Abstract
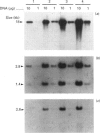
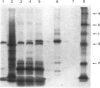
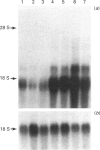
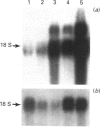
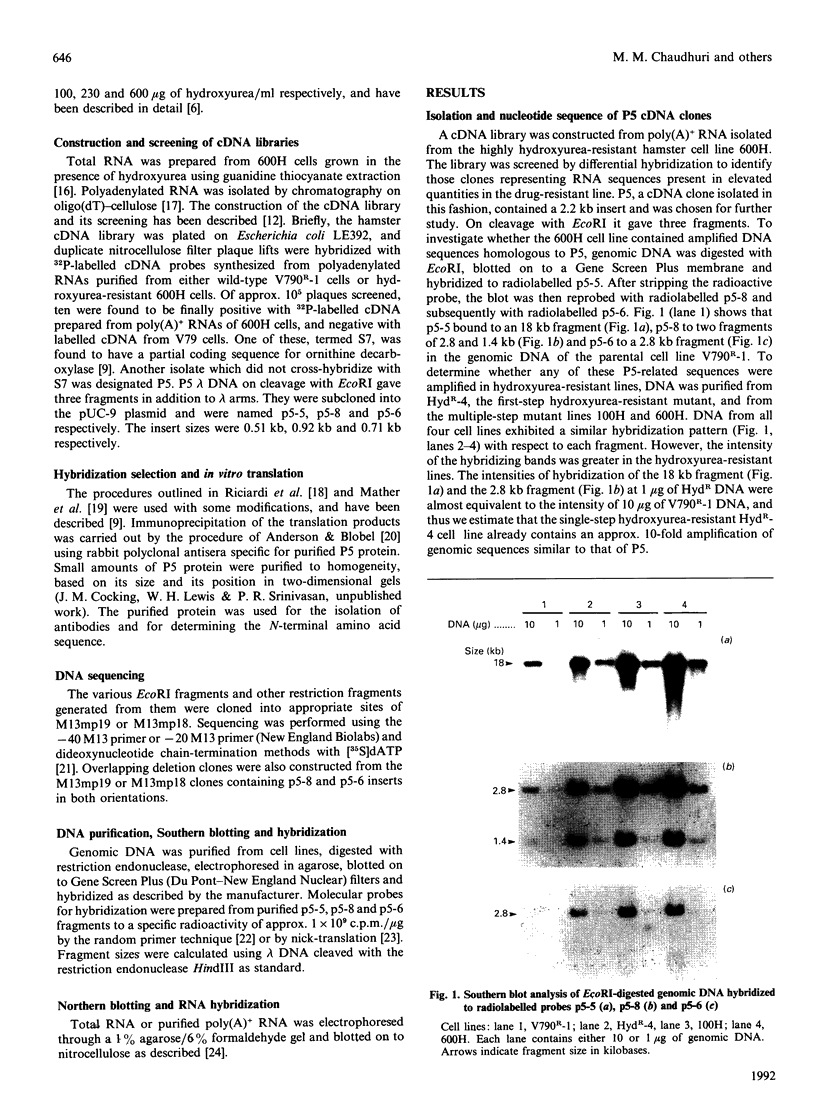
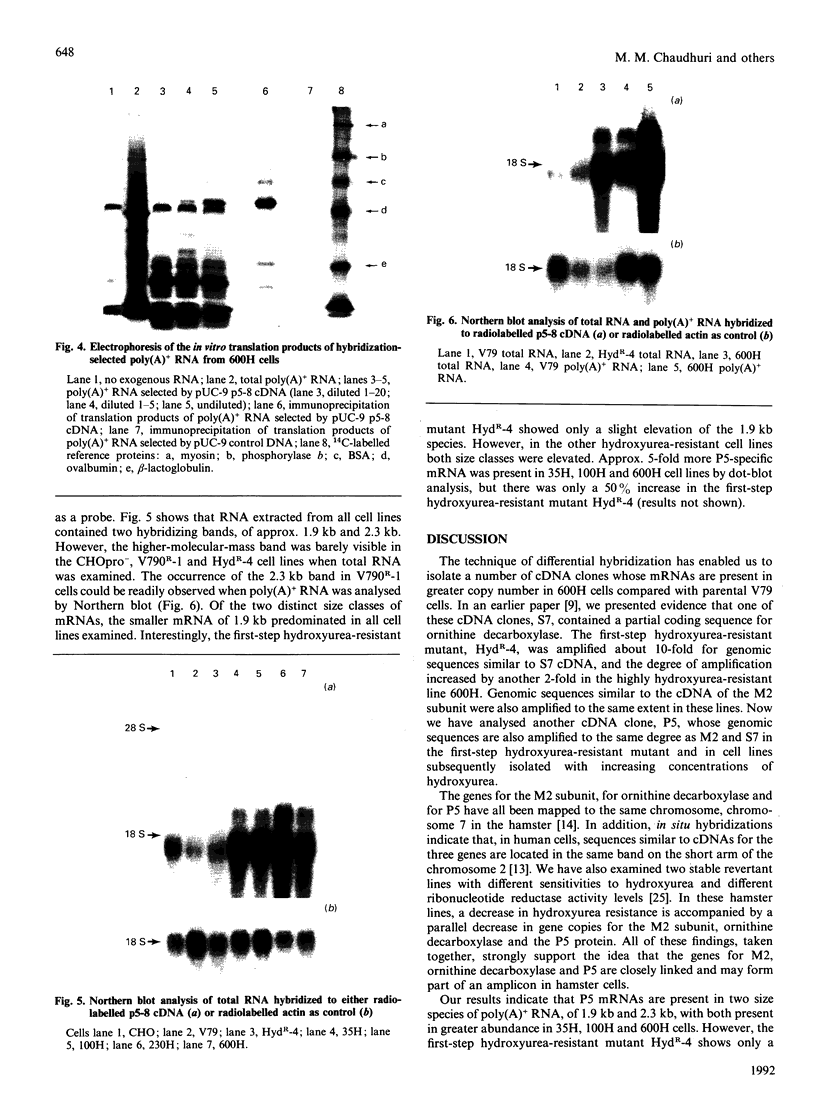
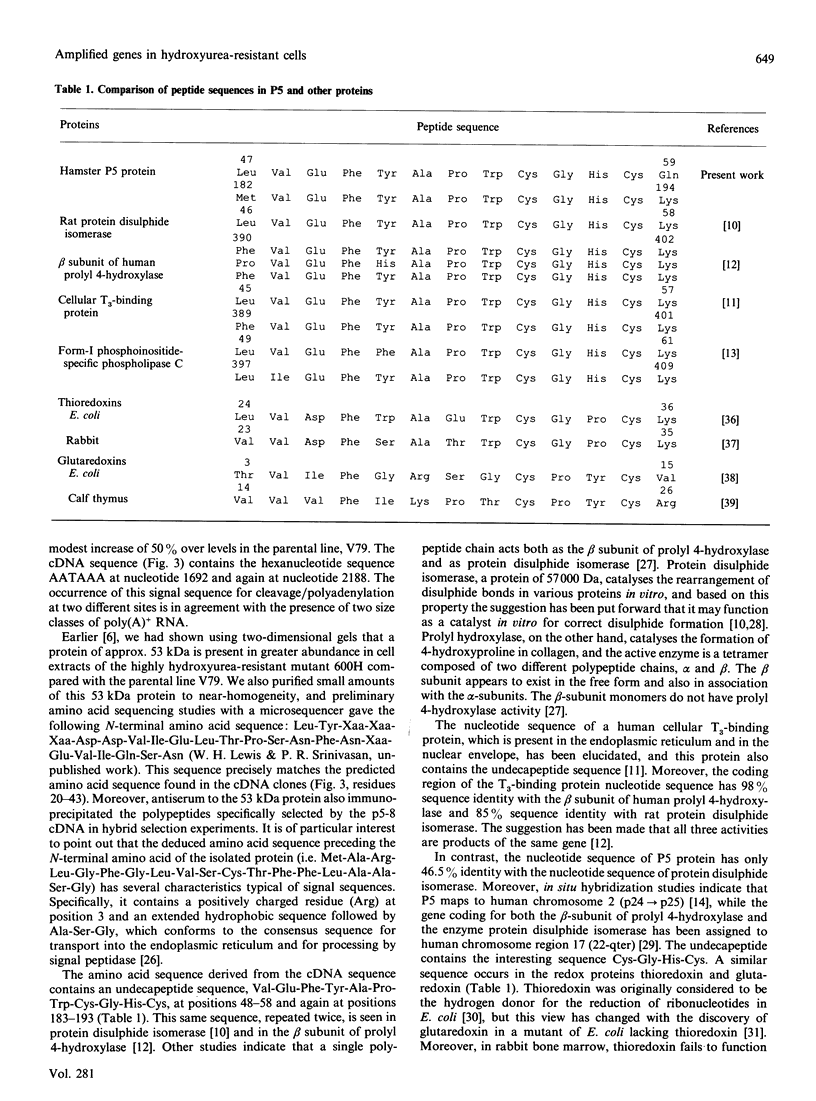
Cell lines selected in multiple steps for increasing resistance to hydroxyurea have been shown to have corresponding increases in ribonucleotide reductase activity. We have isolated a number of cDNA clones from a cDNA library constructed from a highly hydroxyurea-resistant hamster cell line, 600H, in which the activity of ribonucleotide reductase is elevated more than 80-fold. These clones correspond to genomic DNA sequences amplified in the 600H cell line compared with the V79 parental line. One of these cDNA clones, termed P5, codes for a 50 kDa protein detected by in vitro translation of poly(A)+ RNA isolated by hybridization/selection. The cDNA sequence contains a single open reading frame of 1317 nucleotides which encodes a polypeptide of 439 amino acids. The amino acid sequence deduced from the cDNA insert contains two copies of the 11-amino-acid sequence Val-Glu-Phe-Tyr-Ala-Pro-Trp-Cys-Gly-His-Cys. Duplicate copies of this sequence also occur in the active site of rat and human protein disulphide isomerase (also known as the beta-subunit of human prolyl 4-hydroxylase, tri-iodothyronine-binding protein) and in Form I phosphoinositide-specific phospholipase C, indicating that P5 falls into this newly defined superfamily of proteins. Genomic sequences similar to the cDNA clone are amplified 10-20-fold in hamster cells selected for resistance to increasing concentrations of hydroxyurea, a phenomenon observed earlier with cDNA clones for the M2 subunit of ribonucleotide reductase and ornithine decarboxylase. RNA blots probed with P5 cDNA show two poly(A)+ RNA species which are elevated in hydroxyurea-resistant cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerblom L., Ehrenberg A., Gräslund A., Lankinen H., Reichard P., Thelander L. Overproduction of the free radical of ribonucleotide reductase in hydroxyurea-resistant mouse fibroblast 3T6 cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2159–2163. doi: 10.1073/pnas.78.4.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. J., Blobel G. Immunoprecipitation of proteins from cell-free translations. Methods Enzymol. 