Abstract
Cardiac allograft vasculopathy is a leading cause of allograft failure and death among heart transplant recipients. Routine coronary angiography and intravascular ultrasound in the early posttransplant period are widely accepted as the current standard-of-care diagnostic modalities. However, many studies have now demonstrated that invasive coronary physiological assessment provides complementary long-term prognostic data and helps identify patients who are at risk of accelerated cardiac allograft vasculopathy and acute rejection.
Keywords: cardiac allograft vasculopathy, coronary flow reserve, coronary physiology, fractional flow reserve, heart transplantation, index of microcirculatory resistance
Central Illustration
Highlights
-
•
Invasive coronary physiology soon after heart transplant predicts adverse outcomes.
-
•
Posttransplant microvascular dysfunction (high index of microcirculatory resistance) is a major prognostic indicator.
-
•
Prospective studies are needed to validate whether physiological assessment impacts outcomes.
Introduction
Although significant advances in the field of heart transplantation over the past few decades have led to considerable improvements in short-term survival, acute allograft rejection (AAR), and cardiac allograft vasculopathy (CAV) remain the leading causes of a need for retransplantation and death.1 In particular, CAV has been coined the “Achilles’ Heel” of contemporary heart transplantation.2 Risk factors for CAV include immune-mediated factors (eg, human leukocyte antigen mismatch), transplant-related factors (graft ischemia time and cytomegalovirus mismatch/infection), and donor and recipient cardiovascular risk factors.3 Therefore, efforts to prevent and slow the progression of CAV have included the routine institution of statin therapy and antiviral prophylaxis against cytomegalovirus, the selective use of mammalian target of rapamycin (mTOR) inhibitors, and the development of tools for earlier diagnosis and prognostication.3,4 Since the pivotal 2005 trials demonstrating its diagnostic and prognostic capability, intravascular ultrasound (IVUS) has been routinely used as an adjunct to coronary angiography for CAV surveillance.5,6 However, despite the many benefits of IVUS and other invasive imaging modalities, these technologies have notable limitations.7 Major limitations include the inability to assess the microvasculature and the limited ability to distinguish CAV from donor/native atherosclerosis. In addition, the presence of microvasculopathy on endomyocardial biopsy is associated with decreased long-term survival after heart transplantation.8 Indeed, a growing body of literature has revealed that an invasive physiologic evaluation of both the allograft coronary microcirculation and epicardial circuit can provide additional and perhaps even more powerful prognostic value. Here, we review the evidence supporting the use of invasive hemodynamic indices to predict accelerated CAV, acute rejection, and long-term death or retransplantation (Table 1).
Table 1.
Association of invasive coronary physiologic indices with clinical outcomes after heart transplantation.
| Reference, year | Outcomes | Major findings |
||
|---|---|---|---|---|
| FFR | IMR | CFR | ||
| Ahn et al,43 2021 |
|
|
|
|
| Lee et al,26 2021 |
|
|
|
|
| Ahn et al,39 2021 |
|
|
|
|
| Okada et al,38 2019 |
|
|
|
|
| Kobayashi et al,41 2018 |
|
|
|
|
| Fearon et al,29 2017 |
|
|
|
|
| Lee et al,25 2017 |
|
|
|
|
| Yang et al,42 2016 |
|
|
|
|
| Haddad et al,40 2012 |
|
|
|
|
| Fearon et al,16 2003 |
|
|
|
|
AAR, acute allograft rejection; ACR, acute cellular rejection; aHR, adjusted hazard ratio; CFR, coronary flow reserve; FFR, fractional flow reserve; HR, hazard ratio; IMR, index of microvascular resistance; IVUS, intravascular ultrasound; MACE, major adverse cardiac event; OR, odds ratio; PV, plaque volume.
MACE is defined in both Ahn et al, 2021 papers as “the composite of death from any cause, reheart transplantation, myocardial infarction defined by ischemic symptoms and signs with cardiac enzyme elevation more than the upper reference limit, coronary revascularization including percutaneous coronary intervention or coronary bypass surgery, stroke, graft dysfunction defined by newly developed left ventricular dysfunction (ejection fraction ≤45%), or readmission due to a cardiac cause.”
Invasive coronary physiologic indices
Fractional flow reserve
First described in 1996, fractional flow reserve (FFR) is a coronary wire-based physiologic index that assesses the hemodynamic significance of epicardial stenoses.9 FFR is defined as the maximal myocardial blood flow in the presence of a stenosis (distal coronary pressure) divided by the theoretical normal maximal flow in the absence of a stenosis (aortic pressure). Several assumptions are made when deriving FFR from Ohm’s law, such as the following: (1) venous pressure is negligible compared with coronary pressure, and (2) resistance in the microvascular circuit is minimal and constant and rendered trivial by vasodilators (eg, nitroglycerin and adenosine). For these reasons, FFR is specific to the epicardial vessel (Figure 1). A series of landmark randomized controlled trials, including the DEFER (Deferral versus performance of percutanous coronary intervention of functionally non-significant coronary stenosis) and FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) family of trials, have established the safety, efficacy, and cost-effectiveness of FFR-guided percutaneous coronary intervention compared with angiography-guided intervention, with an FFR value of ≤0.80 indicating hemodynamic significance.10, 11, 12
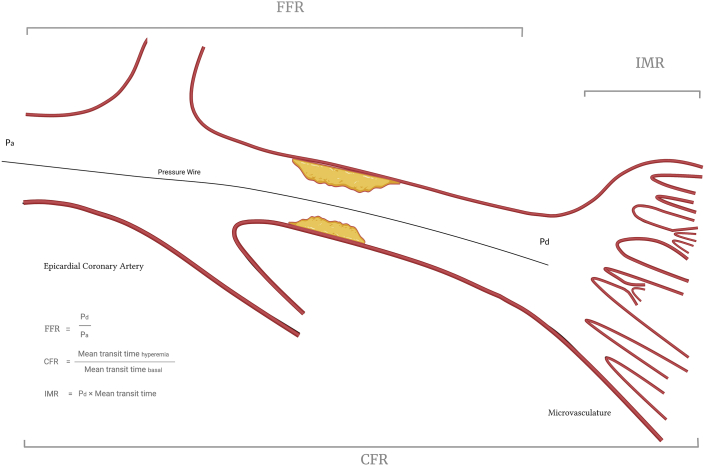
Figure 1.
