Abstract
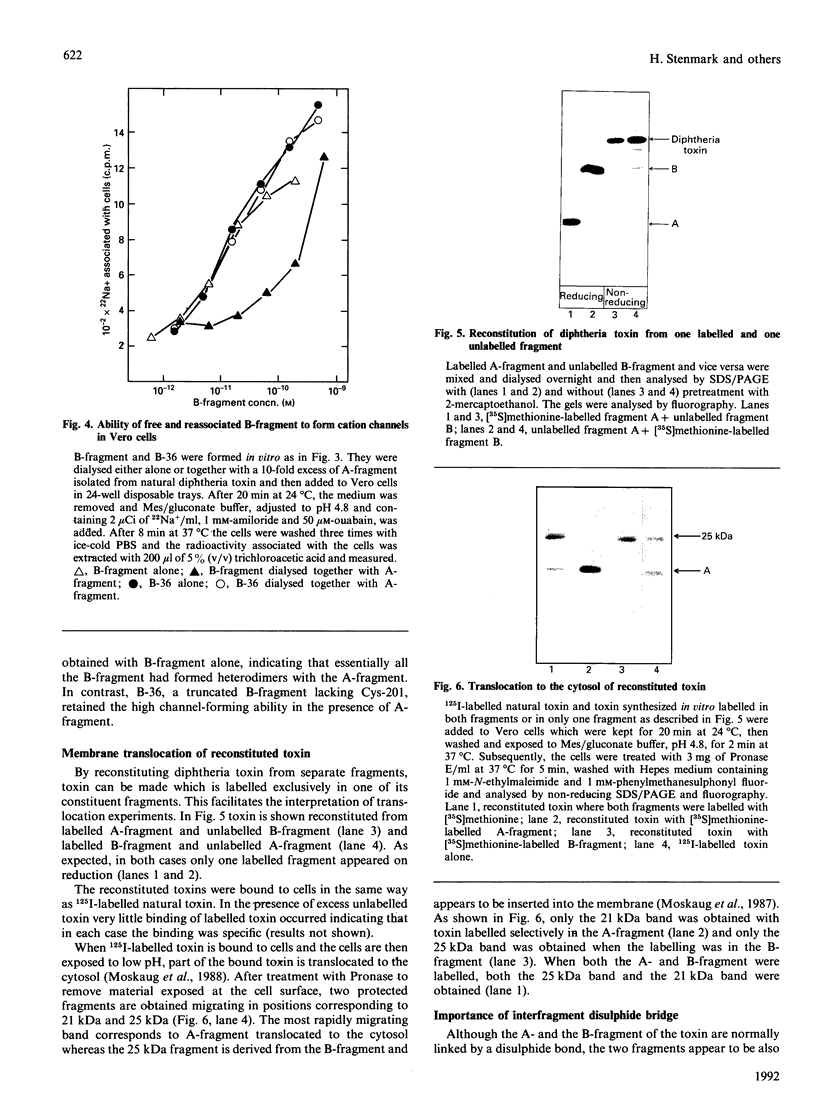
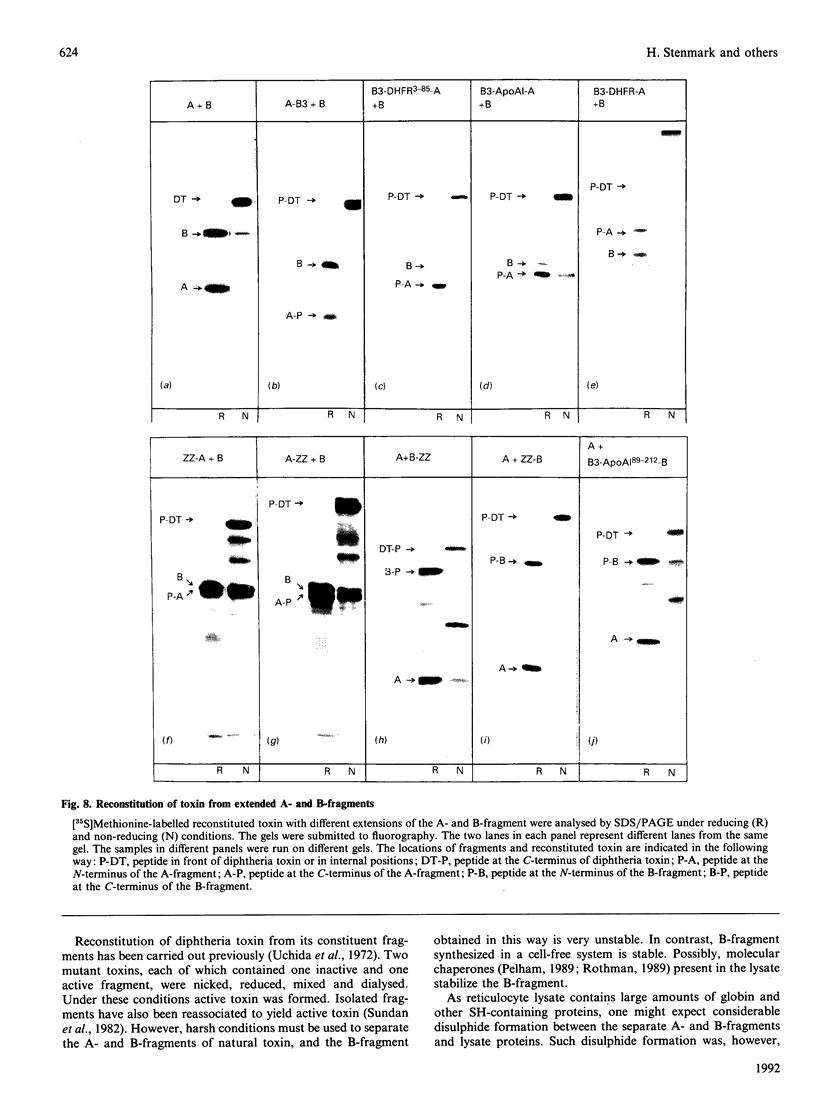
Natural diphtheria toxin is synthesized as a single polypeptide chain that is activated by cleavage into an A- and a B-fragment, which are linked by a disulphide bond. In the present work the ability of independently translated A- and B-fragments to associate was investigated. Low amounts of A- and B-fragments synthesized in vitro were mixed under conditions that allowed formation of a disulphide bridge between the fragments. Under these conditions toxin was reconstituted in close to 100% yield and found to be as toxic to Vero cells as natural diphtheria toxin. Efficient association between the A- and B-fragment was dependent on the formation of a disulphide bridge. Reconstituted toxin obtained from one [35S]methionine-labelled fragment and one unlabelled fragment proved useful in translocation studies. Addition of a number of different polypeptides to the N- and C-termini of either fragment did not, in most cases, prevent reconstitution. The ready reconstitution allows easy manipulations with the toxin to form targeted molecules and to develop diphtheria toxin as a vector for translocation of peptides to the cytosol. The fact that the reconstituted toxin does not need to be nicked with proteinases to be active allows experimentation with proteinase-sensitive constructs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Drazin R., Kandel J., Collier R. J. Structure and activity of diphtheria toxin. II. Attack by trypsin at a specific site within the intact toxin molecule. J Biol Chem. 1971 Mar 10;246(5):1504–1510. [PubMed] [Google Scholar]
- Gill D. M., Dinius L. L. Observations on the structure of diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1485–1491. [PubMed] [Google Scholar]
