Coronary artery disease (CAD) is the leading cause of morbidity and mortality worldwide. For decades, the gold standard test used to diagnose CAD was invasive coronary angiography (ICA). However, an analysis of patients without prior known CAD within the American College of Cardiology National Cardiovascular Data Registry1 and the Ontario Provincial CorHealth Cardiac Registry (CorHealth QPMM Quarterly Reports, written communication, 2022), demonstrated that 50% to 60% of ICA had results showing nonsignificant CAD or normal coronaries. Although ICA offers a definitive diagnosis, the majority of patients do not receive any therapeutic benefit (ie, revascularization). The procedure carries a small but significant risk (ie, vascular complications and 1/1000 ICA results in stroke, heart attack, and/or death), and ICA is expensive (over double that of other noninvasive investigations). The low-diagnostic yield of ICA in elective patients without known CAD is likely due in large part to the suboptimal performance of conventional clinical risk scores and diagnostic algorithms in the prediction of obstructive CAD.
In the past decade, a noninvasive diagnostic test, cardiac computed tomographic angiography (CCTA), has been shown to be as effective as ICA in diagnosing CAD in the appropriate patient population, while being significantly less expensive and lower risk for patients.2 This has led the United Kingdom (UK)’s National Institute for Health and Care Excellence (NICE) and the American College of Cardiology/American Heart Association guidelines to strongly recommend a CCTA-first approach for patients with stable angina and suspected CAD.3,4 However, without a thoughtful approach, unlimited access to CCTA would result in suboptimal test results (ie, nondiagnostic scans due to calcium burden or heart rate variability), leading to frequent double procedures, which is wasteful, exposing patients to unnecessary radiation.
An optimal solution would involve screening that accurately identifies which patients are suitable candidates for CCTA (ie, likely to have either no or mild CAD and a high-quality scan) vs which patients should proceed directly to ICA to receive both a diagnosis and therapeutic benefit from revascularization. Such a screening tool could then be integrated into a centralized triage model to ensure that every patient receives the test that is best suited for them. This type of intervention could prevent adverse events (eg, stroke/heart attack/death) while saving health care systems millions of dollars each year. Novel research strategies that harness the powers of artificial intelligence (AI) and machine learning could help better inform our screening processes to predict patients who would benefit from ICA vs other noninvasive diagnostic modalities like CCTA. AI has been successfully implemented in other areas of cardiology, including a triage system for ST-elevation myocardial infarction patients to reduce door-to-balloon times.5
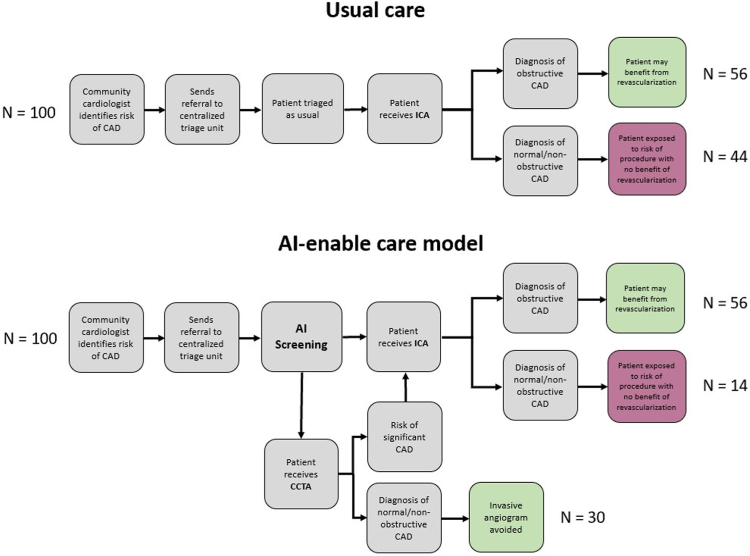
In the past 4 years, our team has extensively explored this area of research. As a first step, the CARDIA study demonstrated that a centralized triage process for CCTA and ICA, if sustained over 3 years at our single cardiac center, could prevent as many as 39 vascular complications and 1 myocardial infarction, stroke, or death, and result in cost savings upward of $1,665,000.6 A noted limitation of this process was the added administrative and physician support required for the triage process. To address this limitation, we developed an AI model to predict obstructive CAD in elective patients without known disease. Retrospective analysis of referral data was used from a provincial cardiac registry between 2008 and 2019, including all outpatients referred for ICA in our health region (>1.4 million individuals in Ontario, Canada). The training set (80% random sample, n = 23,750), including 42 variables, was used to develop 8 prediction models in Python using grid-search cross-validation. The test set (20% random sample, n = 5 5938), evaluated the discrimination performance of each model. Although traditional variables used in existing prediction models were ranked important (ie, sex and age), other nontraditional variables also proved to be important, including, the need for a translator, referring physician, and the month of referral for ICA. The machine learning model, trained using the LightGBM