Endorsement: This expert consensus statement was endorsed by the American College of Cardiology (ACC), British Cardiovascular Intervention Society (BCIS), Canadian Association of Interventional Cardiologists (CAIC), and Outpatient Endovascular and Interventional Society (OEIS).
Key Points.
-
1.
Elective percutaneous coronary intervention (PCI) in settings without surgery on site (no-SOS) has increased in volume and complexity (extending beyond the simple lesion recommendations in the 2014 document). In addition, PCI is now being performed outside of the hospital setting, in office-based laboratories (OBLs) and ambulatory surgery centers (ASCs).
-
2.
Several new studies in the United States and abroad have demonstrated that PCIs performed at no-SOS centers have very low rates of complications and similar outcomes to PCIs performed at surgical centers.
-
3.
Despite increase in age, comorbidities, and lesion complexity, the rate of periprocedural complications has remained constant, or declined, with rates of emergency surgery as low as 0.1% in many series.
-
4.
Complex PCI, including unprotected left main, is being performed in some no-SOS centers, with no increase in major adverse cardiovascular events or emergency coronary artery bypass graft surgery compared with PCI at surgical centers. There have been no comparative studies in other complex PCI subgroups such as chronic total occlusion and atherectomy; however, observational studies demonstrate reasonable outcomes and suggest feasibility with experienced interventional cardiologists.
-
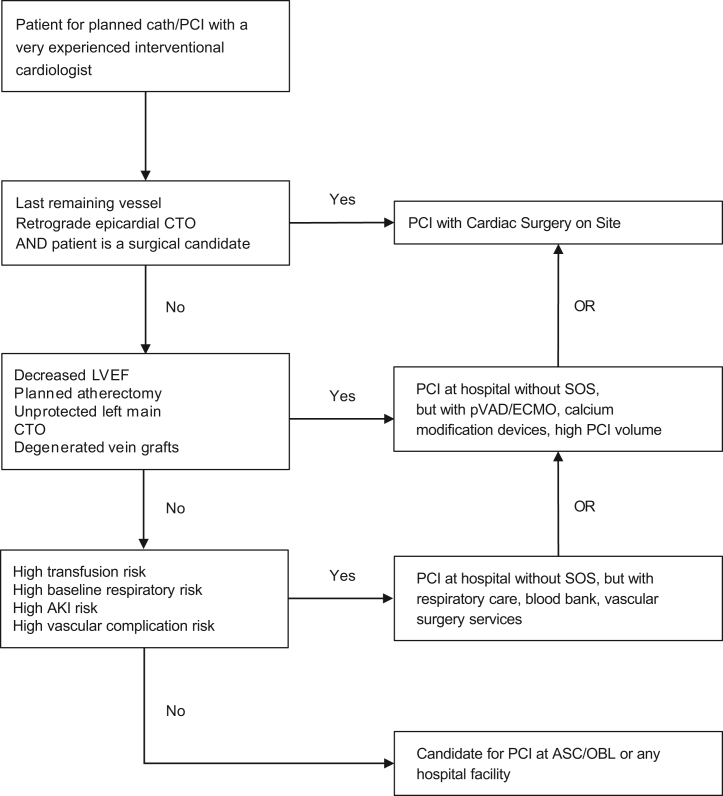
5.We propose a new PCI treatment algorithm (Figure 1) that expands the type of cases that can be performed with no-SOS compared with the 2014 document, with consideration of the patients’ clinical and lesion risk, operator experience (both recent and accumulated), and the experience and rescue capabilities of the site.
Figure 1.
Simplified algorithm for case selection for elective PCI at different facilities, assuming an experienced interventional cardiologist. AKI, acute kidney injury; ASC, ambulatory surgery centers; CTO, chronic total occlusions; ECMO, extracorporeal membrane oxygenation; LVEF, left ventricular ejection fraction; OBL, office-based laboratories; PCI, percutaneous coronary intervention; pVAD, percutaneous ventricular assist device; SOS, surgery on site. -
6.
In the United States, there are considerable financial savings (to insurers and Medicare) for PCI to be performed in ASC and OBL settings, thus out-migration of procedures from hospitals should be anticipated.
Introduction
Although once considered high-risk and below the standard of care, percutaneous coronary intervention (PCI) without on-site surgical backup has been performed with acceptable outcomes since the 1980s.1 An initial consensus document on PCI without surgery on site (no-SOS) was published by Society for Cardiovascular Angiography & Interventions (SCAI) in 2007 and updated in 2014.2,3 The 2014 document summarized new literature, reviewed existing guidelines and other publications related to PCI with no-SOS, and recommended best practices and requirements for facilities performing PCI with no-SOS. At the time, the research and practice of PCI with no-SOS were still limited, and as a result, the recommendations for case selection and practice were conservative.
