Introduction
Each year at the Society for Cardiovascular Angiography & Interventions (SCAI) Annual Scientific Sessions meeting collaborative think tanks involving interventional cardiologists, administrative partners, and members of industry are convened for each SCAI clinical council to discuss topics of particular interest to the group. This document presents the proceedings of the 2023 Coronary Think Tank session, which focused on the topic of coronary microvascular dysfunction (CMD). The goals of this discussion were to identify barriers to diagnosis and treatment and promote actions by the participants, leading to a positive impact on patient care.
Coronary microvascular dysfunction
CMD is increasingly recognized among patients with acute and chronic coronary syndromes and is associated with impaired quality of life and adverse clinical outcomes.1 The assessment of CMD has been identified by SCAI as a key area where there are diagnostic and therapeutic opportunities to improve patient care. It is our understanding that the approaches to the invasive diagnosis of CMD are sufficiently robust that they can be incorporated and used more widely across percutaneous coronary intervention capable cardiac catheterization laboratories. There are, however, several barriers to caring for patients with this diagnosis, including variability in the recognition of CMD by the medical community, a lack of standardized approaches to its diagnosis and treatment, and misunderstandings of the prognostic implications.
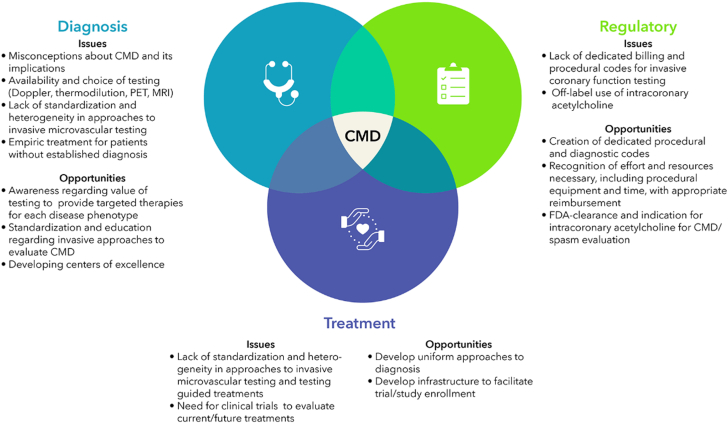
In large part because of randomized data from the CorMicA (CORonary MICrovascular Angina) trial,2 both the 2023 guidelines for the management of patients with chronic coronary disease and the 2021 clinical practice guidelines on the evaluation and diagnosis of chest pain provide class 2a recommendations to perform CMD testing in patients with ischemia and nonobstructive coronary arteries (INOCA).3 Our collaborative SCAI Think Tank, addressing how to improve diagnosis and treatment of CMD and INOCA, identified 3 major areas with unmet needs: (1) diagnostic, (2) regulatory and reimbursement, and (3) treatment issues (Central Illustration).
Central Illustration.
Opportunities and challenges with coronary microvascular disease. CMD, coronary microvascular dysfunction.
Diagnostic issues
For patients with suspected CMD, despite clinical practice guideline recommendations to consider noninvasive or invasive evaluations to assess microvascular function, testing is often not performed. Recognized barriers include lack of awareness and understanding of CMD and its implications, availability and/or expertise of testing, and the use of empiric treatments such as calcium channel blockers or nitrates in patients without an established diagnosis. These issues often contribute to delayed diagnosis in patients who often undergo multiple functional or anatomical tests and empiric or no attempts at medical therapy. There is consensus, however, that testing and definitive diagnosis improve patient care and quality of life by providing targeted therapies for each of the INOCA phenotypes, including those with impaired microvasculature, epicardial coronary spasm, or myocardial bridging, and in those with associated conditions such as heart failure with preserved ejection fraction. Individualized therapies can be tailored to each phenotype once a precise diagnosis is made.1,2
Access to both noninvasive testing modalities, such as positron emission tomography, and invasive assessment via pressure wire measurements are currently not widely available. Noninvasive modalities can only quantify myocardial blood flow and provide insights about nonendothelium–dependent microvascular function, whereas invasive modalities, such as Doppler or thermodilution in combination with coronary spasm provocation using intracoronary acetylcholine allow assessment of both nonendothelium and endothelium-dependent microvascular function.1,3 In CorMicA, 17% of patients had vasospastic angina during acetylcholine testing, and ∼21% had mixed microvascular and vasospastic angina,2 which underscores how noninvasive modalities can provide an assessment of myocardial blood flow but miss abnormal endothelium-dependent microvascular function with epicardial and/or microvascular spasm, which can only be diagnosed with intracoronary acetylcholine provocation in the cardiac catheterization laboratory. Beyond educational efforts to increase awareness and understanding of CMD, there also remains a major unmet need and an opportunity to demonstrate the benefit of invasive testing.
Regulatory and reimbursement issues
There are also recognized regulatory and reimbursement challenges to CMD assessment with respect to issues such as billing and coding. These are important issues given that comprehensive invasive studies often have longer procedural times because of the need to interrogate both the nonendothelium–dependent microvasculature with intracoronary or intravenous adenosine and the endothelium-dependent function with intracoronary acetylcholine. There are billing codes for physiologic wire-based assessments and ergonovine testing. Additional reimbursement is available if intravascular imaging is performed, such as to evaluate for a myocardial bridge. If an intracoronary drug such as adenosine is administered during the same procedure as a pressure wire assessment, then a bundled payment occurs, which negates the charge for the drug infusion. This leads to considerable institutional variability in billing and coding. There is currently no added reimbursement for the additional time, effort, and medications that coronary function testing entails.
