Abstract
Background and Aims
Barrett’s esophagus (BE) screening is not highly utilized in the United States, and there are few data describing providers’ approach to screening. To fill this gap and guide the implementation of future BE screening strategies, we studied evaluation practice patterns for gastroesophageal reflux disease (GERD) by nongastroenterologists.
Methods
We performed a retrospective cohort study of patients with chronic GERD using health claims data from the United States between 2005 and 2019. We used up to 5 years of data after the diagnosis of chronic GERD to determine patient factors associated with completion of a gastroenterology encounter. We also identified patient factors associated with whether the first gastroenterology encounter was a direct-access upper endoscopy or an office visit.
Results
We identified 484,023 patients diagnosed with chronic GERD by a nongastroenterology provider. The cumulative incidence of completing a gastroenterology encounter within 5 years was 38.7%. Gastrointestinal symptoms, such as dysphagia (adjusted hazard ratio [aHR] = 2.11, 95% confidence interval [CI] = 1.94–2.30), abdominal pain (aHR = 1.89, 95% CI = 1.85–1.94), and melena (aHR = 1.73, 95% CI = 1.65–1.82), were strongly associated with completion of a gastroenterology encounter. The patient factors strongly associated with direct-access upper endoscopy included dysphagia (aHR = 1.68, 95% CI = 1.52–1.85), weight loss (aHR = 1.46, 95% CI = 1.28–1.63), and melena (aHR = 1.42, 95% CI = 1.28–1.56).
Conclusion
A total of 38.7% of patients with chronic GERD complete a gastroenterology encounter within 5 years of diagnosis, and gastrointestinal alarm symptoms are the most strongly associated factors for receiving gastroenterology care. These findings highlight the importance of incorporating primary care providers in the development of new BE screening programs.
Keywords: Barrett’s Esophagus, Esophageal Adenocarcinoma, Upper Endoscopy, Health Care Utilization
Introduction
The incidence of esophageal adenocarcinoma (EAC) in the United States has increased 7-fold since 1975, and the 5-year survival rate of EAC is only 20%.1,2 To reduce the morbidity and mortality of EAC, several gastroenterology societies suggest screening and surveillance programs to identify and monitor Barrett’s esophagus (BE), the only identifiable premalignant condition for EAC.3, 4, 5, 6 Because gastroesophageal reflux disease (GERD) is considered the etiologic trigger of BE, contemporary guidelines focus on using upper endoscopy to screen patients with a combination of symptomatic GERD and other risk factors, such as age 50 years and older, male sex, family history of BE/EAC, obesity, and history of tobacco smoking.7
Although the level of adoption of BE screening programs in the United States has not been directly measured, it is estimated to be low based on surveys of primary care providers (PCPs).8,9 Consequently, fewer than 15% of patients who are diagnosed with EAC have undergone upper endoscopy by the time of diagnosis, and only 10% have an established diagnosis of BE.10,11 A potential explanation for the low utilization of BE screening is that PCPs, who are often the first to diagnose and manage GERD, are not aware of BE screening guidelines from gastroenterology societies. In a survey of PCPs of an academic health system, two-thirds of participants expressed difficulty in knowing which patients to screen for BE.12 This difficulty may be because there are no current GERD management guidelines from general medicine societies. This lack of guidance is in part due to the low incidence of EAC relative to other cancers in the United States and uncertainties about the cost effectiveness of upper endoscopy–based screening.13
There is a concerted international effort to develop novel paradigms to improve the effectiveness and adoption of BE screening. These include upper endoscopy risk stratification algorithms and nonendoscopic BE screening modalities, such as minimally invasive cell collection devices, unsedated transnasal endoscopy, and exhaled volatile organic acids.14, 15, 16 As 40% of patients with EAC present at a distant stage, which has a survival rate 9 times worse than that of localized EAC,1 these novel BE screening strategies have the potential to vastly improve the morbidity and mortality of EAC by inducing a stage shift. As PCPs are often the first practitioners to diagnose and manage GERD, understanding their current practice patterns may provide key insights about knowledge, attitudes, and barriers to BE screening that could guide the development and implementation of future BE screening programs. For example, understanding which patient factors influence PCPs to order a direct-access upper endoscopy may inform the types of GERD patient presentations for which they have established diagnostic strategies vs those they refer to gastrointestinal specialists for management.
In this study, we describe GERD evaluation practice patterns of nongastroenterologists in the United States. To do so, we used longitudinal, patient-level data to conduct a retrospective cohort study of patients diagnosed with chronic GERD by nongastroenterologists. Using this cohort, we identified patient factors associated with time to an outpatient medical encounter with a gastroenterology provider. Additionally, among patients with GERD who had an encounter with a gastroenterology provider, we identified patient factors associated with whether the initial gastroenterology encounter was a direct-access upper endoscopy or an office visit.
Methods
Data Source
We used Optum’s deidentified Clinformatics Data Mart Database (Optum) to perform this retrospective cohort study. Optum is a patient-level database consisting of the inpatient, outpatient, pharmacy, and procedure claims of 88 million unique enrollees of large commercial and Medicare Advantage health plans in the United States. Optum has been used to study epidemiology of acute and chronic conditions.17, 18, 19, 20 We used data from January 1, 2005 to December 31, 2019, to derive the study results. To allow for adequate time to assess covariates and exclusions, we limited the cohort to individuals with at least 365 days of continuous follow-up.21 For those with multiple discontinuous enrollments, only the first enrollment was considered to avoid misclassification of exposures, outcomes, and covariates that may have occurred during gaps in enrollment. We chose 2005 as the study start year because it was 1 year after Optum first captured complete inpatient data. We chose December 31, 2019, as the study end date because it was the final date of the last complete quarter prior to COVID-19–related shutdowns in the United States. We also used data from May 1, 2000 to December 31, 2004, to assess exclusion criteria.
