Abstract
Background
Acute and complete unilateral vestibular deafferentation induces a significant change in ipsilateral vestibuloocular reflex gain, making the patient unable to stabilize gaze during active or passive head movements. This inability creates the illusion that the visual environment is moving, resulting in persistent visual discomfort during rapid angular or linear acceleration of the head. This is known as oscillopsia. Our objective was to understand if the spontaneous sensation of oscillopsias after complete unilateral vestibular deafferentation by vestibular neurotomy at 5 days (D5) and at 3 months (M3) is correlated with the loss of vestibuloocular reflex gain and dynamic visual acuity.
Methods
Retrospective cohort study was conducted in an otolaryngology tertiary care center (2019-2022) on patients with complete unilateral vestibular loss by vestibular neurotomy. They were divided into 2 groups according to the presence (group G1) or absence (group G2) of a spontaneous complaint of oscillopsia assessed at M3. Severity of oscillopsias evaluated by Oscillopsia Severity Questionnaire. Vestibuloocular reflex gain based on video head impulse test (vHIT) and the dynamic visual acuity were measured for each group at D5 and M3. Categorical variables were compared using χ2 test and quantitative variables using the nonparametric Wilcoxon-Mann-Whitney test.
Results
All patients have a complete vestibular deafferentation at D5 and M3. At D5 (G1 = 8 patients, G2 = 5 patients), there is no significant difference for ipsilateral and contralateral vestibuloocular reflex gains and dynamic visual acuity losses. The Oscillopsia Severity Questionnaire was 2.68 ± 1.03 in G1 and 1.23 ± 1.03 in G2 (P < .05). At M3 (G1 = 9 patients, G2 = 6 patients), there is no significant difference between groups for epidemiologic and clinical data and for vestibuloocular reflex and dynamic visual acuity losses. The Oscillopsia Severity Questionnaire was 2.10 ± 0.63 in G1 and 1.24 ± 0.28 in G2 (P < .05).
Conclusions
The spontaneous disabling sensation of oscillopsia after complete unilateral vestibular loss is well assessed by the Oscillopsia Severity Questionnaire but cannot be explained by objective vestibular tests assessing vestibuloocular reflex gain (vHIT) or dynamic visual acuity loss at D5 or M3. Further studies are needed to measure the sensation of oscillopsia under real-life conditions and to identify the factors responsible for its persistence.
Trial registration:
Retrospectively registered.
Keywords: oscillopsia, dynamic visual acuity, vestibuloocular reflex, vOR gain, video head impulse test, vHIT, vestibular neurectomy
Graphical Abstract.
Background
The sudden and unilateral loss of vestibular function secondary to surgery (otological complication, removal of a vestibular schwannoma, treatment of Meniere’s disease by vestibular neurotomy, etc) or to a vascular/infectious event (vestibular neuritis, labyrinthine hemorrhage, etc) induces the sensation of a true vertigo, more or less intense depending on the state of the initial vestibular function. 1
It also causes a significant alteration of the ipsilateral vestibuloocular reflex (VOR) gain making the patient unable to stabilize the gaze during active or passive head movements.2-4 This inability gives the illusion that the visual environment is moving, leading to persistent visual discomfort during rapid angular or linear accelerations of the head. This is known as oscillopsia.
Reported by Brickner as early as 1936, oscillopsia limits dynamic visual acuity (DVA) of patients.5-8 The impact of oscillopsia varies from patient to patient. Some do not report it and others experience its presence as a permanent and severe handicap. 9 The reasons for this difference are unknown. It may be related to the degree of DVA loss in patients whose immediate and long-term effects of complete loss of vestibular functions are not well understood.4,9-11
The aim of our study was to evaluate whether the spontaneous complaint of oscillopsia experienced by patients at 5 days (D5) and 3 months (M3) after complete unilateral vestibular loss surgery was correlated with the measure of postoperative VOR gain assessed by the video head impulse test (vHIT) and the measure of DVA loss.
Methods
A retrospective cohort study was conducted in an otolaryngology tertiary care center, covering a period between January 2019 and December 2022. This retrospective study follows the requirements of the French authorities and was built according to the MR004 protocol of the Commission Nationale de l’Informatique et des Libertés (n°2206749-13/09/2018). Our institution does not require an Institutional Review Board or an ethics committee for this type of study. The STROBE guidelines were used for reporting.
Population
All adult patients with preoperative vestibular function partially preserved who underwent a definitive complete unilateral vestibular loss by vestibular neurotomy performed for disabling Meniere’s disease, 12 Tumarkin syndrome, or vestibular schwannoma (Koos stage I and II) between January 2019 and December 2022 were included.
The complete and definitive unilateral vestibular deafferentation was defined by the absence of nystagmus responses to the 18° caloric test on the affected side, by the presence of a horizontal nystagmus contralateral to the affected side triggered during stimulation by a high-frequency vibrator (100 Hz) on the right and left mastoid (NIV or vibration-induced nystagmus), by the collapse of the preoperative ipsilateral VOR gains (vHIT), by the conservation of the VOR and COR (cervico-ocular reflex) gains during kinetics sinusoid (0.1 Hz) tests.
The disabling character of Meniere’s disease corresponded to the inability of the subjects to have an acceptable daily life 13 (repetition of vertiginous attacks several times a week; severity of vertigo and neurovegetative symptoms; Tumarkin’s attack).
Tumarkin attacks or vestibular drop attacks occur when patient with Meniere’s disease suddenly collapses to the ground without loss of consciousness, malaise, or cardiogenic disorder. This fall could induce injury because it is impossible for the patient to prevent it.
Eligible patients were identified from the surgical procedure register of our hospital. All patients were operated and explored before and after the surgery at D5 and M3 under the same conditions by the same surgical teams.
Variable
Epidemiological, subjective, and objective variables were collected from hospital medical records.
Epidemiological variable
The patient’s sex, age, dominant hand, glasses and their characteristics, and the etiology of the pathology requiring surgery are recorded.
Subjective evaluation
Vertigo/instability and their disabilities, spontaneous oscillopsia and its disability, oscillopsia severity assessment by Oscillopsia Severity Questionnaire (OSQ), hospital anxiety and depression scale (HAD), return to work, and driving were recorded.
Objective evaluation
Spontaneous nystagmus characteristics, gain and preponderance of kinetic test (sinusoidal 0.1 Hz), reflexivity and deficit of caloric tests, VOR gain and first saccade latency of vHIT, DVA loss pre- and postoperatively at D5 and M3 were collected.
The Ulmer Synapsis® VNG system (Synapsis), the Framiral® system measuring the DVA (Framiral), and the Ulmer III Synapsys® vHIT (Synapsis) were used according to the manufacturer’s standards [vHIT: 0.71 for the lateral semicircular canal (SCC), 0.81 for the superior SCC, 0.71 for the posterior SCC].
Study Outcomes
The primary endpoint was the spontaneous expression of oscillopsias related to the loss of DVA and/or the VOR gain at vHIT after a complete and definitive unilateral vestibular deafferentation by vestibular neurotomy at D5 and M3. Oscillopsia had to meet the criteria described by Bender in 1965 14 and is assessed on the basis of a subjective assessment made M3 after surgery. Patients with oscillopsia corresponded to group 1 (G1) and those without to group 2 (G2).