Since the publication of the 2014 consensus statement, same-day discharge after elective PCI has increased to 28.6% of all PCIs and 39.7% of radial PCIs in the United States in 2017.4 Elective PCI in no-SOS settings has increased in volume and complexity. Concurrently, there have been interventional cardiologists performing PCI in office-based laboratories (OBLs) and ambulatory surgery centers (ASCs). Although comprising a small percentage of annual PCI procedures, this setting has garnered increased attention, notably with the 2020 expansion of coverage by the US Centers for Medicare & Medicaid Services (CMS) to include PCI in the ASC setting.5 PCI at ASCs may improve access, patient satisfaction, and reduce costs. Several new studies in the United States and abroad have demonstrated that PCIs performed at no-SOS centers have very low rates of complications and similar outcomes to PCIs performed with surgery on site. Moreover, recent consolidation of surgical services within health systems have resulted in some well-established, experienced, and high-quality PCI centers being restricted from performing complex PCI because of the perceived need for on-site surgery.
Thus, the writing committee has revised the 2014 document to (1) update the available data, (2) reconsider the types of cases that could be undertaken without on-site surgical backup, (3) review data regarding which patients are at higher risk, and (4) recommend patient selection criteria based on patient risk, operator experience, and facility capabilities. Importantly, as PCI with no-SOS is often the predominant mode of delivery globally, we expanded the document to include international experience, perspectives, and outcomes.
Methodology
This statement has been developed according to SCAI Publications Committee policies for writing group composition, disclosure, and management of relationships with industry, internal and external review, and organizational approval.
The writing group has been organized to ensure diversity of perspectives and demographics, multistakeholder representation, and appropriate balance of relationships with industry. Relevant author disclosures are included in Supplemental Material 1. Before appointment, members of the writing group were asked to disclose financial and intellectual relationships from the 12 months prior to their nomination. A majority of the writing group disclosed no relevant, significant financial relationships. Disclosures were periodically reviewed during document development and updated as needed. SCAI policy requires that writing group members with a current, relevant financial interest are recused from participating in related discussions or voting on recommendations. The work of the writing committee was supported exclusively by SCAI, a nonprofit medical specialty society, without commercial support. Writing group members contributed to this effort on a volunteer basis and did not receive payment from SCAI.
Literature searches were performed by group members designated to lead each section, and initial section drafts were authored primarily by the section leads in collaboration with other members of the writing group. Recommendations were discussed by the full writing group until a majority of group members agreed on the text and qualifying remarks. All recommendations are supported by a short summary of the evidence or specific rationale.
The draft manuscript was posted for public comment in May 2022 and the document was revised to address pertinent comments. The writing group unanimously approved the final version of the document. SCAI Publications Committee and Executive Committee endorsed the document as official society guidance in November 2022.
SCAI statements are primarily intended to help clinicians make decisions about treatment alternatives. Clinicians also must consider the clinical presentation, setting, and preferences of individual patients to make judgments about the optimal approach.
Improvements in PCI safety over time
Advances in procedural techniques, equipment, and pharmacological treatments have enhanced the safety of PCI over the last decade, despite increasing patient age and comorbidities. 6,7 Coronary anatomic complexity has similarly increased over time as providers embark on revascularization of patients with complex multivessel coronary artery disease according to newer comparative data, surgical ineligibility, or patient preference.8,9 Despite this increase in complexity, the rate of periprocedural complications has remained constant or declined over the last decade. The National Cardiovascular Data Registry (NCDR) describes static rates of coronary perforations (0.4%) and serious vascular access complications (1.4%) in the most recent years analyzed.10,11 In the Veterans Administration (VA) Healthcare System, complications continue to decline and remain below 1%.7 Development of multidisciplinary conferences12 and national peer review systems13 may also serve to ensure better case selection and management of complications. Increased use of radial access,7,11 changes in procedural methods, and improvements in PCI equipment including physiological assessment and intravascular imaging may all be associated with this reduction in periprocedural complications. Finally, evolution in the equipment to rescue complications such as more deliverable covered stents and more widely available mechanical support options may reduce the need for emergent bypass. Collectively, these advances suggest that percutaneous revascularization can be performed safely with a very low complication rate in the contemporary era.
Emergency cardiac surgery
Surgical intervention may be required after complications such as coronary perforation with tamponade, aortic root dissection, recurrent acute vessel closure or retained devices that cannot be managed with percutaneous approaches. The NCDR defines emergency surgery as operative intervention required without delay for patients with ongoing, refractory cardiac compromise unresponsive to therapy other than cardiac surgery.14
The rates of emergent bypass performed for a periprocedural complication after PCI have remained extremely low. After randomizing patients to receive PCI at facilities with or without on-site cardiac surgery, the MASS COMM trial found no difference in the need for emergency surgery, with an incidence of 0.3% vs 0.1%, respectively.15 Data from the British Cardiovascular Intervention Society between 2006 and 2012 revealed emergency surgery was required for 0.04% of patients at centers with no-SOS, compared with 0.1% at centers with on-site surgery.16 A propensity-matched comparison of nonprimary PCI found surgery was performed in 0.5% at centers with surgery, and 0.3% at centers without.17 Further, a meta-analysis encompassing several clinical trials and registries found a rate of emergent bypass surgery of 0.5%,18 with more contemporary data from Michigan17 and the VA Healthcare System19 suggesting rates below 0.1%.