Medical diagnoses were identified using International Classification of Diseases (ICD) codes in any encounter. ICD-9 was used prior to October 1, 2015, and ICD-10 was used thereafter. To convert ICD-9 codes to ICD-10, we used general equivalence mappings from the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Program supplemented with manual review. Procedure and tests were identified by ICD and Current Procedural Terminology codes. Medication use was identified by National Drug Codes, and it was classified into pharmacologic classes using American Hospital Formulary Service codes.
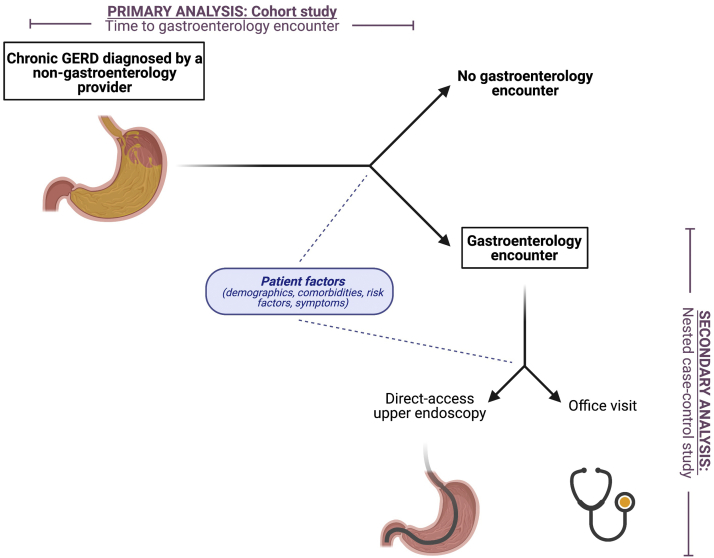
The Institutional Review Board of the University of Pennsylvania has classified research using Optum as exempt. The overall study design is illustrated in Figure 1. Figure A1 illustrates the inclusion and exclusion criteria, covariates, and outcome assessment periods discussed in the following sections.
Figure 1.
Study design. Patients with diagnoses of chronic GERD were identified using ICD codes from health insurance claims. Patients with chronic GERD who were diagnosed by a nongastroenterology provider were followed to determine patient factors associated with completion of a gastroenterology encounter (cohort study). Of the patients who completed a gastroenterology encounter within 5 years of the chronic GERD diagnosis, patient factors associated with direct-access upper endoscopy vs office visit were examined (nested case-control study).
GERD Cohort Inclusion and Exclusion Criteria
Because outpatient diagnoses of GERD may be nonspecific and include nonesophageal entities, such as chronic cough, atypical chest pain, and dyspepsia, we focused on patients likely to have chronic GERD. To do so, we classified patients as being diagnosed with GERD if they had an ICD diagnosis code of GERD in an outpatient encounter and had no encounters for GERD within the first 365 days of enrollment (codes in Table A1). Patients with an initial GERD diagnosis in an inpatient encounter or prior to the age of 18 years were excluded as management for these patients may not reflect ambulatory management of adult patients with GERD. Those with encounters for BE, EAC, dysphagia, or upper endoscopy; prescription claims for proton-pump inhibitors or H2-receptor antagonists; or an outpatient office visit with a gastroenterology provider prior to GERD diagnosis were excluded to avoid including patients with delayed documentation of GERD (codes in Tables A2 and A3).
Of the patients meeting these inclusion/exclusion criteria for GERD, those with a subsequent diagnosis of GERD at least 365 days after the first diagnosis were classified as having chronic GERD and included in the cohort. The date of this subsequent diagnosis was considered the chronic GERD diagnosis date. Patients who had an upper endoscopy or outpatient office visit with a gastroenterology provider between the first GERD diagnosis and the diagnosis of GERD that qualified them for the cohort were censored. Diagnosing provider specialty was determined from National Uniform Claim Committee Taxonomy codes, which are self-identified by providers when applying for a National Provider Identifier, and Optum provider category codes for the service provider of the outpatient visit (codes in Table A3).
Outcomes
The outcome of interest in the primary analysis was time to a direct-access upper endoscopy or outpatient office visit with a gastroenterology provider (collectively referred to as a “gastroenterology encounter” from here forward, codes in Tables A2 and A3). Non–upper endoscopy outpatient procedures such as colonoscopy and endoscopic retrograde cholangiopancreatography were not considered to be gastroenterology encounters for this analysis as they are unlikely to be related to the patient’s GERD diagnosis.
Potential Patient Factors Associated With GERD Management
To identify patient factors that influence providers’ decision-making regarding GERD management, we analyzed the covariates below for association with the outcomes of interest.
-
•
Patient demographics: age (categorized into decades, reference age 50–59 years based on guidelines identifying age ≥50 years as a BE risk factor),4 sex, year of GERD diagnosis, race/ethnicity, and U.S. census division.
-
•
Charlson-Deyo score for comorbid diagnoses.22
-
•
Upper gastrointestinal malignancy risk factors/signs: family history of gastrointestinal cancer (surrogate for family history of EAC), Helicobacter pylori infection, iron deficiency anemia, obesity, and tobacco smoking (codes in Table A4).
-
•
Gastrointestinal signs/symptoms: abdominal pain, diarrhea, dysphagia, nausea/vomiting, melena/rectal bleeding, and weight loss (codes in Table A5).
Upper gastrointestinal malignancy risk factors/signs and gastrointestinal signs/symptoms were unidirectional time-updating covariates. Patients were considered to have malignancy risk factors/signs from the time of the first claim for the condition. Patients were considered to have gastrointestinal signs/symptoms after the first recording of the code on or after the initial GERD diagnosis code date to include only signs and symptoms concurrent with GERD.
