Abstract
Background:
Candida auris is an emerging fungal threat that has been spreading in the United States since it was first reported in 2016.
Objective:
To describe recent changes in the U.S. epidemiology of C auris occurring from 2019 to 2021.
Design:
Description of national surveillance data.
Setting:
United States.
Patients:
Persons with any specimen that was positive for C auris.
Measurements:
Case counts reported to the Centers for Disease Control and Prevention by health departments, volume of colonization screening, and antifungal susceptibility results were aggregated and compared over time and by geographic region.
Results:
A total of 3270 clinical cases and 7413 screening cases of C auris were reported in the United States through 31 December 2021. The percentage increase in clinical cases grew each year, from a 44% increase in 2019 to a 95% increase in 2021. Colonization screening volume and screening cases increased in 2021 by more than 80% and more than 200%, respectively. From 2019 to 2021, 17 states identified their first C auris case. The number of C auris cases that were resistant to echinocandins in 2021 was about 3 times that in each of the previous 2 years.
Limitation:
Identification of screening cases depends on screening that is done on the basis of need and available resources. Screening is not conducted uniformly across the United States, so the true burden of C auris cases may be underestimated.
Conclusion:
C auris cases and transmission have risen in recent years, with a dramatic increase in 2021. The rise in echinocandin-resistant cases and evidence of transmission is particularly concerning because echinocandins are first-line therapy for invasive Candida infections, including C auris. These findings highlight the need for improved detection and infection control practices to prevent spread of C auris.
Primary Funding Source:
None.
Since being initially reported in the United States in 2016 (1), the emerging fungus Candida auris has continued to cause illness and death nationwide. The Centers for Disease Control and Prevention (CDC) rated C auris as an “urgent threat,” the highest level of concern, because it is often multidrug-resistant; spreads easily in health care facilities; and can cause severe, invasive infections with high mortality rates (2).
Whereas many of the early C auris cases in the United States reflected importation from abroad, most of the recent cases reflect local transmission (3). Although it was initially limited primarily to the New York City and Chicago metropolitan areas (4), C auris has now been detected in more than half of U.S. states. C auris has become endemic in some areas, with ongoing transmission within and across health care facilities connected via patient transfers. Health care transmission is responsible for most, if not all, cases (5, 6). Most spread in the United States has occurred in high-acuity post–acute care facilities, specifically long-term acute care hospitals (LTACHs) and ventilator-capable skilled-nursing facilities (7–9). C auris cases tend to occur in patients who have multiple or prolonged health care encounters or indwelling devices, including those receiving mechanical ventilation (5).
In recent years, reports of infections that are associated with health care or caused by multidrug-resistant organisms (MDROs) have increased (10–12). In this article, we describe changes in the U.S. epidemiology of C auris that occurred during 2019 to 2021.
METHODS
Data Sources
We examined clinical and screening C auris cases (confirmed and probable cases, but not suspected cases [13]) reported to state and local health departments and CDC during the period from the first U.S. case—which occurred in 2013 but was reported retrospectively in 2016—through 31 December 2021. Health departments send monthly reports of cases identified by facilities in their jurisdiction to CDC (14). Clinical cases are those with C auris–positive specimens (for example, blood or urine) collected as part of routine clinical care, whereas screening cases are those with C auris–positive skin swabs collected during colonization screening (13). Clinical cases became nationally notifiable to CDC in 2019, reinforcing reporting practices that existed before then. Screening cases have not been nationally notifiable, so reporting to health departments may vary; however, public health laboratories have performed most testing of screening specimens, making reporting to health departments likely.
We also examined data from CDC’s Antimicrobial Resistance Laboratory Network (AR Lab Network), whose 7 regional public health laboratories test for C auris using culture-dependent and molecular methods to confirm clinical laboratory identifications and test colonization screening swabs (15). Colonization screening is based on epidemiology and is typically conducted for people who are at risk for colonization because they are a health care contact of a known case, have stayed in a high-acuity post–acute care facility, or have a history of health care in another country or U.S. region with high C auris burden (16). Screening is often done for high-risk persons or by performing point prevalence surveys involving all patients on targeted units.
Data Analysis
The AR Lab Network performs antifungal susceptibility testing on most clinical C auris isolates and a subset of screening isolates based on epidemiologic importance and capacity. Antifungal susceptibility to C auris was assessed for azoles (fluconazole), polyenes (amphotericin B), and echinocandins (anidulafungin and micafungin). Because C auris–specific susceptibility breakpoints have not been established, this analysis used tentative breakpoints defined by CDC (17). Isolates that were resistant to either echinocandin were considered to be resistant to that class. Isolates that were resistant to all 3 antifungal classes were considered to be pan-resistant.