1983;96:111–120. doi: 10.1016/s0076-6879(83)96012-3. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C. F., Balcarek J. M., Varrichio A., Crooke S. T. Molecular cloning and complete amino-acid sequence of form-I phosphoinositide-specific phospholipase C. Nature. 1988 Jul 21;334(6179):268–270. doi: 10.1038/334268a0. [DOI] [PubMed] [Google Scholar]
- Cheng S. Y., Gong Q. H., Parkison C., Robinson E. A., Appella E., Merlino G. T., Pastan I. The nucleotide sequence of a human cellular thyroid hormone binding protein present in endoplasmic reticulum. J Biol Chem. 1987 Aug 15;262(23):11221–11227. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Edman J. C., Ellis L., Blacher R. W., Roth R. A., Rutter W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature. 1985 Sep 19;317(6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Freedman R. B. Protein disulfide isomerase: multiple roles in the modification of nascent secretory proteins. Cell. 1989 Jun 30;57(7):1069–1072. doi: 10.1016/0092-8674(89)90043-3. [DOI] [PubMed] [Google Scholar]
- Gräslund A., Ehrenberg A., Thelander L. Characterization of the free radical of mammalian ribonucleotide reductase. J Biol Chem. 1982 May 25;257(10):5711–5715. [PubMed] [Google Scholar]
- Holmgren A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2275–2279. doi: 10.1073/pnas.73.7.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. 6. The amino acid sequence of the protein from escherichia coli B. Eur J Biochem. 1968 Dec 5;6(4):475–484. doi: 10.1111/j.1432-1033.1968.tb00470.x. [DOI] [PubMed] [Google Scholar]
- Hopper S., Iurlano D. Properties of a thioredoxin purified from rabbit bone marrow which fails to serve as a hydrogen donor for the homologous ribonucleotide reductase. J Biol Chem. 1983 Nov 25;258(22):13453–13457. [PubMed] [Google Scholar]
- Hopper S., Johnson R. S., Vath J. E., Biemann K. Glutaredoxin from rabbit bone marrow. Purification, characterization, and amino acid sequence determined by tandem mass spectrometry. J Biol Chem. 1989 Dec 5;264(34):20438–20447. [PubMed] [Google Scholar]