Because a patient’s level of prior health care utilization could influence provider decision-making about whether to refer to gastroenterology, we also analyzed the association of health care utilization intensity on GERD management. To quantify health care utilization intensity, we adapted methodology from high-dimensional propensity scores using all diagnoses, procedures, and prescriptions recorded in the database.23 Among patients in the GERD cohort, we identified the 200 most prevalent ICD-9 diagnoses, the 200 most prevalent ICD-10 diagnoses, the 50 most prevalent procedures, and the 200 most prevalent prescriptions. From these 650 codes, the 200 codes with the strongest associations with an encounter with a gastroenterology provider were selected. The final health care utilization intensity score was the total number of the final 200 codes that the patient was documented to have in the 365 days preceding the initial GERD diagnosis code.
Statistical Analyses
Stata, version 16, (College Station, TX, USA) was used for data extraction and all statistical analyses. Diagrams were drawn using BioRender and diagrams.net. The Forest plot was generated using the ggplot2 package of the R statistical computing environment (Vienna, Austria).
Primary Analysis (Cohort Study)—Time to Encounter With a Gastroenterology Provider
To determine patient factors associated with time to an encounter with a gastroenterology provider, patients in the chronic GERD cohort were followed from the time that they met criteria for chronic GERD to the earliest of upper endoscopy, outpatient gastroenterology office visit, exiting the database, or 5 years from chronic GERD diagnosis. We used a time-to-event outcome instead of a binary outcome of whether a patient ever completed a gastroenterology encounter to account for bias from loss to follow-up and to incorporate time-varying patient factors that reflect dynamic changes in patient presentation that may influence provider decision-making more strongly than baseline patient factors at the time of GERD diagnosis. The time to an encounter with a gastroenterology provider was illustrated using Kaplan-Meier failure curves. Cox proportional hazards regression was used to identify etiologic patient factors associated with time to a gastroenterology encounter. These associations are reported as adjusted hazard ratios (aHRs). To identify the regression model that optimally balanced control of confounding, parsimony, and goodness of fit, we used best subset regression.24 All candidate regression models included age, sex, race/ethnicity, census division, year of GERD diagnosis, Charlson-Deyo score, and health care utilization intensity. The regression subsets were composed of combinations of the eleven upper gastrointestinal malignancy risk factors/signs and gastrointestinal signs/symptoms described in the previous section. The subset with the lowest Bayesian information criteria was selected as the best-fitting regression model. We accounted for correlation of outcomes by provider practice patterns by correcting standard errors for group clustering by the individual provider who diagnosed GERD. From this Cox proportional hazards model, we calculated the aHR of completing a gastroenterology encounter for gastrointestinal signs/symptoms and combinations of BE risk factors identified by recent guidelines (age 50 years or older, non-Hispanic white race/ethnicity, male sex, family history, tobacco use, and obesity).3,4
Secondary Analysis (Nested Case-Control Study)—Direct-Access Upper Endoscopy or Outpatient Office Visit as the First Gastroenterology Encounter
To determine patient factors associated with whether an individual with chronic GERD who receives care from a gastroenterology provider undergoes a direct-access upper endoscopy vs an outpatient gastroenterology office visit, we developed a nested case-control study from the cohort of patients with chronic GERD. Patients whose first gastroenterology encounter was a direct-access upper endoscopy were defined as cases, and patients whose first gastroenterology encounter was an outpatient office visit were defined as controls. We used mixed-effects logistic regression to identify etiologic patient factors at the time of initial gastroenterology encounter associated with direct-access upper endoscopy. These associations are reported as adjusted odds ratios. We accounted for correlation of outcomes by provider practice patterns by incorporating the individual provider who diagnosed GERD as a random effect. All other covariates were fixed effects. To identify the best-fitting regression model, we used best subset regression as described in the statistical analysis section of the primary analysis. Among patients who completed an upper endoscopy, we calculated the proportion who were subsequently diagnosed with BE or EAC.
Sensitivity Analysis
Contrary to prior guidelines that suggested BE screening only for patients with chronic GERD,4,5 the most recent U.S. gastroenterology society BE guideline, published by the American Society for Gastrointestinal Endoscopy in 2019, suggests BE screening for patients with GERD of any duration and another risk factor.3 To assess whether the study results are generalizable to these BE screening criteria, we performed a sensitivity analysis that expanded the GERD cohort to include patients who had only less than 1 year of diagnosed GERD.
Results
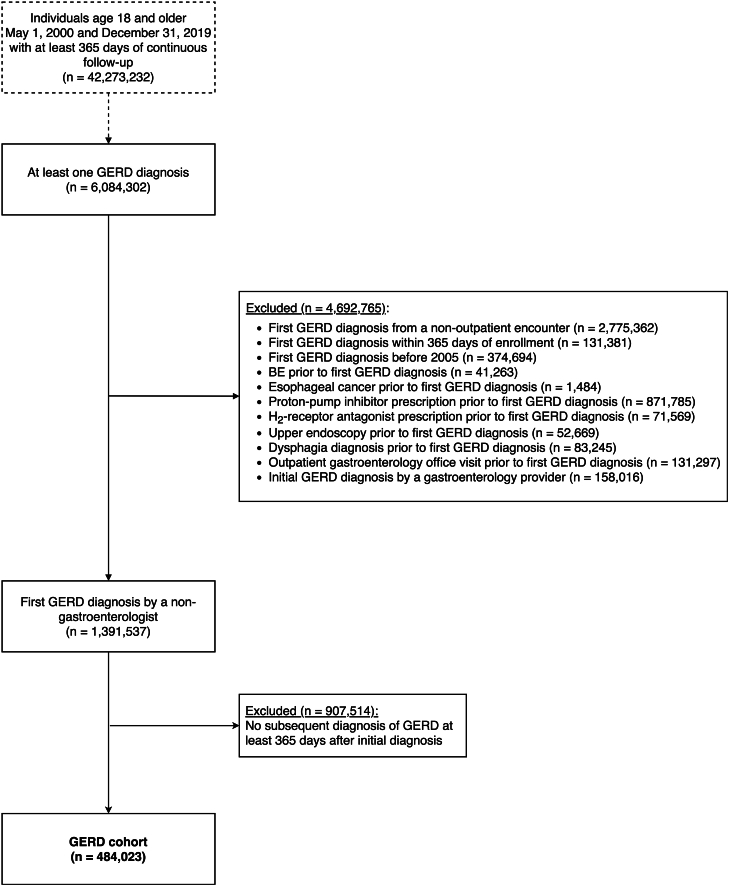
The final cohort consisted of 484,023 patients diagnosed with chronic GERD by a nongastroenterology provider (study flow diagram in Figure 2). Notable cohort characteristics include 70.4% aged 50 years or older, 41.8% male, and 70.1% white (Table 1).