algorithm, significantly outperformed existing risk scores and current clinical practice (area under the receiver operating characteristic curve 0.81 vs 0.62; net reclassification improvement 27.84% [95%CI, 24.85%-30.83%]).7 Based on these results, if this AI-enabled model were to be implemented, 30 ICA per 100 patients referred could be avoided as compared with usual care (Figure 1). External validation using provincial data to confirm the generalizability of our model to an entire province is well underway. Furthermore, we have incorporated the AI model into a decision support software tool that not only predicts obstructive disease but also suggests the appropriate test (CCTA versus ICA) based on characteristics that may affect CCTA study quality (ie, atrial fibrillation). The AI tool has been deployed in our local cardiac center using a silent trial design, to establish feasibility and address future implementation issues. Plans to evaluate this centralized triage and AI-supported decision support for elective patients with suspected CAD in a randomized controlled trial are in the development stages. We hypothesize that this model will optimize patient selection for CCTA vs ICA, and reduce the rate of normal or nonobstructive CAD in patients undergoing ICA, which in turn may reduce risk to patients, improve equity, and reduce costs to the health care system.
Figure 1.
Proportion of patients referred for invasive coronary angiography (ICA) who would benefit from potential revascularization and ICA avoided in usual care as compared to artificial intelligence (AI)-enabled prediction model. CAD, coronary artery disease; CCTA, computed tomographic angiography.
Although the centralized triage model we evaluated is generalizable to many health care systems, the AI model as described is unique to the data set that informed its development. That being said, similar data sets to the one used to develop this AI model exist across North America and many places around the world. Therefore, this concept of centralized triage, coupled with an AI model to best select patients for CCTA vs ICA is a feasible means to improve patient safety and health care efficiencies.
Declaration of competing interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments
Funding sources
The AI model development and the CARDIA study were funded by the Hamilton Academic Health Sciences Organization (HAHSO) AFP Innovation Grant (HAH-18-002, 2018). Support for the AI model development was also made available from the Hamilton Health Sciences Foundation.
Ethics statement and patient consent
This research included a retrospective analysis of referral data. Patient consent was not required.
References
- 1.Patel M.R., Peterson E.D., Dai D., et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362(10):886–895. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haase R., Schlattmann P., Gueret P., et al. Diagnosis of obstructive coronary artery disease using computed tomography angiography in patients with stable chest pain depending on clinical probability and in clinically important subgroups: meta-analysis of individual patient data. BMJ. 2019;365:l1945. doi: 10.1136/bmj.l1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence Recent-onset chest pain of suspected cardiac origin: assessment and diagnosis. https://www.nice.org.uk/guidance/cg95 Updated November 30, 2016. [PubMed]
- 4.Gulati M., Levy P.D., Mukherjee D., et al. AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144(22):e368–e454. doi: 10.1161/CIR.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 5.Schwalm J.D., Bouck Z., Natarajan M.K., et al. Centralized triage of suspected coronary artery disease using coronary computed tomographic angiography to optimize the diagnostic yield of invasive angiography. CJC Open. 2023;5(2):148–157. doi: 10.1016/j.cjco.2022.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y.C., Chen K.W., Tsai B.Y., et al. Implementation of an all-day artificial intelligence-based triage system to accelerate door-to-balloon times. Mayo Clin Proc. 2022;97(12):2291–2303. doi: 10.1016/j.mayocp.2022.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Schwalm J.D., Di S., Sheth T., et al. A machine learning-based clinical decision support algorithm for reducing unnecessary coronary angiograms. CardioVasc Digit Health J. 2022;3(1):21–30. doi: 10.1016/j.cvdhj.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]