Overview of invasive physiological indices. CFR, coronary flow reserve; FFR, fractional flow reserve; IMR, index of microcirculatory resistance.
Coronary flow reserve
In contrast to FFR, coronary flow reserve (CFR)—the ratio of hyperemic coronary flow to resting coronary flow—evaluates the entire coronary tree, including the microcirculation (Figure 1). Hence, CFR has traditionally been utilized to detect microvascular disease in the setting of angiographically normal epicardial vessels. However, its inability to distinguish between epicardial stenoses and microvascular dysfunction when the obstructive or diffuse epicardial disease is present is its fundamental limitation. Additionally, CFR is affected by hemodynamic variations and lacks a uniform normal cutoff value, which makes CFR difficult to interpret and compare between patients.13 A systematic review found that the mean normal CFR among 18 studies was 3.61 and the mean abnormal CFR was 2.14; indeed, the generally accepted threshold for a normal CFR is >2.14 Historically measured with a Doppler coronary wire, CFR is currently most commonly measured by thermodilution using a pressure–thermistor tipped wire, which allows for CFR and FFR measurements using a single wire. Given that the flow is inversely proportional to time, CFR is calculated as the resting mean transit time divided by the hyperemic mean transit time. Of note, the International Society for Heart and Lung Transplantation guidelines provide a class IIa recommendation for using CFR to detect CAV manifesting as a microvascular disease.15
Index of microcirculatory resistance
The index of microcirculatory resistance (IMR) was first described in 2003 and was developed to interrogate the coronary microvasculature independent of the epicardial circuit and of hemodynamic changes.16 Similar to FFR, IMR is derived from Ohm’s law, the principle that current is directly proportional to the voltage across 2 points and inversely proportional to the resistance across those points (voltage = current × resistance). Using the same pressure–thermistor-tipped wire, coronary flow (the “current”) can readily be calculated because it is inversely proportional to the hyperemic mean transit time. The pressure difference across the microvasculature (the “voltage”) is reflected by the mean distal coronary pressure (similar to the derivation of FFR, venous pressure is disregarded because it is assumed to be negligible compared with aortic pressure). Subsequently, IMR can be calculated via the following equation: IMR = distal coronary pressure × hyperemic mean transit time (Figure 1). In healthy individuals, a normal IMR value has been demonstrated to be <25.17, 18, 19 Figure 2 shows an example of a comprehensive physiological assessment in a heart transplant recipient.
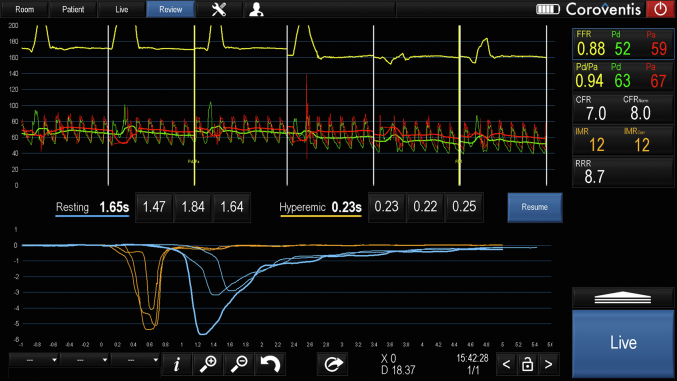
Figure 2.
An example of a standard physiological assessment of the left anterior descending coronary artery in a heart transplant recipient.