Predictors of need for emergency surgery
Patients presenting acutely, with impaired left ventricular function and cardiogenic shock, are at higher risk for emergency surgery20, 21, 22 as are female patients and patients with chronic total occlusions (CTOs) and proximal lesions.23 Other anatomic factors, such as vessel tortuosity and severe calcification, also contribute to risk. Interventions on CTOs, bifurcation lesions, and complex right coronary arteries have been recognized as being higher risk for root dissection, perforation, and need for emergency surgery.20 From an analysis of the National Inpatient Sample database, risk factors for emergency surgery included complex anatomy, peripheral vascular disease, heart failure, stroke, hypertension, hemodialysis, connective tissue disease, lung disease, and obesity.21 Scores have been developed to predict the need for emergent surgical support, but even the highest tertile of patients in these scoring systems required emergent surgery in only 0.6% of cases.21
Outcomes after emergency surgery
Emergency coronary bypass surgery (CABG) after PCI is associated with high mortality rates, ranging between 7.4% and 21%.21,22,24,25 UK registry data reported in-hospital major adverse cardiovascular and cerebrovascular events (MACCE) of 14%, with a prolonged in-hospital stay of 9 days longer in those surviving surgery.26 Despite the high perioperative risk of mortality, it appears survivors have a good long-term prognosis.22
Not surprisingly, a longer time to surgery occurred in patients transferred from centers with no-SOS (306 minutes) compared with patients at centers with surgical capability (160 minutes). Paradoxically, despite more rapid emergency CABG in surgical hospitals, the in-hospital mortality rate was 12-fold higher.22 The explanation for this finding is not clear, but along with patient and procedure-related factors, it is possible that those from no-SOS centers in extremis may have died before getting to an operation or that they underwent successful bailout PCI, which may not have been attempted had the surgical option been more readily available. These times to surgery are important considerations as even with on-site surgery, patients with complications must be stabilized sufficiently in the catheterization laboratory with mechanical support to survive the 2 to 3 hours before surgery can be performed.
Updated publications comparing PCI at no-SOS vs surgical centers
The outcome of PCI performed at no-SOS centers has been studied in only 2 randomized controlled studies, both of which excluded patients requiring primary PCI (PPCI) or high-risk features such as poor left ventricular function. The CPORT-E trial showed noninferiority of PCI at hospitals with no-SOS compared with surgical centers at 6 weeks and 9 months.27 As described previously, the MASS-COM trial showed no significant differences in the rates of death, myocardial infarction (MI), repeat revascularization, and stroke between the 2 hospital settings.15
A meta-analysis of 23 studies comparing PCI outcomes in centers with and without on-site surgical backup including 1,101,123 patients was published in 2015.18 For PPCI (133,574 patients), all-cause mortality (odds ratio [OR], 0.99; 95% CI, 0.91-1.07; P = .729) and emergency CABG (OR, 0.76; 95% CI, 0.56-1.01; P = .062) did not differ by the presence of on-site surgery. Similarly, for non-PPCI (967,549 patients), all-cause mortality (OR, 1.15; 95% CI, 0.94-1.41; P = .172) and emergency CABG (OR, 1.14; 95% CI, 0.62-2.13; P = .669) were not significantly different. Importantly, the pooled-effect size for all-cause mortality after PPCI did not shift over time, despite the differences in practice patterns or patient populations from 1995 to 2014.
Much of the more recent data for PCI procedures undertaken in no-SOS centers have been derived from observational studies (Table 1). Analysis of Blue Cross Blue Shield of Michigan Cardiovascular Consortium data including all non-PPCI cases performed at 47 hospitals (14 without and 33 with surgery) between 2016 and 2018 revealed that 4721 of 50,817 (9.3%) PCI procedures were undertaken in no-SOS centers, with an increase over time.17 Patients undergoing PCI in no-SOS sites were younger with fewer comorbidities and were more likely to present with non-ST elevation MI (34.7% vs 28.4%; P < .001). In contrast, PCI of left main (4.0% vs 1.0%; P < .001), bypass grafts (6.4% vs 3.5%; P < .001), and CTOs (4.8% vs 1.9%; P < .001) were more likely to be undertaken in surgical centers, in keeping with the prior SCAI recommendations. Major adverse cardiovascular events (2.6% vs 2.8%; P = .443), and all-cause in-hospital mortality (0.6% vs 0.5%; P = .465) were similar, as was major bleeding, transfusion, other vascular complications, subacute stent thrombosis, target lesion revascularization, dialysis, urgent/emergent CABG, contrast nephropathy, and length of stay. Rates of stroke and heart failure were lower in no-SOS centers although absolute differences were small and likely reflect a lower-risk population. In a smaller subgroup of Medicare fee for service patients where post discharge outcomes could be tracked, 90-day readmission rates (18.8% vs 20.0%; P = .400) and costs ($26,457.25 vs $26,279.80; P = .902) were similar at sites with and without cardiac surgery. A separate analysis from the same registry reported similar outcomes in patients undergoing PPCI, with mortality (5.4% vs 5.8%; P = .442) as well as composite and individual outcomes of in-hospital mortality, contrast-induced nephropathy, bleeding, and stroke between surgical and nonsurgical centers.28
Table 1.
