Abstract
Transcatheter atrial shunt therapies, designed to dynamically lower left atrial (LA) pressure by shunting blood into the larger reservoir of the right atrium and central veins, have been developed as a novel treatment for heart failure (HF) over the past 10+ years. Several atrial shunt devices and procedures are currently in development with several pivotal randomized clinical trials (RCT) underway; however, only 2 sham-controlled RCT (both with the Atrial Shunt Device [Corvia Medical] in HF with EF ≥ 40%) have been reported thus far; a mechanistic RCT (n = 44) that demonstrated a reduction in exercise LA pressure at 1 month and a pivotal RCT (n = 626) that was neutral with no difference in outcomes or health status between shunt and sham groups. Subsequent analyses of the single completed pivotal RCT found that peak exercise pulmonary vascular resistance <1.74 WU plus the absence of a cardiac rhythm management device identified a responder group that benefited from LA unloading with atrial shunt implantation, a finding that is currently being confirmed in a follow-up RCT. Here we provide a comprehensive review of the field of atrial shunt therapeutics with a description of the following: (1) current HF treatment; (2) rationale and history of atrial shunt development; (3) design of and accumulated evidence for the various atrial shunt devices and procedures under investigation; (4) unanswered questions in the field; and (5) future considerations. Atrial shunts represent a potential innovative therapeutic for HF but the optimal design/approach and phenotype of HF most likely to benefit are yet to be determined.
Keywords: clinical trials, heart failure with preserved ejection fraction, interventional cardiology, left atrium, physiology
Central Illustration
Highlights
-
•
Atrial shunt therapy to lower left atrium pressure is a novel potential treatment for heart failure.
-
•
Several different atrial shunt devices and procedures are currently in development.
-
•
The first completed large pivotal trial of an atrial shunt device was neutral overall.
-
•
Low exercise pulmonary vascular resistance may be necessary for a beneficial response to atrial shunt therapy.
-
•
Forthcoming results of ongoing atrial shunt trials will provide additional insights.
Introduction
Despite advances in the treatment of heart failure (HF) across the spectrum of ejection fraction (EF), morbidity and mortality for HF patients remain high.1 In addition, while there are now 4 established pillars of guideline-directed medical therapy (GDMT) for HF with reduced EF ([HFrEF] EF < 40%), with 3 of the 4 treatments effective in HF with mildly reduced EF ([HFmrEF] EF 40%-50%), there are still limited treatment options for HF with preserved EF ([HFpEF] EF > 50%), with sodium-glucose co-transport protein-2 (SGLT2) inhibitors as the only clearly proven treatment that reduces HF hospitalizations.2, 3, 4 Medical therapy for HF is also challenging because polypharmacy (≥5 medications) and hyperpolypharmacy (≥10 medications) are very common due to the several medications often needed for the treatment of HF regardless of EF, and because HF patients often have a high comorbidity burden, resulting in even more medications. Both hyperpolypharmacy5 and medication nonadherence6 have been associated with worse outcomes in HF patients. For these reasons, device-based therapeutics in HF are an attractive potential option to reduce symptoms, improve quality of life (QOL) and exercise capacity, and prevent adverse outcomes without having to add to the pill burden in HF patients.
For the past 10+ years, transcatheter atrial shunt therapies (either with devices or device-less procedures), which are designed to create a passageway from the left atrium (LA) to the right atrium (RA), have been developed and investigated as a novel treatment for HF that can dynamically unload the LA and reduce LA pressure at rest and during exertion, thereby potentially benefiting HF patients.7 What started out as a nascent field has exploded into a crowded space with multiple companies, devices, and procedures in the space, and several trials, including pivotal clinical trials, underway or completed. Nevertheless, several questions regarding atrial shunt therapies, including the best device/procedure; the best clinical trial design (including the need for invasive exercise hemodynamics); the HF phenotype that derives the most benefit and the least harm (eg, across the EF spectrum or HFpEF only, absence of latent pulmonary vascular disease [PVD]); the optimal size of the shunt (including whether 1 size fits all, or whether multiple sizes are needed); the ideal timing for treatment (with regard to severity of HF); and whether shunting from the LA into the right heart is safe in the long-term remain key unanswered questions at the present time. Here we begin by providing an overview of the current treatment of HF, followed by the historical context of atrial shunt therapeutic development, a detailed review of each device/procedure currently under investigation, a discussion of the unanswered questions in the field, and conclude with considerations for the design and conduct of ongoing and future clinical trials.
Current treatment of HF
Significant contemporary advancement in the medical treatment of HF across the spectrum of EF has led to improved functional capacity and symptom burden while decreasing morbidity and mortality. HFrEF has enjoyed the most success over the last 25 years with foundational, practice-changing pharmacologic and device therapies. Four pillars of GDMT, composed of β-blockers, mineralocorticoid receptor antagonists, angiotensin receptor-neprilysin inhibitors, and SGLT2 inhibitors form the backbone of medical therapy for HFrEF with device therapy, including implantable cardioverter-defibrillators, cardiac resynchronization therapy (CRT), implantable hemodynamic monitoring, and transcatheter edge-to-edge mitral valve repair (for severe mitral regurgitation) functioning in concert with GDMT to reduce HF hospitalizations, promote myocardial recovery, and prevent sudden cardiac death.2 Although the evidence base for GDMT in HFmrEF is still in development, in general, therapies that are beneficial for HFrEF (particularly angiotensin receptor blockers, mineralocorticoid receptor antagonists, angiotensin receptor-neprilysin inhibitors, and SGLT2 inhibitors) appear to confer similar benefits in HFmrEF.
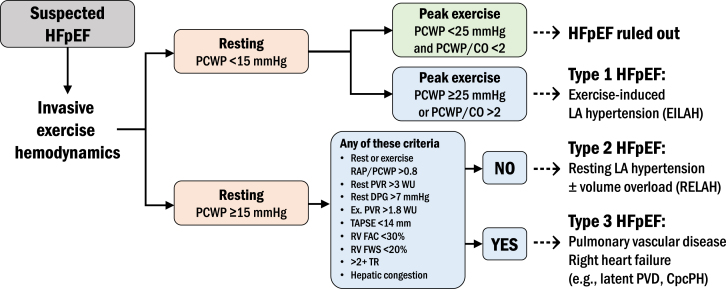
In contrast with HFrEF and HFmrEF, HFpEF has suffered from a dearth of evidence-based treatments until very recently. The diagnosis of HFpEF is typically based on the following: (1) symptoms of dyspnea and exercise intolerance; (2) evidence of abnormal cardiac structure/function (eg, concentric left ventricular [LV] hypertrophy, LA enlargement); (3) pulmonary or systemic congestion by physical examination, chest radiography, echocardiography (eg, elevated E/e′ ratio or elevated estimated pulmonary artery [PA] systolic pressure in the setting of other signs of left heart disease), and/or elevated natriuretic peptide levels; and (4) an EF > 50%.8 Importantly, alternate causes for the patient’s symptoms and “masqueraders” (ie, etiologies of the syndrome of HF and a preserved EF such as infiltrative cardiomyopathies, constrictive pericarditis, severe valvular disease, etc., which have specific treatments) should be excluded.8 While such criteria are often sufficient, the gold standard test for the HFpEF diagnosis is invasive hemodynamic exercise testing. The definitive diagnosis of HFpEF, from a hemodynamic standpoint, rests on an elevated pulmonary capillary wedge pressure (PCWP) at rest ≥ 15 mm Hg or supine exercise PCWP ≥ 25 mm Hg at end-expiration. If performing upright exercise, a peak exercise PCWP ≥ 20 mm Hg or PCWP/cardiac output (CO) slope > 2 mm Hg/L/min is also considered diagnostic.9, 10, 11 Once the diagnosis of HFpEF has been established and HFpEF masqueraders have been excluded, it is helpful to further classify the patient into specific subtypes given the heterogeneity of the HFpEF syndromes. Although several HFpEF classification systems have been proposed, a classification system based on invasive exercise hemodynamics is attractive for transcatheter therapeutics given the interventional, procedural nature of the treatment, and because invasive hemodynamic-based phenotyping may be particularly relevant to interventional HF therapeutics. We have proposed, but not yet validated, a classification system for HFpEF subtypes based on invasive exercise hemodynamic testing (Figure 1) that includes 3 distinct HFpEF subtypes that may respond differentially to various medical and device therapies. Type I HFpEF is exercise-induced LA hypertension,12 defined by a resting PCWP < 15 mm Hg but with peak exercise PCWP ≥ 25 mm Hg. Type II HFpEF is resting LA hypertension with or without clinical signs of volume overload, defined by resting PCWP ≥ 15 mm Hg, without evidence of significant PVD, right ventricular (RV) dysfunction/failure, or tricuspid regurgitation (TR). Type III HFpEF is characterized by a resting PCWP ≥ 15 mm Hg with features of PVD or RV dysfunction/failure, or greater than moderate TR.7
Figure 1.
Proposed heart failure with preserved ejection fraction (HFpEF) subtypes defined by invasive hemodynamic testing. CO, cardiac output; CpcPH, combined postcapillary and precapillary pulmonary hypertension; EILAH, exercise-induced left atrial hypertension; Ex, exercise; FAC, fractional area change; FWS, free wall strain; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RELAH, resting left atrial hypertension; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Until recently, evidence-based treatments for HFpEF were lacking due to a long track record of neutral phase 3 clinical trials. Both the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) and PARAGON-HF (Prospective Comparison of Angiotensin Receptor–Neprilysin Inhibitor With Angiotensin-Receptor Blocker Global Outcomes in Heart Failure With Preserved Ejection Fraction) suggested a benefit in HFpEF patients with EF < 55% to 60%, and both drugs are now class 2b guideline recommendations for HFpEF.13 Recently, practice-changing clinical trials (EMPEROR [Empagliflozin Outcome Trial in Patients With Chronic Heart Failure]-Preserved3 and DELIVER [Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure]4), which compared SGLT2 inhibitors (empagliflozin and dapagliflozin, respectively) with placebo, resulted in approximately 20% reduction in the primary end point of HF hospitalization and cardiovascular (CV) death, which led to a class 2a guideline recommendation.2 Finally, results of the STEP-HFpEF (Effect of Semaglutide 2.4 mg Once Weekly on Function and Symptoms in Subjects with Obesity-related Heart Failure with Preserved Ejection Fraction) trial14 were recently published; in this trial, patients with HFpEF (and body mass index > 30 kg/m2) but without diabetes were randomized to semaglutide, a glucagon-like peptide receptor agonist vs placebo. After 52 weeks, semaglutide resulted in significant weight loss and improvement in health status (change from baseline –11% and +7.8 point [both placebo-corrected values] for weight loss and Kansas City Cardiomyopathy Questionnaire [KCCQ] overall summary score, respectively).15
Despite these significant advancements in the treatment of HF across the range of EF, morbidity and mortality remain high. Even when on optimal GDMT, HF patients may be at risk of suffering from adverse effects of complex, nuanced pharmacotherapy some of which include renal dysfunction, electrolyte imbalance, polypharmacy, and dehydration leading to possible hypotension. At its core, GDMT is an outpatient pursuit, which ultimately places the onus on the patient to monitor their vital signs, daily weights, and adhere to frequent blood testing in the ambulatory setting. Alternatively, interventional device and procedure-based therapy provides an opportunity to reduce pill burden and management complexity, while potentially improving QOL and reducing hospitalizations.16 Initial devices investigated in HFpEF were wireless implantable PA pressure monitoring systems such as CardioMEMS (Abbott), which, in the CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients) trial was associated with approximately 50% reduction in HF hospitalization across the EF spectrum, including in HFpEF.17,18 However, implantable PA monitoring requires constant vigilance from the patient (to take daily readings) and clinicians (to act on the data).