Invasive coronary physiology and CAV
Several early studies in the 1990s have used Doppler-derived invasive CFR to characterize allograft coronary vasodilatory reserve in heart transplant recipients.20,21 However, modern invasive hemodynamic indices, such as FFR and thermodilution-derived CFR were first studied in the heart transplant population in the Physiologic Investigation for Transplant Arteriopathy (PITA) study in 2003.22 Among 46 heart transplant recipients who underwent either baseline or annual screening angiography and exhibited no angiographic evidence of CAV, 75% had an FFR ≤0.94 (previously reported lower limit of normal) in the left anterior descending coronary artery, whereas 15% had values ≤0.80, suggesting hemodynamically significant silent ischemia.22 These FFR measurements correlated with several IVUS parameters of plaque burden; for example, patients with FFR ≤0.80 had a mean plaque volume of approximately 40% noted by IVUS, a significantly greater amount than those with FFR of >0.80.22 CFR was also measured and noted to be ≤2.0 in 47% of the cases, with 14% demonstrating hemodynamic findings consistent with microvascular dysfunction (ie, reduced CFR and normal FFR).22
The subsequent PITA II study evaluated FFR and IMR in 25 heart transplant recipients at baseline and 1 year posttransplant.23 Mean FFR decreased significantly from 0.90 at baseline to 0.85 at 1 year, reflecting plaque progression and, thus, greater epicardial ischemia. Furthermore, as in the original PITA study, FFR correlated with IVUS indices of plaque burden.23 Notably, IMR also decreased during the first year after transplant, signifying that microvascular function—in contrast to epicardial function—actually improved in the early posttransplant period.23 The authors hypothesized that early improvement in microvascular dysfunction likely reflected the resolution of allograft ischemia and reperfusion injury, postoperative inflammation, and immune-mediated activation. The same group later published corroborating data in 92 posttransplant patients, in which they again observed that FFR significantly decreased from baseline to 1 year posttransplant and inversely correlated with plaque burden.24 Interestingly, they also found that IMR increased at 2 years and beyond, suggesting that microvascular dysfunction is a later manifestation of CAV.24 A subsequent study of 44 heart transplant recipients found that a baseline IMR of ≥20 was associated with higher plaque volume on IVUS at 1-year follow-up, indicating that a greater degree of microvascular dysfunction soon after transplant portends accelerated CAV.25 Finally, in a 2021 prospective study, all heart transplant patients with moderate-to-severe angiographic CAV at 2-year follow-up had IMR of ≥15 on baseline assessment.26
FFR has also been used to establish the hemodynamic impact of negative coronary remodeling after heart transplantation. Negative remodeling, or reduction in vessel size, has been recognized as a key feature of CAV,27,28 and in 1 study of 34 heart transplant recipients, 19 (56%) had negative remodeling (defined as ≥10% loss of vessel volume on IVUS) at 1 year posttransplant.29 Intriguingly, these patients had significantly decreased FFR despite no significant plaque progression or improvement in microvascular function, suggesting that negative remodeling can indeed independently affect epicardial coronary flow by effectively reducing luminal size.29
Finally, these physiologic indices have also been used to evaluate the efficacy of therapies for CAV. For example, a 2008 study comparing mycophenolate mofetil to the mTOR inhibitor rapamycin revealed that FFR significantly declined at 1 year in patients treated with mycophenolate mofetil but did not substantially change among those treated with rapamycin.30 Furthermore, both CFR and IMR improved significantly in the rapamycin cohort, indicating improved microvascular function.30 Prior studies of mTOR inhibitors have reported slowing of CAV progression on IVUS compared with other immunosuppressants.4,31,32 Taken together, these data indicate that mTOR inhibitors attenuate both epicardial and microvascular CAV, resulting in the corresponding coronary physiologic changes described earlier. Another study examining the role of the renin-angiotensin system on the development of CAV found that heart transplant recipients randomized to receive ramipril compared with placebo had significant improvement in microvascular function from baseline to 1-year posttransplant (ie, lower IMR and higher CFR).33
Invasive coronary physiology and AAR
Invasive coronary physiologic indices have also been studied for their ability to predict the risk of AAR. Acute rejection is an important risk factor for the development or progression of CAV and long-term outcomes, including graft failure, death, and retransplantation.34, 35, 36, 37 Therefore, it has been hypothesized that using coronary physiology to identify patients who are at greater risk for acute rejection and its long-term sequelae may allow for the earlier institution of targeted therapies and ultimately improve outcomes.
A 2019 retrospective study of 88 heart transplant recipients reported that those who experienced AAR (defined as acute cellular rejection [ACR] grade ≥2R and/or grade ≥pAMR2 [pathological antibody-mediated rejection]) during the first year posttransplant were more likely to have had baseline microvascular dysfunction (CFR ≤3.90 and/or IMR ≥16) than those without AAR.38 Notably, all patients who had ACR grade 3R/3B or multiple episodes of ACR had a baseline IMR of ≥16. AAR was also associated with greater intimal growth, reduction in FFR, and negative remodeling within the first year.38 A prospective, single-center study demonstrated similar findings in a larger cohort of 154 heart transplant patients.26 Those with IMR of ≥15 were significantly more likely to sustain grade ≥2R ACR (negative predictive value = 96.2%). Additionally, CFR of ≤3.0 was associated with a significantly higher risk of ACR. The investigators also found that the addition of IMR to clinical variables added significant incremental prognostic value for assessing the risk of ACR.26
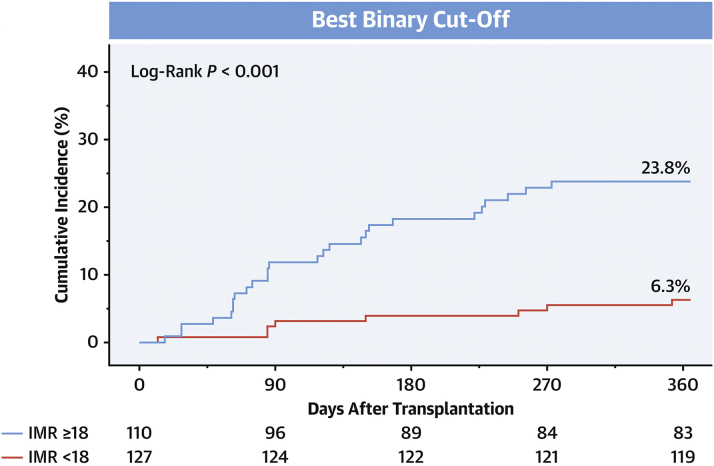
More recently, these data were validated in a multicenter, prospective, pooled-cohort study of 237 heart transplant recipients.39 The authors showed that a baseline IMR of ≥18 was significantly associated with the development of AAR during the first year posttransplant (adjusted hazard ratio [aHR], 3.93; 95% CI, 1.77-8.73; P = .001). Conversely, an IMR of <18 was associated with a low risk of incurring AAR (negative predictive value = 93.7%, Figure 3). Furthermore, a baseline IMR of ≥12 was associated with a significantly increased risk of 10-year major adverse cardiac events (aHR, 2.60; 95% CI, 1.24-5.42; P = .011).39 Collectively, these studies indicate that a larger burden of microvascular dysfunction early after heart transplantation confers a substantially higher risk of future AAR. One potential hypothesis for these findings is that a high IMR soon after transplantation may reflect a low level of rejection that is not yet detectable by biopsy or other noninvasive studies.