Studies on nonprimary PCI at centers with no-SOS published since 2014.
| Author | Study type | No. patients | Mortality | Em CABG | Comments |
|---|---|---|---|---|---|
| Lee et al,18 2015 | Meta-analysis 4 RCT, 19 registries |
No-SOS = 58,670 | 1.6% | 0.5% | No difference death (OR, 1.15; 95% CI, 0.94-1.41), EmCABG (OR, 1.14; 95% CI, 0.62-2.13), CVA, reMI, tamponade |
| SOS = 908,879 | 2.1% | 0.8% | |||
| Garg et al,16 2015 | UK Registry 2006-2012. (79% stable angina or NSTEMI) | No-SOS = 119,096 | 0.3% SA, 1.6% NSTEMI |
0.04% | Lower rates EmCABG at no-SOS (P < .001) No difference in death. 3-fold increase in No-SOS cases. |
| SOS = 264,917 | 0.4% SA, 1.7% NSTEMI |
0.1% | |||
| Akasaka et al,32 2017 | 3241 ACS patients from the Kumamoto Intervention Conference Study, Japan. | No-SOS = 477 | 2.9% | 0% | No difference in in-hospital mortality, cardiac death, nonfatal MI or stroke. Greater re-PCI at SOS for culprit vessels 12.9% vs 8.4% and nonculprit vessels 7.1% vs 4.6% compared with no-SOS. |
| SOS = 2764 | 3.7% | 0.1% | |||
| Goel et al,30 2017 | National Inpatient Sample (NIS) database 2003-2012 | No-SOS = 396,471 | 0.5% Elective 0.9% NSTEMI 4.2% STEMI |
NA | No difference TIA, CVA, transfusion. Less vascular injury with PCI at no-SOS (0.9% vs 1.1%, P < .001). 7-fold increase in no-SOS cases. |
| SOS = 6,515,491 | 0.4% Elective 0.9% NSTEMI 4.6% STEMI |
NA | |||
| Afana et al,28 2018 | PPCI at 47 hospitals in Michigan from January 2010 to December 2015 | No-SOS = 4091 (propensity score-matched population) |
5.8% | 1.9% | No difference in primary end point of all-cause, in-hospital mortality, contrast-induced nephropathy, NCDR defined bleeding, major bleeding, and stroke. Significant difference in emergency CABG (2.9% vs 1.9%, P = .0008). |
| SOS = 4091 | 5.4% | 2.9% | |||
| Dziewierz et al,36 2018 | 66,707 patients presenting with STEMI undergoing primary PCI from 154 centers in Poland | No-SOS = 51,667 | Whole cohort 1.6% Matched cohort 1.67% |
Lower mortality, no reflow and coronary perforation in matched cohort in SOS. | |
| SOS = 15,040 | Whole cohort 1.09% Matched cohort 1.04% |
||||
| Hannan et al,29 2019 | New York PCI registry 2013-2015 | No-SOS = 10,962 | 0.7% NSTEMI 2.8% STEMI |
NA | Adjusted mortality similar in all subgroups (STEMI, NSTEMI, and elective PCI). No difference in CVA or transfusion but less vascular injury at no-SOS (0.9% vs 1.1%, OR, 1.31; 95% CI, 1.26-1.35). |
| SOS = 65,735 | 0.7% NSTEMI 2.6% STEMI |
NA | |||
| Afana et al,17 2020 | Michigan BCBS PCI registry, nonprimary PCI 2016-2018 | No-SOS = 4721 | 0.5% | 0.3% | No difference in any clinical outcome in propensity-matched population. 3-fold increase in volume at no-SOS sites. |
| SOS = 46,096 | 0.6% | 0.5% | |||
| Waldo et al,19 2021 | VA CART registry | No-SOS = 21,856 | Overall rate 0.05% | No difference in death, CVA, EmCABG. No difference in high-risk lesions (31% vs 36%, P = .126). Decrease in no-SOS volume attributed to SCAI document in 2014. | |
| SOS = 53,708 | |||||
| Li et al,46 2021 | Claims database outpatient PCI, 2007-2016 propensity matched | ASC PCI = 849 | NA | NA | No difference in MI or hospitalization. ASC PCI increased risk of bleeding (location and severity of bleeding not noted). |
| Hosp OP PCI = 95,492 | NA | NA | |||
| Hanson et al,37 2022 | Victorian Cardiac Outcomes Registry data, Australia, unprotected LMS PCI | No-SOS = 136 | 30-day mortality = 24% | 1.5% | On-site cardiac surgery was not associated with in-hospital mortality (OR 0.68; 95% CI, 0.32-1.43; P = .31) or 30-day mortality (OR, 0.70; 95% CI, 0.33-1.48; P = .35). |
| SOS = 594 | 30-day mortality = 12% | 2.2% | |||
| Rashid et al,38 2022 | British Cardiovascular Interventional Society registry, LMS PCI, 2006-2020 | No-SOS = 13,922 | In-hospital mortality = 5.7% | 0.2% | No-SOS was not associated with in-hospital mortality (OR, 0.92; 95% CI, 0.69-1.22), in-hospital MACCE (OR, 1.00; 95% CI, 0.79-1.25) or emergency CABG (OR 1.00 95% CI 0.95-1.06). No-SOS had lower BARC 3-5 bleeding complications (OR, 0.53; 95% CI, 0.34-0.82). |
| SOS = 26,822 | In-hospital mortality = 7.0% | 0.1% |