- Hög J. O., Jörnvall H., Holmgren A., Carlquist M., Persson M. The primary structure of Escherichia coli glutaredoxin. Distant homology with thioredoxins in a superfamily of small proteins with a redox-active cystine disulfide/cysteine dithiol. Eur J Biochem. 1983 Oct 17;136(1):223–232. doi: 10.1111/j.1432-1033.1983.tb07730.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. S., Mathews W. R., Biemann K., Hopper S. Amino acid sequence of thioredoxin isolated from rabbit bone marrow determined by tandem mass spectrometry. J Biol Chem. 1988 Jul 15;263(20):9589–9597. [PubMed] [Google Scholar]
- Klintrot I. M., Hög J. O., Jörnvall H., Holmgren A., Luthman M. The primary structure of calf thymus glutaredoxin. Homology with the corresponding Escherichia coli protein but elongation at both ends and with an additional half-cystine/cysteine pair. Eur J Biochem. 1984 Nov 2;144(3):417–423. doi: 10.1111/j.1432-1033.1984.tb08481.x. [DOI] [PubMed] [Google Scholar]
- Koivu J., Myllylä R., Helaakoski T., Pihlajaniemi T., Tasanen K., Kivirikko K. I. A single polypeptide acts both as the beta subunit of prolyl 4-hydroxylase and as a protein disulfide-isomerase. J Biol Chem. 1987 May 15;262(14):6447–6449. [PubMed] [Google Scholar]
- LAURENT T. C., MOORE E. C., REICHARD P. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES. IV. ISOLATION AND CHARACTERIZATION OF THIOREDOXIN, THE HYDROGEN DONOR FROM ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3436–3444. [PubMed] [Google Scholar]
- Lewis W. H., Srinivasan P. R. Chromosome-mediated gene transfer of hydroxyurea resistance and amplification of ribonucleotide reductase activity. Mol Cell Biol. 1983 Jun;3(6):1053–1061. doi: 10.1128/mcb.3.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis W. H., Wright J. A. Isolation of hydroxyurea-resistant CHO cells with altered levels of ribonucleotide reductase. Somatic Cell Genet. 1979 Jan;5(1):83–96. doi: 10.1007/BF01538788. [DOI] [PubMed] [Google Scholar]
- Mather E. L., Alt F. W., Bothwell A. L., Baltimore D., Koshland M. E. Expression of J chain RNA in cell lines representing different stages of B lymphocyte differentiation. Cell. 1981 Feb;23(2):369–378. doi: 10.1016/0092-8674(81)90132-x. [DOI] [PubMed] [Google Scholar]
- McClarty G. A., Tonin P. N., Srinivasan P. R., Wright J. A. Relationships between reversion of hydroxyurea resistance in hamster cells and the co-amplification of ribonucleotide reductase M2 component, ornithine decarboxylase and P5-8 genes. Biochem Biophys Res Commun. 1988 Aug 15;154(3):975–981. doi: 10.1016/0006-291x(88)90235-5. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Pajunen L., Myllylä R., Helaakoski T., Pihlajaniemi T., Tasanen K., Höyhtyä M., Tryggvason K., Solomon E., Kivirikko K. I. Assignment of the gene coding for both the beta-subunit of prolyl 4-hydroxylase and the enzyme disulfide isomerase to human chromosome region 17p11----qter. Cytogenet Cell Genet. 1988;47(1-2):37–41. doi: 10.1159/000132501. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T., Helaakoski T., Tasanen K., Myllylä R., Huhtala M. L., Koivu J., Kivirikko K. I. Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 1987 Mar;6(3):643–649. doi: 10.1002/j.1460-2075.1987.tb04803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan P. R., Tonin P. N., Wensing E. J., Lewis W. H. The gene for ornithine decarboxylase is co-amplified in hydroxyurea-resistant hamster cells. J Biol Chem. 1987 Sep 15;262(26):12871–12878. [PubMed] [Google Scholar]
- Thelander L., Berg P. Isolation and characterization of expressible cDNA clones encoding the M1 and M2 subunits of mouse ribonucleotide reductase. Mol Cell Biol. 1986 Oct;6(10):3433–3442. doi: 10.1128/mcb.6.10.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander L., Eriksson S., Akerman M. Ribonucleotide reductase from calf thymus. Separation of the enzyme into two nonidentical subunits, proteins M1 and M2. J Biol Chem. 1980 Aug 10;255(15):7426–7432. [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Tonin P. N., Stallings R. L., Carman M. D., Bertino J. R., Wright J. A., Srinivasan P. R., Lewis W. H. Chromosomal assignment of amplified genes in hydroxyurea-resistant hamster cells. Cytogenet Cell Genet. 1987;45(2):102–108. doi: 10.1159/000132438. [DOI] [PubMed] [Google Scholar]
- Wright J. A., Alam T. G., McClarty G. A., Tagger A. Y., Thelander L. Altered expression of ribonucleotide reductase and role of M2 gene amplification in hydroxyurea-resistant hamster, mouse, rat, and human cell lines. Somat Cell Mol Genet. 1987 Mar;13(2):155–165. doi: 10.1007/BF01534695. [DOI] [PubMed] [Google Scholar]
- Yang-Feng T. L., Barton D. E., Thelander L., Lewis W. H., Srinivasan P. R., Francke U. Ribonucleotide reductase M2 subunit sequences mapped to four different chromosomal sites in humans and mice: functional locus identified by its amplification in hydroxyurea-resistant cell lines. Genomics. 1987 Sep;1(1):77–86. doi: 10.1016/0888-7543(87)90108-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]