Figure 3.
Patients with baseline IMR of ≥18 in this multicenter prospective study had significantly higher rates of acute allograft rejection at 1 year than those with a lower IMR. IMR, index of microcirculatory resistance. Reproduced with permission from Ahn et al, 2021, J Am Coll Cardiol.39
Invasive coronary physiology and hard outcomes
Similar to the previously described landmark IVUS studies, investigators have examined the utility of invasive coronary physiology for predicting long-term hard outcomes in heart transplant recipients. In a 2012 study of 63 heart transplant recipients, those with an IMR of >20 at 1-year posttransplant (approximately 50% of the cohort) had a significantly higher likelihood of death, graft failure, or CAV at 5 years than those without microvascular dysfunction.40 These data were corroborated by 2 later studies. In 2018, investigators demonstrated that an IMR value of 19.3 at 1 year was the best cutoff for accurately predicting long-term death or retransplantation (aHR, 2.50; 95% CI, 1.05-5.96; P = .04).41 A subsequent 2019 retrospective study found that, although baseline physiologic indices were not associated with long-term survival, increased IMR at 1-year was associated with significantly higher mortality (HR, 2.81; 95% CI, 0.99-7.65; P = .04).38 Another study of 74 patients showed that a baseline FFR of <0.90 was associated with a significantly higher rate of the composite end point of death or retransplantation (aHR, 0.13; 95% CI, 0.02-0.81; P = .03) at a mean follow-up of 4.5 years.42 Additionally, IMR of ≥20 at 1 year was also a predictor of death or retransplantation (aHR, 3.93; 95% CI, 1.08-14.27; P = .04).42
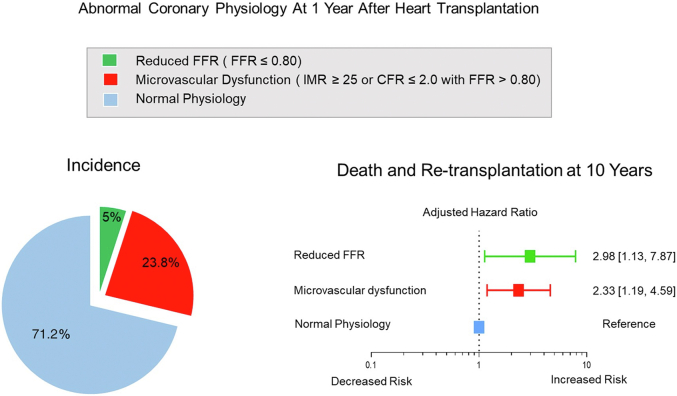
Finally, a more recent 2021 study assessed outcomes at a 10-year follow-up in an international multicenter cohort of 199 heart transplant recipients who underwent baseline and 1-year invasive physiologic assessment.43 The authors found that abnormal physiologic indices at 1 year but not baseline portended worse long-term outcomes (Figure 4). Specifically, hemodynamically significant epicardial disease (FFR ≤0.80) at 1 year was associated with a significantly increased risk of death or retransplantation (aHR, 2.98; 95% CI, 1.13-7.87; P = .028). Furthermore, microvascular dysfunction (defined as IMR of ≥25 or CFR of ≤2.0 with an FFR of >0.80) was also associated with a significantly higher risk of death or retransplantation (aHR, 2.33; 95% CI, 1.19-4.59; P = .015) as well as increased risk of major adverse cardiovascular events (aHR, 2.52; 95% CI, 1.45-4.35; P <.001).43 Interestingly, similar to a few recent studies, the authors also found that changes in maximal intimal thickness on IVUS were not predictive of long-term outcomes, challenging the validity of this historical parameter for prognostication in the modern era of heart transplantation.43,44 Taken together, although invasive intracoronary imaging remains essential for the early diagnosis of CAV, the contemporary literature suggests that invasive coronary physiology early after transplant may carry more prognostic value than invasive imaging in current practice. Furthermore, among the physiological indices studied, IMR appears to have the most robust data supporting its association with hard outcomes in heart transplant recipients, suggesting that microvascular dysfunction, in particular, is an important prognostic indicator.