- Greenfield L., Bjorn M. J., Horn G., Fong D., Buck G. A., Collier R. J., Kaplan D. A. Nucleotide sequence of the structural gene for diphtheria toxin carried by corynebacteriophage beta. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6853–6857. doi: 10.1073/pnas.80.22.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley V. E., Bacha P., Pankewycz O., Nichols J. C., Murphy J. R., Strom T. B. Interleukin 2-diphtheria toxin fusion protein can abolish cell-mediated immunity in vivo. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3980–3984. doi: 10.1073/pnas.85.11.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Löwenadler B., Jansson B., Paleus S., Holmgren E., Nilsson B., Moks T., Palm G., Josephson S., Philipson L., Uhlén M. A gene fusion system for generating antibodies against short peptides. Gene. 1987;58(1):87–97. doi: 10.1016/0378-1119(87)90032-1. [DOI] [PubMed] [Google Scholar]
- McGill S., Stenmark H., Sandvig K., Olsnes S. Membrane interactions of diphtheria toxin analyzed using in vitro synthesized mutants. EMBO J. 1989 Oct;8(10):2843–2848. doi: 10.1002/j.1460-2075.1989.tb08431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B., Leppla S. H. Association of diphtheria toxin with Vero cells. Demonstration of a receptor. J Biol Chem. 1978 Oct 25;253(20):7325–7330. [PubMed] [Google Scholar]
- Moskaug J. O., Sandvig K., Olsnes S. Cell-mediated reduction of the interfragment disulfide in nicked diphtheria toxin. A new system to study toxin entry at low pH. J Biol Chem. 1987 Jul 25;262(21):10339–10345. [PubMed] [Google Scholar]
- Moskaug J. O., Sandvig K., Olsnes S. Low pH-induced release of diphtheria toxin A-fragment in Vero cells. Biochemical evidence for transfer to the cytosol. J Biol Chem. 1988 Feb 15;263(5):2518–2525. [PubMed] [Google Scholar]
- Moskaug J. O., Sletten K., Sandvig K., Olsnes S. Translocation of diphtheria toxin A-fragment to the cytosol. Role of the site of interfragment cleavage. J Biol Chem. 1989 Sep 15;264(26):15709–15713. [PubMed] [Google Scholar]
- Olsnes S., Stenmark H., McGill S., Hovig E., Collier R. J., Sandvig K. Formation of active diphtheria toxin in vitro based on ligated fragments of cloned mutant genes. J Biol Chem. 1989 Aug 5;264(22):12747–12751. [PubMed] [Google Scholar]