Given the high morbidity and mortality in HF across the EF spectrum despite GDMT, the limited medical treatment options for HFpEF, and issues with polypharmacy and poor medication adherence, transcatheter HF therapeutics have gained considerable attention as potential solutions to improve the lives of patients with HF. One such transcatheter-based treatment is atrial shunting, which has been intensively studied over the past 10+ years for HF across the EF spectrum, particularly for HFpEF.
Atrial shunt therapies for HF
Historical perspective
In 1916, the French physician René Lutembacher published the first comprehensive description of a patient with coexisting atrial septal defect (ASD) and mitral stenosis, in which he details a 61-year-old patient who was found to have both a primum ASD and mitral stenosis, both thought to be congenital in etiology. Lutembacher hypothesized that the ASD resulted in amelioration of LA overload by decompressing the high-pressure LA by shunting blood to the lower-pressure RA and systemic veins, but his case description showed that prolonged shunting through a large ASD causes severe right heart enlargement and failure. The rare combination of a congenital ASD (typically secundum) and mitral stenosis (either congenital or acquired) has since been called Lutembacher syndrome, although it was initially described in 1750 in a letter by Johann Friedrich Meckel,19 an anatomist, and subsequently described periodically in the 19th century. Lutembacher and others hypothesized that in cases of congenital mitral stenosis, incomplete closure of the interatrial septum was in fact caused by the increased LA pressure that resulted in obstruction of transmitral blood flow. Alternatively, in patients with acquired mitral stenosis, a patent foramen ovale may become stretched due to increased LA pressure and LA enlargement, thereby resembling a secundum ASD.
Whether an ASD in the presence of elevated LA pressure due to mitral stenosis (or HF in general) is beneficial or detrimental is thought to be dependent on the size of the ASD, the health of the RV, and the compliance of the RA and systemic veins. The benefits of ASD in the setting of elevated LA pressure occur due to decompression of the LA, thereby reducing symptoms of pulmonary congestion. Reducing chronic LA pressure elevation also reduces the load on the pulmonary vasculature, thereby preventing pulmonary venous and arterial remodeling which can delay or reduce the severity of pulmonary hypertension. However, if the ASD is too large, RA and RV enlargement can occur, ultimately leading to tricuspid annular dilation, significant TR, and right-sided HF, which has been described in patients with Lutembacher syndrome.20 Furthermore, worsening of RV failure can lead to reversal of the pressure gradient between the RA and LA, resulting in right-to-left shunting, hypoxemia, and hypoxic pulmonary vasoconstriction, which leads to worsening pulmonary hypertension. Reversal of the shunt can also occur intermittently or acutely, such as in the setting of obstructive sleep apnea, acute pulmonary embolism, or if there is a paradoxical increase in pulmonary vascular resistance (PVR) during exercise (instead of the normal vasodilatory response). In these settings, paradoxical emboli are of primary concern. Finally, it is possible that decompression of the LA and loading of the RA in the setting of an ASD (particularly if large) could predispose some patients to atrial arrhythmias.
Different in its core pathophysiology but similar in resultant hemodynamic consequence, HFpEF, regardless of its etiology or phenotype, is characterized by elevated LA pressure at rest and/or with exertion. Over the past 10 to 15 years, inspired by the hemodynamic advantages observed in some cases of Lutembacher syndrome, coupled with cases of ASD closure associated with unmasking HFpEF in older individuals with significant LV diastolic dysfunction,21 contemporary interest has grown in the controlled creation of iatrogenic atrial shunts as a therapeutic for HF. Key to the early development of atrial shunts was the recognition of the need for optimal sizing of atrial shunts, balancing the creation of a shunt large enough to allow for effective LA decompression into the RA, thereby leading to a clinically meaningful reduction in pulmonary venous pressures, while avoiding too large a shunt, with resultant adverse consequences leading to right heart overload, clinically significant TR, and right-sided HF.
Overview of atrial shunt therapy development
Several approaches to atrial shunting have been developed, the majority using the interatrial septum, and one using the coronary sinus (CS). The basic procedural technique for atrial shunt creation involves a percutaneous, endovascular catheter with or without device delivery through common femoral venous access. Transseptal puncture is then performed under transesophageal echocardiography (TEE) or intracardiac echocardiography guidance, after which there are 3 general approaches to shunting: (1) placement of an interatrial shunt device (IASD) across the interatrial septum; (2) creation of an interatrial shunt through removal of septal tissue or radiofrequency ablation of the septum; and (3) creation of a shunt from the LA to the CS.
Atrial shunt therapies are in varying stages of development and clinical trial progression (Table 1,22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 Central Illustration). Most have cleared bench testing, animal studies, and first-in-human ([FIH] pilot, early feasibility) studies. Larger open-label studies for several atrial shunt therapies have concluded, and within the last year, 1 large-scale randomized clinical trial (RCT) has been published, and several additional industry-sponsored pivotal RCT are underway. Device-less atrial shunt therapeutic procedures strive to create an interatrial shunt without leaving a permanent implant. Potential advantages of a device-less approach include the following: (1) lower infection, thromboembolic, and device embolization risks; (2) avoidance of potential difficulties in future transseptal access for left-sided cardiac percutaneous procedures (an advantage also shared by the LA to CS shunt); and (3) easier closure of the interatrial shunt should the need arise clinically. A potential disadvantage of device-less procedures that create an interatrial shunt is anatomic or functional reduction in shunt orifice size due to tissue ingrowth or partial collapse of the orifice during the cardiac cycle (particularly during atrial contraction), respectively.
Table 1.
Completed and ongoing clinical trials of atrial shunt devices and procedures.
| Atrial shunt therapy | Status | Trial/study name | Study design | EF range | Sample size | Follow-up durationa | Key findings/notes |
|---|---|---|---|---|---|---|---|
| Corvia IASD System II (LA→RA, implant) | Published in 201622 | REDUCE LAP-HF | Open-label study (exercise RHC) | ≥40% | 64 | 12 mo | Reduced exercise PCWP; improved NYHA, 6MWD, and QOL compared with baseline |
| Published in 201823,24 | REDUCE LAP-HF I | Randomized, sham-controlled trial (exercise RHC) | ≥40% | 44 | 1 mo | Reduced exercise PCWP compared with sham; numerically HF hosp., NYHA but no effect on KCCQ or 6MWD | |
| Published in 202225 | REDUCE LAP-HF II | Randomized, sham-controlled trial (exercise RHC) | ≥40% | 626 | 12-24 mo | Win ratio = 1.0 (neutral results) Potential responder group identified |
|
| Enrolling | RESPONDER-HF | Randomized, sham-controlled trial (exercise RHC) | ≥40% | 260 | 12-24 mo | Excludes patients with pacemaker/ICD or peak exercise PVR ≥ 1.75 WU | |
| V-Wave Ventura (LA→RA, implant) | Published in 201826 | — | Open-label study (resting RHC only) | All EF | 38 | 12 mo | Improved NYHA class, KCCQ, 6MWD; No change in PCWP, RAP, or PAP; 14% occluded, 36% stenotic by 12 mo |
| Completed enrollment in 2022 | RELIEVE-HF | Open-label roll-in → randomized, sham-controlled trial (resting RHC only) | All EF | 508 | 12 mo | Roll-in (open-label) results: improved KCCQ and echocardiographic parameters (follow-up to conclude by the end of 2023) |
|
| Occlutech Atrial Flow Regulator (LA→RA, implant) | Published in 201927 | PRELIEVE | Open-label study (exercise RHC) | All EF | 34 | 12 mo | Improved NYHA, KCCQ, 6MWD |
| Enrolling | FROST-HF | Randomized, sham-controlled trial (exercise RHC) | All EF | 588 | 12 mo | Active treatment group: 2 shunt sizes (6 and 8 mm) depending on resting PCWP | |
| Alleviant Medical (LA→RA, no implant) | Published in 202328 | ALLEVIATE-HF 1,2 | Open-label study (exercise RHC) | ≥40% | 28 | 6 mo | Decreased exercise PCWP and NTproBNP; increased 6MWD and KCCQ |
| Enrolling | ALLAY-HF | Randomized, sham-controlled trial (exercise RHC) | ≥40% | 400-700 (adaptive) | 12 mo | Excludes patients with pacemaker/ICD or peak exercise PVR ≥ 1.75 WU | |
| Edwards APTURE Shunt (LA→CS, implant) | Published in 202329 | ALT-FLOW | Open-label study (exercise RHC) | ≥40% | 87 | 6 mo | Improved exercise PCWP, NYHA, and KCCQ |
| Enrolling | ALT-FLOW II | Randomized, sham-controlled trial (exercise RHC) | ≥40% | 100 | 6 mo | Primary efficacy end point = PCWP at 20W exercise at 6 mo |
|
| InterShunt (LA→RA, no implant) | Presented in 202330 | EASE-HF | Open-label study (resting RHC only) | All EF | 10 | 6 mo | Favorable safety and shunt patency |
| NoYA (LA→RA, no implant) | Presented in 201931 | — | Open-label study (resting RHC only) | >35% | 10 | 1-3 mo | 6MWD increased, NTproBNP decreased |
| Published in 202232 | RAISE | Open-label study (resting RHC only) | >15% | 10 | 1-3 mo | 3/10 shunts closed | |
| Enrolling | RAISE II | Open-label study (resting RHC only) | ≥15% | 120 | 12 mo | Primary outcome = cardiac death + HF hospitalizations |
6MWD, 6-minute walk distance; EF, ejection fraction; HF, heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; LA, left atrium; NTproBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; QOL, quality of life; RA, right atrium; RAP, right atrial pressure; RHC, right heart catheterization.
Follow-up duration is for the primary end point.