Figure 4.
Abnormal coronary physiology 1 year after heart transplantation predicts long-term outcomes at 10 years. FFR, fractional flow reserve; IMR, index of microcirculatory resistance. Reproduced with permission from Ahn et al, 2021, Eur Heart J.43
Future directions
Although invasive coronary physiology may provide important prognostic information in the heart transplant population, its use is not without risk. Specifically, patients undergoing a comprehensive invasive physiologic assessment are exposed to increased albeit fairly minimal procedural risks, including coronary dissection and/or perforation of the vessel while wiring the vessel, nephrotoxicity because of the additional contrast use, and transient hypotension, heart block, or cardiopulmonary symptoms with adenosine infusion. Coronary angiography-derived FFR was developed as a potential means for eliminating these aforementioned risks. This technology utilizes various algorithms to reconstruct the epicardial coronary tree, estimate maximal flow and resistance, and then calculate a virtual FFR. In 2020, a study demonstrating the feasibility of using an angiography-derived FFR in heart transplant recipients showed that it classified hemodynamic impairment in 32% of cases where angiography alone did not.45 A follow-up study found that angiography-derived FFR correlated well with traditional wire-based FFR in heart transplant patients (r = 0.92; P <.001).46 Larger studies validating this modality and assessing its ability to predict long-term outcomes in heart transplant recipients are needed.
Additionally, with the advent of coronary computed tomography angiography-derived FFR (FFRCT), noninvasive hemodynamic coronary assessment is now commercially available and has been increasingly used in nontransplant patients. FFRCT, which uses anatomical computed tomography images to derive functional data, was recently used to evaluate coronary physiology in heart transplant recipients participating in the Assessing Diagnostic Value of Noninvasive FFRCT in Coronary Care registry.47 Among 73 patients, 25% had at least 1 moderate focal coronary lesion (>30% diameter stenosis) with an FFRCT of ≤0.80; these patients were significantly farther out from their heart transplantation at the time of evaluation, had greater CAV burden and were more likely to have received a coronary stent. They were also more likely to undergo additional testing to assess the coronaries during 1-year follow-up.47 Despite these promising preliminary data, larger studies are needed to validate FFRCT against invasive FFR in heart transplant patients. Lastly, although FFRCT avoids the risks of invasive assessment, it still requires contrast use and therefore does not forego all of the aforementioned risks associated with an invasive approach.
Conclusion
CAV is a major cause of long-term adverse outcomes following heart transplantation. However, although coronary angiography and invasive imaging with IVUS are the current mainstays of diagnostic screening, physiological evaluation of the epicardial circulation with FFR and the microcirculation with CFR, particularly, IMR, early after heart transplantation provides important complementary diagnostic and prognostic information (Central Illustration), despite these encouraging and robust data, future studies must demonstrate that assessing coronary physiology in this challenging population can lead to appreciable changes in care (eg, more aggressive upfront immunosuppression) and ultimately improved long-term outcomes.
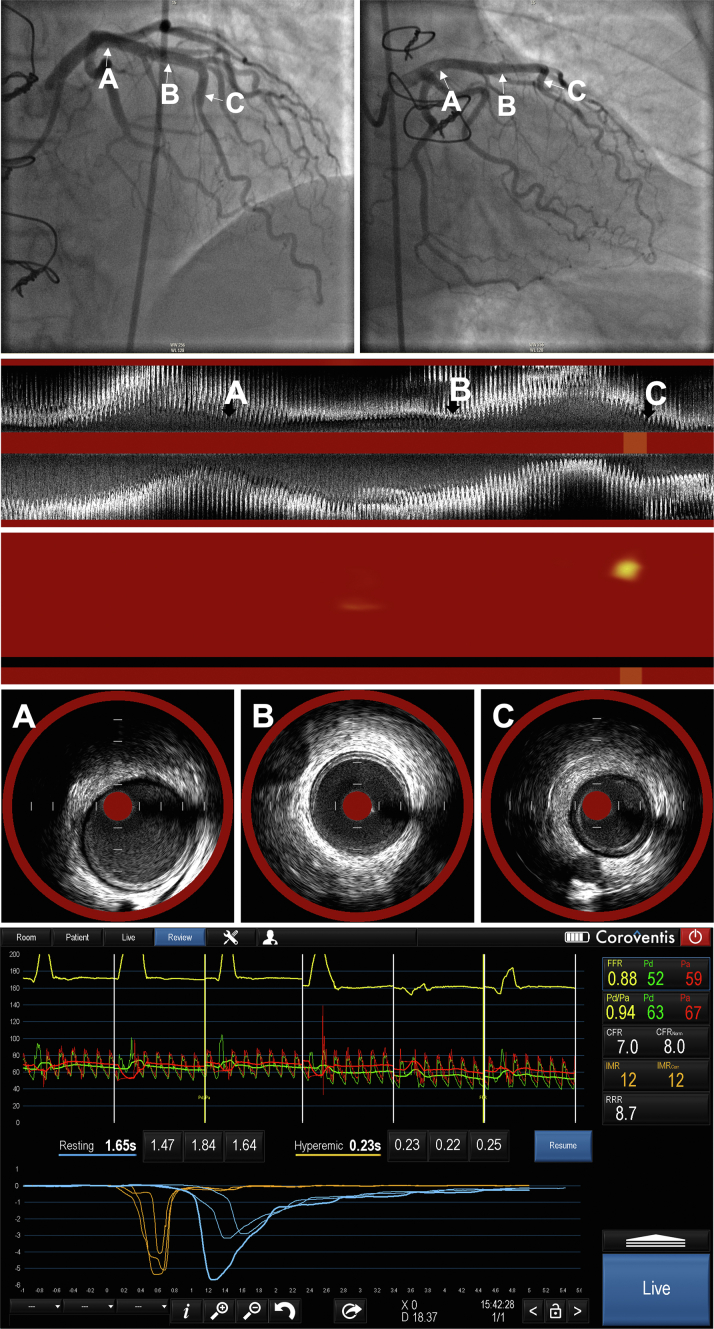
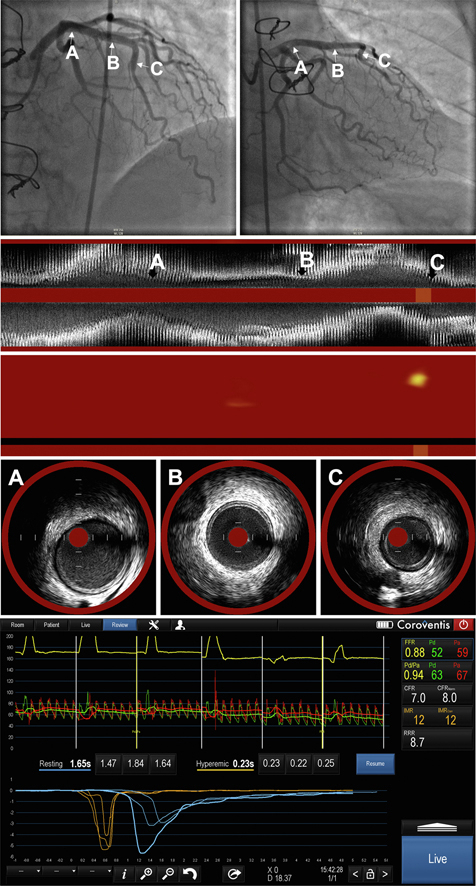
Central Illustration.
A representative case of physiological and intravascular assessment in patients with CAV. Upper panel: angiogram in the right anterior oblique caudal and cranial angulations. Arrows designate the proximal (A), proximal-mid (B), and mid-LAD segments. Lower panel: physiological assessment of the same case. Middle panels: longitudinal IVUS images and chemogram derived from NIRS in the LAD. Panels A-C depict the IVUS and NIRS findings that correspond to the vessel segments designated by the arrows in the angiogram. CAV, cardiac allograft vasculopathy; IVUS, intravascular ultrasound; LAD, left anterior descending coronary artery; NIRS, near-infrared spectroscopy.