Analyses were conducted using SAS, version 4.0 (SAS Institute). For all analyses, we used the specimen collection date to classify cases by time; when this was unavailable, we used the date of reporting to CDC. Some patients had multiple specimens or isolates.
This activity was reviewed by CDC and was conducted in accordance with applicable federal law and CDC policy (for example, 45 CFR §46.102(l)(2), 21 CFR §56, 42 USC §241(d), 5 USC §552a, and 44 USC §3501 et seq.).
Role of the Funding Source
No funding was received specifically for this analysis.
RESULTS
Increasing Burden of C auris
A total of 3270 clinical cases and 7413 screening cases of C auris were reported in the United States through 31 December 2021. Probable cases comprised 36% of cases before 2017, but only 3 such cases have been reported since. Annual clinical case counts, which are not subject to differences in screening practices, increased from 53 in 2016—the year in which cases were first reported—to 330 in 2018 and subsequently increased by 44% to 476 in 2019 (Figure 1). Annual clinical cases then increased by 59% to 756 in 2020 and by an additional 95% to 1471 in 2021.
Figure 1.
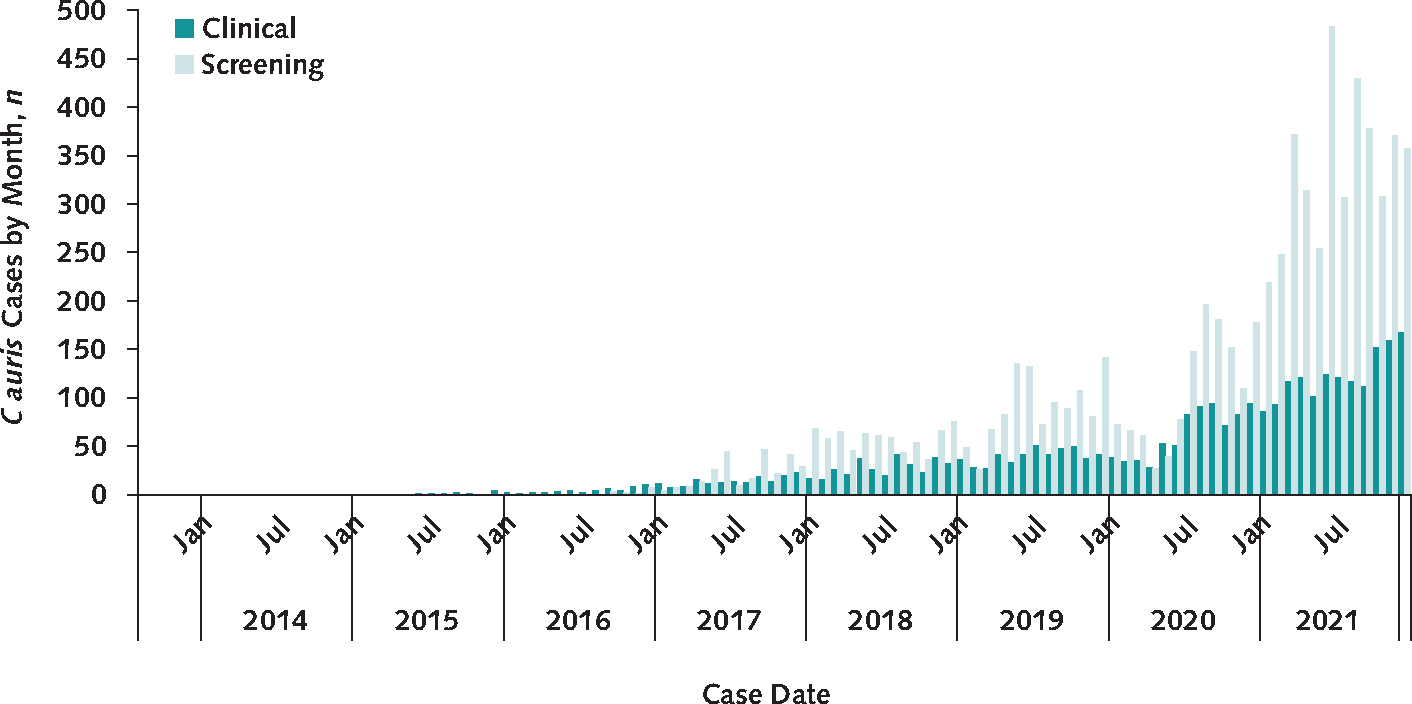
Number of clinical and screening C auris cases reported to the Centers for Disease Control and Prevention during 2013 to 2021.
C auris = Candida auris.