To avoid bias, the following situations lead to exclusion of the patients from the analysis: patients with oculomotor disorders or neurological pathology or otological history which modify the performance of the objectives tests, those with bilateral vestibular involvement, those with a schwannoma affecting the brainstem, those whose surgery was marked by a cerebellar trauma (at the surgeon’s discretion) or followed by pre/postoperative neurological complications and those for whom no clinical and paraclinical examination (vHIT and DVA) was performed at the third month because of the Covid-19 pandemic (confinement) or a technical failure.
Statistical Analysis
For descriptive analysis, continuous variables are expressed as mean and standard deviation or median. A χ 2 test was conducted to compare preoperative and postoperative categorical variables like epidemiological data. A nonparametric Wilcoxon-Mann-Whitney test was conducted to compare preoperative and postoperative quantitative one like patients age, characteristics of spontaneous nystagmus, gain and preponderance of kinetic tests, VOR gain and saccade latency, and DVA loss. All statistical analyses were conducted using Prism8 program, and P < .05 was considered statistically significant.
Results
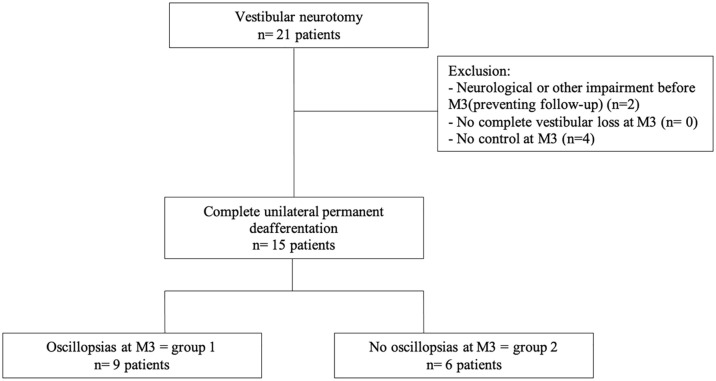
Twenty-one patients underwent a complete unilateral vestibular loss by vestibular neurotomy between February 2019 and January 2022. A total of 80% were for disabling Meniere’s disease (66.7% Tumarkin syndrome, 33.3% repeated and disabling attacks) and 20% following surgery for vestibular schwannoma. Six patients were excluded of our study because they had a neurological history (n = 2: stroke, psychiatric pathology) or could not attend the consultation at M3 because of COVID19 pandemic (n = 4) to evaluated if the unilateral vestibular loss was complete (Figure 1).
Figure 1.
Flowchart.
Fifteen patients were included. Thirteen underwent vestibular evaluation at D5 and all were evaluated at M3. Two patients were not evaluated at D5 because the intensity of the postoperative vertigo made it impossible to perform tests at that time.
Clinical and Vestibular Comparison of G1 and G2 in the Immediate Postoperative Period (D5)
Baseline patient characteristics
Thirteen patients were including à D5. A total of 53.8% were female. The vestibular neurotomy was on the left side for 46.15% of patients. The vestibular evaluation was done at 5 ± 1 day. The mean age was 57 ± 12 years for this population.
None of the patients had complete loss of vestibular function at preoperative evaluation. The preoperative spontaneous nystagmus velocity was 1.17 ± 2.06°/second; the mean gain on kinetic tests was 0.67 ± 0.31 and the preponderance was 4.53 ± 3.46°/second. The caloric preoperative threshold was normal at the contralateral side of the surgery and lowered on the ipsilateral side. Preoperative VOR gain obtained at vHIT for each of the SCCs was normal on the ipsilateral side (respectively for anterior/lateral/posterior SCC: 0.92 ± 0.13, 0.84 ± 0.15, 0.87 ± 0.16) and on the contralateral side (respectively for anterior/lateral/posterior SCC: 1 ± 0.13, 0.94 ± 0.08, 0.91 ± 0.15). Preoperative DVA loss was also normal on the ipsilateral (0.13 ± 0.11 logMAR) and contralateral side (0.07 ± 0.12 logMAR) of the surgery. We observed that it was slightly lower in the downward gaze (0.21 ± 0.19 logMAR).
After the surgery, the postoperative spontaneous nystagmus velocity was significantly higher than the preoperative velocity (10.84 ± 6.1 vs 1.17 ± 2.06; P < .05) at D5. The VOR gain at vHIT was also significantly decreased on the affected side on the anterior SCC (0.92 ± 0.12 vs 0.37 ± 0.15), the lateral SCC (0.84 ± 0.14 vs 0.07 ± 0.12), and the posterior one (0.87 ± 0.16 vs 0.37 ± 0.19); P < .05 for each. We also observed a slightly decreased gain for each contralateral SCC (for anterior SCC: 1 ± 0.13 vs 0.75 ± 0.18; for lateral SCC 0.94 ± 0.08 vs 0.85 ± 0.14; for posterior SCC 0.91 ± 0.15 vs 0.62 ± 0.16; P < .05 for each).
Pre- and postoperative comparison characteristics of the G1 group and the G2 group
At D5, 5 patients spontaneously reported and complained of oscillopsia and instability (G1 group), while 8 patients did not spontaneously report it (G2 group). The OSQ was statistically different between these groups, being higher in G1 (SQO = 2.68 ± 1.03) than in G2 (SQO = 1.23 ± 1.03); P = .03*. We found that the only statistically significant epidemiological difference between G1 and G2 was age, which was higher in G2 (62 ± 13 vs 50 ± 13 years; P = .004). We observed that the sensation of rotatory vertigo induced by surgery decreased and was comparable between groups (60% in G1 and 75% in G2; P = 1) at D5.
The contralateral preoperative VOR gain of SCC posterior to vHIT was significantly higher in the G1 group (0.99 ± 0.25 vs 0.87 ± 0.25; P = .009). Other homolateral and contralateral VOR SCC gain were comparable. There was also no statistically significant difference in preoperative DVA loss between groups.
At postoperative day 5, the only statistically significant outcome in videonystagmography (VNG) between G1 and G2 was the severity of caloric deficit, which was higher in the G2 group than in the G1 group (45.42% vs 26.75%; P = .04). The other criteria were not statistically different, but mean spontaneous nystagmus velocity (14.1 ± 6.1 vs 8.84 ± 6.1; P = .18) and directional preponderance in kinetics (14.1 ± 5.78 vs 9.75 ± 5.78; P = .18) appeared to be higher in G1.
Comparison of the VOR gains (vHIT) at D5 for each SCC ipsilateral or contralateral to surgery showed no significant difference between G1 and G2.
The covert saccades were present from D5 in both groups with no statistical difference but the latency appeared to be a little bit shorter in G1 (148.1 ± 20.91 vs 158.4 ± 24.27; P = .41).
