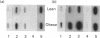Abstract
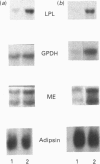
The genetically obese Zucker rat displays excessive fat storage capacity which is due to a tissue-specific increase in the activities of a number of lipid storage-related enzymes in adipose tissue. The aim of this study was to investigate the molecular mechanism responsible for this phenomenon. Lean (Fa/fa) and obese (fa/fa) Zucker rats were studied during the early stages of adipose tissue overdevelopment, both before (at 16 days of age) and after (at 30 days of age) the emergence of hyperinsulinaemia, in order to delineate the effects of the fatty genotype independently of those of hyperinsulinaemia. Lipoprotein lipase (LPL), glycerophosphate dehydrogenase (GPDH), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and malic enzyme (ME) mRNA levels in the adipose tissue of lean and obese rats were assessed by Northern blot analysis, and the relative transcription rates of the corresponding genes were compared in the two genotypes by a nuclear run-on assay. In normoinsulinaemic 16-day-old pre-obese rats, mRNA levels were increased over control values (LPL, 5-fold; ME, 2-fold; GAPDH, 3-fold), in close correlation with genotype-mediated differences in enzyme activities. Stimulation of the transcription rates of the ME and GAPDH genes was observed in obese rats, which could fully account for differences in steady-state mRNA levels. At this age, GPDH activity, mRNA level and transcription rate were similar in the two genotypes. In hyperinsulinaemic 30-day-old obese rats, a 6-7-fold increase in both mRNA and the transcription rate of GPDH emerged, together with an amplification of the genotype-mediated differences observed in younger animals (GAPDH, 6-fold; ME, 7.9-fold; LPL, 10-fold). These results demonstrate that the obese genotype exerts a co-ordinated control on the expression of these genes in adipose tissue, mainly at the transcriptional level. This genotype effect is greatly amplified by the development of hyperinsulinaemia.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazin R., Lavau M. Development of hepatic and adipose tissue lipogenic enzymes and insulinemia during suckling and weaning on to a high-fat diet in Zucker rats. J Lipid Res. 1982 Aug;23(6):839–849. [PubMed] [Google Scholar]
- Boulangé A., Planche E., de Gasquet P. Onset and development of hypertriglyceridemia in the Zucker rat (fa/fa). Metabolism. 1981 Nov;30(11):1045–1052. doi: 10.1016/0026-0495(81)90046-9. [DOI] [PubMed] [Google Scholar]
- Boulangé A., Planche E., de Gasquet P. Onset of genetic obesity in the absence of hyperphagia during the first week of life in the Zucker rat (fa/fa). J Lipid Res. 1979 Sep;20(7):857–864. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Dugail I., Le Liepvre X., Quignard-Boulangé A., Pairault J., Lavau M. Adipsin mRNA amounts are not decreased in the genetically obese Zucker rat. Biochem J. 1989 Feb 1;257(3):917–919. doi: 10.1042/bj2570917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugail I., Quignard-Boulange A., Bazin R., Le Liepvre X., Lavau M. Adipose-tissue-specific increase in glyceraldehyde-3-phosphate dehydrogenase activity and mRNA amounts in suckling pre-obese Zucker rats. Effect of weaning. Biochem J. 1988 Sep 1;254(2):483–487. doi: 10.1042/bj2540483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugail I., Quignard-Boulange A., Brigant L., Etienne J., Noe L., Lavau M. Increased lipoprotein lipase content in the adipose tissue of suckling and weaning obese Zucker rats. Biochem J. 1988 Jan 1;249(1):45–49. doi: 10.1042/bj2490045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerbäck S., Semb H., Tavernier J., Bjursell G., Olivecrona T. Tissue-specific regulation of guinea pig lipoprotein lipase; effects of nutritional state and of tumor necrosis factor on mRNA levels in adipose tissue, heart and liver. Gene. 1988 Apr 15;64(1):97–106. doi: 10.1016/0378-1119(88)90484-2. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsurada A., Iritani N., Fukuda H., Matsumura Y., Noguchi T., Tanaka T. Effects of insulin and fructose on transcriptional and post-transcriptional regulation of malic enzyme synthesis in diabetic rat liver. Biochim Biophys Acta. 1989 Jul 17;1004(1):103–107. doi: 10.1016/0005-2760(89)90219-1. [DOI] [PubMed] [Google Scholar]