C auris screening cases also increased, with a 21% increase in 2020 compared with the previous year and a 209% increase to 4041 cases in 2021 (essentially tripling from 1310 cases in 2020) (Figure 1). In April and May 2020, during the initial months of the COVID-19 pandemic, the volume of screening swabs collected and screening cases identified decreased. After that, some regions identified new C auris transmission, prompting health departments in these areas to increase screening and response efforts, which resulted in higher overall screening volumes than in previous years. The total number of C auris colonization screening tests performed through CDC’s AR Lab Network increased from 19 756 in 2019 to 21 567 in 2020 (9% increase) to more than 40 000 (preliminary data) in 2021, which reflects an approximate 100% increase compared with 2020 (Figure 2). Meanwhile, the positivity rate of these screenings remained consistent at about 8% each year from 2019 to 2021.
Figure 2.
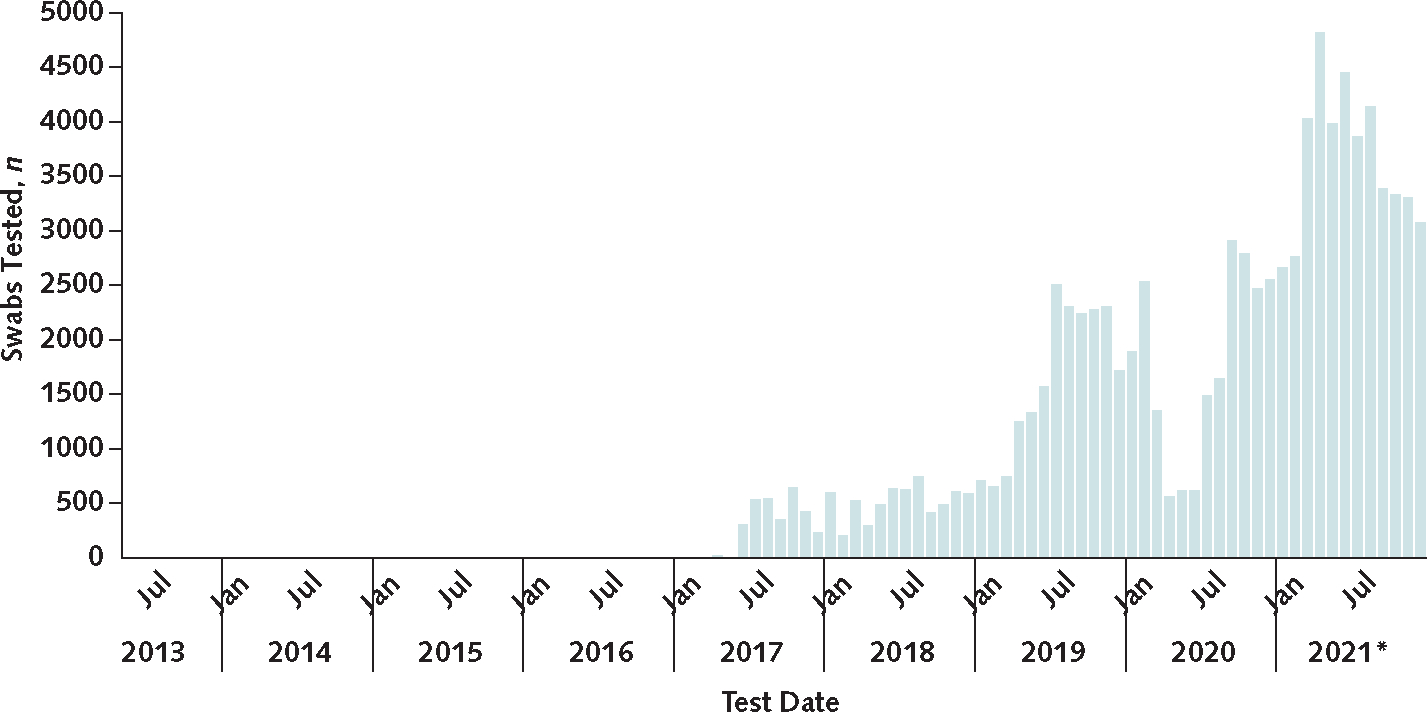
Volume of C auris screening through the Antimicrobial Resistance Laboratory Network, 2013 to 2021.
C auris screening was onboarded to the network in 2017, but the test date axis aligns with that of Figure 1 for ease of comparison. C auris = Candida auris.
* Data for 2021 are preliminary.
The geographic spread of C auris also increased, with 2020 having the highest number of new states affected (n = 8), compared with 6 in 2019, 2 in 2018, and 10 in all previous years combined (Figure 3). In 2021, 3 states reported their first case. During this time, some areas with previous cases but minimal spread (parts of California, the Mid-Atlantic, the Midwest, Texas, and Florida) also had new and growing transmission.
Figure 3.