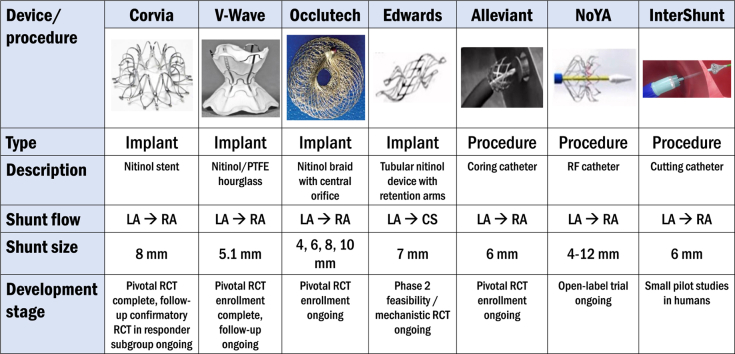
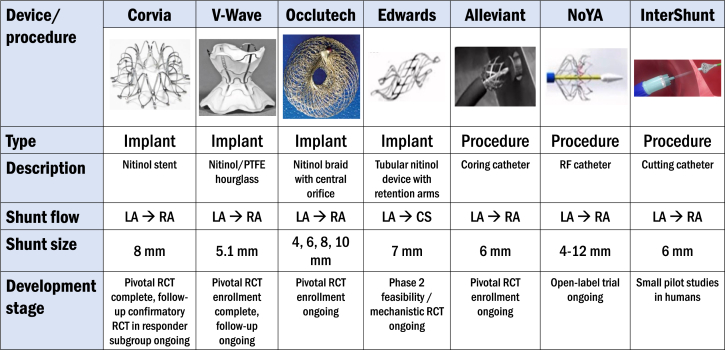
Central Illustration.
Interatrial shunt therapies currently under investigation in humans. CS, coronary sinus; LA, left atrium; PTFE, polytetrafluoroethylene; RA, right atrium; RCT, randomized clinical trial.
Corvia atrial shunt device
To date, the largest volume of evidence—including the only completed RCT—comes from studies using the IASD System II, a nitinol-based double-disc, 8-mm atrial shunt device (Corvia Medical) (Central Illustration). Placement involves a 16F catheter system introduced into the LA after transseptal puncture. Patients randomized to the atrial shunt who are not already on dual antiplatelet therapy or therapeutic anticoagulation are treated with dual antiplatelet therapy for 6 months postprocedure.33 After promising results from an initial single-center, 11-patient, open-label pilot study,34 the multicenter, open-label REDUCE LAP-HF (Reduce Left Atrial Pressure in Patients with Heart Failure) study of 64 patients with HF patients, EF > 40%, and exercise PCWP ≥ 25 mm Hg was conducted. It demonstrated 2 to 3 mm Hg reductions in exercise PCWP with an average shunt fraction (Qp:Qs) of 1.27 and no reduction in CO at the 3-month follow-up.22 After 12 months, there were no significant changes in LVEF LA or RA size. There was a mild increase in RV end-diastolic volume and a mild decrease in LV end-diastolic volume that plateaued at 6 months, and shunt patency was confirmed in all patients through 12 months.35
The follow-up REDUCE LAP-HF I trial—the first RCT conducted in the atrial shunt therapy field—was a mechanistic study aimed at determining the initial efficacy and safety of the Corvia atrial shunt device.33 Forty-four patients with HF, EF ≥ 40%, elevated exercise PCWP (≥25 mm Hg), and PCWP to RA pressure gradient ≥ 5 mm Hg were randomized 1:1 to the atrial shunt device or a sham-control procedure. The trial met its primary efficacy end point; at 1 month of follow-up, patients in the IASD group had a statistically significant (P = .028) greater reduction in exercise PCWP compared with the sham group, using data across all stages of exercise through a modified mixed model for repeated measures analysis.23 PCWP could be evaluated in most patients at rest, legs up, and at 20W of exercise because most patients were quite debilitated and could not exercise beyond 20W. Mean placebo-corrected differences in 1-month change in PCWP were 1.7, 5.0, and 4.1 mm Hg greater reduction in PCWP at rest, legs up, and at 20W of exercise, respectively. All shunts were patent at 1 month, with left-to-right shunting visualized in all patients, and right-to-left shunting visualized in 3 patients at rest. There were no between-group differences in safety outcomes at 1 month. After 12 months of follow-up, there were no between-group differences in major adverse CV, cerebral, and renal events. There were also fewer HF hospitalizations in the IASD group (0.22 per year) compared with the sham-control group (0.63 per year), and greater improvements in the New York Heart Association (NYHA) class in the IASD group (median: –1 class) compared with the sham group (median: no change), but these differences did not meet statistical significance; however, the sample size was small. Furthermore, there were no between-group differences in KCCQ or 6-minute walk distance (6MWD) at 12 months, though the trial was underpowered for these outcomes.24
Based on the promising mechanistic efficacy results of the REDUCE LAP-HF I RCT, a multicenter, sham-controlled, pivotal RCT (REDUCE LAP-HF II36) was conducted at 89 sites in the United States, Europe, Australia, and Japan.25 As in the REDUCE LAP-HF I RCT, patients with HF and EF > 40% underwent rigorous noninvasive screening, followed by invasive exercise hemodynamic testing, to confirm a peak exercise PCWP ≥ 25 mm Hg. Key exclusion criteria, which were based primarily on factors that were predicted to lower chances of a beneficial outcome, included severe HF (eg, cardiac index < 2.0 L/min/m2); evidence of right heart dysfunction (including RV enlargement/dysfunction, moderate or greater TR, and elevated RA pressure [>14 mm Hg] or PVR [>3.5 WU] at rest); and recent thromboembolic event (eg, deep vein thrombosis, pulmonary embolism, stroke, or transient ischemic attack). The primary end point was a hierarchical composite composed of CV death or nonfatal ischemic stroke at 12 months; followed by HF events up to 24 months; and finally change in KCCQ at 12 months. A total of 626 patients were randomized, making it the largest therapeutic interventional HF trial in HFpEF to date. From a safety standpoint, there appeared to be an early increase in cumulative HF events and a statistically significant increase in major adverse cardiac events in the shunt group compared with the sham group (4% vs 1%, respectively; P = .025). There were no between-group differences in the primary hierarchical composite end point or any of its components, with a win ratio of 1.0, signifying a “tie” between groups with no overall benefit or harm with IASD vs sham treatment.25
A balanced result in REDUCE LAP-HF II could have either meant no effect of the intervention at all, or it could have meant that there was a subgroup that responded well to the device while the rest of the patients worsened with the device. Indeed, PA systolic pressure at 20W exercise > 70 mm Hg emerged as the most significant prespecified subgroup characteristic that differentiated responders from nonresponders (interaction P = .01).25 Subsequent post hoc analyses identified peak exercise PVR < 1.74 WU and no cardiac rhythm management (CRM) device (ie, no pacemaker or implantable cardioverter-defibrillator) at baseline as characteristics that differentiated responders (those with the greatest beneficial treatment response in response to IASD) compared with nonresponders.37 The necessity of having a low PVR during exercise to allow for left-to-right interatrial shunting and unloading of the LA, and the possibility that the presence of a CRM device was an indicator of a sicker right heart (or a tricuspid valve vulnerable to worsening regurgitation due to lead-associated valve impingement) rendering the patient more susceptible to RV overload in response to left-to-right shunting, were hypothesized to be the reasons underlying the identified responder characteristics. However, given the post hoc nature of these analyses, a follow-up RCT (RESPONDER-HF [Reevaluation of Atrial Shunt Device in a Precision Medicine Trial to Determine Efficacy in Mildly Reduced or Preserved Ejection Fraction Heart Failure]) in patients with the responder phenotype (peak exercise PVR < 1.75 WU and no CRM device) is currently underway to validate these findings.
V-Wave Ventura interatrial shunt system
The V-Wave interatrial shunt system (V-Wave) is a different IASD platform with a unique, nitinol hour-glass shape skeleton covered with polytetrafluoroethylene (Central Illustration). The funnel shape in the central part of the device is designed to provide adequate shunt flow (based on a Venturi effect) in the setting of a smaller diameter of 5.1 mm, which theoretically could lower the risk of paradoxical emboli and excessive RV overload. The open-label V-wave FIH study, which utilized the original V-Wave device (which included a 1-way valve to prevent right-to-left shunting) included 38 total HF patients with varying EF (8/38 with HFpEF) and NYHA class III or IV symptoms.26,38 At 3- and 12-month follow-ups, there were improvements in overall health status quantified by NYHA class, QOL scores, and 6MWD. However, over one-third of patients had stenosis of the shunt at 12 months due to pannus formation raising concern for shunt durability due to its incorporation of a valve. The second iteration of the device without a valve is under investigation in the larger, randomized, sham-controlled pivotal Reducing Lung Congestion Symptoms in Advanced Heart Failure trial (RELIEVE-HF [Reducing Lung Congestion Symptoms in Advanced Heart Failure], NCT03499236) which completed enrollment in late 2022 with a total of 508 patients. The primary end point is a hierarchical composite death, cardiac transplantation, mechanical circulatory support, HF hospitalization, outpatient worsening HF events, and change in KCCQ with a follow-up of 12 to 24 months.
Unlike the REDUCE LAP-HF I and II trials, RELIEVE-HF included an initial roll-in, open-label cohort (n = 97, 52% of which had LVEF ≥ 40%). Results from the roll-in cohort were promising, with KCCQ scores improving from 46 to 58 at 1 month, remaining durable to 12 months in those who had available follow-up data at the time of reporting. Besides including HF patients across the EF spectrum and a roll-in cohort in their pivotal trial, additional key differences between the RELIEVE-HF trial and the REDUCE LAP-HF I and II trials were the inclusion of sicker patients with more advanced HF and only resting right heart catheterization without exercise (which is significant, because 29% of the REDUCE LAP-HF II trial patients did not have an elevated PCWP at rest and therefore would not have qualified for the RELIEVE-HF trial which required elevated PCWP at rest).
Occlutech Atrial Flow Regulator
The Atrial Flow Regulator (Occlutech) is a dual-disk nitinol IASD (Central Illustration) available in 3 orifices sizes of 6, 8, and 10 mm. The AFR-PRELIEVE trial was a prospective, nonrandomized FIH study that enrolled 53 HF patients, 29 of whom had HFpEF.27 Exercise invasive hemodynamic testing was required for trial inclusion. Two sizes of the device were utilized; patients with resting PCWP ≥ 15 mm Hg received an 8 mm sized IASD whereas patients with PCWP < 15 mm Hg but exercise PCWP ≥ 25 mm Hg received a 10 mm sized IASD. Immediate postprocedural outcomes included 1 device embolization that required surgical removal and 1 major procedure-related adverse event that involved syncope and bleeding. Device patency was 92% at 12 months.39 Outcomes showed improvement in NYHA class by 1, KCCQ improvement by 15 points, and 6MWD increase by 50 meters. Three-month follow-up demonstrated a significant reduction in PCWP by 5 mm Hg across both cohorts and in HFpEF patients, there was an increase in RV diameter but preserved RV systolic function.
Flow Regulation by Opening the SepTum in patients with Heart Failure (FROST-HF [Flow Regulation by Opening the Septum in Patients With Heart Failure], NCT05136820) is the pivotal, prospective, randomized, double-blind, sham-controlled trial currently underway with the goal of randomizing 700 HF patients across the EF spectrum to various sizes of the Occlutech IASD. Of note, the FROST-HF trial does not include exercise invasive hemodynamics, and it also does not exclude patients with elevated exercise PVR or CRM devices. The primary end point is a hierarchical composite of CV mortality, cardiac transplantation, LVAD, and recurrent HF events accrued until the last patient enrolled completes the 12-month follow-up, along with a change in KCCQ at 6 months.