Declaration of competing interest
William F. Fearon receives institutional research support from Abbott Vascular, Boston Scientific, and Medtronic, consults with Siemens and CathWorks and has stock options with HeartFlow. Rushi Parikh receives research support from Bayer, Infraredx, and Abbott Vascular and consulting fees from Abbott Vascular. Negeen Shahandeh, Justin Song, Kan Saito, Yasuhiro Honda, Frederik M. Zimmermann, and Jung-Min Ahn reported no financial interests.
Acknowledgments
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Khush K.K., Cherikh W.S., Chambers D.C., et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-sixth adult heart transplantation report — 2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38(10):1056–1066. doi: 10.1016/j.healun.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chih S., Chong A.Y., Mielniczuk L.M., Bhatt D.L., Beanlands R.S.B. Allograft vasculopathy: the Achilles’ heel of heart transplantation. J Am Coll Cardiol. 2016;68(1):80–91. doi: 10.1016/j.jacc.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Lee F., Nair V., Chih S. Cardiac allograft vasculopathy: insights on pathogenesis and therapy. Clin Transplant. 2020;34(3) doi: 10.1111/ctr.13794. [DOI] [PubMed] [Google Scholar]
- 4.Eisen H.J., Tuzcu E.M., Dorent R., et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349(9):847–858. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]
- 5.Kobashigawa J.A., Tobis J.M., Starling R.C., et al. Multicenter intravascular ultrasound validation study among heart transplant recipients: outcomes after five years. J Am Coll Cardiol. 2005;45(9):1532–1537. doi: 10.1016/j.jacc.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 6.Tuzcu E.M., Kapadia S.R., Sachar R., et al. Intravascular ultrasound evidence of angiographically silent progression in coronary atherosclerosis predicts long-term morbidity and mortality after cardiac transplantation. J Am Coll Cardiol. 2005;45(9):1538–1542. doi: 10.1016/j.jacc.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 7.Shahandeh N., Kashiyama K., Honda Y., et al. Invasive coronary imaging assessment for cardiac allograft vasculopathy: state-of-the-art review. J Soc Cardiovasc Angiogr Interv. 2022;1(4) [Google Scholar]
- 8.Hiemann N.E., Wellnhofer E., Knosalla C., et al. Prognostic impact of microvasculopathy on survival after heart transplantation: evidence from 9713 endomyocardial biopsies. Circulation. 2007;116(11):1274–1282. doi: 10.1161/CIRCULATIONAHA.106.647149. [DOI] [PubMed] [Google Scholar]
- 9.Pijls N.H.J., de Bruyne B., Peels K., et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334(26):1703–1708. doi: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- 10.Pijls N.H.J., van Schaardenburgh P., Manoharan G., et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49(21):2105–2111. doi: 10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 11.Tonino P.A.L., De Bruyne B., Pijls N.H.J., et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 12.De Bruyne B., Pijls N.H.J., Kalesan B., et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 13.De Bruyne B., Bartunek J., Sys S.U., Pijls N.H., Heyndrickx G.R., Wijns W. Simultaneous coronary pressure and flow velocity measurements in humans. Feasibility, reproducibility, and hemodynamic dependence of coronary flow velocity reserve, hyperemic flow versus pressure slope index, and fractional flow reserve. Circulation. 1996;94(8):1842–1849. doi: 10.1161/01.cir.94.8.1842. [DOI] [PubMed] [Google Scholar]
- 14.Kelshiker M.A., Seligman H., Howard J.P., et al. Coronary flow reserve and cardiovascular outcomes: a systematic review and meta-analysis. Eur Heart J. 2022;43(16):1582–1593. doi: 10.1093/eurheartj/ehab775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costanzo M.R., Dipchand A., Starling R., et al. The international society of heart and lung transplantation guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29(8):914–956. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 16.Fearon W.F., Balsam L.B., Farouque H.M.O., et al. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107(25):3129–3132. doi: 10.1161/01.CIR.0000080700.98607.D1. [DOI] [PubMed] [Google Scholar]