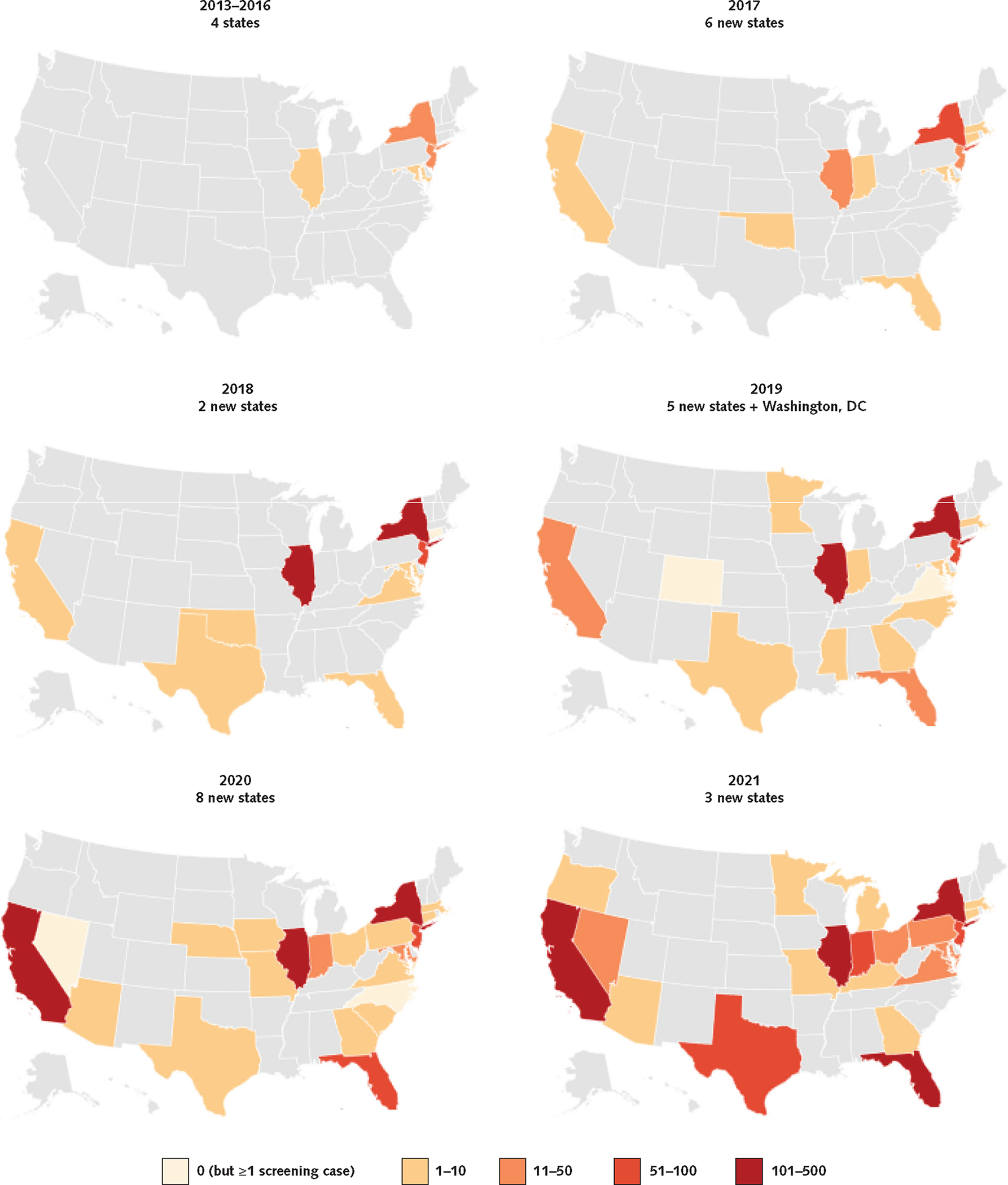
Geographic distribution of clinical C auris cases in the United States reported to the Centers for Disease Control and Prevention by state during 2013 to 2021.
C auris = Candida auris.
Drug Resistance
During 2020, 86% of isolates tested by the AR Lab Network were resistant to azoles and 26% were resistant to amphotericin B (Table). Most notably, azole resistance increased by about 7% between 2019 and 2020. Susceptibility patterns varied by geographic region due to local circulation of specific clades. Azole resistance is common (>90%) in isolates from the Northeast (predominantly clade I, originally detected in South Asia), the Southeast and West (predominantly clade III, originally detected in Africa), and the Mid-Atlantic and Mountain regions (both clade I and clade III) (Table). Isolates in the Midwest were primarily clade IV (originally detected in South America), which have had greater susceptibility to antifungals, including azoles (11% resistance). Amphotericin B resistance trends were similarly regional, with 85% of Mid-Atlantic isolates and 45% of Northeast isolates resistant compared with 5% in the Southeast and fewer than 5% in the Midwest, Mountain, and West regions. Few isolates were reported from the Central region.
Table.