Alleviant
The Alleviant system (Alleviant Medical) utilizes radiofrequency ablation technology to cauterize the interatrial septum and excise a precise disk of septal tissue 7 mm in diameter (Central Illustration). Early preclinical swine models demonstrate adequate margin healing, endothelialization, and lack of trauma to adjacent tissue on postmortem histological analysis.40 The multicenter, open-label, early feasibility pilot studies (ALLEVIATE-HF [Evaluation of the Safety and Feasibility of a Percutaneously Created Interatrial Shunt to Alleviate Heart Failure Symptoms in Patients With Chronic Heart Failure and Preserved or Mid-Range Left Ventricular Ejection Fraction]-1 and -2) included 31 HFpEF patients; results of follow-up through 3 months in all patients and through 6 months in 15 patients have been reported.28 In this trial, procedure success was 100%, safety events were minimal, and there was 100% 6-month shunt patency by TEE. Favorable early efficacy signals were seen with respect to exercise PCWP, HF hospitalizations, KCCQ, 6MWD, and NTproBNP.
ALLAY-HF (Safety and Efficacy of the Alleviant System for No-Implant Interatrial Shunt Creation in Patients With Chronic Heart Failure), a large, multicenter, sham-controlled pivotal RCT of the Alleviant atrial shunt system in patients with HF and EF ≥ 40%, is currently underway and seeks to enroll 400 to 700 patients with an adaptive design based on accrual of events. Of note, as in the aforementioned RESPONDER-HF trial, ALLAY-HF requires both an exercise PVR < 1.80 WU (absence of latent PVD) and no CRM device. The primary efficacy outcome is a hierarchical end point comprising CV death, HF events, and KCCQ.
Edwards Lifesciences APTURE shunt
An alternative, novel shunting approach is the APTURE shunt (Edwards Lifesciences), which uses a nitinol-based frame to create a shunt between the LA and CS with an internal shunt diameter of 7 mm (Central Illustration). Theoretical advantages of this approach include preservation of the interatrial septum for future interventions (eg, atrial fibrillation [AF] ablation, LA appendage occlusion, mitral valve procedures, etc.), reduced possibility of paradoxical embolization, and maintenance of normal RA flow dynamics. The APTURE device requires preprocedural cardiac computed tomography to verify eligible anatomy and identify landmarks for implantation. The implantation procedure is performed under general anesthesia with TEE guidance and uses a transjugular approach. A 20F guide sheath is inserted into the RA through which a guide wire is inserted into the distal CS. Using an anchoring balloon, an atriotomy is performed with a solid core needle from the CS into the LA, and a supportive 0.035" wire allows placement of the distal end into the LA. Balloon dilatation with a 6 to 8 mm angioplasty balloon is then performed to facilitate the delivery of the device.
The open-label ALT-FLOW (Early Feasibility Study of the Edwards Transcatheter Atrial Shunt System) early feasibility study enrolled 87 HF patients (93% with EF ≥ 40%) who underwent attempted device implantation.29 Exercise invasive hemodynamic testing was mandated to ensure that all patients had PCWP >15 mm Hg at rest or ≥25 mm Hg with exercise and a PCWP-RA gradient ≥5 mm Hg at rest or ≥10 mm Hg during exercise. Seventy-eight (90%) of the patients had successful device implantation. At 30 days, 2 patients underwent emergent cardiac surgery—1 for CS perforation and 1 for device retrieval due to embolization. At 6-month follow-up, 100% patency was demonstrated, and 68% of the treated patients improved to NYHA I to II class with a 23-point mean improvement in KCCQ scores. Invasive hemodynamic assessment at 6 months demonstrated a reduction of 20-watt exercise PCWP by 7 mm Hg compared with baseline. CS shunting did not show evidence of adverse effects on right heart function or RV-PA coupling.
While the APTURE device represents a novel and potentially advantageous approach to LA decompression, the significant adverse safety events in the early feasibility study require further examination in larger numbers of patients and enrolling sites though iterative improvements in device design and delivery continue. A follow-up phase 2 sham-controlled, multicenter RCT, ALT-FLOW II, which includes exercise invasive hemodynamics to verify elevated PCWP during exercise (≥25 mm Hg) and PCWP-RA pressure gradient ≥ 10 mm Hg, is randomizing 100 patients with HF and EF > 40% to the APTURE device vs a sham procedure. Patients with elevated exercise PVR and those with CRM devices are not excluded. ALT-FLOW II (A Randomized, Sham-controlled Trial of the Edwards APTURE Transcatheter Shunt System) will allow for greater experience with the device, evaluation of feasibility and safety of device deployment, and assessment of efficacy compared with a sham procedure. The primary safety outcome is 30-day freedom from major adverse cardiovascular, cerebrovascular, and renal events (MACCRE) or reintervention for study device-related complications, and the primary efficacy end points is the mean change in PCWP at 20W exercise from baseline to the 6-month follow-up visit.
NoYA
The NoYA interatrial shunt (NoYA MedTech) is an adjustable radiofrequency ablation-based atrial shunt procedure system that allows for the creation of an interatrial shunt with a waist diameter that ranges from 4 to 12 mm (Central Illustration).30 Early data from swine (n = 11) and an HFpEF FIH study (n = 10) showed no procedure-related safety events and shunt patency rate at 6 months was 70%.32 In the FIH study, median shunt diameter was 5.0 mm immediately postprocedure and in those with shunt patency, there was a mild attrition in shunt diameter to 4.0 mm at 6 months. Statistically significant improvements were seen in NTproBNP levels (mean reduction = 2149 pg/mL) and 6MWD (mean increase = 88 m), and NYHA class improved in 8 of the 10 patients. NoYA is currently conducting the RAISE II trial, which is an open-label study of 120 patients with EF ≥ 15%, resting PCWP ≥ 15 mm Hg, and PCWP-RA pressure gradient ≥ 5 mm Hg. The primary efficacy end point is a composite of CV mortality and HF hospitalization.
Intershunt
The InterShunt Percutaneous Atrial Shunt Catheter System (InterShunt Technologies, Inc) is another device-less atrial shunt procedure system that excises a 6-mm circular section of tissue in the atrial septum (Central Illustration). An early, open-label, pilot study of 10 patients demonstrated favorable safety at 30 days and durability of left-to-right shunting at 90 days.31
Adona Medical
Adona Medical has created a device, still early in development, which combines a novel, adjustable interatrial shunt design with biatrial pressure sensors. The shunt's design allows its size to be adjusted even after implantation in the cardiac catheterization laboratory by momentarily heating the shunt device, which resets its orifice size to its minimum, after which it can be redilated to the desired orifice size. The adaptability of the atrial shunt orifice size allows for fine-tuning shunt flow individualized to each patient’s unique hemodynamic requirements both during the initial procedure and later as needed clinically. The built-in hemodynamic sensors, positioned on both the LA and RA side of the interatrial septum allow, for the first time, automated ambulatory LA and RA pressure measurements several times a day, without the need for patient involvement. These frequent hemodynamic measurements could offer unprecedented insight into HF pathophysiology, enhance atrial shunt treatment, and facilitate improvements in the medical management of HF; however, human studies with the Adona device are yet to be reported.
Unanswered questions and ongoing controversies
Limitations of currently available evidence
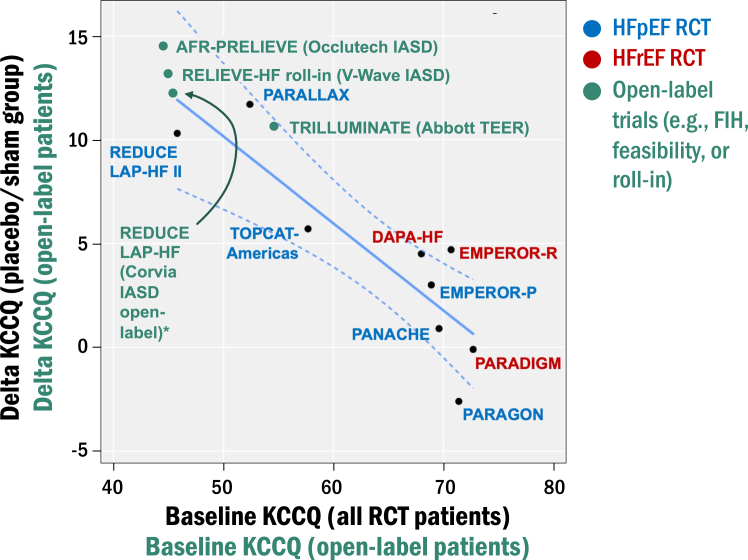
There are >30 prior and active studies involving at least 8 different companies in the atrial shunt therapeutic space. Thus far, only 2 RCT have been completed, both with the Corvia device.23,25 As a result, the current status of the field remains reliant on single-arm, open-label studies with relatively low sample sizes. This is not unexpected in the natural history of new device development. Nevertheless, it is important to understand the limitations of these early, uncontrolled studies. One main consideration is the placebo effect in open-label trials where patients and staff are unblinded. Publications and presentations of the REDUCE-LAP, AFR-PRELIEVE, RELIEVE-HF, and ALT-FLOW (all open-label, unblinded early feasibility, roll-in, or FIH studies) have demonstrated at least 10 to 15 point increases in KCCQ during follow-up, findings that are often promoted by investigators and sponsors. However, in REDUCE-LAP II, a randomized double-blind RCT, KCCQ scores increased by a mean value of 10 points in the sham-control group.25 As shown in Figure 2, in previously completed HF trials, baseline KCCQ scores correlate inversely with delta KCCQ scores in the placebo group, which suggests that baseline KCCQ score is a major determinant of the change in KCCQ scores over time in the placebo group (lower baseline scores = higher delta change). Baseline KCCQ scores tend to be lower for interventional HF trials compared with most pharmacological HF trials, and therefore, in open-label studies, the placebo/sham effect alone can explain the improvements in KCCQ seen in these trials (also shown in Figure 2). Open-label trials are also plagued by a lack of clarity on whether clinical improvement is driven by the atrial shunt procedure vs the effect of impactful, contemporary GDMT for HF across the EF spectrum. For these reasons, early on in the development of atrial shunt therapeutics, after demonstrating initial safety and feasibility, sponsors and investigators should pivot to sham-controlled RCT.
Figure 2.