Figure 2.
Chronic GERD cohort inclusion and exclusion criteria. Exclusions applied in the listed order.
Table 1.
Patient Characteristics at GERD Diagnosis
| Characteristic | Main analysis: 2 or more GERD diagnoses at least 365 d apart |
-Sensitivity analysis: 1 or more GERD diagnosis |
|---|---|---|
| n = 484,023 | n = 1,391,537 | |
| Demographics | ||
| Age (%) | ||
| 18–29 | 4.6 | 9.8 |
| 30–39 | 9.5 | 14.2 |
| 40–49 | 15.6 | 18.1 |
| 50–59 | 18.9 | 18.5 |
| 60–69 | 22.8 | 18.7 |
| 70–79 | 20.4 | 14.3 |
| 80+ | 8.3 | 6.3 |
| Female (%) | 58.2 | 56.3 |
| Race/ethnicity (%) | ||
| Asian | 3.1 | 3.8 |
| Black | 10.6 | 9.7 |
| Hispanic | 8.6 | 9.7 |
| Unknown | 7.6 | 11.1 |
| White | 70.1 | 65.7 |
| Total follow-up (mean y) | 7.4 | 5.6 |
| Enrollment to GERD diagnosis (mean y) | 4.4 | 2.5 |
| Charlson-Deyo score (mean) | 2.2 | 1.9 |
| High-dimensional utilization score (mean) | 1.8 | 1.7 |
| Upper gastrointestinal malignancy risk factors/signs (%) | ||
| Family history of gastrointestinal cancer | 1.1 | 1.1 |
| Helicobacter pylori infection | 0.1 | 0.1 |
| Iron deficiency anemia | 7.0 | 7.0 |
| Obesity | 7.8 | 9.1 |
| Tobacco use | 7.5 | 8.6 |
| Gastrointestinal signs/symptoms (%) | ||
| Abdominal pain | 4.9 | 6.3 |
| Diarrhea | 0.8 | 1.0 |
| Nausea/vomiting | 1.1 | 1.6 |
| Melena/rectal bleeding | 0.6 | 0.7 |
| Weight loss | 0.4 | 0.4 |
Patient Factors Associated With Time to an Encounter With a Gastroenterology Provider
Of the patients in the GERD cohort, 91,492 completed a gastroenterology encounter within 5 years of the chronic GERD diagnosis (cumulative incidence = 38.7%, incidence rate = 106.8 per 1000 person-years, 95% confidence interval [CI] 106.1–107.5, Figure A2). The best-fitting Cox proportional hazards regression model contained all eleven of the evaluated upper gastrointestinal malignancy risk factors/signs and gastrointestinal signs/symptoms (Table 2). Dysphagia (aHR = 2.11, 95% CI = 1.94–2.30), abdominal pain (aHR = 1.89, 95% CI = 1.85–1.94), and melena/rectal bleeding (aHR = 1.73, 95% CI = 1.65–1.82) were the patient factors most strongly associated with completion of a gastroenterology encounter. In general, the aHRs of completion of a gastroenterology encounter for gastrointestinal signs/symptoms were higher than those of individual BE risk factors. The aHRs of combinations of 2 or more BE risk factors (family history of gastrointestinal cancer, obesity, or tobacco use) were roughly equivalent to the aHRs of abdominal pain, melena/rectal bleeding, or dysphagia (Table 3).
Table 2.
Multivariable Cox Proportional Hazards Regression Model Describing Patient Factors Associated With Completion of a Gastroenterology Encounter After a Diagnosis of Chronic GERD (n = 484,023)
| Characteristic | aHR | 95% CI |
|---|---|---|
| Demographics | ||
| Age (ref: 50–59) | ||
| 18–29 | 0.74 | (0.70–0.78) |
| 30–39 | 0.74 | (0.72–0.77) |
| 40–49 | 0.84 | (0.83–0.85) |
| 60–69 | 0.92 | (0.91–0.93) |
| 70–79 | 0.81 | (0.81–0.82) |
| 80+ | 0.63 | (0.58–0.69) |
| Female | 1.00 | (0.99–1.01) |
| Race/ethnicity (ref: white) | ||
| Asian | 1.04 | (1.02–1.07) |
| Black | 0.90 | (0.89–0.91) |
| Hispanic | 0.91 | (0.89–0.93) |
| Unknown | 1.05 | (1.02–1.09) |
| Year of GERD diagnosis | 1.03 | (1.00–1.05) |
| Charlson-Deyo score | 0.99 | (0.99–1.00) |
| High-dimensional utilization score | 0.99 | (0.99–1.00) |
| Upper gastrointestinal malignancy risk factors/signs | ||
| Family history of gastrointestinal cancer | 1.30 | (1.22–1.38) |
| Helicobacter pylori infection | 1.36 | (1.24–1.49) |
| Iron deficiency anemia | 1.45 | (1.43–1.48) |
| Obesity | 1.31 | (1.29–1.34) |
| Tobacco use | 1.37 | (1.34–1.39) |
| Gastrointestinal signs/symptoms | ||
| Abdominal pain | 1.89 | (1.85–1.94) |
| Diarrhea | 1.31 | (1.29–1.34) |
| Dysphagia | 2.11 | (1.94–2.30) |
| Nausea/vomiting | 1.43 | (1.39–1.48) |
| Melena/rectal bleeding | 1.73 | (1.65–1.82) |
| Weight loss | 1.51 | (1.47–1.54) |
The model was also adjusted for U.S. census division (data not shown).