Percentage Resistance of Candida auris Isolates Tested by the Antimicrobial Resistance Laboratory Network, 2018 to 2020*
| Year or Region | Azoles† | Amphotericin B‡ | Echinocandins§ |
|---|---|---|---|
|
| |||
| Year | |||
| 2018 (n = 463) | 372 (80.3) | 151 (32.6) | 2 (0.4) |
| 2019 (n = 1006) | 787 (78.2) | 242 (24.1) | 14 (1.4) |
| 2020 (n = 1294) | 1109 (85.7) | 331 (25.6) | 15 (1.2) |
| Region ∥ | |||
| Mid-Atlantic (n = 135) | 133 (98.5) | 115 (85.2) | 4 (3.0) |
| Midwest (n = 156) | 17 (10.9) | 2 (1.4) | 0 (0.0) |
| Mountain (n = 25) | 24 (96.0) | 1 (4.0) | 0 (0.0) |
| Northeast (n = 1051) | 1046 (99.5) | 468 (44.5) | 22 (2.1) |
| Southeast (n = 172) | 170 (99.4) | 9 (5.2) | 0 (0.0) |
| West (n = 556) | 553 (99.5) | 17 (3.1) | 1 (0.2) |
Data are numbers (percentages). Numbers are based on records with any minimum inhibitory concentrations (MICs). About 1% of all records for all times were missing MICs for 1 or 2 drug classes.
The tentative MIC breakpoint for fluconazole was ≥32 mcg/mL.
The tentative MIC breakpoint for amphotericin B was ≥2 mcg/mL.
The tentative MIC breakpoint for echinocandins was ≥4 mcg/mL (anidulafungin or micafungin).
The Central region is excluded because of the small number of isolates.
Although echinocandin resistance across all clades and geographic areas has been low (<5%), the number of patients with echinocandin-resistant and pan-resistant isolates increased in 2021. Before 2020, 4 patients with pan-resistant isolates and 6 others with echinocandin-resistant isolates had been reported in the United States; investigation suggested that these patients developed resistance during echinocandin treatment and had no epidemiologic links to other resistant cases (18). However, in 2021, 7 patients with pan-resistant isolates and 19 other patients with echinocandin-resistant isolates were detected compared with 6 and 3, respectively, in 2020. Epidemiologic investigation of cases identified 2 independent outbreaks of echinocandin-resistant and pan-resistant C auris among patients with shared health care exposures and no previous use of echinocandins, suggesting the first U.S. health care transmission of echinocandin-resistant C auris (19).
DISCUSSION
C auris cases and transmission in the United States have continued to increase in recent years, not only in areas with established transmission but also in areas with minimal previous transmission and areas with no prior cases. The United States is not the only country to observe notable increases in recent years; other countries have reported additional cases since 2020 (20) or reported their first cases or outbreaks (21–25).
Although echinocandin resistance is still uncommon, the number of cases with echinocandin resistance is slowly increasing, with a substantial increase in 2021 and multiple outbreaks of these resistant strains raising concerns about transmission (19). Even this subtle increase is concerning because echinocandins are the first-line therapy for invasive Candida infections and most C auris infections. Several new antifungal medications are in development (26–28), but more research is needed to understand outcomes for patients with these highly resistant strains and to guide treatment.
The reasons for the steady increase in C auris case burden are multifactorial and reflect deficiencies in early identification of cases and implementation of infection prevention and control (IPC) measures. Although infection control gaps existed and caused transmission before the COVID-19 pandemic, the timing of this increased C auris spread and findings from public health investigations suggest it may have been exacerbated by pandemic-related strain on the health care and public health systems, which included staff and equipment shortages, increased patient burden and disease severity, increased antimicrobial use, changes in patient movement patterns, and poor implementation of non–COVID-19 IPC measures (12, 29). Although attention to certain aspects of IPC grew during the pandemic (30, 31), focus on COVID-19 precautions seems to have occurred in some facilities at the expense of proper implementation of standard and contact precautions and environmental disinfection needed to reduce transmission of C auris and other MDROs (12, 32–34).
Similar to previous years, most U.S. cases continue to be found in high-acuity post–acute care facilities (8, 9, 35), especially LTACHs. Outbreaks in ACHs have historically been uncommon, perhaps because of more robust infection control and shorter lengths of stay compared with long-term care facilities (36–39). However, several large C auris outbreaks occurred in ACHs in recent years (29), showing that all facilities are vulnerable to transmission. During the COVID-19 pandemic, ACHs experienced challenges that are familiar in long-term care facilities and accordingly made changes in infection control practices (for example, extended use or reuse of personal protective equipment or inappropriate use of multiple gowns and gloves at once), which may have contributed to transmission (29, 40).