ACS, acute coronary syndrome; MI, myocardial infarction; ASC, ambulatory surgery center; CART, Clinical Assessment Reporting and Tracking; CVA, cerebrovascular accident; EmCABG, emergency coronary artery bypass surgery; NA, not applicable; NCDR, National Cardiovascular Data Registry; MACCE, major adverse cardiovascular and cerebrovascular events; NSTEMI, non-ST elevation myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; PPCI, primary percutaneous coronary intervention; RCT, randomized controlled trial; reMI, recurrent myocardial infarction; SA, stable angina; SOS, surgery on site; STEMI, ST-elevation myocardial infarction; TIA, transient ischemic attack.
The New York PCI registry reported no significant difference in mortality or 2-year repeat target lesion PCI between no-SOS and surgical centers, with similar findings reported in the ST-elevation MI (STEMI) subgroup, except for 2-year repeat target lesion PCI which was lower in surgical centers.29 Similarly, an analysis from the National Inpatient Sample reported no significant difference in the rate of in-hospital mortality between no-SOS and surgical centers (OR, 1.01; 95% CI, 0.98-1.03) for acute coronary syndrome and elective PCIs, with similar odds of in-hospital transient ischemic attack or stroke.30 In contrast, the incidence of vascular injury was higher at centers with on-site surgery (1.1% vs 0.9%; adjusted OR, 1.31; 95% CI, 1.26-1.35), although there was no difference in the incidence of blood transfusion (0.7% vs 0.8%; adjusted OR, 1.02; 95%CI, 0.98-1.06).
Overall clinical complexity, as assessed by the NCDR CathPCI score, was greater for patients treated with PCI at VA surgical facilities (18.4) compared with those at sites without (17.9, P < .001). However, over time, anatomic complexity increased more in patients treated at no-SOS sites, such that by the end of the study, VA SYNTAX scores were similar between the 2 groups. Complications and mortality rates were similar across the subgroups at sites with and without cardiothoracic surgery.19 In summary, the most recent data fail to find any clinically significant differences in outcomes of PCI at surgical versus non-SOS PCI centers.
International data
Much of the data derived from the United States is from healthcare systems where no-SOS centers represent the minority of PCI cases undertaken. In contrast, there are no formal criteria regarding which patients cannot be treated at a no-SOS center in the United Kingdom, and PCI at these centers is the norm, and in fact represents the majority of PCI activity (74 of the 118 centers [63%] in 2020).16 Unlike in the United States, patients undergoing PCI at no-SOS sites were older, had a higher prevalence of previous PCI or CABG, and were more likely to undergo PCI for stable angina. Up to 40% of left main cases and 27% of cases using circulatory support (predominantly with the intra-aortic balloon pump) were undertaken in no-SOS centers. No significant differences in mortality were observed between surgical and nonsurgical centers following adjustments for differences in baseline covariates in the overall cohort, as well as in patients undergoing PCI for stable angina, non-STEMI, or STEMI.16
Dutch studies have shown that PPCI undertaken in no-SOS centers (14 of the 30 total PCI centers) is safe and associated with shorter door to balloon times, with similar major adverse cardiovascular event (MACE) rates to surgical centers (7.9% and 8.1%, respectively).31 Further data from Japan suggests no significant difference in clinical outcomes following PCI for acute coronary syndrome between hospitals with and without on-site cardiac surgery backup.32 Furthermore, in a recent report from Australia of 1179 cardiogenic shock patients, there was no difference in in-hospital MACCE and mortality if treated at a no-SOS hospital compared with a surgical center.33
A national report from Canada (excluding Quebec) using medico-administrative databases between 2016 and 2018 also confirmed the short-term safety of performing PCI without SOS.34 However, a study from Ontario on patients who were diagnosed with severe multivessel disease and were subsequently revascularized within 90 days, found a potential adverse association if diagnostic angiography was performed at no-SOS centers (hazard ratio, 1.09; 95% CI, 1.02-1.18 for death and hazard ratio, 1.10; 95% CI, 1.03-1.17 for MI).35 The mechanism of this poor outcome is uncertain because institutional capability was not predictive of referral for PCI vs CABG.