There was no significant difference in DVA loss between groups ipsilateral or contralateral or downward at D5. Only the mean upward DVA loss was lower in G1 (0.19 ± 0.13 vs 0.30 ± 0.13; P = .03). The delta of DVA loss was comparable between groups (Table 1).
Table 1.
Clinical and Vestibular Comparison Between Patients With and Without Oscillopsia (Group 1/Group 2) at 5 Postoperative Days.
| Variable | Group 1 (n = 5) | Group 2 (n = 8) | P value |
|---|---|---|---|
| Sex (male/female) | 2/3 (40%/60%) | 4/4 (50%/50%) | 1 |
| Mean age (y) | 50 ± 13 | 62 ± 13 | .04* |
| Dominant hand (right/left) | 5/0 (100%/0%) | 2/6 (25%/75%) | / |
| HAD anxiety scale | 7.2 ± 3.74 | 7 ± 3.5 | .91 |
| HAD depression scale | 4.2 ± 2.9 | 2.5 ± 3.4 | .46 |
| Optical correction | |||
| Progressive glasses | 3/5 (60%) | 4/8 (50%) | 1 |
| Port all day | 3/3 (100%) | 4/4 (100%) | / |
| Initial etiology | |||
| Meniere’s disease | 4/5 (80%) | 7/8 (87.5%) | 1 |
| Multiples crisis | 2/4 (50%) | 2/7 (28.6%) | .57 |
| Tumarkin syndrome | 2/4 (50%) | 5/7 (71.4%) | .57 |
| Others (schwannoma) | 1/5 (20%) | 1/8 (12.5%) | / |
| Age of the disease (vestibular) | 3.13 ± 5.45 | 3.6 ± 5.3 | .70 |
| Neurectomy side (right/left) | 2/3 (40%/60%) | 5/3 (62.5%/37.5%) | .59 |
| Postoperative vertigo attack | 2 (40%) | 2 (25%) | 1 |
| Instability | 5 (100%) | 6 (75%) | / |
| Disabling nature of instability | |||
| Little or not disabling | 0 (0%) | 6 (75%) | / |
| Quite disabling | 3 (60%) | 1 (12.5%) | .21 |
| Disabling | 1 (20%) | 1 (12.5%) | / |
| Very disabling | 1 (20%) | 0 (0%) | / |
| Oscillopsia | 5 (100%) | 0 (0%) | / |
| Instability + oscillopsia | 5 (100%) | 0 (0%) | / |
| OSQ (mean) | 2.68 ± 1.03 | 1.23 ± 1.03 | .03* |
| Disabling nature of oscillopsia | |||
| Little or not disabling | 1 (20%) | 0 (0%) | / |
| Quite disabling | 3 (60%) | 0 (0%) | / |
| Disabling | 0 (0%) | 0 (0%) | / |
| Very disabling | 1 (20%) | 0 (0%) | / |
| Spontaneous nystagmus (°/s) (preoperative) | 0.56 ± 2.12 | 1.56 ± 2.12 | .4 |
| Spontaneous nystagmus (°/s) (postoperative) | 14.1 ± 6.1 | 8.84 ± 6.1 | .16 |
| Kinetic tests (sinusoïde 0.1 Hz) (preoperative) | |||
| Gain | 0.7 ± 0.3 | 0.66 ± 0.3 | .63 |
| Preponderance (AV) | 4.04 ± 3.52 | 4.83 ± 3.52 | .69 |
| Kinetic tests (sinusoïde 0.1 Hz) (postoperative) | |||
| Gain | 0.47 ± 0.23 | 0.47 ± 0.23 | .97 |
| Preponderance (AV) | 14.2 ± 5.78 | 9.75 ± 5.78 | .18 |
| Caloric tests (preoperative) | |||
| Ipsilateral reflexivity | 27.33 | 9.17 | .19 |
| Contralateral reflexivity | 34.64 | 24.65 | .44 |
| Deficit (%) ipsilateral | 26.75 | 45.42 | .04* |
| Caloric tests (postoperative) | Not performed at J5 | Not performed at J5 | |
| vHIT | |||
| Preoperative VOR gain of ipsilateral SCC | |||
| Anterior (A) | 0.98 ± 0.13 | 0.88 ± 0.22 | .11 |
| Lateral (L) | 0.92 ± 0.15 | 0.80 ± 0.21 | .14 |
| Posterior (P) | 0.95 ± 0.16 | 0.83 ± 0.22 | .07 |
| Preoperative VOR gain of contralateral SCC | |||
| Anterior (A) | 1 ± 0.28 | 1 ± 0.28 | 1 |
| Lateral (L) | 0.98 ± 0.25 | 0.92 ± 0.25 | .08 |
| Posterior (P) | 0.99 ± 0.25 | 0.87 ± 0.25 | .009* |
| Postoperative VOR gain of ipsilateral SCC (D5) | |||
| Anterior (A) | 0.34 ± 0.16 | 0.39 ± 0.16 | .63 |
| Lateral (L) | 0.08 ± 0.13 | 0.07 ± 0.13 | .89 |
| Posterior (P) | 0.33 ± 0.14 | 0.41 ± 0.15 | .56 |
| Postoperative VOR gain of contralateral SCC (D5) | |||
| Anterior (A) | 0.77 ± 0.18 | 0.75 ± 0.19 | .89 |
| Lateral (L) | 0.85 ± 0.15 | 0.86 ± 0.15 | .93 |
| Posterior (P) | 0.64 ± 0.15 | 0.60 ± 0.15 | .74 |
| First saccade latency | 148.8 ± 18.07 | 154.13 ± 18.07 | .69 |
| DVA | |||
| Preoperative DVA loss | |||
| Static flash | 0.17 ± 0.11 | 0.11 ± 0.11 | .5 |
| Ipsilateral | 0.06 ± 0.12 | 0.17 ± 0.11 | .15 |
| Contralateral | 0.02 ± 0.12 | 0.11 ± 0.12 | .1 |
| Up | 0.05 ± 0.1 | 0.16 ± 0.1 | .07 |
| Down | 0.18 ± 0.2 | 0.23 ± 1.19 | .58 |
| Postoperative DVA loss (D5) | |||
| Static flash | 0.16 ± 0.1 | 0.15 ± 0.1 | .84 |
| Ipsilateral | 0.31 ± 0.12 | 0.42 ± 0.12 | .06 |
| Contralateral | 0.14 ± 0.13 | 0.21 ± 0.13 | .23 |
| Up | 0.19 ± 0.13 | 0.30 ± 0.13 | .03* |
| Down | 0.29 ± 0.15 | 0.36 ± 0.15 | .48 |
| Postoperative delta DVA loss (D5) | |||
| Ipsilateral | −0.24 ± 0.11 | −0.20 ± 0.14 | .57 |
| Contralateral | −0.13 ± 0.09 | −0.08 ± 0.14 | .43 |
| Up | −0.14 ± 0.09 | −0.1 ± 0.15 | .58 |
| Down | −0.11 ± 0.24 | −0.07 ± 0.19 | .76 |
DVA losses are expressed in logMAR. Delta DVA loss is (preoperative DVA loss—postoperative DVA loss). Data are expressed as n (%) and mean standard deviation unless otherwise indicated.