Effect of placebo/sham on health status (Kansas City Cardiomyopathy Questionnaire [KCCQ] scores) in completed heart failure (HF) randomized clinical trial (RCT) compared with open-label interventional HF trials. For the pharmacological HF trials, baseline KCCQ scores correlated inversely with delta KCCQ scores in the placebo group (R2 = 0.76, P = .002), suggesting that the change in KCCQ scores over time is dependent on the starting (baseline) KCCQ score. This is particularly relevant to open-label interventional HF trials because baseline KCCQ scores tend to be lower than most pharmacological HF trials except those such as PARALLAX, which have an upper threshold for KCCQ values. In the RCT, patients in the placebo group are blinded, whereas patients in the open-label interventional HF trials are unblinded, which may enhance placebo effects, as suggested by the higher delta KCCQ in the unblinded (open-label) vs blinded (sham control) patients in the Corvia REDUCE LAP-HF vs REDUCE LAP-HF II trials, respectively. FIH, first-in-human.
What is the ideal HF phenotype to benefit from IASD therapy?
CRT is a very relevant example of device-based therapy that took years of iterative clinical trial design to clarify the optimal patient population to derive the most clinical benefit. These seminal trials had slightly different inclusion criteria with respect to QRS duration, NYHA class, presence of left bundle branch block (LBBB), and LVEF. In 2004, the MIRACLE (Multicenter InSync Implantable Cardioversion Defibrillation Randomized Clinical Evaluation) trial demonstrated significant improvement in event-free survival in patients with LVEF ≤ 35% and QRS duration ≥ 130 milliseconds.41 However, subgroup analyses from the subsequently published COMPANION (Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure) trial showed that patients with QRS duration > 168 milliseconds and LBBB derived the greatest benefit from CRT.42 Subsequent trials verified the COMPANION findings, leading to current class I guideline recommendation for CRT in patients with HF, EF ≤ 35%, LBBB, and QRS duration ≥ 150 milliseconds.2
Comparatively, evidence for atrial shunt therapeutics is in its infancy and challenged by heterogeneity in both HF phenotypes and in trial design (eg, the variable requirement for invasive exercise hemodynamic testing for enrollment). Intuitively, if HFrEF patients have more prevalent or are at increased risk for right heart dysfunction, atrial shunt therapy in these patients could lead to worse outcomes. In REDUCE LAP HF-II, lower LVEF and lower LV global longitudinal strain patients trended toward more frequent recurrent HF events compared with sham, although interaction P values for each parameter did not meet statistical significance, and only 7% of patients had HFmrEF in the trial.25 Three-month follow-up data in the open-label Occlutech AFR-PRELIEVE-HF study showed a statistically significant mean 5 mm Hg decrease in PCWP in HFpEF patients whereas the reduction in PCWP in HFrEF patients was lower (4 mm Hg) and did not meet statistical significance. In the V-Wave open-label RELIEVE-HF roll-in cohort, KCCQ analysis showed improvement in scores across EF, with a trend for greater improvement in HFpEF vs HFrEF at 12 months not meeting statistical significance. In future clinical trial design, if the study population is to include HF of all types, adequate numbers of subjects will be necessary to allow for meaningful prespecified analyses stratified by HF subtype (eg, EF < 40% vs EF ≥ 40%) for accurate delineation of potential differential treatment effects in each type of HF. Whether patients with CRM devices or elevated exercise PVR should receive atrial shunt therapies is also a question of optimal HF phenotype, as discussed below.
Is invasive exercise hemodynamic testing mandatory for trial inclusion?
Invasive exercise hemodynamic testing provides information on dynamic changes in PVR and PCWP-RA pressure gradient in response to exercise, and in HFpEF can assist with hemodynamic subtype classification as outlined above.43,44 Arguments against the requirement for invasive exercise hemodynamic testing mainly have to do with the lack of widespread use of exercise testing in cardiac catheterization laboratories. However, the REDUCE LAP-HF II trial showed that with training and experience, exercise right heart catheterization could be conducted successfully at numerous sites in several countries. Given the complexity of transcatheter valve therapeutics that are now routine in cardiac catheterization laboratories, the incorporation of exercise testing does not seem overly burdensome, especially considering the beneficial spillover effect of the ability to use exercise invasive hemodynamic testing for the evaluation of patients with dyspnea during exertion who may be misdiagnosed if only studied at rest. Indeed, as shown in REDUCE LAP-HF II, 29% of patients had PCWP < 15 mm Hg at rest and would have been excluded if only resting hemodynamic testing had been done.12 Nevertheless, only PA systolic pressure during 20W exercise and no other prespecified invasive hemodynamic parameter (including the presence or absence of elevated PCWP at rest or change in PCWP-RA pressure gradient during exercise) differentiated responders vs nonresponders in REDUCE LAP-HF II. However, there is considerable variability in PCWP readings during exercise; thus, the comparative ease of measuring PA pressure tracings during exercise could have contributed to these findings. Thus, the importance of PCWP-RA pressure gradient at rest and/or during exercise for the identification of patients most likely to benefit from atrial shunt therapy remains unknown. The results of pivotal RCTs of other atrial shunt therapies will hopefully shed further light on this issue.
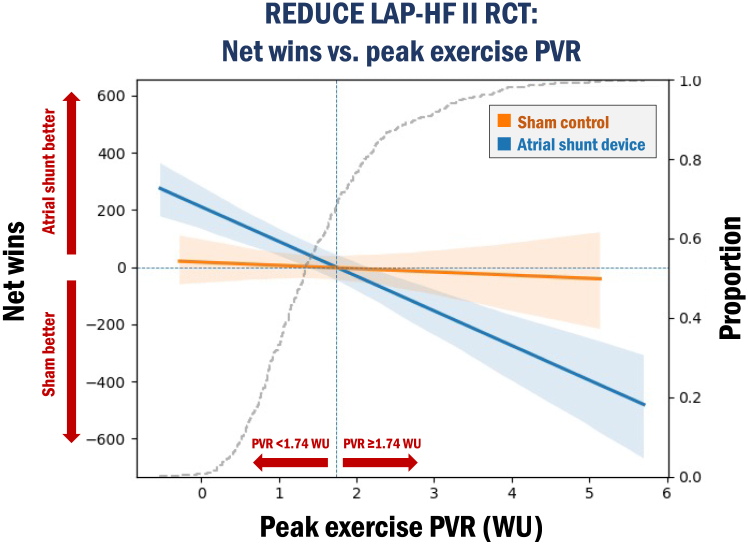
An important advantage of exercise testing during right heart catheterization is the ability to evaluate for the presence of latent PVD (ie, peak exercise PVR ≥ 1.74 WU). As shown in Figure 3, in the REDUCE LAP-HF II trial, patients without evidence of latent PVD had a favorable response to atrial shunt device compared with sham, whereas those with latent PVD fared worse with atrial shunting.37 The identification of this “responder subgroup” without latent PVD implies that the ability to either maintain a low PVR during exercise (without exercise-induced pulmonary vasoconstriction) or appropriately vasodilate the pulmonary vasculature during exercise (in patients with higher PVR at rest) may be necessary to derive benefit from atrial shunting.45 During exercise, if PVR increases to (or remains elevated above) a threshold of 1.75 to 1.80 WU, blood flow through the pulmonary vasculature is impeded, thereby leading to a greater increase in RA pressure relative to LA pressure. If RA pressure rises to a greater extent than LA pressure during exertion, the LA cannot decompress across the atrial shunt into the RA and systemic veins; even worse, the exaggerated rise in RA pressure relative to LA pressure could lead to intermittent reversal of shunt flow from the RA to LA, resulting in hypoxemia and further increases in PVR (due to hypoxic pulmonary vasoconstriction). Even in those who are still able to shunt from the LA to the RA, the increase in RV afterload due to elevated exercise PVR may inhibit the ability of the right ventricle to remain compensated from the increased volume load of blood shunted from the LA to the RA resulting in RV-PA “uncoupling” which has been associated with worse adverse outcomes across in HF across the EF spectrum.46,47 Based on our experience with REDUCE LAP-HF II and other interventional HF studies, the important additional pathophysiological insights gained, and the proven feasibility of invasive exercise testing, we strongly advocate for its use in ongoing and future trials of atrial shunt therapies.
Figure 3.
Net wins for the composite hierarchical efficacy end point in the atrial shunt device vs sham-control groups across values of peak exercise pulmonary vascular resistance (PVR) in the REDUCE LAP-HF II trial. In patients enrolled in the Corvia REDUCE LAP-HF II trial, there were divergent treatment effects in the atrial shunt group vs sham-control group depending on whether patients had a baseline peak exercise PVR < 1.74 Wood units (WU) (no latent pulmonary vascular disease [PVD]) vs PVR ≥ 1.74 WU (latent PVD). In the win ratio analysis of the composite efficacy end point (hierarchy cardiovascular death, nonfatal ischemic stroke, total (first and recurrent) heart failure (HF) events, and Kansas City Cardiomyopathy Questionnaire [KCCQ]), each patient in the atrial shunt group was compared with each patient in the sham group. There were more wins in the atrial shunt group in patients with no latent PVD (suggesting a beneficial effect with shunting) whereas there were more wins in the sham group in patients with latent PVD (suggesting a detrimental effect with shunting); P = .0006. Note: patients with pacemakers and defibrillators were excluded from this analysis. RCT, randomized clinical trial.
Are there absolute contraindications to atrial shunt therapy?
At the onset of the first atrial shunt therapy trials, it was recognized that patients with HF who had significant RV dysfunction, RA pressure elevation, significant (eg, greater than moderate) TR, or severe PVD would not benefit from atrial shunt therapies due to the following: (1) inability to unload the LA (due to a low LA-RA pressure gradient at rest and during exertion); (2) increased susceptibility to right-sided HF (due to a sicker RV); and (3) the potential for reversal of the shunt (RA to LA shunt flow), which would be deleterious. Thus, atrial shunt therapy trials have included exclusion criteria to avoid patients with these risk factors for a poor outcome in response to atrial shunting. However, the exact specifications for contraindications to shunt therapy are yet to be established. For example, as noted above, ongoing trials are mixed in terms of approach to latent PVD and CRM devices, with some trials excluding patients with either of these potential risk factors for poor response to atrial shunting, whereas others allow enrollment of such patients. Regardless of these differing approaches, if future atrial shunt clinical trials show a clinical benefit, clinicians using atrial shunt therapies will need to be very careful to avoid enrolling patients with risk factors for right-sided HF to avoid harming patients.
Recent studies have shed light on the HFpEF latent PVD phenotype using cardiopulmonary exercise testing (CPET) and exercise cardiac magnetic resonance imaging (MRI). CPET can assist in HFpEF subphenotyping based on its ability to accurately measure multiple organ systems relevant for adequate tissue oxygen delivery and therefore can pinpoint the cause(s) of exercise limitation in the individual patient (ie, personalized oxygen pathway analysis).48 Caravita et al49 retrospectively studied 86 HFpEF patients who underwent exercise invasive hemodynamic testing with simultaneous CPET. In this study, patients with HFpEF and latent PVD had impaired CO augmentation during exercise due to impaired increases in RV stroke volume, leading to worse event-free survival. Furthermore, the CPET data revealed increased levels of carbon dioxide (CO2) during exercise due to increased dead space fraction in patients with latent PVD. Ventilatory inefficiency leading to higher arterial CO2 may explain the elevation in PVR during exercise because CO2 is a pulmonary vasoconstrictor, which occurs as a homeostatic response to optimize ventilation/perfusion mismatch. Whether pulmonary vasodilators or drugs that treat pulmonary vascular remodeling (eg, sotatercept) can lower exercise PVR and could potentially improve response to atrial shunt therapies in patients with latent PVD requires further study. Some have argued that reducing the size of the shunt is necessary for patients with exercise-induced elevations in PVR to avoid excessive shunting and RV overload. However, as explained above, data from REDUCE LAP-HF II indicate that the LA-RA pressure gradient increases to a lesser extent in patients with high exercise PVR; thus, a smaller shunt may not effectively unload the LA.