The Polish National Registry reported 66,707 patients undergoing PPCI from 154 centers, of which 22.6% were treated in surgical centers.36 On-site surgical backup was associated with a higher PCI annual volume (1098.7 ± 483.5 vs 662.4 ± 301.8; P < .001) but a lower operator PCI volume (207.8 ± 96.6 vs 226.7 ± 126.0; P < .001). Periprocedural mortality was lower in patients undergoing PPCI at surgical centers, and surgical backup (OR, 0.62; 95% CI, 0.52-0.74; P < .001) was independently associated with reduced periprocedural death.
PCI of complex lesions in no-SOS centers
Although previous analyses have focused on outcomes associated with overall PCI, there have been more limited data regarding high-risk lesion subsets. An analysis of 32 centers (17 surgical centers, 15 nonsurgical centers) that contribute to the Victorian Cardiac Outcomes Registry in Australia reported that 19% of unprotected left main procedures (136 of 730) were undertaken in no-SOS centers.37 Patients treated at no-SOS sites had a higher prevalence of left ventricular dysfunction, STEMI, and/or cardiogenic shock or required intubation, and had higher mortality and MACE rates. Importantly, however, on-site cardiac surgery was not independently associated with in-hospital mortality (OR, 0.68; 95% CI, 0.32-1.43; P = .31) or 30-day mortality (OR, 0.70; 95% CI, 0.33-1.48; P = .35). A large series of 40,744 left main PCIs from the United Kingdom reported these procedures were commonly being performed at no-SOS centers (36.7% of all left main PCIs in 2020).38 There was no association between surgery backup status and risk of death, MACCE, or emergency CABG, and interestingly, bleeding complications were lower at no-SOS centers. Other single center registries have shown the feasibility of PCI for unprotected left main at no-SOS sites but have lacked comparative data from surgical centers, making interpretation of outcomes challenging.
There have been no comparative studies in other complex PCI subgroups such as CTO and atherectomy; however, several observational studies have been reported. A retrospective analysis of 221 cases using orbital atherectomy at no-SOS sites reported an in-hospital MACE rate of 0.5% (1 MI) and 30-day MACE rate of 1.4%.39 In-hospital coronary perforation and no reflow were reported at 0.5% and procedural success was 97.3%. A retrospective analysis of 531 patients undergoing rotational atherectomy (RA) in 3 no-SOS centers in Australia noted 11 (2.1%) procedure-related deaths (of which 5 were directly attributable to RA) within 30 days. Complications directly attributable to RA included coronary dissection (1%), perforation (0.5%), tamponade (0.4%), and burr entrapment (1.3%). Only 2 patients (0.4%) were referred to off-site cardiac surgery for bailout.40 These complication rates are comparable to other case series with atherectomy, suggesting that atherectomy procedures can be safely performed in non-SOS centers.
One series of 20 antegrade CTO cases (mean J-CTO score 1.65 ± 1.2) reported an 85% success rate with 3 minor procedural complications.41 Only 2 patients had post-PCI MI and there were no in-hospital or 30-day deaths. A UK retrospective analysis of 276 CTO cases undertaken over a 5-year period from a single no-SOS center42 reported antegrade wire escalation was used in 82.2% (n = 227), retrograde wire escalation in 2.2% (n = 6), antegrade dissection re-entry in 8.7% (n = 24) and retrograde dissection re-entry in 6.9% (n = 19) of CTO cases. Success rates were 76% at first attempt by all operators. Complications included side branch occlusion in 3.5%, perforation in 4%, and cardiac tamponade in 1%. Death occurred in 1.4%, MI in 1.1%, target lesion revascularization in 1.8%, and cerebrovascular accident in 1.1%. Although such outcome data are undoubtedly difficult to interpret in the absence of a comparator group, they suggest that CTO procedures are feasible in centers with experienced operators but with higher complication rates than with other anatomic subsets.
PCI standards at hospitals without SOS, ASCs, and OBLs
Potential no-SOS settings
In the United States, there are several settings where no-SOS PCI may take place. Similar to Europe, there are no-SOS acute care hospitals performing PCI on an outpatient basis with same-day discharge. These hospitals provide the safety net of conversion to an inpatient stay if necessary as well as additional support services including an intensive care unit, anesthesia support, medical imaging (computed tomography, magnetic resonance imaging, and ultrasound), transfusions, renal replacement therapy, and emergency vascular surgery.