Previous experiences suggest that containment efforts can mitigate and even contain spread of C auris, as evidenced by the smaller increase in clinical cases from 2018 to 2019 and the fact that some facilities have stopped widespread transmission (41–43). The response to initial cases of C auris in Orange County, California, in 2019 illustrates how early detection followed by screening and IPC interventions can minimize spread (44). The subsequent increase in cases reveals how hard-won and fragile this progress can be in the absence of continued interventions. Effectively reducing the spread of C auris and other MDROs across the health care system will require investment to improve case detection and infection control, particularly in long-term care facilities. Although targeted IPC improvements after detection of C auris can mitigate transmission, proactive implementation of high-quality IPC measures is most effective because C auris can spread extensively before detection, and it will also contribute to reducing spread of many other pathogens.
C auris was not a reportable condition in many jurisdictions and screening cases were not nationally notifiable, but the effect of this on case reporting seems to be minimal. Screening varies across the United States on the basis of public health need and available resources. C auris cases may go undetected where screening is not occurring, which may result in an underestimate of the true burden. Data from the AR Lab Network do not reflect all C auris susceptibility or colonization testing performed nationally, as clinical, commercial, and public health laboratories may test independently of the AR Lab Network.
C auris remains an ongoing health threat in the United States. Public health and health care facilities already have limited resources and IPC capacity, and they experience further challenges with MDRO surveillance and prevention when those limited resources shift to fight other threats, such as the COVID-19 pandemic. Still, mitigation and even regional containment are possible, as facilities have shown that C auris transmission can be controlled. The Centers for Medicare & Medicaid Services is increasing guidance and accountability for infection control in nursing homes, providing an important incentive to ensure sustained IPC improvements (45); similar tools might help improve patient safety and C auris control in LTACHs. In addition to the fundamentals of MDRO containment (for example, surveillance, screening, and infection control), new tools are needed, such as faster and more accessible colonization testing, improved disinfection methods, increased capacity for antifungal susceptibility testing, and new antifungal drugs. Targeting interventions to the weakest links in the health care system’s infection control network, specifically LTACHs and ventilator-capable skilled-nursing facilities, will have benefits beyond C auris, including reducing the spread of other MDROs and improving preparedness for future epidemics. The spread of C auris provides motivation to refocus on public health fundamentals to prevent illness and save lives.
Acknowledgment:
The authors acknowledge the contribution of the AR Lab Network in providing testing for C auris detection and drug susceptibility.
Financial Support:
No funding was received specifically for this analysis. The Centers for Disease Control and Prevention provides federal funding that is used for ongoing surveillance activities, specifically to health care–associated infection programs of local health departments that conduct surveillance and to AR Lab Network laboratories that perform testing.
Footnotes
Disclosures: Authors have reported no disclosures of interest. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-3469.
Reproducible Research Statement: Study protocol, statistical code, and data set: Available to approved persons through written agreements (yeo4@cdc.gov).
References
- 1.Vallabhaneni S, Kallen A, Tsay S, et al. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus - United States, May 2013-August 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1234–7. doi: 10.15585/mmwr.mm6544e1 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. Centers for Disease Control and Prevention; 2019. doi: 10.15620/cdc:82532 [DOI] [Google Scholar]
- 3.Chow NA, Gade L, Tsay SV, et al. ; US Candida auris Investigation Team. Multiple introductions and subsequent transmission of multi-drug-resistant Candida auris in the USA: a molecular epidemiological survey. Lancet Infect Dis. 2018;18:1377–84. doi: 10.1016/S1473-3099(18)30597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsay S, Welsh RM, Adams EH, et al. Notes from the field: ongoing transmission of Candida auris in health care facilities - United States, June 2016-May 2017. MMWR Morb Mortal Wkly Rep. 2017;66:514–5. doi: 10.15585/mmwr.mm6619a7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forsberg K, Woodworth K, Walters M, et al. Candida auris: the recent emergence of a multidrug-resistant fungal pathogen. Med Mycol. 2019;57:1–12. doi: 10.1093/mmy/myy054 [DOI] [PubMed] [Google Scholar]
- 6.Snyder GM, Wright SB. The epidemiology and prevention of Candida auris. Curr Infect Dis Rep. 2019;21:19. doi: 10.1007/s11908-019-0675-8 [DOI] [PubMed] [Google Scholar]
- 7.Rossow J, Ostrowsky B, Adams E, et al. ; New York Candida auris Investigation Workgroup. Factors associated with Candida auris colonization and transmission in skilled nursing facilities with ventilator units, New York, 2016–2018. Clin Infect Dis. 2021;72:e753–e760. doi: 10.1093/cid/ciaa1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pacilli M, Kerins JL, Clegg WJ, et al. Regional emergence of Candida auris in Chicago and lessons learned from intensive follow-up at 1 ventilator-capable skilled nursing facility. Clin Infect Dis. 2020;71:e718–e725. doi: 10.1093/cid/ciaa435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams EH, Quinn M, Ostrowsky B, et al. 385. The value added from Candida auris point prevalence and environmental studies in New York State. Open Forum Infect Dis. 2018;5(Suppl 1):S149. doi: 10.1093/ofid/ofy210.396 [DOI] [Google Scholar]
- 10.Baker MA, Sands KE, Huang SS, et al. ; CDC Prevention Epicenters Program. The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections. Clin Infect Dis. 2022;74:1748–54. doi: 10.1093/cid/ciab688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiner-Lastinger LM, Pattabiraman V, Konnor RY, et al. The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections in 2020: a summary of data reported to the National Healthcare Safety Network - ADDENDUM. Infect Control Hosp Epidemiol. 2022;43:137. doi: 10.1017/ice.2022.10 [DOI] [PubMed] [Google Scholar]
- 12.Witt LS, Howard-Anderson JR, Jacob JT, et al. The impact of COVID-19 on multidrug-resistant organisms causing healthcare-associated infections: a narrative review. JAC Antimicrob Resist. 2023;5:dlac130. doi: 10.1093/jacamr/dlac130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Candida auris: 2019 Case Definition. Accessed at https://ndc.services.cdc.gov/case-definitions/candida-auris-2019on8February2023.