Schuster et al50 investigated exercise cardiac MRI in HFpEF, examining long-axis strain and filling volumes of all cardiac chambers at rest and during exercise, to determine differences between patients with vs without latent PVD. Those with latent PVD were noted to have worse baseline RV function and with stress, RV stroke volume was reduced leading to impaired LA filling, and ultimately lower LV CO. Interestingly, RV dysfunction during peak stress quantified by RV peak flow area under the curve was predictive of latent PVD. Though extremely insightful, the lack of widespread availability of exercise cardiac MRI is a major limitation. Nevertheless, the study provided important pathophysiological insights that support the findings of REDUCE LAP-HF II.
One of the problems with the assessment of the presence of latent PVD and susceptibility to right-sided HF is that echocardiography and right heart catheterization are predominantly done at rest. Even in the setting of trials like REDUCE LAP-HF II, RESPONDER, ALLAY-HF, and ALT-FLOW II, which are systematically performing exercise invasive hemodynamics, it would be ideal to develop a noninvasive proxy. Exercise echocardiography, which is widely available and much more feasible than exercise MRI, can determine dynamic worsening of RV function (eg, lack of augmentation or worsening of RV free wall strain); exercise-induced RV overload or ventricular interdependence (eg, septal flattening or interatrial septal bowing from RA to LA); worsening TR; elevation in PA systolic pressure (or noninvasive PVR, which can be roughly estimated using the ratio of peak TR velocity/RV outflow tract velocity time integral); and lack of CO augmentation during exercise, and could therefore be extremely helpful in evaluating HF patients prior to atrial shunt therapy. In patients with symptomatic HF, the aforementioned findings, if present, are typically apparent at low workloads (eg, 20-25W), thereby increasing the feasibility of obtaining these parameters in the clinical setting. However, further research is required to determine the feasibility and accuracy of stress echocardiography in this setting and is being explored as a substudy in RESPONDER-HF to evaluate noninvasive prediction of exercise PVR.
In REDUCE LAP-HF II, patients in the highest tertile of RA volume index also had a worse response to the atrial shunt compared with those in the lower 2 tertiles.25 Advanced RA myopathy results in stiffening of the RA, which reduces its ability to effectively accept blood that is shunted from the LA to the RA, which would be expected to result in reduced benefit (or even clinical worsening) in response to atrial shunt therapy.
Lastly, the creation of an atrial shunt creates a potential passage for venous thrombi to reach the systemic (arterial) circulation if RA pressure exceeds the LA pressure event intermittently. Thus, patients with a history of a hypercoagulable state, recent venous thromboembolism, or risk factors for recurrent venous thromboembolism should not receive atrial shunt therapy. Reassuringly, the risk of paradoxical embolism appears to be low at least in the first few years after atrial shunt therapy; in the REDUCE LAP-HF II trial, there were no paradoxical embolism events in the atrial shunt device-treated patients during the first 12 months of follow-up.25
What is the optimal size of atrial shunts?
Given the need to balance adequate LA decompression while avoiding excessive right heart overload and right-sided HF, one of the first considerations in atrial shunt therapeutic development was orifice size. Will a one-size-fits-all approach work, or will shunt size need to be individualized based on factors such as body/heart size, LA-RA pressure gradient, and/or the health of the right heart and pulmonary vasculature? Early on during the conception of atrial shunts as a possible therapeutic option for HF, a study utilizing a computer simulation to model the effect of an interatrial shunt on hemodynamics at rest and during exercise in HFpEF was conducted using data from real patients.51 By plotting varying shunt diameters against rest and exercise hemodynamic measurements, the optimal beneficial effect was found to be at 8 to 9 mm diameter orifice size, with less LA decompression at smaller sizes and a greater chance for RV overload at larger sizes. Accordingly, the first atrial shunt to be tested in humans (Corvia) was designed to be a fixed 8 mm orifice, which resulted in a Qp:Qs of approximately 1.3 to 1.4 in computer simulations. The study was limited by demonstrating acute changes in hemodynamics without being able to predict long-term consequences. Furthermore, aggregate data (eg, mean values) from a cohort of “real-world” HFpEF patients with exercise invasive hemodynamics were used as inputs into the simulation, which may have reduced the ability to determine the heterogeneity of patients encountered in clinical trials and clinical practice. Future studies on the optimal orifice size may benefit from more comprehensive simulation modeling, but ultimately, clinical experience with the atrial shunt devices that come in a variety of sizes will be most helpful in a better understanding of optimal shunt size.
How should atrial shunt therapies be monitored (for benefit and harm) after treatment delivery, and are there long-term adverse effects of iatrogenic creation of an atrial shunt?
There are salient physiologic changes after atrial shunt therapy that need to be monitored closely because they can lead to adverse clinical outcomes if left unchecked. Examples include RA and RV enlargement/dysfunction, PVD, worsening of TR, shunt closure, reversal of flow across the shunt (which may occur intermittently [eg, at night in patients with sleep apnea or during exertion in patients with right heart dysfunction], which would likely still be deleterious); paradoxical embolism; and new-onset or worsening atrial arrhythmias. However, the optimal frequency of follow-up testing is unclear. In addition, the development of mild RA or RV enlargement, or mild increase in TR, which appears to plateau after 6 months, may be a sign that the shunt is beneficial because these are signs that the LA is effectively unloading, particularly if RV function remains preserved. Still, differentiating mild, subclinical RV dysfunction from physiological increases in RV size may be difficult to distinguish in the individual patient, even if sensitive imaging techniques such as speckle-tracking strain analysis of the RV are performed on echocardiography. Regardless, if RV dilation is observed after shunt therapy, to what degree is acceptable vs pathologic that may portend a poor prognosis in terms of an increased risk of future RV failure?
What are the long-term effects of atrial shunting? In a rat HFpEF model, the introduction of an iatrogenic atrial shunt resulted in a decrease in LA volume and an increase in PA wall shear stress, elastin density, and endothelial nitric oxide synthase expression.52 In addition, a pooled analysis of REDUCE LAP-HF and REDUCE LAP-HF I (both of which excluded those with resting PVR > 4 WU or mild RV dysfunction at baseline) demonstrated 17% reduction in PVR, 12% reduction in PA elastance, and 24% increase in PA compliance.53 These beneficial pulmonary vascular changes were thought to be due to the following: (1) lowering of pulmonary venous pressures (ie, PCWP), with a resultant reduction in the magnitude of the PA reflected wave which would lead to less PA systolic pressure augmentation, lower PA systolic pressure and PA pulse pressure, and reduced PVR; (2) delivery of oxygenated blood to the pulmonary vasculature, which has vasodilatory properties; and (3) an increase in right-sided CO, leading to recruitment of pulmonary vasculature and lowering of PVR. The aforementioned study also found that patients receiving shunt therapy who had the greatest degree of beneficial pulmonary vascular response had the greatest improvements in exercise capacity.
These initial analyses of early-phase Corvia trials, which suggested that LA to RA shunting could have beneficial effects on the pulmonary vasculature, contrasted with the much larger REDUCE LAP-HF II trial, which, as discussed above, found that patients with latent PVD at baseline did not benefit from atrial shunt therapy. If true, these results suggest that in patients with elevated peak exercise PVR ≥ 1.74 WU, the pulmonary vasculature is either unable to respond favorably to left-to-right shunting or that given the relative lack of LA-RA pressure gradient, shunting does not occur appropriately. Thus, patients with less advanced HF could derive greater benefit in the long-term from atrial shunt therapies due to improved pulmonary vascular function whereas patients with more advanced HF (who typically have more PVD) do not.
The Corvia trials show relative increases in RA and RV size, but these changes remained stable after 6 months.35 A publication describing a patient who participated in the original open-label REDUCE LAP study reported 6-year follow-up data showing stable hemodynamics on right heart catheterization, a Qp:Qs that increased from 1.18 to 1.36 WU and a CO that increased from 6.8 to 10.5 L/minute. RV end-diastolic volume remained stable but RV end-systolic volume increased, suggesting the development of RV dysfunction.54 The patient’s daily doses of furosemide and spironolactone were quadrupled to maintain euvolemia and prevent symptom worsening. This case highlights the potential of developing high-output HF in some patients from shunting that can lead to progressive fluid retention. Whether the shunt itself led to a high output state or whether a high output state, coupled with the presence of an atrial shunt, was the cause of this patient’s decompensation is unknown. Regardless, cases such as this should be investigated further and reported in the literature so that such situations can be avoided or appropriately treated in the future.
Is there relevance in monitoring the Qp:Qs in follow-up after atrial shunt therapy delivery as a way of monitoring patients? By Doppler echocardiography, data from open-label V-Wave studies showed that Qp:Qs increased from 1.0 to 1.2 at 3 months and then decreased to 1.1 by 12 months. However, Qp:Qs is difficult to measure by Doppler echocardiography due to challenges in measuring the PA diameter, and is therefore often inaccurate with high measurement variability. Nevertheless, other studies cite similar increases in shunt fraction over trial follow-up. Beyond just an interval assessment of shunt patency and integrity, its prognostic importance generates further questions. Is there a ratio above which the shunting degree is thought to be too high and may increase the risk of right heart dysfunction? What is the minimal Qp:Qs threshold for a beneficial treatment effect? Is Qp:Qs during exercise more prognostically important than resting Qp:Qs? In the case of no-implant strategies, is the excision of additional septal tissue or the creation of another septal defect a feasible future option for patients with inadequate shunting? Are there medical therapies that can modify Qp:Qs by selectively vasodilating or vasoconstricting the pulmonary or systemic vasculature? Data from ongoing randomized pivotal trials of existing devices and future studies of adjustable shunts (eg, Adona Medical) that are in development may shed some light on these questions.
Finally, given the possibility that atrial shunting can have effects on atrial arrhythmogenicity, whether patients with atrial shunts should be monitored for atrial arrhythmias postprocedurally (and how frequently, and for how long) remain important questions. Fundamentally, HFpEF is typically characterized by some level of baseline LA myopathy, which can be either secondary (due to a stiff left ventricle ± abnormalities in long-axis LV systolic function, which reduces the ability of the LA to fill [ie, reducing LA reservoir function]) or primary (due to primary LA myopathy). Regardless of the type, LA myopathy is associated with a higher prevalence of AF. Therefore, unloading the LA with atrial shunt therapy could result in a lower AF burden. However, abrupt change in LA size or RA size (which can occur with the delivery of atrial shunt therapy) and overloading a vulnerable RA chronically could both result in the worsening of atrial arrhythmias. In REDUCE LAP-HF II, the majority of randomized patients were evaluated prior to and periodically after their index procedure, regardless of treatment assignment.55 Overall, there were no differences between groups in incidence or burden of AF which was reassuring, but such arrhythmia monitoring follow-up studies should be repeated in trials of other atrial shunts to verify these findings.