Abbreviations: °/s, velocity in °/second; AV, absolute value; D5, 5 days; DVA, dynamic visual acuity; HAD, hospital anxiety and depression scale; OSQ, Oscillopsia Severity Questionnaire; SCC, semicircular canal; VOR, vestibuloocular reflex; *, statistically significant P value.
Epidemiological, Clinical, and Vestibular Comparison of G1 and G2 at M3
Baseline patient characteristics
Fifteen patients were including à M3. A total of 53.3% were female. The vestibular neurotomy was on the left side for 53.3% of patients. The vestibular evaluation was done at 4 ± 1 months. The mean age was 59.3 ± 13 years for this population. Most of patients were driving (73.3%) or had resumed their usual and/or professional activities with self-vestibular rehabilitation (86.7%). Vertiginous attacks in Meniere’s disease never recurred.
None of the patients had complete loss of vestibular function at preoperative evaluation.
The preoperative spontaneous nystagmus velocity was 1.02 ± 2.91°/second; the mean gain on kinetic tests was 0.71 ± 0.3 and the preponderance was 4.46 ± 3.69°/second. The caloric preoperative threshold was normal at the contralateral side of the surgery and lowered on the ipsilateral side.
Preoperative VOR gain obtained at vHIT for each of the SCCs was normal on the ipsilateral side (respectively for anterior/lateral/posterior SCC: 0.89 ± 0.21, 0.84 ± 0.21, 0.82 ± 0.21) and on the contralateral side (respectively for anterior/lateral/posterior SCC: 1 ± 0.23, 0.93 ± 0.2, 0.86 ± 0.21). Preoperative DVA loss was also normal on the ipsilateral (0.13 ± 0.11 logMAR) and contralateral side (0.08 ± 0.12 logMAR) of the surgery. We observed that it was slightly lower in the downward gaze (0.20 ± 0.19 logMAR).
After the surgery, the postoperative spontaneous nystagmus velocity was not significantly higher than the preoperative velocity (1.65 ± 1.29 vs 1.02 ± 1.29; P = .22) at M3. The VOR gain at vHIT was also significantly decreased on the affected side on the anterior SCC (0.89 ± 0.21 vs 0.36 ± 0.2), the lateral SCC (0.84 ± 0.21 vs 0.07 ± 0.14), and the posterior one (0.82 ± 0.21 vs 0.32 ± 0.17); P < .05 for each. We also observed a slightly decreased gain for each contralateral SCC (for anterior SCC 1 ± 0.23 vs 0.84 ± 0.10; for lateral SCC 0.93 ± 0.2 vs 0.84 ± 0.14; for posterior SCC 0.86 ± 0.21 vs 0.60 ± 0.22; P < .05 for each).
Pre- and postoperative comparison characteristics of the G1 group and the G2 group
At M3, 9 patients (60%) spontaneously reported the presence of oscillopsia at M3 (G1) with a mean OSQ that was statistically higher than the G2 OSQ (2.10 ± 0.63 vs 1.24 ± 0.28; P < .05).
There were no statistical clinical or epidemiological differences between the 2 groups. We observed that the visual correction was more frequent in G1(n = 7, 77.8%) than in G2 (n = 1, 16.7%) and the resumption of normal professional and/or daily activities was comparable and very high (G1: 88.9%; G2: 83.3%; P = .76). A total of 50% of G2 patients had resumed driving before M3, whereas 100% G1 patients had already resumed driving.
The contralateral preoperative VOR gain of SCC lateral to vHIT was significantly higher in the G1 group (0.98 ± 0.04 vs 0.87 ± 0.09; P = .007). Other homolateral and contralateral VOR SCC gain were comparable. There was also no statistically significant difference in DVA loss before the surgery between groups.
After the surgery, at M3, all patients had complete unilateral vestibular loss on the affected side. There was no significant difference between G1 and G2 regarding the results of the VNG. The velocity of spontaneous nystagmus was comparable (1.52 ± 1.45 vs 1.85 ± 1.12; P = .31) as the gain (respectively 0.53 ± 0.17 vs 0.54 ± 0.23; P = .67) or the preponderance (respectively 2.98 ± 1.65 vs 2.75 ± 2.11; P = .19) of kinetic tests.
At M3, there was no statistical difference between G1 and G2 groups regarding the VOR gain at vHIT on the operated side. However, we note that the VOR gain at vHIT of the SCCs on the unoperated side were significantly decreased on the anterior (0.89 ± 0.11 vs 0.79 ± 0.07; P = .049) and posterior SCCs (0.7 ± 0.2 vs 0.47 ± 0.21; P = .049) of the G2 group.
The latency of the covert saccade was not significantly different between group at M3 (136.9 ± 19.51 vs 158.8 ± 25.25; P = .14).
At M3, the DVA loss and the delta of DVA loss (pre- and postoperative difference) were not significantly difference between G1 and G2. We also observed that the scores were a little bit higher in the G2 group than in the G1 group (Table 2).
Table 2.
Clinical and Vestibular Comparison Between Patients With and Without Oscillopsia (Group 1/Group 2) at 3 Postoperative Months.