- Lavau M., Bazin R., Guerre-Millo M. Increased capacity for fatty acid synthesis in white and brown adipose tissues from 7-day-old obese Zucker pups. Int J Obes. 1985;9 (Suppl 1):61–66. [PubMed] [Google Scholar]
- Lavau M., Bazin R. Inguinal fat pad weight plotted versus body weight as a method of genotype identification in 16-day-old Zucker rats. J Lipid Res. 1982 Aug;23(6):941–943. [PubMed] [Google Scholar]
- Magnuson M. A., Nikodem V. M. Molecular cloning of a cDNA sequence for rat malic enzyme. Direct evidence for induction in vivo of rat liver malic enzyme mRNA by thyroid hormone. J Biol Chem. 1983 Oct 25;258(20):12712–12717. [PubMed] [Google Scholar]
- Moustaid N., Lasnier F., Hainque B., Quignard-Boulange A., Pairault J. Analysis of gene expression during adipogenesis in 3T3-F442A preadipocytes: insulin and dexamethasone control. J Cell Biochem. 1990 Apr;42(4):243–254. doi: 10.1002/jcb.240420407. [DOI] [PubMed] [Google Scholar]
- Nasrin N., Ercolani L., Denaro M., Kong X. F., Kang I., Alexander M. An insulin response element in the glyceraldehyde-3-phosphate dehydrogenase gene binds a nuclear protein induced by insulin in cultured cells and by nutritional manipulations in vivo. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5273–5277. doi: 10.1073/pnas.87.14.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson-Ehle P., Schotz M. C. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res. 1976 Sep;17(5):536–541. [PubMed] [Google Scholar]
- Raynolds M. V., Awald P. D., Gordon D. F., Gutierrez-Hartmann A., Rule D. C., Wood W. M., Eckel R. H. Lipoprotein lipase gene expression in rat adipocytes is regulated by isoproterenol and insulin through different mechanisms. Mol Endocrinol. 1990 Sep;4(9):1416–1422. doi: 10.1210/mend-4-9-1416. [DOI] [PubMed] [Google Scholar]
- Semenkovich C. F., Wims M., Noe L., Etienne J., Chan L. Insulin regulation of lipoprotein lipase activity in 3T3-L1 adipocytes is mediated at posttranscriptional and posttranslational levels. J Biol Chem. 1989 May 25;264(15):9030–9038. [PubMed] [Google Scholar]
- Spiegelman B. M., Frank M., Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983 Aug 25;258(16):10083–10089. [PubMed] [Google Scholar]
- Spydevold S. O., Greenbaum A. L., Baquer N. Z., McLean P. Adaptive responses of enzymes of carbohydrate and lipid metabolism to dietary alteration in genetically obese Zucker rats (fa/fa). Eur J Biochem. 1978 Sep 1;89(2):329–339. doi: 10.1111/j.1432-1033.1978.tb12534.x. [DOI] [PubMed] [Google Scholar]
- Wion K. L., Kirchgessner T. G., Lusis A. J., Schotz M. C., Lawn R. M. Human lipoprotein lipase complementary DNA sequence. Science. 1987 Mar 27;235(4796):1638–1641. doi: 10.1126/science.3823907. [DOI] [PubMed] [Google Scholar]
- Yoo-Warren H., Monahan J. E., Short J., Short H., Bruzel A., Wynshaw-Boris A., Meisner H. M., Samols D., Hanson R. W. Isolation and characterization of the gene coding for cytosolic phosphoenolpyruvate carboxykinase (GTP) from the rat. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3656–3660. doi: 10.1073/pnas.80.12.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner R., Newman T. C., Sherry B., Cerami A., Breslow J. L. Recombinant human cachectin/tumor necrosis factor but not interleukin-1 alpha downregulates lipoprotein lipase gene expression at the transcriptional level in mouse 3T3-L1 adipocytes. Mol Cell Biol. 1988 Jun;8(6):2394–2401. doi: 10.1128/mcb.8.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker L. M., Antoniades H. N. Insulin and obesity in the Zucker genetically obese rat "fatty". Endocrinology. 1972 May;90(5):1320–1330. doi: 10.1210/endo-90-5-1320. [DOI] [PubMed] [Google Scholar]