Table 3.
aHRs for Completion of a Gastroenterology Encounter for Selected Patient Factors After a Diagnosis of Chronic GERD (n = 484,023)
| Characteristic | aHR | 95% CI |
|---|---|---|
| Demographics | ||
| Age 50–59 y | 1.00 | (Ref.) |
| Male sex | 1.00 | (Ref.) |
| Non-Hispanic white race/ethnicity | 1.00 | (Ref.) |
| BE risk factors | ||
| Age 50–59 y, male, white, and … | ||
| Family history of gastrointestinal cancer | 1.30 | (1.22–1.38) |
| Obesity | 1.31 | (1.29–1.34) |
| Tobacco use | 1.37 | (1.34–1.39) |
| Family history of gastrointestinal cancer and obesity | 1.70 | (1.57–1.85) |
| Family history of gastrointestinal cancer and tobacco use | 1.78 | (1.63–1.92) |
| Obesity and tobacco use | 1.79 | (1.73–1.86) |
| Family history of gastrointestinal cancer, obesity, and tobacco use | 2.33 | (2.11–2.57) |
| Gastrointestinal signs/symptoms | ||
| Age 50–59 y, male, white, and … | ||
| Abdominal pain | 1.89 | (1.85–1.94) |
| Diarrhea | 1.31 | (1.29–1.34) |
| Dysphagia | 2.11 | (1.94–2.30) |
| Nausea/vomiting | 1.43 | (1.39–1.48) |
| Melena/rectal bleeding | 1.73 | (1.65–1.82) |
| Weight loss | 1.51 | (1.47–1.54) |
Direct-Access Upper Endoscopy or Outpatient Office Visit as the First Gastroenterology Encounter
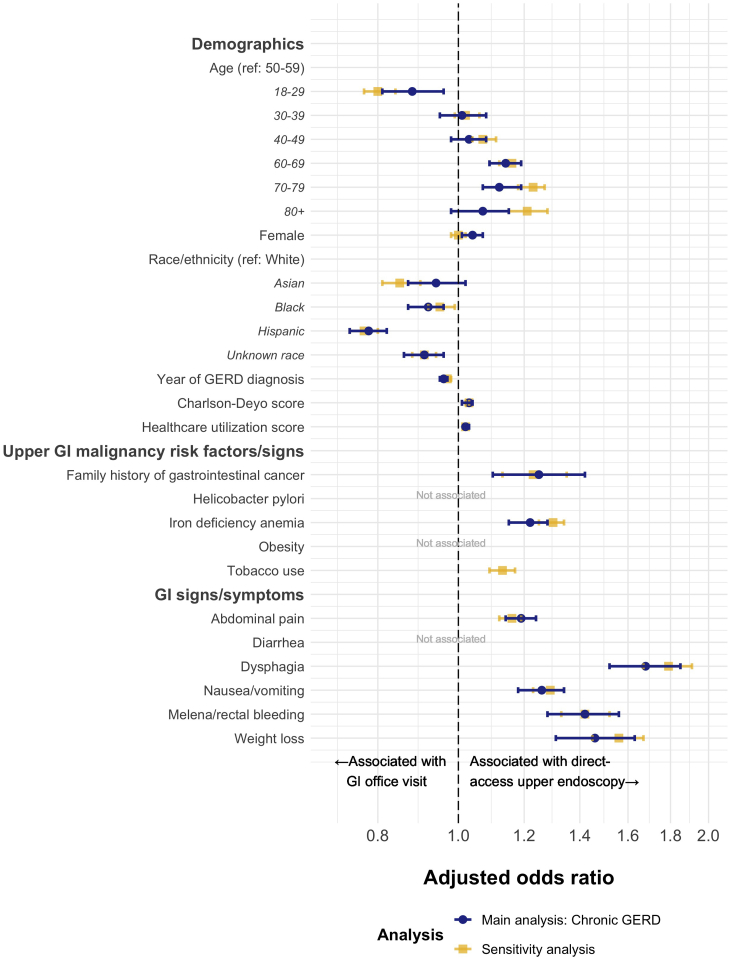
The best-fitting mixed-effects logistic regression model demonstrated that family history of gastrointestinal cancer, history of iron deficiency anemia, abdominal pain, dysphagia, nausea/vomiting, melena/rectal bleeding, and weight loss were associated with direct-access upper endoscopy (Figure 3). Dysphagia (aHR = 1.68, 95% CI = 1.52–1.85), weight loss (aHR = 1.46, 95% CI = 1.28–1.63), and melena/rectal bleeding (aHR = 1.42, 95% CI = 1.28–1.56) were the most strongly associated factors.
Figure 3.