| Variable | Group 1 (n = 9) | Group 2 (n = 6) | P value |
|---|---|---|---|
| Sex (male/female) | 4/5 (44.4%/55.6%) | 3/3 (50%/50%) | .05 |
| Mean age (y) | 56.11 ± 10.97 | 62.50 ± 10.37 | .34 |
| Dominant hand (R/L) | 9/0 (100%/0%) | 4/2 (66.7%/33.3%) | .06 |
| HAD anxiety scale | 8.22 ± 4.02 | 8.0 ± 2.94 | >.99 |
| HAD depression scale | 4.00 ± 3.74 | 3.25 ± 3.95 | .90 |
| Optical correction | 7 (77.8%) | 1 (16.7%) | / |
| Progressive glasses | 7/7 (100%) | 1 (16.7%) | / |
| Port all day | 7/7 (100%) | 1 (16.7%) | / |
| Initial etiology | |||
| Meniere’s disease | 7 (77.8%) | 5 (83.3%) | .79 |
| Multiples crisis | 2/7 (28.6%) | 2/5 (40%) | .68 |
| Tumarkin syndrome | 5/7 (71.4%) | 3/5 (60%) | .68 |
| Others (schwannoma) | 2 (22.2%) | 1 (16.7%) | / |
| Age of the disease (vestibular) | 3.19 ± 2.04 | 7.14 ± 9.09 | .54 |
| Neurectomy side (R/L) | 4/5 (44.4%/55.6%) | 3/3 (50%/50%) | .83 |
| Vertigo attack | 0 (0%) | 0 (0%) | / |
| Instability | 7 (77.8%) | 5 (83.3%) | .79 |
| Disabling nature of instability | |||
| Little or not disabling | 4 (44.4%) | 4 (66.7%) | .4 |
| Quite disabling | 5 (55.6%) | 1 (16.7%) | / |
| Disabling | 0 (0%) | 0 (0%) | / |
| Very disabling | 0 (0%) | 1 (16.7%) | / |
| OSQ (mean) | 2.10 ± 0.63 | 1.24 ± 0.28 | .01* |
| Disabling nature of oscillopsia | |||
| Little or not disabling | 5 (55.6%) | 6 (100%) | .06 |
| Quite disabling | 3 (33.3%) | 0 (0%) | / |
| Disabling | 1 (11.1%) | 0 (0%) | / |
| Very disabling | 0 (0%) | 0 (0%) | / |
| Resumption of driving | 8 (100%) (n = 8) | 3 (50%) | .1 |
| Resumption of activities/walking >1 h/d | 8 (88.9%) | 5 (83.3%) | .76 |
| Spontaneous nystagmus (°/s) (preoperative) | 0.8 ± 2.06 | 1.35 ± 2.03 | .71 |
| Spontaneous nystagmus (°/s) (postoperative) | 1.52 ± 1.45 | 1.85 ± 1.12 | .31 |
| Kinetic tests (sinusoïde 0.1 Hz) (preoperative) | |||
| Gain | 0.78 ± 0.3 | 0.59 ± 0.31 | .22 |
| Preponderance (AV) | 4.2 ± 3.52 | 4.81 ± 3.5 | .8 |
| Kinetic tests (sinusoïde 0.1 Hz) (postoperative) | |||
| Gain | 0.53 ± 0.17 | 0.54 ± 0.23 | .67 |
| Preponderance (AV) | 2.98 ± 1.65 | 2.75 ± 2.11 | .19 |
| Caloric tests (preoperative) | |||
| Ipsilateral reflexivity | 15.84 | 9.04 | .28 |
| contralateral reflexivity | 26.37 | 26.16 | .98 |
| Deficit (%) ipsilateral | 36 | 46.5 | .14 |
| Caloric tests (postoperative) | 8 (100%) (n = 8) | 6 (100%) | NC |
| Preoperative vHIT | |||
| Preoperative VOR gain of ipsilateral SCC | |||
| Anterior (A) | 0.95 ± 0.10 | 0.82 ± 0.11 | .06 |
| Lateral (L) | 0.87 ± 0.11 | 0.81 ± 0.21 | .93 |
| Posterior (P) | 0.85 ± 0.16 | 0.80 ± 0.20 | .40 |
| Preoperative VOR gain of contralateral SCC | |||
| Anterior (A) | 1.04 ± 0.09 | 0.94 ± 0.13 | .18 |
| Lateral (L) | 0.98 ± 0.04 | 0.87 ± 0.09 | .007* |
| Posterior (P) | 0.92 ± 0.10 | 0.79 ± 0.22 | .14 |
| Postoperative vHIT | |||
| Postoperative VOR gain of ipsilateral SCC (M3) | |||
| Anterior (A) | 0.44 ± 0.16 | 0.25 ± 0.22 | .07 |
| Lateral (L) | 0.06 ± 0.13 | 0.08 ± 0.17 | .97 |
| Posterior (P) | 0.38 ± 0.18 | 0.24 ± 0.13 | .26 |
| Postoperative VOR gain of contralateral SCC (M3) | |||
| Anterior (A) | 0.89 ± 0.11 | 0.79 ± 0.07 | .0496* |
| Lateral (L) | 0.91 ± 0.09 | 0.75 ± 0.15 | .03 |
| Posterior (P) | 0.7 ± 0.2 | 0.47 ± 0.21 | .0496* |
| First saccade latency | 136.9 ± 19.51 | 158.8 ± 25.25 (n = 5) | .14 |
| Preoperative DVA | |||
| Preoperative DVA loss | |||
| Static flash | 0.15 ± 0.12 | 0.13 ± 0.14 | .75 |
| Ipsilateral | 0.10 ± 0.13 | 0.17 ± 0.06 | .15 |
| Contralateral | 0.07 ± 0.11 | 0.11 ± 0.13 | .76 |
| Up | 0.10 ± 0.11 | 0.16 ± 0.08 | .28 |
| Down | 0.18 ± 0.14 | 0.24 ± 0.25 | .71 |
| Postoperative DVA | |||
| Postoperative DVA loss (M3) | |||
| Static flash | 0.10 ± 0.12 | 0.13 ± 0.17 | .77 |
| Ipsilateral | 0.32 ± 0.14 | 0.38 ± 0.15 | .46 |
| Contralateral | 0.25 ± 0.10 | 0.35 ± 0.26 | .77 |
| Up | 0.24 ± 0.09 | 0.29 ± 0.22 | .11 |
| Down | 0.25 ± 0.12 | 0.38 ± 0.23 | .46 |
| Postoperative delta DVA loss (M3) | |||
| Ipsilateral | −0.22 ± 0.13 | −0.21 ± 0.12 | .98 |
| Contralateral | −0.18 ± 0.10 | −0.24 ± 0.21 | .84 |
| Up | −0.14 ± 0.12 | −0.24 ± 0.20 | .44 |
| Down | −0.07 ± 0.16 | −0.14 ± 0.19 | .44 |
DVA losses are expressed in logMAR. Delta DVA loss is (preoperative DVA loss—postoperative DVA loss). Data are expressed as n (%) and mean standard deviation unless otherwise indicated.
Abbreviations: °/s, velocity in °/second; AV, absolute value; DVA, dynamic visual acuity; HAD, hospital anxiety and depression scale; L, left; M3, 3 months; OSQ, Oscillopsia Severity Questionnaire; R, right; SCC, semicircular canal; VOR, vestibuloocular reflex; *, statistically significant P value.
Discussion
Our findings showed that vestibular assessments do not explain why there was a difference in the sensation of oscillopsia between patients at D5 or M3 after vestibular deafferentation. Technically, all patients were operated on in the same way, by a single team and without postoperative complications.
On the vestibular level, unlike other publications based on models with potentially incomplete or even reversible deafferentation (vestibular neuritis),15,16 vestibular neurotomy induces a complete, unilateral, irreversible,17,18 clinically effective (total disappearance of Meniere’s attacks 19 ), and homogeneous complete vestibular loss between all patients. In accordance with the literature,6,7,11,20 the postoperative VOR gain of deafferented SCCs was collapsed and comparable between G1 and G2 at D5/M3. Passive rotations ipsilateral to surgery result in greater loss of VOR gain. The other postoperative vestibular explorations showed no difference between groups. Our model therefore does not include any partial deafferentation and does not generate a bias explaining the difference in the sensation of oscillopsia.