- 14.Centers for Disease Control and Prevention. Tracking Candida auris. Accessed at www.cdc.gov/fungal/candida-auris/tracking-c-auris.htmlon8February2023.
- 15.Centers for Disease Control and Prevention. About the AR Lab Network. Accessed at www.cdc.gov/drugresistance/ar-lab-networks/domestic.htmlon8February2023.
- 16.Centers for Disease Control and Prevention. Interim Guidance for a Public Health Response to Contain Novel or Targeted Multidrug-resistant Organisms (MDROs). January 2019. Accessed at www.cdc.gov/hai/pdfs/containment/Health-Response-Contain-MDRO-H.pdfon8February2023. [Google Scholar]
- 17.Centers for Disease Control and Prevention. Antifungal Susceptibility Testing and Interpretation. Accessed at www.cdc.gov/fungal/candida-auris/c-auris-antifungal.htmlon8February2023.
- 18.Ostrowsky B, Greenko J, Adams E, et al. ; C. auris Investigation Work Group. Candida auris isolates resistant to three classes of antifungal medications - New York, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:6–9. doi: 10.15585/mmwr.mm6901a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyman M, Forsberg K, Reuben J, et al. Notes from the field: transmission of pan-resistant and echinocandin-resistant Candida auris in health care facilities - Texas and the District of Columbia, January–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1022–3. doi: 10.15585/mmwr.mm7029a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez JY, Le Pape P, Lopez O, et al. Candida auris: a latent threat to critically ill patients with coronavirus disease 2019 [Letter]. Clin Infect Dis. 2021;73:e2836–e2837. doi: 10.1093/cid/ciaa1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan American Health Organization. Epidemiological alert: Candida auris outbreaks in health care services in the context of the COVID-19 pandemic (6 February 2021). Pan American Health Organization; 2021. [Google Scholar]
- 22.Kömeç S, Karabiçak N, Ceylan AN, et al. [Three Candida auris case reports from Istanbul, Turkey]. Mikrobiyol Bul. 2021;55:452–60. doi: 10.5578/mb.20219814 [DOI] [PubMed] [Google Scholar]
- 23.de Almeida JN Jr, Francisco EC, Hagen F, et al. Emergence of Candida auris in Brazil in a COVID-19 intensive care unit. J Fungi (Basel). 2021;7. doi: 10.3390/jof7030220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villanueva-Lozano H, Treviño-Rangel RJ, González GM, et al. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico [Letter]. Clin Microbiol Infect. 2021;27:813–6. doi: 10.1016/j.cmi.2020.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allaw F, Kara Zahreddine N, Ibrahim A, et al. First Candida auris outbreak during a COVID-19 pandemic in a tertiary-care center in Lebanon. Pathogens. 2021;10. doi: 10.3390/pathogens10020157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghannoum M, Arendrup MC, Chaturvedi VP, et al. Ibrexafungerp: a novel oral triterpenoid antifungal in development for the treatment of Candida auris infections. Antibiotics (Basel). 2020;9. doi: 10.3390/antibiotics9090539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiederhold NP, Najvar LK, Olivo M, et al. Ibrexafungerp demonstrates in vitro activity against fluconazole-resistant Candida auris and in vivo efficacy with delayed initiation of therapy in an experimental model of invasive candidiasis. Antimicrob Agents Chemother. 2021;65. doi: 10.1128/AAC.02694-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arendrup MC, Chowdhary A, Jørgensen KM, et al. Manogepix (APX001A) in vitro activity against Candida auris: head-to-head comparison of EUCAST and CLSI MICs. Antimicrob Agents Chemother. 2020;64. doi: 10.1128/AAC.00656-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prestel C, Anderson E, Forsberg K, et al. Candida auris outbreak in a COVID-19 specialty care unit - Florida, July–August 2020. MMWR Morb Mortal Wkly Rep. 2021;70:56–57. doi: 10.15585/mmwr.mm7002e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roshan R, Feroz AS, Rafique Z, et al. Rigorous hand hygiene practices among health care workers reduce hospital-associated infections during the COVID-19 pandemic. J Prim Care Community Health. 