Unique to the United States, no-SOS PCI may also take place in a freestanding facility completely detached and geographically separate from a hospital. Staffing is often streamlined, consisting of an interventional cardiologist, nurses, technologists, and support staff. Two types of facilities exist: ASCs and OBLs. The 2 are primarily distinguished by the level of regulatory requirements and oversight. ASCs must meet requirements set forth by Medicare at the federal level as well as specific state requirements. Consistent with the name, OBLs are legally indistinct from a medical office and may exist within the physician’s medical office building or at a freestanding separate site. Compared with ASCs, OBLs have lower regulatory standards that are governed by state-specific policies.
Equipment and supplies
High-quality image acquisition and digital archive systems should be present in all catheterization laboratories regardless of setting. Fixed, mounted fluoroscopy systems should be the standard rather than mobile C-arm fluoroscopy machines. Portable ultrasound machines should be available with trained staff or physicians to obtain and interpret images, to facilitate vascular access, and for emergency assessment of ventricular dysfunction and to exclude pericardial tamponade. General resuscitation equipment including emergency airway kits, cardiac arrest and vasoactive medications, and defibrillators are mandatory.
Equipment for intravascular imaging and physiologic assessment are required for hospital facilities and strongly recommended for ASCs given the benefit for guiding PCI and reducing complications. Current Medicare reimbursement policy makes the use of these valuable adjunctive technologies in the ASC environment economically challenging but is scheduled to be corrected in 2023.
Catheterization laboratories should have an appropriate inventory of interventional and rescue equipment, including guide catheters, guide extension catheters, balloons, and stents in multiple sizes; thrombectomy and distal protection devices (if treating vein grafts); covered stents; temporary pacemakers; and pericardiocentesis trays. At minimum, an intra-aortic balloon pump should be available for mechanical support, and facilities that perform more complex PCI procedures should also have a percutaneous left ventricular assist device available.
Transfer agreements
Facilities without SOS should have transfer arrangements and protocols in place with a cardiac surgery facility to provide emergency surgery and ongoing care when necessary. A transfer protocol should outline communications between the ambulatory facility, Emergency Medical Services, and the receiving facility (see Supplemental Material 2 for example). Patients undergoing emergency or salvage cardiac surgery are high risk and may be financially unprofitable for the receiving institution, potentially causing delays in transfers in the absence of well-established transfer agreements (despite legal and ethical requirements to accept patients to a higher level of care). Collaborating institutions may be financially or organizationally tied, contractually obligated, or linked by memoranda of understanding.
Rapid transfer of critically ill patients requires appropriate ground or air transportation with appropriate support. Ambulances should be large enough to accommodate a balloon pump or ventricular assist device with an optimal goal to arrive at the no-SOS center within 30 minutes. Some facilities may choose to invest in their own transportation on site, should ambulance services fail to meet these requirements. If intensive care is necessary in transit, this may require members of the referring team to assist or travel with the patient. Transport protocols should be tested a minimum of 2 times per year involving both the referring and receiving facility.
Quality assurance
There is clearly the need for a standardized mechanism by which both ASC and OBL facilities—and those who provide patient care in such facilities—can be evaluated and credentialed.
Comparison with national benchmarks is critical to identify program deficiencies and opportunities for improvement. Quality and outcomes including procedural indications and complications must be reviewed regularly and entered into a national registry such as the NCDR and/or state-specific registries. The Outpatient Endovascular and Interventional Society is developing a registry specific to the ambulatory setting that should be available soon. In the future, registries may provide a pathway for the safe expansion of practice within the ambulatory setting and for assisting in the credentialing of interventional cardiologists.
A robust quality program is essential. Ideally, internal peer review should occur regularly with access to external review available. In programs with a single interventional cardiologist, a process for external peer review should be defined. There should be ongoing audits for MACE with predefined correction plans. Mock codes and bailout drills are strongly recommended to prepare staff for the possibility of serious, but infrequent, complications. An ideal practice is to conduct next-day and 30-day follow up calls to identify late presenting complications.
Informed consent
Respect for patient autonomy demands that patients receive full informed consent for their procedures. A need for emergency surgery, although rare in the modern era (0.2%), remains a potential complication. Patients must be informed that should emergency surgery be required during their PCI procedure, a transfer would be necessary. Documentation of this detail may be protective should legal action arise after an emergency transfer.
Operator requirements
Interventional cardiologists in non-SOS hospitals should be experienced and fully trained in resuscitation and the treatment of complications including vascular damage with bleeding, arrhythmias, acute vessel closure, cardiorespiratory arrest, pericardial tamponade, and shock. Advanced cardiovascular life support resuscitation certification and significant experience with mechanical circulatory support device insertion is required. New interventional cardiologists require mentorship and oversight and should generally avoid ASCs and complex procedures at a non-SOS facility. Operators should be board-certified in interventional cardiology and unless experienced or very experienced, should average at least 50 PCI procedures annually. Individual operator volume is only one of several factors that should be considered in assessing operator competence, which include lifetime experience, experience with other cardiovascular interventions, quality assessment of ongoing performance, and institutional volume.
