mediated neutralization of primary isolates of human immunodeficiency virus type 1 in peripheral blood mononuclear cells is not affected by the initial activation state of the cells. J Virol. 1997;71:2512–2517. doi: 10.1128/jvi.71.3.2512-2517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]