Future clinical trial design and regulatory/payer considerations
The nature and magnitude of atrial shunt treatment efficacy and safety that will be required for eventual future regulatory approval is unknown. Regulatory bodies typically expect symptom improvement, a reduction in HF hospitalizations, and/or improvement in life expectancy when evaluating a new therapy. For example, symptom improvement quantified by NYHA class and/or patient-reported outcomes (PRO) such as KCCQ may not accurately capture the full QOL spectrum. The focus on a more comprehensive assessment of patient health status that includes symptom burden, physical/mental/emotional/social status, and health-related QOL (discrepancy between actual and desired well-being) may reveal the true benefit of a given therapy,56 and PRO have been shown to improve the accuracy of NYHA assessment.57 HF patients are also known to willingly concede longevity for an improvement in functional health status, a concept known as time trade-off.58 Therefore, in 2019, the U.S. Food and Drug Administration came out with a statement stating that substantial persistent symptomatic or functional improvement would be an acceptable end point for drug/device approval.59 This is particularly advantageous in HFpEF due to the relative paucity of treatments compared with HFrEF and no treatment that lowers mortality.
An interesting, novel emerging concept is “patient-centric” clinical trial design which incorporates patient perspectives at all steps of trial design and execution, including family member/caregiver input in trial end points and inclusion of patients on clinical trial steering committees to help inform clinical trial decisions. Atrial shunt therapy clinical trials are at an early enough stage to allow for potential novel clinical trial design with end points that may more accurately reflect symptom/functional status/QOL improvement that could increase chances of regulatory approval. Most of the pivotal trials in the field have already taken advantage of a hierarchical composite end point and win ratio statistic as a way of increasing statistical power to achieve sample sizes that are 10-fold lower than pharmacological HF trials, thereby increasing efficiency and lowering costs.
While benefits in symptomatology/QOL may be sufficient to achieve regulatory approval, payers prioritize other aspects that may impede the widespread uptake of new therapies in routine clinical practice, particularly devices. For example, payers may still favor the competing, relatively lower-cost modern GDMT pharmacotherapies that boast improvement in morbidity and mortality. Beyond clinical efficacy, payers, according to the “reasonable and necessary” subjective ethos, value cost-effectiveness, generalizability, and accountability that is based on the weighing of the incremental health benefit of an intervention against its incremental long-term net cost.60 Economic analyses will be critically important if atrial shunt therapy succeeds in trials and is supported by an increasing evidence base, especially in value-based reimbursement models. For these reasons, treatment with atrial shunt therapy would ideally demonstrate both an improvement in health status and a reduction in HF hospitalization to win both regulatory approval and payer reimbursement.
Future needs and directions
There is a significant need for accurate, validated noninvasive methods to diagnose HFpEF and latent PVD. Resting transthoracic echocardiography provides valuable information on cardiac structure, function, and hemodynamics, but given the equilibration of LA and RA pressure through a shunt at rest, evaluation of the dynamic changes during exertion, both preatrial and postatrial shunt therapy delivery, is critical. As described in detail above, CPET may also provide valuable information both to ensure that the cause of exercise limitation can be addressed by LA unloading and also to evaluate for overt or latent PVD.
Although most atrial shunt devices involve creating an iatrogenic defect in the interatrial septum, novel devices such as the Edwards APTURE shunt may have advantages as described above, though much more investigation is necessary to determine the physiological effects and clinical benefits of LA to CS shunting given the increased complexity of its delivery procedure. Continued advances in engineering will also undoubtedly be important in future atrial shunt therapeutic development, as evidenced by the advent of an adjustable shunt that can measure both LA and RA pressure, such as the device created by Adona Medical. Different altogether is a novel right PA to superior vena cava shunt designed to reduce pulmonary blood flow thereby decreasing LA filling, leading to a reduction in LA pressure.61 This novel approach, which has been tested in an ongoing early feasibility study, could avoid RV pressure/volume overload and in theory obviates the need for identifying latent PVD, but the clinical and physiological effects of reducing pulmonary blood flow in patients with HF require further investigation.
Conclusions
Significant advances over the last 2 decades have improved morbidity and mortality in HF, more so in HFrEF compared with HFpEF. Despite this progress, continued suboptimal QOL, persistent symptom burden, adverse side effects, and polypharmacy remain challenges in HF. Atrial shunt therapeutics for HF is a new and exciting frontier that may serve an important role in addressing therapeutic gaps for certain HF patients. Open-label, single-arm studies have demonstrated promising safety and feasibility results with cautiously optimistic clinical end points given the potential for significant placebo effects and optimized GDMT in trial settings. Initial RCT data with a single device and shunt size suggested that a specific responder group with low peak exercise PVR and no CRM device may derive the most benefit, a finding that must be confirmed in follow-up trials. Additional contemporary pivotal RCT data will be essential to elucidate the efficacy and safety of the several atrial shunt sizes and designs currently in development. Importantly, these studies will assist in the identification of the ideal HF subtype that will benefit most from dynamic decompression of the LA with an atrial shunt. In the meantime, atrial shunt treatments will continue to evolve, and analysis of completed and ongoing trials should continue to provide important pathophysiological insights into the HF syndrome.
Acknowledgments
Declaration of competing interest
Vikrant Jagadeesan has no disclosures or conflicts of interest to report. William A. Gray is a principal investigator with the Edwards Life Sciences sponsored ALT-FLOW clinical trial. Sanjiv J. Shah is the international principal investigator for the Corvia REDUCE LAP-HF I, REDUCE LAP-HF II, and RESPONDER-HF trials; has received research grants from AstraZeneca, Corvia, and Pfizer; and has received consulting fees from Abbott, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer-Ingelheim, Boston Scientific, Bristol Myers Squibb, Corvia, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Imara, Impulse Dynamics, Intellia, Ionis, Lilly, Merck, Metabolic Flux, MyoKardia, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sardocor, Shifamed, Tenax, and Tenaya.
Funding sources
Sanjiv J. Shah is supported by research grants from the National Institutes of Health (U54 HL160273, R01 HL140731, and R01 HL149423).
Ethics statement and patient consent
All research reported here has adhered to relevant ethical guidelines according to primary publication sources.
References
- 1.Roger V.L. Epidemiology of heart failure: a contemporary perspective. Circ Res. 2021;128(10):1421–1434. doi: 10.1161/CIRCRESAHA.121.318172. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022;145(18):e895–e1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 3.Anker S.D., Butler J., Filippatos G., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 4.Solomon S.D., McMurray J.J.V., Claggett B., et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089–1098. doi: 10.1056/NEJMoa2206286. [DOI] [PubMed] [Google Scholar]
- 5.Minamisawa M., Claggett B., Suzuki K., et al. Association of hyper-polypharmacy with clinical outcomes in heart failure with preserved ejection fraction. Circ Heart Fail. 2021;14(11) doi: 10.1161/CIRCHEARTFAILURE.120.008293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J.R., Moser D.K. Medication adherence mediates the relationship between heart failure symptoms and cardiac event-free survival in patients with heart failure. J Cardiovasc Nurs. 2018;33(1):40–46. doi: 10.1097/JCN.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Litwin S.E., Borlaug B.A., Komtebedde J., Shah S.J. Update on atrial shunt therapy for treatment of heart failure. Struct Heart. 2022;6(6) doi: 10.1016/j.shj.2022.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borlaug B.A., Sharma K., Shah S.J., Heart H.J.E. Failure with preserved ejection fraction: JACC scientific statement. J Am Coll Cardiol. 2023;81(18):1810–1834. doi: 10.1016/j.jacc.2023.01.049. [DOI] [PubMed] [Google Scholar]
- 9.Reddy Y.N.V., Kaye D.M., Handoko M.L., et al. Diagnosis of heart failure with preserved ejection fraction among patients with unexplained dyspnea. JAMA Cardiol. 2022;7(9):891–899. doi: 10.1001/jamacardio.2022.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emani S., Burkhoff D., Lilly S.M. Interatrial shunt devices for the treatment of heart failure. Trends Cardiovasc Med. 2021;31(7):427–432. doi: 10.1016/j.tcm.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Reddy Y.N.V., Carter R.E., Obokata M., Redfield M.M., Borlaug B.A., Simple A. A Simple, Evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138(9):861–870. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Litwin S.E., Komtebedde J., Hu M., et al. Exercise-induced left atrial hypertension in heart failure with preserved ejection fraction. JACC Heart Fail. 2023;11(8 Pt 2):1103–1117. doi: 10.1016/j.jchf.2023.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitt B., Pfeffer M.A., Assmann S.F., et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 14.Kosiborod M.N., Abildstrøm S.Z., Borlaug B.A., et al. Design and baseline characteristics of STEP-HFpEF program evaluating semaglutide in patients with obesity HFpEF phenotype. JACC Heart Fail. 2023;11(8 Pt 1):1000–1010. doi: 10.1016/j.jchf.2023.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Kosiborod M.N., Abildstrøm S.Z., Borlaug B.A., et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023;389(12):1069–1084. doi: 10.1056/NEJMoa2306963. [DOI] [PubMed] [Google Scholar]
- 16.Shah S.J. Interventional heart failure: a new field. EuroIntervention. 2016;12(Suppl X):X85–X88. doi: 10.4244/EIJV12SXA16. [DOI] [PubMed] [Google Scholar]
- 17.Adamson P.B., Abraham W.T., Bourge R.C., et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7(6):935–944. doi: 10.1161/CIRCHEARTFAILURE.113.001229. [DOI] [PubMed] [Google Scholar]
- 18.Abraham W.T., Adamson P.B., Bourge R.C., et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377(9766):658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 19.Wiedemann H.R. Earliest description by Johann Friedrich Meckel Senior (1750) of what is known today as Lutembacher syndrome (1916) Am J Med Genet. 1994;53(1):59–64. doi: 10.1002/ajmg.1320530113. [DOI] [PubMed] [Google Scholar]