- 17.Melikian N., Vercauteren S., Fearon W.F., et al. Quantitative assessment of coronary microvascular function in patients with and without epicardial atherosclerosis. EuroIntervention. 2010;5(8):939–945. [PubMed] [Google Scholar]
- 18.Luo C., Long M., Hu X., et al. Thermodilution-derived coronary microvascular resistance and flow reserve in patients with cardiac syndrome X. Circ Cardiovasc Interv. 2014;7(1):43–48. doi: 10.1161/CIRCINTERVENTIONS.113.000953. [DOI] [PubMed] [Google Scholar]
- 19.Solberg O.G., Ragnarsson A., Kvarsnes A., et al. Reference interval for the index of coronary microvascular resistance. EuroIntervention. 2014;9(9):1069–1075. doi: 10.4244/EIJV9I9A181. [DOI] [PubMed] [Google Scholar]
- 20.Wolford T.L., Donohue T.J., Bach R.G., et al. Heterogeneity of coronary flow reserve in the examination of multiple individual allograft coronary arteries. Circulation. 1999;99(5):626–632. doi: 10.1161/01.cir.99.5.626. [DOI] [PubMed] [Google Scholar]
- 21.Kern M.J., Bach R.G., Mechem C.J., et al. Variations in normal coronary vasodilatory reserve stratified by artery, gender, heart transplantation and coronary artery disease. J Am Coll Cardiol. 1996;28(5):1154–1160. doi: 10.1016/S0735-1097(96)00327-0. [DOI] [PubMed] [Google Scholar]
- 22.Fearon W.F., Nakamura M., Lee D.P., et al. Simultaneous assessment of fractional and coronary flow reserves in cardiac transplant recipients: Physiologic Investigation for Transplant Arteriopathy (PITA study) Circulation. 2003;108(13):1605–1610. doi: 10.1161/01.CIR.0000091116.84926.6F. [DOI] [PubMed] [Google Scholar]
- 23.Fearon W.F., Hirohata A., Nakamura M., et al. Discordant changes in epicardial and microvascular coronary physiology after cardiac transplantation: Physiologic Investigation for Transplant Arteriopathy II (PITA II) study. J Heart Lung Transplant. 2006;25(7):765–771. doi: 10.1016/j.healun.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Hirohata A., Nakamura M., Waseda K., et al. Changes in coronary anatomy and physiology after heart transplantation. Am J Cardiol. 2007;99(11):1603–1607. doi: 10.1016/j.amjcard.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J.H., Okada K., Khush K., et al. Coronary endothelial dysfunction and the index of microcirculatory resistance as a marker of subsequent development of cardiac allograft vasculopathy. Circulation. 2017;135(11):1093–1095. doi: 10.1161/CIRCULATIONAHA.116.025268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J.M., Choi K.H., Choi J.O., et al. Coronary microcirculatory dysfunction and acute cellular rejection after heart transplantation. Circulation. 2021;144(18):1459–1472. doi: 10.1161/CIRCULATIONAHA.121.056158. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell R.N., Libby P. Vascular remodeling in transplant vasculopathy. Circ Res. 2007;100(7):967–978. doi: 10.1161/01.RES.0000261982.76892.09. [DOI] [PubMed] [Google Scholar]
- 28.Pethig K., Heublein B., Wahlers T., Haverich A. Mechanism of luminal narrowing in cardiac allograft vasculopathy: inadequate vascular remodeling rather than intimal hyperplasia is the major predictor of coronary artery stenosis. Working group on cardiac allograft vasculopathy. Am Heart J. 1998;135(4):628–633. doi: 10.1016/s0002-8703(98)70278-9. [DOI] [PubMed] [Google Scholar]
- 29.Fearon W.F., Felix R., Hirohata A., et al. The effect of negative remodeling on fractional flow reserve after cardiac transplantation. Int J Cardiol. 2017;241:283–287. doi: 10.1016/j.ijcard.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Sinha S.S., Pham M.X., Vagelos R.H., et al. Effect of rapamycin therapy on coronary artery physiology early after cardiac transplantation. Am Heart J. 2008;155(5):889.e1–889.e6. doi: 10.1016/j.ahj.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Mancini D., Pinney S., Burkhoff D., et al. Use of rapamycin slows progression of cardiac transplantation vasculopathy. Circulation. 2003;108(1):48–53. doi: 10.1161/01.CIR.0000070421.38604.2B. [DOI] [PubMed] [Google Scholar]
- 32.Keogh A., Richardson M., Ruygrok P., et al. Sirolimus in de novo heart transplant recipients reduces acute rejection and prevents coronary artery disease at 2 years: a randomized clinical trial. Circulation. 2004;110(17):2694–2700. doi: 10.1161/01.CIR.0000136812.90177.94. [DOI] [PubMed] [Google Scholar]