The forest plot depicting mixed-effects logistic regression models describing patient factors associated with direct-access upper endoscopy vs outpatient office visit as the first gastroenterology encounter among patients with chronic GERD (main analysis, n = 91,492) or any GERD (sensitivity analysis, n = 210,962). Models selected using best subset regression (patient factors not associated in the best-fitting models are not depicted). Models also adjusted for the U.S. census division and GERD-diagnosing provider (data not shown). Upper gastrointestinal malignancy risk factors/signs and gastrointestinal signs/symptoms were unidirectional time-updating variables. GI, gastrointestinal.
Sensitivity Analysis: Expanded GERD Cohort
The results of the sensitivity analysis expanding the cohort to patients with less than 1 year of documented GERD were consistent with the main analyses. The expanded GERD cohort consisted of 1,391,537 patients whose GERD was initially documented by a nongastroenterology provider (Table 1). In the analysis assessing patient factors associated with time to an encounter with a gastroenterology provider, 210,962 completed a gastroenterology encounter within 5 years (cumulative incidence = 29.9%, incidence rate = 79.1 per 1000 person-years, 95% CI = 78.8–79.5, Figure A3). Like the main analysis, the best-fitting Cox proportional hazards regression model contained all eleven of the evaluated upper gastrointestinal malignancy risk factors/signs and gastrointestinal signs/symptoms (Table A6). In the analysis assessing patient factors associated with direct-access upper endoscopy vs office visit at the first gastroenterology encounter, direct-access upper endoscopy was the first encounter for 104,907 (49.7%) of the 210,962 patients. The best-fitting mixed-effects logistic regression model was consistent with the main analysis with the exception that a history of tobacco smoking was significantly associated in the sensitivity analysis but not in the main analysis (Figure 3). Of the 106,055 patients whose first gastroenterology encounter was an office visit, 38,174 (36.0%) later went on to have an upper endoscopy (median 19 days later, interquartile range = 8–36 days). Of the 143,081 patients who underwent either direct-access upper endoscopy or upper endoscopy after a gastroenterology office visit, 6603 (4.6%) were subsequently diagnosed with BE and 243 (0.2%) were subsequently diagnosed with EAC.
Discussion
In this retrospective cohort study of nearly than 500,000 patients with chronic GERD diagnosed by nongastroenterology providers, we demonstrate that approximately 40% of patients complete either an upper endoscopy or gastroenterology outpatient office visit within 5 years of diagnosis. Additional, gastrointestinal symptoms, such as abdominal pain, diarrhea, and nausea/vomiting, and gastrointestinal alarm symptoms, such as dysphagia, melena/rectal bleeding, and weight loss, are strongly associated with completion of a gastroenterology encounter. Established BE risk factors such as family history of gastrointestinal cancer, obesity, and history of tobacco smoking were also associated with completion of a gastrointestinal encounter, but less strongly than gastrointestinal symptoms. Together, these results complement conclusions from a recent study that surveyed PCPs from 4 tertiary care centers and 2 affiliated safety net health systems about their practice patterns and attitudes related to BE screening.9 PCPs in this study described that BE risk factors such as race, sex, and obesity did not strongly influence their decision to order upper endoscopy.
Together, these results highlight the importance of developing new BE screening paradigms that can be implemented in primary care settings. One potential approach is to offer screening only to patients at high risk for future BE or EAC. Although current gastroenterology society BE screening criteria have poor discrimination for distinguishing who will develop BE from those who will not,25 several prediction tools developed using regression techniques, such as M-BERET, the HUNT tool, and the Kunzmann tool, have demonstrated promise.14,26, 27, 28, 29 These or similar tools could be incorporated into electronic medical records to provide automated prompts to PCPs to highlight which patients may benefit from BE screening.30 Furthermore, if these risk stratification tools are coupled with emerging novel screening modalities that do not require sedation, it may be possible to offer cost-effective BE screening to more patients.16 For example, a recent randomized control trial demonstrated that a BE screening program based on the cell collection device cytosponge trefoil factor 3 identified 10 times more cases of BE compared to usual care in general practices in the United Kingdom.31
This study has several strengths that lend credibility to its conclusions. First, by using longitudinal, patient-level data from large health plans, we were able to identify over 500,000 patients with chronic GERD. This sample size provides high statistical power and degrees of freedom to perform analyses that carefully adjust for confounding by demographics, health care utilization, comorbidities, and provider factors. These analyses included time-updating covariates to study the influence of patient factors throughout follow-up, not just at cohort entry. Second, we used strict inclusion criteria such as a 365-day lead-in period and no prescriptions for proton-pump inhibitors or H2-receptor antagonists prior GERD diagnosis to avoid including patients with prevalent GERD in the cohort. This is important as inclusion of individuals who were diagnosed with GERD prior to registration in the database could introduce bias to the measures of association of patient factors with GERD management strategies. Third, the results of this study are consistent with conclusions of a prior study from the Veterans Health Administration that demonstrated upper gastrointestinal symptoms are strongly associated with upper endoscopy within 1 year of GERD diagnosis.32 This study generalizes those conclusions that were drawn among predominately elderly, male patients of a national health care system to patients of both sexes with commercial health insurance or Medicare Advantage. Additionally, this study follows patients for 5 years after chronic GERD diagnosis and distinguishes between direct-access upper endoscopy and gastroenterology office visits.