Concerning the preoperative vestibular state, no oscillopsia sensation was reported. On the affected side, preoperative VOR gains and mean DVA losses were normal and comparable to healthy patients.4,7,21-23 There was no difference between groups. On the contralateral side of the operation, the finding was identical except for the posterior SCC at D5 and the lateral SCC at M3, where the VOR gain of G2 was lower. It seems unlikely that this difference could explain the oscillopsia sensations between G1 and G2. The lateral SCC encodes horizontal information and the oscillopsia is more related to vertical movement. The posterior SCC VOR gain at M3 was comparable between groups. Moreover, the preoperative DVA values were comparable between groups of the operated and contralateral sides, which excludes the presence of a preoperative unconscious compensatory mechanism of the DVA limiting the oscillopsia sensations. We note that there was a decrease in postoperative VOR gains on the contralateral SCC and an increase in contralateral DVA loss. This is the postoperative push-pull effect20,24,25: the VOR gain at the vHIT of a normal SCC always corresponds to the gain of the tested channel and a small part of the contralateral channels. It is therefore logical to have a decrease in gain after vestibular neurotomy on the nonaffected side. It seems to be higher in G2 than G1. The contralateral vestibule and its postoperative push-pull function are therefore not involved in the difference in oscillopsia sensation. The group with the best contralateral VOR gains was the one with the most oscillopsia sensation at M3.
Concerning the VOR gain to the vHIT, the compensation system relies on the appearance of catch-up ocular saccades triggered during (covert saccade) and after (overt saccade) the rapid head movements of the deafferented side. These catch-up saccades limit eye position errors and improve dynamic visual performance.26-29 It was possible to compare the characteristics of saccades between patients because the vHIT system only records them from a minimum head movement velocity (minimum velocity 200°/second, maximum velocity 250°/second and acceleration 3000°/second). They appeared in both groups (G1/G2) at D5/M3 without any significant difference in their latencies (148.8 ± 18.07 ms vs 154.13 ± 18.07 ms and 136.9 ± 19.51 ms vs 158.8 ± 25.25 ms, respectively). Their values are consistent with the literature.5,30 This equivalent process between G1 and G2, does not explain the difference in oscillopsia sensation experienced. One hypothesis concerning the catch-up saccades would be related to the patient’s ability to calibrate his saccade in advance because of the knowledge of the expected final position. These central mechanisms could lead to a decrease in the sensation of spontaneous oscillopsia despite a large loss of DVA.11,20,31,32
No difference was demonstrated between groups regarding the loss of DVA or the delta of postoperative loss on the both side at D5/M3. The loss of DVA does not explain the difference in oscillopsia felt, especially since the patients with the least discomfort were those with the most DVA loss (nonsignificant difference). This result is counter-intuitive but shared by other authors who find no correlation between severity of oscillopsia and the degree of DVA loss.9,11,30 One explanation is thought to be related to the conditions under which DVA was assessed.24,33 In daily life, patients are constantly making active vertical head movements. The assessment of the DVA is based on passive, horizontal, and unpredictable movements of the head. These movements are useful in the diagnosis of vestibular loss 20 because they sensitize the loss of DVA. Regarding active vertical movements, there is an inverse correlation between the spontaneous complaint of oscillopsia and the measured DVA loss in case of unilateral or bilateral vestibular loss. This finding is likely related to a central adaptation strategy that would increase tolerance to retinal slip during active movement by altering cortical perception or suppressing visual perception of movement to reduce the impact of oscillopsia and visual blur.11,33,34 This hypothesis is supported by the decrease in retinal slip during active movement. This hypothesis is supported by the decrease in visual cortex activity on functional MRI after uni/bilateral complete vestibular loss 35 and by the observation of oculocephalic coordination disorders during oculomotor paresis inducing a dissociation between retinal gliding and oscillopsia.29,36,37 A second hypothesis would be related to head stabilization by vestibulocolic reflexes generated by the saccules and vertical SCCs (medial vestibulospinal pathway). However, the unilateral saccular deficit is not very symptomatic and the VOR gains of the vertical channels are less deficient than those of the horizontal channels because of the co-activation of agonists combined with the push-pull effect. Moreover, saccular function is not assessed by the vertical DVA, which tests the stabilization of the head and not the eyes. The third hypothesis would be behavioral, as patients avoid any visual focusing task while walking to avoid oscillopsia.
DVA measurement, unlike vHIT, evolves over time and improves with vestibular compensation5,38 especially if it is early.39,40 Vestibular rehabilitation seems to be the main factor by promoting the use of centrally preprogrammed eye movements and thus replacing the VOR gain.11,22,41,42 The DVA measurements at M3 between G1 and G2 were comparable although none of the patients included had received vestibular rehabilitation, the patients doing self-rehabilitation. The DVA measurement is therefore not a marker to explain the sensation of oscillopsia.
Proprioception can be altered by cervical disorders or by muscle fatigue. The weight of the latter may increase after surgery and generate an impact on gaze stability, on the DVA and thus on the perception of oscillopsia. 43 However, this process is probably in the minority because oscillopsia was experienced immediately after surgery. This is too early for the daily physiological cervical movements that generate oscillopsia to have been reset.
Clinically, the age of our patients is comparable at M3 but not at D5. At D5, the youngest patients spontaneously felt oscillopsias the most. It is well known that vestibular performance declines with age (especially after 75 years) and that DVA fluctuates. Therefore, age does not seem to be a factor in explaining differences in the sensation of chronic oscillopsia.
On visual level, the wearing of progressive lenses could be an explanatory factor. Many patients wearing them complained of oscillopsia. These lenses require a high capacity to modulate the gain according to the height of the eyes, independently of any deficit. The compensation time for these patients may be prolonged and the oscillopsia may persist. 44
The definition of oscillopsia is difficult to differentiate from instability (77.8% in G1, 83.3% in G2), especially since these 2 symptoms can be added. 10 Patients may overlook oscillopsia in favor of instability. To take this factor into account, our patients completed a validated questionnaire 9 on oscillopsia and its impact (OSQ). The higher the score, the greater the activity restriction, the severity, and the frequency of oscillopsia. 10 The OSQ was statistically higher in G1, showing the impact of oscillopsia in these patients. However, this score was much lower than in bilateral complete vestibular loss suggesting that a normal contralateral VOR gain reduces the severity of oscillopsia.9,10 This is the case in our population where contralateral VOR gains were normal. The asymmetry of the VOR gain on the posterior SCC at D5 or lateral SCC at M3 has no impact because patients with oscillopsia have better contralateral VOR gain at VHIT.
If the difference experienced by the patients is not explained by the surgical technique, by a partial deafferentation, by a contralateral vestibular impairment, by the compensatory processes, by the characteristics of the population studied, an alternative process must exist. A rapid reorganization of the neuronal circuits could occur as early as the fourth postoperative day. This is a hypothesis without any known scientific basis because all the patients describe this precise delay to see their sensations improve significantly. An earlier evaluation of the patients remains difficult but could provide answers to this difference in sensation. An in vivo study of these sensations for each patient could also provide answers to the differences in sensation and better orient the management of these patients.