- 33.Fearon W.F., Okada K., Kobashigawa J.A., et al. Angiotensin-converting enzyme inhibition early after heart transplantation. J Am Coll Cardiol. 2017;69(23):2832–2841. doi: 10.1016/j.jacc.2017.03.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lund L.H., Edwards L.B., Kucheryavaya A.Y., et al. The registry of the international society for heart and lung transplantation: thirty-second official adult heart transplantation report—2015; focus theme: early graft failure. J Heart Lung Transplant. 2015;34(10):1244–1254. doi: 10.1016/j.healun.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Dong L., Maehara A., Nazif T.M., et al. Optical coherence tomographic evaluation of transplant coronary artery vasculopathy with correlation to cellular rejection. Circ Cardiovasc Interv. 2014;7(2):199–206. doi: 10.1161/CIRCINTERVENTIONS.113.000949. [DOI] [PubMed] [Google Scholar]
- 36.Jimenez J., Kapadia S.R., Yamani M.H., et al. Cellular rejection and rate of progression of transplant vasculopathy: a 3-year serial intravascular ultrasound study. J Heart Lung Transplant. 2001;20(4):393–398. doi: 10.1016/s1053-2498(00)00249-7. [DOI] [PubMed] [Google Scholar]
- 37.Raichlin E., Edwards B.S., Kremers W.K., et al. Acute cellular rejection and the subsequent development of allograft vasculopathy after cardiac transplantation. J Heart Lung Transplant. 2009;28(4):320–327. doi: 10.1016/j.healun.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Okada K., Honda Y., Luikart H., et al. Early invasive assessment of the coronary microcirculation predicts subsequent acute rejection after heart transplantation. Int J Cardiol. 2019;290:27–32. doi: 10.1016/j.ijcard.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 39.Ahn J.M., Zimmermann F.M., Gullestad L., et al. Microcirculatory resistance predicts allograft rejection and cardiac events after heart transplantation. J Am Coll Cardiol. 2021;78(24):2425–2435. doi: 10.1016/j.jacc.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Haddad F., Khazanie P., Deuse T., et al. Clinical and functional correlates of early microvascular dysfunction after heart transplantation. Circ Heart Fail. 2012;5(6):759–768. doi: 10.1161/CIRCHEARTFAILURE.111.962787. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi Y., Kobayashi Y., Yang H.M., et al. Long-term prognostic value of invasive and non-invasive measures early after heart transplantation. Int J Cardiol. 2018;260:31–35. doi: 10.1016/j.ijcard.2018.01.070. [DOI] [PubMed] [Google Scholar]
- 42.Yang H.M., Khush K., Luikart H., et al. Invasive assessment of coronary physiology predicts late mortality after heart transplantation. Circulation. 2016;133(20):1945–1950. doi: 10.1161/CIRCULATIONAHA.115.018741. [DOI] [PubMed] [Google Scholar]
- 43.Ahn J.M., Zimmermann F.M., Arora S., et al. Prognostic value of comprehensive intracoronary physiology assessment early after heart transplantation. Eur Heart J. 2021;42(48):4918–4929. doi: 10.1093/eurheartj/ehab568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okada K., Kitahara H., Yang H.M., et al. Paradoxical vessel remodeling of the proximal segment of the left anterior descending artery predicts long-term mortality after heart transplantation. JACC Heart Fail. 2015;3(12):942–952. doi: 10.1016/j.jchf.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 45.Nagumo S., Gallinoro E., Candreva A., et al. Vessel fractional flow reserve and graft vasculopathy in heart transplant recipients. J Interv Cardiol. 2020;2020 doi: 10.1155/2020/9835151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mileva N., Nagumo S., Gallinoro E., et al. Validation of coronary angiography-derived vessel fractional flow reserve in heart transplant patients with suspected graft vasculopathy. Diagnostics (Basel) 2021;11(10):1750. doi: 10.3390/diagnostics11101750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Budde R.P.J., Nous F.M.A., Roest S., et al. CT-derived fractional flow reserve (FFRCT) for functional coronary artery evaluation in the follow-up of patients after heart transplantation. Eur Radiol. 2022;32(3):1843–1852. doi: 10.1007/s00330-021-08246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]