- 20.Scherlis L., Cowley R.A. The Lutembacher syndrome: a physiologic study and case report. Ann Intern Med. 1955;43(3):575–590. doi: 10.7326/0003-4819-43-3-575. [DOI] [PubMed] [Google Scholar]
- 21.Ewert P., Berger F., Nagdyman N., et al. Masked left ventricular restriction in elderly patients with atrial septal defects: a contraindication for closure? Catheter Cardiovasc Interv. 2001;52(2):177–180. doi: 10.1002/1522-726x(200102)52:2<177::aid-ccd1043>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 22.Hasenfuß G., Hayward C., Burkhoff D., et al. A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trial. Lancet. 2016;387(10025):1298–1304. doi: 10.1016/S0140-6736(16)00704-2. [DOI] [PubMed] [Google Scholar]
- 23.Feldman T., Mauri L., Kahwash R., et al. Transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP-HF I [reduce elevated left atrial pressure in patients with heart failure]): a phase 2, randomized, sham-controlled trial. Circulation. 2018;137(4):364–375. doi: 10.1161/CIRCULATIONAHA.117.032094. [DOI] [PubMed] [Google Scholar]
- 24.Shah S.J., Feldman T., Ricciardi M.J., et al. One-year safety and clinical outcomes of a transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction in the reduce elevated left atrial pressure in patients with heart failure (REDUCE LAP-HF I) trial: a randomized clinical trial. JAMA Cardiol. 2018;3(10):968–977. doi: 10.1001/jamacardio.2018.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah S.J., Borlaug B.A., Chung E.S., et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomised, multicentre, blinded, sham-controlled trial. Lancet. 2022;399(10330):1130–1140. doi: 10.1016/S0140-6736(22)00016-2. [DOI] [PubMed] [Google Scholar]
- 26.Rodés-Cabau J., Bernier M., Amat-Santos I.J., et al. Interatrial shunting for heart failure: early and late results from the first-in-human experience with the V-Wave System. JACC Cardiovasc Intv. 2018;11(22):2300–2310. doi: 10.1016/j.jcin.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Paitazoglou C., Özdemir R., Pfister R., et al. The AFR-PRELIEVE trial: a prospective, non-randomised, pilot study to assess the Atrial Flow Regulator (AFR) in heart failure patients with either preserved or reduced ejection fraction. EuroIntervention. 2019;15(5):403–410. doi: 10.4244/EIJ-D-19-00342. [DOI] [PubMed] [Google Scholar]
- 28.Udelson J.E., Barker C.M., Wilkins G., et al. No-implant interatrial shunt for HFpEF: 6-month outcomes from multicenter pilot feasibility studies. JACC Heart Fail. 2023;11(8 Pt 2):1121–1130. doi: 10.1016/j.jchf.2023.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Hibbert B., Zahr F., Simard T., et al. Left atrial to coronary sinus shunting for treatment of symptomatic heart failure. JACC Cardiovasc Intv. 2023;16(11):1369–1380. doi: 10.1016/j.jcin.2023.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Lotan C. Presented at TCT 2019; September 26, 2019. A novel, stentless RF-based shunt solution. The NoYA System. [Google Scholar]
- 31.Vardi G. Presented at TVT 2023; June 8, 2023. EASE-HF: Evaluation of an implant free interatrial shunt to improve heart failure: 6 month results. [Google Scholar]
- 32.Sun W., Zou H., Yong Y., et al. The RAISE trial: a novel device and first-in-man trial. Circ Heart Fail. 2022;15(4) doi: 10.1161/CIRCHEARTFAILURE.121.008362. [DOI] [PubMed] [Google Scholar]
- 33.Feldman T., Komtebedde J., Burkhoff D., et al. Transcatheter interatrial shunt device for the treatment of heart failure: rationale and design of the randomized trial to REDUCE elevated left atrial pressure in heart failure (REDUCE LAP-HF I) Circ Heart Fail. 2016;9(7) doi: 10.1161/CIRCHEARTFAILURE.116.003025. [DOI] [PubMed] [Google Scholar]
- 34.Søndergaard L., Reddy V., Kaye D., et al. Transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel interatrial implant to lower left atrial pressure. Eur J Heart Fail. 2014;16(7):796–801. doi: 10.1002/ejhf.111. [DOI] [PubMed] [Google Scholar]
- 35.Kaye D.M., Hasenfuß G., Neuzil P., et al. One-year outcomes after transcatheter insertion of an interatrial shunt device for the management of heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9(12) doi: 10.1161/CIRCHEARTFAILURE.116.003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berry N., Mauri L., Feldman T., et al. Transcatheter interatrial shunt device for the treatment of heart failure: rationale and design of the pivotal randomized trial to REDUCE Elevated Left Atrial Pressure in Patients with Heart Failure II (REDUCE LAP-HF II) Am Heart J. 2020;226:222–231. doi: 10.1016/j.ahj.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Borlaug B.A., Sharma K., Shah S.J., Heart H.J.E. Failure with preserved ejection fraction. J Am Coll Cardiol. 2023;81(18):1810–1834. doi: 10.1016/j.jacc.2023.01.049. [DOI] [PubMed] [Google Scholar]
- 38.Guimarães L., Bergeron S., Bernier M., et al. Interatrial shunt with the second-generation V-Wave system for patients with advanced chronic heart failure. EuroIntervention. 2020;15(16):1426–1428. doi: 10.4244/EIJ-D-19-00291. [DOI] [PubMed] [Google Scholar]
- 39.Paitazoglou C., Bergmann M.W., Özdemir R., et al. One-year results of the first-in-man study investigating the Atrial Flow Regulator for left atrial shunting in symptomatic heart failure patients: the PRELIEVE study. Eur J Heart Fail. 2021;23(5):800–810. doi: 10.1002/ejhf.2119. [DOI] [PubMed] [Google Scholar]
- 40.Barker C.M., Meduri C.U., Fail P.S., et al. Feasibility of a no-implant approach to interatrial shunts: preclinical and early clinical studies. Struct Heart J Heart Team. 2022;6(4) doi: 10.1016/j.shj.2022.100078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abraham W.T., Fisher W.G., Smith A.L., et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346(24):1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 42.Bristow M.R., Saxon L.A., Boehmer J., et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 43.Borlaug B.A., Blair J., Bergmann M.W., et al. Latent pulmonary vascular disease may alter the response to therapeutic atrial shunt device in heart failure. Circulation. 2022;145(21):1592–1604. doi: 10.1161/CIRCULATIONAHA.122.059486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah S.J., Borlaug B.A., Kitzman D.W., et al. Research priorities for heart failure with preserved ejection fraction: National Heart, Lung, and Blood Institute working group summary. Circulation. 2020;141(12):1001–1026. doi: 10.1161/CIRCULATIONAHA.119.041886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oakland H.T., Shah S.J. Comprehensive investigation of latent pulmonary vascular disease: an important exercise for a novel HFpEF phenotype. JACC Heart Fail. 2023;11(10):1439–1442. doi: 10.1016/j.jchf.2023.05.032. [DOI] [PubMed] [Google Scholar]
- 46.Guazzi M., Villani S., Generati G., et al. Right ventricular contractile reserve and pulmonary circulation uncoupling during exercise challenge in heart failure: pathophysiology and clinical phenotypes. J Am Coll Cardiol HF. 2016;4(8):625–635. doi: 10.1016/j.jchf.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Guazzi M., Dixon D., Labate V., et al. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. J Am Coll Cardiol Img. 2017;10(10 Pt B):1211–1221. doi: 10.1016/j.jcmg.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 48.Houstis N.E., Eisman A.S., Pappagianopoulos P.P., et al. Exercise intolerance in heart failure with preserved ejection fraction: diagnosing and ranking its causes using personalized O(2) pathway analysis. Circulation. 2018;137(2):148–161. doi: 10.1161/CIRCULATIONAHA.117.029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caravita S., Baratto C., Filippo A., et al. Shedding light on latent pulmonary vascular disease in heart failure with preserved ejection fraction. J Am Coll Cardiol HF. 2023;11(10):1427–1438. doi: 10.1016/j.jchf.2023.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Schuster A., Schulz A., Lange T., et al. Concomitant latent pulmonary vascular disease leads to impaired global cardiac performance in HFpEF. Eur J Heart Fail. 2023;25(3):322–331. doi: 10.1002/ejhf.2781. [DOI] [PubMed] [Google Scholar]
- 51.Kaye D., Shah S.J., Borlaug B.A., et al. Effects of an interatrial shunt on rest and exercise hemodynamics: results of a computer simulation in heart failure. J Card Fail. 2014;20(3):212–221. doi: 10.1016/j.cardfail.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Danial P., Dupont S., Escoubet B., Osborne-Pellegrin M., Jondeau G., Michel J.B. Pulmonary haemodynamic effects of interatrial shunt in heart failure with preserved ejection fraction: a preclinical study. EuroIntervention. 2020;16(5):434–440. doi: 10.4244/EIJ-D-18-01100. [DOI] [PubMed] [Google Scholar]
- 53.Obokata M., Reddy Y.N.V., Shah S.J., et al. Effects of interatrial shunt on pulmonary vascular function in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2019;74(21):2539–2550. doi: 10.1016/j.jacc.2019.08.1062. [DOI] [PubMed] [Google Scholar]
- 54.Dujka L., Neužil P., Reddy V., et al. Long-term follow-up of a patient undergoing interatrial shunt device implantation for treatment of heart failure with a preserved ejection fraction. J Int Med Res. 2022;50(5) doi: 10.1177/03000605221098178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel R.B., Reddy V.Y., Komtebedde J., et al. Atrial fibrillation burden and atrial shunt therapy in heart failure with preserved ejection fraction. J Am Coll Cardiol HF. 2023;11(10):1351–1362. doi: 10.1016/j.jchf.2023.05.024. [DOI] [PubMed] [Google Scholar]
- 56.Zannad F., Alikhaani J., Alikhaani S., et al. Patient-reported outcome measures and patient engagement in heart failure clinical trials: multi-stakeholder perspectives. Eur J Heart Fail. 2023;25(4):478–487. doi: 10.1002/ejhf.2828. [DOI] [PubMed] [Google Scholar]
- 57.Sandhu A.T., Zheng J., Kalwani N.M., et al. Impact of patient-reported outcome measurement in heart failure clinic on clinician health status assessment and patient experience: a substudy of the PRO-HF trial. Circ Heart Fail. 2023;16(2) doi: 10.1161/CIRCHEARTFAILURE.122.010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kraai I.H., Vermeulen K.M., Luttik M.L., Hoekstra T., Jaarsma T., Hillege H.L. Preferences of heart failure patients in daily clinical practice: quality of life or longevity? Eur J Heart Fail. 2013;15(10):1113–1121. doi: 10.1093/eurjhf/hft071. [DOI] [PubMed] [Google Scholar]
- 59.United States Food and Drug Administration (FDA) Patient-reported outcome measures: use in medical product development to support labeling claims. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims [DOI] [PMC free article] [PubMed]
- 60.Gheorghiade M., Shah A.N., Vaduganathan M., et al. Recognizing hospitalized heart failure as an entity and developing new therapies to improve outcomes: academics’, clinicians’, industry’s, regulators’, and payers’ perspectives. Heart Fail Clin. 2013;9(3):285–290. doi: 10.1016/j.hfc.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 61.Fudim M., Howard E., Taft R., Lewis G. A novel right-to-right cardiac shunt for left atrial pressure decompression in heart failure and secondary pulmonary hypertension. J Card Fail. 2023;29(4):595. doi: 10.1016/j.cardfail.2022.10.123. [DOI] [Google Scholar]