There are also potential limitations to consider when interpreting these study results. First, because the exposures were determined from diagnostic codes originally intended for medical billing instead of research, there could be misclassification of some of the covariates. In particular, ICD codes for obesity and tobacco smoking are known to have low sensitivity, so it is possible that some obese patients were misclassified as nonobese and some smokers were classified as nonsmokers.33,34 However, for this issue to have impacted the study conclusions, there would need to be an association between misclassification as nonobese or nontobacco smoking and likelihood of completing a gastroenterology encounter. Additionally, because there are no ICD codes for family history of BE or EAC, we use codes for family history of a gastrointestinal malignancy as a surrogate. Second, because the outcomes were ascertained from diagnostic codes, we could only determine whether patients completed a gastrointestinal encounter—not whether their managing provider referred them to one. This could introduce confounding to the study results if there is an association between the patient factors of interest and completion of health care recommendations. However, a prior systematic review and meta-analysis did not show conclusive associations between several of the patient factors studied here and nonadherence.35 Third, we were not able to assess the severity or response to treatment of GERD. These could be important factors in provider decision-making about whether to send patients for management by a gastroenterology provider. Fourth, the cohort was limited to patients with GERD who were insured by commercial health insurance or Medicare Advantage. While these results may not generalize to patients with public health insurance, 68% of 19- to 64-year-olds in the United States had commercial insurance in 2019.36
In conclusion, this study demonstrates that upper gastrointestinal alarm symptoms such as dysphagia, iron deficiency anemia, melena, and weight loss are strongly associated with completion of a subsequent gastroenterology encounter—particularly direct-access upper endoscopy. These results provide insights into provider decision-making about GERD management strategies for patients potentially at risk for BE and EAC and underscore the importance of providing PCPs with clear BE screening criteria such as risk thresholds based on clinical decision tools.
Footnotes
Authors' Contributions: Amit G. Singal, Ravy K. Vajravelu, and Sachin Wani contributed to conceptualization. Ravy K. Vajravelu and Sachin Wani contributed to funding acquisition. Amit G. Singal, Ravy K. Vajravelu, and Sachin Wani contributed to methodology. Ravy K. Vajravelu is a guarantor and contributed to data curation, formal analysis, visualization, and writing—original draft. All authors contributed to investigation and writing—review and editing. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Conflicts of Interest: These authors disclose the following: Gary W. Falk is a consultant for Cernostics, CDx, Interpace, and Lucid. David A. Katzka is an advisory board member of Celgene and Shire. Sachin Wani is a consultant for Boston Scientific, Cernostics, Interpace, and Medtronic. The remaining authors disclose no conflicts.
Funding: Gary W. Falk: NIH/NIDDK—P30-DK050306 and NIH/NCI—U54-CA163004. Amit G. Singal: NIH/NCI—R01-CA222900. Ravy K. Vajravelu: NIH/NIDDK—K08-DK119475, American College of Gastroenterology—ACG-CR-005-2020. Sachin Wani: NIH/NIDDK—U34-DK124174, American College of Gastroenterology—ACG-CR-005-2020, and University of Colorado Department of Medicine Outstanding Early Scholars Award. This research was funded by the American College of Gastroenterology (ACG-CR-005-2020 to R.K.V. and S.W.) and the National Institutes of Health in the United States (NIH/NIDDK—K08-DK119475 to R.K.V.). The funders of this study had no role in the study design, data collection, data analysis, data interpretation, or presentation of the results.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: All data are available to researchers who establish a data use agreement with Optum. Analytic files are available by contacting the corresponding author.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2022.03.001.
Supplementary Materials
References
- 1.Thrift A.P. Barrett's esophagus and esophageal adenocarcinoma: how common are they really? Dig Dis Sci. 2018;63:1988–1996. doi: 10.1007/s10620-018-5068-6. [DOI] [PubMed] [Google Scholar]
- 2.Kolb J.M., Han S., Scott F.I., et al. Early-onset esophageal adenocarcinoma presents with advanced-stage disease but has improved survival compared with older individuals. Gastroenterology. 2020;159:2238–2240.e4. doi: 10.1053/j.gastro.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qumseya B., Sultan S., Bain P., et al. ASGE guideline on screening and surveillance of Barrett's esophagus. Gastrointest Endosc. 2019;90:335–359.e2. doi: 10.1016/j.gie.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Shaheen N.J., Falk G.W., Iyer P.G., et al. ACG clinical guideline: diagnosis and management of Barrett's esophagus. Am J Gastroenterol. 2016;111:30–50. doi: 10.1038/ajg.2015.322. quiz 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spechler S.J., Sharma P., Souza R.F., et al. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology. 2011;140:1084–1091. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 6.Rubenstein J.H., Shaheen N.J. Epidemiology, diagnosis, and management of esophageal adenocarcinoma. Gastroenterology. 2015;149:302–317.e1. doi: 10.1053/j.gastro.2015.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubenstein J.H., Thrift A.P. Risk factors and populations at risk: selection of patients for screening for Barrett's oesophagus. Best Pract Res Clin Gastroenterol. 2015;29:41–50. doi: 10.1016/j.bpg.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Menezes A., Tierney A., Yang Y.X., et al. Adherence to the 2011 American Gastroenterological Association medical position statement for the diagnosis and management of Barrett's esophagus. Dis Esophagus. 2015;28:538–546. doi: 10.1111/dote.12228. [DOI] [PubMed] [Google Scholar]