Our study has weaknesses because it is retrospective, monocentric, on a small population. Otholitic tests such as cervical vestibular evoked myogenic potential, ocular vestibular evoked myogenic potential, and vertical subjective visual evoked potentials were not performed, so we cannot be sure that the vestibular loss is complete. However, in our experience, when the neurotomy is incomplete, residual fibers are always close to the cochlear nerve. In this situation, the residual vestibular function is always that of the inferior vestibular nerve. This finding is probably related to a tonotopic distribution of vestibular frequencies in the vestibular nerve as in the cochlear nerve. In this situation, the VOR gain (vHIT) of the posterior SCC persists and the patient often experiences in the weeks following surgery signs suggestive of BPPV (Benign paroxysmal positional vertigo) on the posterior SCC. These signs are often associated with positional nystagmus on videonystagmoscopy. In our study, the gain of the posterior SCC was systematically collapsed which seems to confirm that the vestibular loss was complete and that the otholitic efferences are severed. Our results can be extrapolated to all patients with complete unilateral vestibular loss such as complete vestibular neuritis, translabyrinthine fracture, operated Meniere’s disease, or operated vestibular schwannoma.
Conclusion
The results of this cohort support that spontaneous complaints of oscillopsia D5 or M3 after complete unilateral vestibular loss are not explained by VOR gain (vHIT) measurement or DVA loss. There is also no epidemiological or clinical reason to explain why some patients are disabled and others are not. These results suggest that it is necessary to study patients in real conditions to identify independent factors that could explain this oscillopsia sensation (central compensation, head stability, disease experience, anxiety state, etc).
Acknowledgments
Not applicable.
Footnotes
Author Contributions: X.D., E.R., and L.S. coordinated and supervised the study, interpreted the data, drafted the initial manuscript, and reviewed and revised the manuscript. E.D. conceptualized and designed the study, collected data, drafted the initial manuscript, and reviewed and revised the manuscript. C.V.N., M.L., J.C.K., A.B., and E.B. critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Consent for Publication: Not applicable.
Ethics Approval and Consent to Participate: This research has been performed in accordance with the Declaration of Helsinki. Also all participants were informed and gave consent to participate in this study. This is a retrospective study; and in my country, ethics committee is not necessary.
ORCID iD: Xavier Dubernard  https://orcid.org/0000-0001-8776-1934
https://orcid.org/0000-0001-8776-1934
References
- 1. Mantokoudis G, Schubert MC, Tehrani ASS, Wong AL, Agrawal Y. Early adaptation and compensation of clinical vestibular responses after unilateral vestibular deafferentation surgery. Otol Neurotol. 2014;35(1):148-154. doi: 10.1097/MAO.0b013e3182956196 [DOI] [PubMed] [Google Scholar]
- 2. Brickner RM. Oscillopsia: a new symptom commonly occurring in multiple sclerosis. Arch Neurol Psychiat. 1936;36(3):586-589. doi: 10.1001/archneurpsyc.1936.02260090139009 [DOI] [Google Scholar]
- 3. Bronstein AM. Oscillopsia: editorial review. Curr Opin Neurol. 2005;18(1):1-3. doi: 10.1097/00019052-200502000-00002 [DOI] [PubMed] [Google Scholar]
- 4. Badaracco C, Labini FS, Meli A, Tufarelli D. Oscillopsia in labyrinthine defective patients: comparison of objective and subjective measures. Am J Otolaryngol. 2010;31(6):399-403. doi: 10.1016/j.amjoto.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 5. Hermann R, Pelisson D, Dumas O, Urquizar C, Truy E, Tilikete C. Are covert saccade functionally relevant in vestibular hypofunction? Cerebellum. 2018;17(3):300-307. doi: 10.1007/s12311-017-0907-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schubert MC, Migliaccio AA, Santina CCD. Dynamic visual acuity during passive head thrusts in canal planes. J Assoc Res Otolaryngol. 2006;7(4):329-338. doi: 10.1007/s10162-006-0047-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schubert MC, Migliaccio AA, Ng TWC, Shaikh AG, Zee DS. The under-compensatory roll aVOR does not affect dynamic visual acuity. J Assoc Res Otolaryngol. 2012;13(4):517-525. doi: 10.1007/s10162-012-0330-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Nechel C. [The eyes of the labyrinth]. Bull Acad Natl Med. 2014;198(6):1095-1105; discussion 1105-1108. [PubMed] [Google Scholar]
- 9. Guinand N. Visual acuity while walking and oscillopsia severity in healthy subjects and patients with unilateral and bilateral vestibular function loss. Arch Otolaryngol Head Neck Surg. 2012;138(3):301-306. doi: 10.1001/archoto.2012.4 [DOI] [PubMed] [Google Scholar]
- 10. Anson ER, Gimmon Y, Kiemel T, Jeka JJ, Carey JP. A Tool to quantify the functional impact of oscillopsia. Front Neurol. 2018;9:142. doi: 10.3389/fneur.2018.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schubert MC, Herdman SJ, Tusa RJ. Vertical dynamic visual acuity in normal subjects and patients with vestibular hypofunction. Otol Neurotol. 2002;23(3):372-377. doi: 10.1097/00129492-200205000-00025 [DOI] [PubMed] [Google Scholar]
- 12. Lopez-Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Menière’s disease. J Vestib Res. 2015;25(1):1-7. doi: 10.3233/VES-150549 [DOI] [PubMed] [Google Scholar]
- 13. Committee on Hearing and Equilibrium guidelines for the evaluation of results of treatment of conductive hearing loss. American Academy of Otolaryngology—Head and Neck Surgery Foundation, Inc. Otolaryngol Head Neck Surg. 1995;113(3):186-187. doi: 10.1016/S0194-5998(95)70103-6 [DOI] [PubMed] [Google Scholar]
- 14. Bender MB. Oscillopsia. Arch Neurol. 1965;13:204-213. doi: 10.1001/archneur.1965.00470020094013 [DOI] [PubMed] [Google Scholar]
- 15. Allum JHJ. Recovery of vestibular ocular reflex function and balance control after a unilateral peripheral vestibular deficit. Front Neurol. 2012;3:83. doi: 10.3389/fneur.2012.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manzari L, Burgess AM, MacDougall HG, Curthoys IS. Vestibular function after vestibular neuritis. Int J Audiol. 2013;52(10):713-718. doi: 10.3109/14992027.2013.809485 [DOI] [PubMed] [Google Scholar]
- 17. Leveque M, Seidermann L, Tran H, Langagne T, Ulmer E, Chays A. Vestibular function outcomes after vestibular neurectomy in Meniere disease: can vestibular neurectomy provide complete vestibular deafferentation? Auris Nasus Larynx. 2010;37(3):308-313. doi: 10.1016/j.anl.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 18. Véleine Y, Brenet E, Labrousse M, et al. Long-term efficacy of vestibular neurotomy in disabling Ménière’s disease and Tumarkin drop attacks. J Neurosurg. 2022;137:1034-1040. doi: 10.3171/2021.10.JNS21145 [DOI] [PubMed] [Google Scholar]