There are also issues with respect to the use of intracoronary acetylcholine for spasm provocation. There is no dedicated billing code for this procedure, which contributes to variation in whether the procedure is either not coded and thus unbilled or coded as an ergonovine challenge. Acetylcholine is the preferred medication for coronary spasm provocation given its established safety record.4 The 2014 AHA/ACC NSTE-ACS guideline provides a class IIb recommendation for consideration of provocative testing during invasive coronary angiography in patients with suspected vasospastic angina.5 Likewise, the 2021 Chest Pain guidelines proposed a clinical decision pathway for patients with INOCA that incorporates the use of acetylcholine to identify patients with epicardial and/or microvascular spasms.3 Despite its safety record and guideline-recommended use, the intracoronary use of acetylcholine represents the off-label use of an ophthalmic medication. A dedicated indication and clearance by the US Food and Drug Administration to evaluate patients with suspected coronary spasms would be beneficial in standardizing its use and help prevent shortages in availability. Given the anticipated increase in usage, as INOCA becomes more widely recognized, more availability of acetylcholine will be required.
There are also multiple invasive methods for evaluating CMD. Coronary flow reserve (CFR) can be assessed using Doppler or thermodilution.1 Doppler allows the assessment of average peak resting and hyperemic average peak velocities (APV) to calculate CFR. The Doppler wire (Philips) allows the assessment of APV and CFR, while the ComboWire (Philips) allows the assessment of APV, CFR, hyperemic microvascular resistance (HMR), and fractional flow reserve (FFR), but neither of these is currently commercially available. A new iteration of the Doppler wire is in development and expected within the next few years. Thermodilution with the PressureWire X (Abbott Vascular) allows the assessment of resting full-cycle ratio (RFR), FFR, the index of microcirculatory resistance (IMR), and CFR. Consensus regarding normal/abnormal ranges and reproducibility of metrics used to diagnose CMD are necessary.
Treatment issues
The value of evaluating patients for CMD and coronary spasm was demonstrated in the CorMicA trial in which 151 patients without obstructive coronary artery disease. underwent an invasive diagnostic procedure consisting of the assessment of CFR, index of microcirculatory resistance, and FFR, as well vasoreactivity testing with acetylcholine, and were randomized 1:1 to stratified medical therapy (intervention group, results disclosed) or standard care (control group, results not disclosed [blinded]).2 The intervention improved angina scores and quality of life and resulted in a significant improvement in the Seattle Angina Questionnaire summary score at 6 months (primary end point). There were also improvements in secondary end points such as blood pressure control and compliance with cardiac rehabilitation. In this trial, in addition to lifestyle changes and smoking cessation, patients with vasospastic angina were treated with calcium channel blockers and/or long-acting nitrates, whereas those with microvascular angina were treated with beta-blockers and consideration for angiotensin-converting-enzyme inhibitors and statins. Novel approaches being evaluated include enhanced external counterpulsation, coronary sinus reduction, lipid-lowering and anti-inflammatory therapies, and stem cells, among others. For device-based therapies addressing quality of life, study designs involving a sham procedure as in CorMicA would be beneficial.
There are also emerging multicenter registries such as DISCOVER INOCA (NCT05288361) and collaborative efforts such as the Microvascular Network that may provide the infrastructure to enhance education and future clinical trial efforts. These registries may help with the use of similar protocols and standardization efforts that could eventually facilitate the enrollment of patients into clinical trials.
Acknowledgments
Peer review statement
Editor-in-Chief Alexandra J. Lansky had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Associate Editor Cindy L. Grines.
Declaration of competing interest
Yader Sandoval is on the advisory board of Abbott Diagnostics, Phillips, Roche Diagnostics, and Zoll. Alexandra J. Lansky received research support from Abbott Vascular. Samit Shah is a consultant for Abbott Vascular and principal investigator for Abbott and Philips. Jennifer Tremmel received an honorarium from Abbott Vascular and is on the advisory board of Abbott Vascular. Robert Riley is a consultant for Abbott Vascular. Timothy D. Henry is a consultant for Phillips and Abbott Vascular. Connie S. Baumgard, Regina Deible, Melissa Jackson, and Nick E. J. West are employed at Abbott. Ilka Bijoux and Lisa Cavaliere are employed at Terumo. Casey Culbertson and Krish Ramakrishnan are employed at GE. Maya El-Sabban and Chrissy Whalen-Morton are employed at Medtronic. Jeremy Jackson is employed at LivaNova. Bethany Kalich, Daya Perkins, and Cezary Wojcik are employed at Amgen. Vanessa Long is employed at Abiomed and Amy Newell is employed at Corazon. Karen Russell and Kristi Winterfeldt are employed at Shockwave. Vinod Sharma and Paul Underwood are employed at Boston Scientific. Steve Zizzo is employed at Philips Healthcare. The other authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding sources
This work was not supported by funding agencies in the public, commercial, or non-for-profit sectors.
Ethics statement and patient consent
The authors adhered to the relevant ethical guidelines when developing this manuscript.
References
- 1.Kunadian V., Chieffo A., Camici P.G., et al. An EAPCI Expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation endorsed by Coronary Vasomotor Disorders International Study group. Eur Heart J. 2020;41(37):3504–3520. doi: 10.1093/eurheartj/ehaa503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford T.J., Stanley B., Good R., et al. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol. 2018;72(23 Pt A):2841–2855. doi: 10.1016/j.jacc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Gulati M., Levy P.D., Mukherjee D., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;78(22):e187–e285. doi: 10.1016/j.jacc.2021.07.053. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T., Samuels B.A., Li W., et al. Safety of provocative testing with intracoronary acetylcholine and implications for standard protocols. J Am Coll Cardiol. 2022;79(24):2367–2378. doi: 10.1016/j.jacc.2022.03.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amsterdam E.A., Wenger N.K., Brindis R.G., et al. 2014 AHA/ACC Guideline for the Management of Patients with non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]