2020;11:2150132720943331. doi: 10.1177/2150132720943331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo SH, Lin CY, Hung CT, et al. The impact of universal face masking and enhanced hand hygiene for COVID-19 disease prevention on the incidence of hospital-acquired infections in a Taiwanese hospital. Int J Infect Dis. 2021;104:15–18. doi: 10.1016/j.ijid.2020.12.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleisher LA, Schreiber M, Cardo D, et al. Health care safety during the pandemic and beyond - building a system that ensures resilience. N Engl J Med. 2022;386:609–11. doi: 10.1056/NEJMp2118285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thoma R, Seneghini M, Seiffert SN, et al. The challenge of preventing and containing outbreaks of multidrug-resistant organisms and Candida auris during the coronavirus disease 2019 pandemic: report of a carbapenem-resistant Acinetobacter baumannii outbreak and a systematic review of the literature. Antimicrob Resist Infect Control. 2022;11:12. doi: 10.1186/s13756-022-01052-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Jin L, Fisher D. MDRO transmission in acute hospitals during the COVID-19 pandemic. Curr Opin Infect Dis. 2021;34:365–71. doi: 10.1097/QCO.0000000000000735 [DOI] [PubMed] [Google Scholar]
- 35.McKinnell JA, Singh RD, Miller LG, et al. The SHIELD Orange County Project: multidrug-resistant organism prevalence in 21 nursing homes and long-term acute care facilities in southern California. Clin Infect Dis. 2019;69:1566–73. doi: 10.1093/cid/ciz119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee BY, Bartsch SM, Hayden MK, et al. How to choose target facilities in a region to implement carbapenem-resistant Enterobacteriaceae control measures. Clin Infect Dis. 2021;72:438–47. doi: 10.1093/cid/ciaa072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee BY, Bartsch SM, Lin MY, et al. How long-term acute care hospitals can play an important role in controlling carbapenem-resistant Enterobacteriaceae in a region: a simulation modeling study. Am J Epidemiol. 2021;190:448–58. doi: 10.1093/aje/kwaa247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Won SY, Munoz-Price LS, Lolans K, et al. ; Centers for Disease Control and Prevention Epicenter Program. Emergence and rapid regional spread of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis. 2011;53:532–40. doi: 10.1093/cid/cir482 [DOI] [PubMed] [Google Scholar]
- 39.Lee MH, Lee GA, Lee SH, et al. A systematic review on the causes of the transmission and control measures of outbreaks in long-term care facilities: back to basics of infection control. PLoS One. 2020;15:e0229911. doi: 10.1371/journal.pone.0229911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez S, Innes GK, Walters MS, et al. Increase in hospital-acquired carbapenem-resistant Acinetobacter baumannii infection and colonization in an acute care hospital during a surge in COVID-19 admissions - New Jersey, February–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1827–31. doi: 10.15585/mmwr.mm6948e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schelenz S, Hagen F, Rhodes JL, et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control. 2016;5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lesho EP, Bronstein MZ, McGann P, et al. Importation, mitigation, and genomic epidemiology of Candida auris at a large teaching hospital. Infect Control Hosp Epidemiol. 2018;39:53–57. doi: 10.1017/ice.2017.231 [DOI] [PubMed] [Google Scholar]
- 43.Austin L, Guild P, Rovinski C, et al. Novel case of Candida auris in the Veterans Health Administration and in the state of South Carolina. Am J Infect Control. 2022;50:1258–62. doi: 10.1016/j.ajic.2022.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karmarkar EN, O’Donnell K, Prestel C, et al. Rapid assessment and containment of Candida auris transmission in postacute care settings—Orange County, California, 2019. Ann Intern Med. 2021;174:1554–62. doi: 10.7326/M21-2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The White House. Fact Sheet: Protecting Seniors by Improving Safety and Quality of Care in the Nation’s Nursing Homes. 28 February 2022. Accessed at www.whitehouse.gov/briefing-room/statements-releases/2022/02/28/fact-sheet-protecting-seniors-and-people-with-disabilities-by-improving-safety-and-quality-of-care-in-the-nations-nursing-homes on 8 February 2023. [Google Scholar]