- 19. Lemnos L, Aubry K, Moreau JJ, Caire F, Salle H. Postoperative compensation after neurotomy in Meniere’s disease: retrospective study of 15 cases. Neurochirurgie. 2019;65:20-26. doi: 10.1016/j.neuchi.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 20. Tian JR, Shubayev I, Demer JL. Dynamic visual acuity during passive and self-generated transient head rotation in normal and unilaterally vestibulopathic humans. Exp Brain Res. 2002;142(4):486-495. doi: 10.1007/s00221-001-0959-7 [DOI] [PubMed] [Google Scholar]
- 21. Murnane O, Mabrey H, Pearson A, Byrd S, Akin F. Normative data and test-retest reliability of the SYNAPSYS video head impulse test. J Am Acad Audiol. 2014;25(3):244-252. doi: 10.3766/jaaa.25.3.3 [DOI] [PubMed] [Google Scholar]
- 22. Schubert MC, Migliaccio AA, Clendaniel RA, Allak A, Carey JP. Mechanism of dynamic visual acuity recovery with vestibular rehabilitation. Arch Phys Med Rehabil. 2008;89(3):500-507. doi: 10.1016/j.apmr.2007.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Herdman SJ, Tusa RJ, Blatt P, Suzuki A, Venuto PJ, Roberts D. Computerized dynamic visual acuity test in the assessment of vestibular deficits. Am J Otol. 1998;19(6):790-796. [PubMed] [Google Scholar]
- 24. Halmagyi GM, Black RA, Thurtell MJ, Curthoys IS. The human horizontal vestibulo-ocular reflex in response to active and passive head impulses after unilateral vestibular deafferentation. Ann N Y Acad Sci. 2003;1004:325-336. doi: 10.1196/annals.1303.030 [DOI] [PubMed] [Google Scholar]
- 25. Halmagyi GM, Aw ST, Cremer PD, Todd MJ, Curthoys IS. The human vertical vestibuloocular reflex in response to high-acceleration stimulation after unilateral vestibular neurectomy. Ann N Y Acad Sci. 1992;656:732-738. doi: 10.1111/j.1749-6632.1992.tb25251.x [DOI] [PubMed] [Google Scholar]
- 26. Schubert MC, Hall CD, Das V, Tusa RJ, Herdman SJ. Oculomotor strategies and their effect on reducing gaze position error. Otol Neurotol. 2010;31(2):228-231. doi: 10.1097/MAO.0b013e3181c2dbae [DOI] [PubMed] [Google Scholar]
- 27. Ramaioli C, Colagiorgio P, Sağlam M, et al. The effect of vestibulo-ocular reflex deficits and covert saccades on dynamic vision in opioid-induced vestibular dysfunction. PLoS One. 2014;9(10):e110322. doi: 10.1371/journal.pone.0110322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sjögren J, Karlberg M, Hickson C, Magnusson M, Fransson PA, Tjernström F. Short-latency covert saccades—the explanation for good dynamic visual performance after unilateral vestibular loss? Front Neurol. 2021;12:695064. doi: 10.3389/fneur.2021.695064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leigh RJ, Zee DS. The Neurology of Eye Movements. 5th ed. Oxford University Press; 2015. Accessed April 9, 2022. https://oxfordmedicine.com/view/10.1093/med/9780199969289.001.0001/med-9780199969289;jsessionid=AC1A3505FCB7468B013C00CC574FEECE [Google Scholar]
- 30. Pogson JM, Taylor RL, Bradshaw AP, et al. The human vestibulo-ocular reflex and compensatory saccades in schwannoma patients before and after vestibular nerve section. Clin Neurophysiol. 2022;138:197-213. doi: 10.1016/j.clinph.2022.02.014 [DOI] [PubMed] [Google Scholar]
- 31. Herdman SJ, Schubert MC, Tusa RJ. Role of central preprogramming in dynamic visual acuity with vestibular loss. Arch Otolaryngol Head Neck Surg. 2001;127(10):1205-1210. doi: 10.1001/archotol.127.10.1205 [DOI] [PubMed] [Google Scholar]
- 32. Santina CCD, Cremer PD, Carey JP, Minor LB. Comparison of head thrust test with head autorotation test reveals that the vestibulo-ocular reflex is enhanced during voluntary head movements. Arch Otolaryngol Head Neck Surg. 2002;128(9):1044-1054. doi: 10.1001/archotol.128.9.1044 [DOI] [PubMed] [Google Scholar]
- 33. Grunfeld EA, Morland AB, Bronstein AM, Gresty MA. Adaptation to oscillopsia: a psychophysical and questionnaire investigation. Brain. 2000;123(2):277-290. doi: 10.1093/brain/123.2.277 [DOI] [PubMed] [Google Scholar]
- 34. Morland A, Bronstein A, Ruddock K, Wooding D. Oscillopsia: visual function during motion in the absence of vestibulo-ocular reflex. J Neurol Neurosurg Psychiatry. 1998;65(6):828-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahmad H, Roberts RE, Patel M, et al. Downregulation of early visual cortex excitability mediates oscillopsia suppression. Neurology. 2017;89(11):1179-1185. doi: 10.1212/WNL.0000000000004360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wist ER, Brandt T, Krafczyk S. Oscillopsia and retinal slip. Evidence supporting a clinical test. Brain. 1983;106(pt 1):153-168. doi: 10.1093/brain/106.1.153 [DOI] [PubMed] [Google Scholar]
- 37. Grunfeld EA, Shallo-Hoffmann JA, Cassidy L, et al. Vestibular perception in patients with acquired ophthalmoplegia. Neurology. 2003;60(12):1993-1995. doi: 10.1212/01.wnl.0000067992.17185.60 [DOI] [PubMed] [Google Scholar]
- 38. Millar JL, Gimmon Y, Roberts D, Schubert MC. Improvement after vestibular rehabilitation not explained by improved passive VOR gain. Front Neurol. 2020;11:79. doi: 10.3389/fneur.2020.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lacour M, Thiry A, Tardivet L. Two conditions to fully recover dynamic canal function in unilateral peripheral vestibular hypofunction patients. J Vestib Res. 2021;31(5):407-421. doi: 10.3233/VES-201557 [DOI] [PubMed] [Google Scholar]
- 40. Lacour M, Bernard-Demanze L. Interaction between vestibular compensation mechanisms and vestibular rehabilitation therapy: 10 recommendations for optimal functional recovery. Front Neurol. 2015;5:285. doi: 10.3389/fneur.2014.00285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Herdman SJ, Schubert MC, Das VE, Tusa RJ. Recovery of dynamic visual acuity in unilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg. 2003;129(8):819-824. doi: 10.1001/archotol.129.8.819 [DOI] [PubMed] [Google Scholar]
- 42. Lacour M, Tardivet L, Thiry A. Rehabilitation of dynamic visual acuity in patients with unilateral vestibular hypofunction: earlier is better. Eur Arch Otorhinolaryngol. 2020;277(1):103-113. doi: 10.1007/s00405-019-05690-4 [DOI] [PubMed] [Google Scholar]
- 43. Al Saif AA, Al Senany S. Determine the effect of neck muscle fatigue on dynamic visual acuity in healthy young adults. J Phys Ther Sci. 2015;27(1):259-263. doi: 10.1589/jpts.27.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Michaelides E, Schutt CA. The correlation between the vestibulo-ocular reflex and multi-focal ocular correction: implications for vestibular compensation. Am J Otolaryngol. 2014;35(5):572-576. doi: 10.1016/j.amjoto.2014.06.003 [DOI] [PubMed] [Google Scholar]