The safety and effectiveness of transseptal transcatheter mitral valve replacement (TMVR) is being evaluated in clinical trials targeting patients with mitral regurgitation at a high surgical risk or unfavorable anatomy for edge-to-edge repair. Severe mitral leaflet and annular calcification (MAC) may be associated with incomplete expansion of self-expanding prostheses or trauma, including catastrophic annular rupture, following balloon-expandable valve implantation.
We recently described the first use of Shockwave (Shockwave Medical) intravascular lithotripsy (IVL) to facilitate transseptal Intrepid (Medtronic) TMVR in a patient with severe MAC (valve calcium score, 7756 HU) who was enrolled in the Apollo clinical trial.
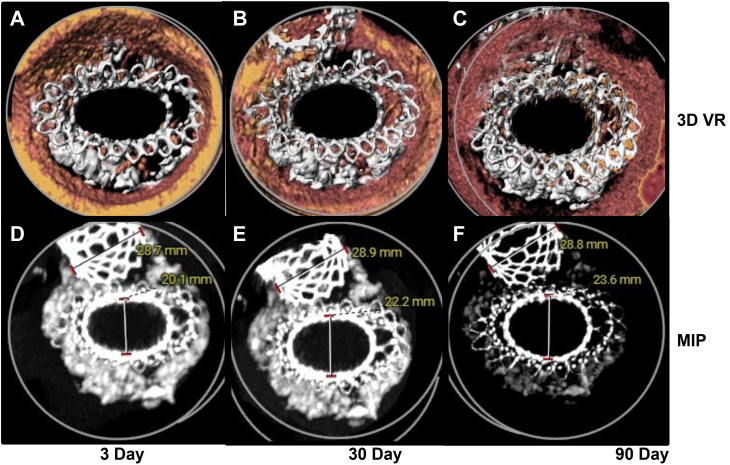
Since that report,1 computed tomography angiography at 90 days was performed, which provides longer-term characterization of valve expansion (Figure 1). These serial computed tomography angiography evaluations over time were consistent with progressive valve expansion in the anteroposterior dimension (>17% from hospital discharge to 90 days), with resulting circularization of the stent frame following IVL modification of severe MAC. This was the first attempt to characterize the intermediate-term safety and effectiveness of IVL-facilitated TMVR with a self-expanding nitinol prosthesis. Although further clinical experience is required, this observation suggests the potential for IVL to expand the role of TMVR in patients with severe MAC.
Figure 1.
Computed tomography angiography studies on days 3, 30, and 90 following Intrepid transcatheter mitral valve replacement demonstrating progressive circularization, with increased anteroposterior dimension of the valve. Maximum-intensity projections (MIP) and 3-dimensional color reconstructions (3D VR) are shown.
Acknowledgments
Declaration of competing interests
Puvi Seshiah, Santiago Garcia, Joseph Choo, J. Michael Smith, Geoffrey A. Answini, and Terri Stewart are subinvestigators for the APOLLO trial, sponsored by Medtronic. Dean J. Kereiakes is a consultant for Shockwave Medical, Inc.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement and patient consent
This research was performed in accordance with ethical guidelines and following The Christ Hospital institutional review board approval. The patient signed informed consent for participation in the APOLLO trial.
Peer review statement
Given his role as Deputy Editor, Dean J. Kereiakes, had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Alexandra J. Lansky.
Reference
- 1.Seshiah P., Garcia G., Choo J., et al. Lithotripsy assisted transcatheter mitral valve replacement for severe mitral annular and valve calcification. J Soc Cardiovasc Angiogr Interv. 2023;2(1):100540. [Google Scholar]