- 9.Kolb J.M., Chen M., Tavakkoli A., et al. Understanding compliance, practice patterns and barriers among gastroenterologists and primary care providers is crucial for developing strategies to improve screening for Barrett's esophagus. Gastroenterology. 2022 doi: 10.1053/j.gastro.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper G.S., Kou T.D., Chak A. Receipt of previous diagnoses and endoscopy and outcome from esophageal adenocarcinoma: a population-based study with temporal trends. Am J Gastroenterol. 2009;104:1356–1362. doi: 10.1038/ajg.2009.159. [DOI] [PubMed] [Google Scholar]
- 11.Tan M.C., Mansour N., White D.L., et al. Systematic review with meta-analysis: prevalence of prior and concurrent Barrett's oesophagus in oesophageal adenocarcinoma patients. Aliment Pharmacol Ther. 2020;52:20–36. doi: 10.1111/apt.15760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M.L., Kolb J., Tavakkoli A., et al. Study of compliance, practice patterns, and barriers regarding established national screening programs for Barrett’s esophagus among primary care providers: SCREEN-BE. Gastroenterology. 2021;160 S-49. [Google Scholar]
- 13.Inadomi J.M., Saxena N. Screening and surveillance for Barrett's esophagus: is it cost-effective? Dig Dis Sci. 2018;63:2094–2104. doi: 10.1007/s10620-018-5148-7. [DOI] [PubMed] [Google Scholar]
- 14.Rubenstein J.H., McConnell D., Waljee A.K., et al. Validation and comparison of tools for selecting individuals to screen for Barrett's esophagus and early neoplasia. Gastroenterology. 2020;158:2082–2092. doi: 10.1053/j.gastro.2020.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spechler S.J., Katzka D.A., Fitzgerald R.C. New screening techniques in Barrett's esophagus: great ideas or great practice? Gastroenterology. 2018;154:1594–1601. doi: 10.1053/j.gastro.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 16.Yusuf A., Fitzgerald R.C. Screening for Barrett's oesophagus: are we ready for it? Curr Treat Options Gastroenterol. 2021:1–16. doi: 10.1007/s11938-021-00342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma G.K., Brensinger C.M., Wu Q., et al. Increasing incidence of multiply recurrent Clostridium difficile infection in the United States: a cohort study. Ann Intern Med. 2017;167:152–158. doi: 10.7326/M16-2733. [DOI] [PubMed] [Google Scholar]
- 18.Vajravelu R.K., Mamtani R., Scott F.I., et al. Incidence, risk factors, and clinical effects of recurrent diverticular hemorrhage: a large cohort study. Gastroenterology. 2018;155:1416–1427. doi: 10.1053/j.gastro.2018.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran L.V., Ongur D., Hsu J., et al. Psychosis with methylphenidate or amphetamine in patients with ADHD. N Engl J Med. 2019;380:1128–1138. doi: 10.1056/NEJMoa1813751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye Y., Yin Y., Huh S.Y., et al. Epidemiology, etiology and treatment of gastroparesis: real-world evidence from a large US national claims database. Gastroenterology. 2022;162:109–121.e5. doi: 10.1053/j.gastro.2021.09.064. [DOI] [PubMed] [Google Scholar]
- 21.Lewis J.D., Bilker W.B., Weinstein R.B., et al. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf. 2005;14:443–451. doi: 10.1002/pds.1115. [DOI] [PubMed] [Google Scholar]
- 22.Deyo R., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 23.Schneeweiss S., Rassen J.A., Glynn R.J., et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20:512–522. doi: 10.1097/EDE.0b013e3181a663cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hocking R.R., Leslie R.N. Selection of the best subset in regression analysis. Technometrics. 1967;9:531–540. [Google Scholar]
- 25.Nguyen T.H., Thrift A.P., Rugge M., et al. Prevalence of Barrett's esophagus and performance of societal screening guidelines in an unreferred primary care population of U.S. veterans. Gastrointest Endosc. 2021;93:409–419.e1. doi: 10.1016/j.gie.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubenstein J.H., Morgenstern H., Appelman H., et al. Prediction of Barrett's esophagus among men. Am J Gastroenterol. 2013;108:353–362. doi: 10.1038/ajg.2012.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie S.H., Ness-Jensen E., Medefelt N., et al. Assessing the feasibility of targeted screening for esophageal adenocarcinoma based on individual risk assessment in a population-based cohort study in Norway (the HUNT study) Am J Gastroenterol. 2018;113:829–835. doi: 10.1038/s41395-018-0069-9. [DOI] [PubMed] [Google Scholar]
- 28.Kunzmann A.T., Thrift A.P., Cardwell C.R., et al. Model for identifying individuals at risk for esophageal adenocarcinoma. Clin Gastroenterol Hepatol. 2018;16:1229–1236.e4. doi: 10.1016/j.cgh.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Rubenstein J.H., Raghunathan T., Doan C., et al. Validation of tools for predicting incident adenocarcinoma of the esophagus or esophagogastric junction. Am J Gastroenterol. 2021;116:949–957. doi: 10.14309/ajg.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 30.Baldwin-Hunter B.L., Knotts R.M., Leeds S.D., et al. Use of the electronic health record to target patients for non-endoscopic Barrett's esophagus screening. Dig Dis Sci. 2019;64:3463–3470. doi: 10.1007/s10620-019-05707-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzgerald R.C., di Pietro M., O'Donovan M., et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett's oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet. 2020;396:333–344. doi: 10.1016/S0140-6736(20)31099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kramer J.R., Shakhatreh M.H., Naik A.D., et al. Use and yield of endoscopy in patients with uncomplicated gastroesophageal reflux disorder. JAMA Intern Med. 2014;174:462–465. doi: 10.1001/jamainternmed.2013.13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ammann E.M., Kalsekar I., Yoo A., et al. Validation of body mass index (BMI)-related ICD-9-CM and ICD-10-CM administrative diagnosis codes recorded in US claims data. Pharmacoepidemiol Drug Saf. 2018;27:1092–1100. doi: 10.1002/pds.4617. [DOI] [PubMed] [Google Scholar]
- 34.Wiley L.K., Shah A., Xu H., et al. ICD-9 tobacco use codes are effective identifiers of smoking status. J Am Med Inform Assoc. 2013;20:652–658. doi: 10.1136/amiajnl-2012-001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gast A., Mathes T. Medication adherence influencing factors-an (updated) overview of systematic reviews. Syst Rev. 2019;8:112. doi: 10.1186/s13643-019-1014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Health Insurance Coverage in the United States: 2019. Current population reports. United States Census Bureau; Washington: 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.