- Pelham H. R. Heat shock and the sorting of luminal ER proteins. EMBO J. 1989 Nov;8(11):3171–3176. doi: 10.1002/j.1460-2075.1989.tb08475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogne S., Myklebost O., Olaisen B., Gedde-Dahl T., Jr, Prydz H. Confirmation of the close linkage between the loci for human apolipoproteins AI and AIV by the use of a cloned cDNA probe and two restriction site polymorphisms. Hum Genet. 1986 Jan;72(1):68–71. doi: 10.1007/BF00278820. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin-induced channels in Vero cells selective for monovalent cations. J Biol Chem. 1988 Sep 5;263(25):12352–12359. [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Rapid entry of nicked diphtheria toxin into cells at low pH. Characterization of the entry process and effects of low pH on the toxin molecule. J Biol Chem. 1981 Sep 10;256(17):9068–9076. [PubMed] [Google Scholar]
- Stenmark H., McGill S., Olsnes S., Sandvig K. Permeabilization of the plasma membrane by deletion mutants of diphtheria toxin. EMBO J. 1989 Oct;8(10):2849–2853. doi: 10.1002/j.1460-2075.1989.tb08432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., Moskaug J. O., Madshus I. H., Sandvig K., Olsnes S. Peptides fused to the amino-terminal end of diphtheria toxin are translocated to the cytosol. J Cell Biol. 1991 Jun;113(5):1025–1032. doi: 10.1083/jcb.113.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundan A., Olsnes S., Sandvig K., Pihl A. Preparation and properties of chimeric toxins prepared from the constituent polypeptides of diphtheria toxin and ricin. Evidence for entry of ricin A-chain via the diphtheria toxin pathway. J Biol Chem. 1982 Aug 25;257(16):9733–9739. [PubMed] [Google Scholar]
- Uchida T., Pappenheimer A. M., Jr, Harper A. A. Reconstitution of diphtheria toxin from two nontoxic cross-reacting mutant proteins. Science. 1972 Feb 25;175(4024):901–903. doi: 10.1126/science.175.4024.901. [DOI] [PubMed] [Google Scholar]
- Wilson B. A., Reich K. A., Weinstein B. R., Collier R. J. Active-site mutations of diphtheria toxin: effects of replacing glutamic acid-148 with aspartic acid, glutamine, or serine. Biochemistry. 1990 Sep 18;29(37):8643–8651. doi: 10.1021/bi00489a021. [DOI] [PubMed] [Google Scholar]