Staff requirements
Facility administrative leadership support is necessary to maintain minimum staffing requirements in the cardiac catheterization laboratory. Required roles include administration of sedation and airway monitoring, recorder, and circulator. Nurses and technicians should have appropriate training and certification to work in a critical care catheterization laboratory environment, including advanced cardiovascular life support training, electrocardiogram recognition, airway management, hemodynamic monitoring, and management of temporary pacemakers, balloon pumps, and ventricular assist devices. All staff should be fully trained in algorithms for cardiopulmonary resuscitation and patient emergency evacuation/transfer protocols. As with interventional cardiologists, novice staff should not be placed in high-risk situations until they have received adequate mentorship and experience.
Surgical consultation
Patients often undergo ad hoc elective PCI without consultation with a cardiac surgeon, both at SOS and non-SOS facilities. Although there are limited direct data supporting the Heart Team approach, clinical guidelines strongly endorse the practice to ensure the best care of the patient. Prior documents3 attempted to formalize the role of the cardiac surgeon by recommending that the surgeon have privileges at the referring facility and regular meetings with the referring interventional cardiologists. In the current era, formal staff privileges for off-site cardiac surgeons are rarely extended or necessary. However, the principles of the Heart Team approach should be operationalized through regular communication of referrals, reviews of cases performed, and comparison with guideline recommendations and appropriate use criteria. Ad hoc elective PCI should be performed primarily in patients where the guidelines and good judgment are clearly in favor of PCI, whereas in borderline cases, especially including patients with intermediate or high SYNTAX scores, we strongly recommend that a Heart Team approach, and at a minimum a surgical consultation, should follow the diagnostic angiogram.
Case selection and management
Notably, the United Kingdom and Canada have no formal criteria restricting the type of PCI or patient subgroup that can be treated with PCI with no-SOS. By contrast, prior US statements recommended avoidance of long, calcified, or angulated lesions, nonculprit lesions, and unprotected left main cases, and explicitly precluded the performance of CTO-PCI without cardiac surgery backup.3,5 However, despite the goal of protecting patients, these recommendations may have restricted practice, limited patient choice, and exposed interventional cardiologists to legal risk. Such prohibitions have become outdated as the skill of interventional cardiologists and technological advances have expanded treatment options, outcomes data show no harm with PCI with no-SOS, and government policies actively encourage moving care to lower-cost areas. In particular, the prohibition of rotational and other atherectomy devices can paradoxically result in increased risk to the patient when balloon angioplasty is attempted in a calcified vessel. Similarly, CTO-PCI tools and techniques have advanced significantly and the risk of antegrade wire escalation, antegrade dissection-reentry, and retrograde septal approaches may be acceptable at selected, experienced no-SOS facilities, although operator experience and available rescue equipment must factor into decision making.
We propose a new algorithm that takes into account not only the patients’ clinical risk and lesion risk but also the rescue capabilities of the site (Table 2). Equally important is the experience (both recent and accumulated) of the interventional cardiologists on site, as such experience is essential for accurate risk assessment, complication identification and management, and knowledge of rescue options (Table 3).
Table 2.
Case selection.
| ASC/OBL | Level 1 No-SOS Hospital | Level 2 No-SOS Hospital | Cardiac Surgery Facility | |
|---|---|---|---|---|
| Typical characteristics | No ICU, Code team, blood bank. | Low volume (<200 PCI) cath lab | Experienced interventional cardiologists Well-staffed team (4/room) Well-resourced Often multiple cath labs and ORs 24/7 ICU/anesthesia/radiology/OR support |
Experienced interventional cardiologists High-volume cath lab Structural heart procedures Well-staffed, resourced, on-call cath lab team Multiple operating rooms On-call cardiac surgeon and perfusionist Shock team |
| Rescue/support capabilities | IABP | IABP | IABP pVAD or ECMO Vascular/thoracic surgery |
IABP pVAD Cardiopulmonary bypass +/-ECMO +/- RVAD +/- LVAD +/- transplant |
| Plaque modification devices | Often cutting balloon or IVL | Often cutting balloon or IVL | Cutting balloon Rotational atherectomy Orbital atherectomy IVL |
Cutting balloon Rotational atherectomy Orbital atherectomy IVL |
| Cases that may be higher risk to avoid | High transfusion risk Calcified lesions Atherectomy Low EF CTO Unprotected left main Degenerated vein grafts |
Calcified lesions Atherectomy Low EF CTO Unprotected left main Degenerated vein grafts |
Epicardial retrograde CTO Last remaining vessel/conduit |
Cath lab, catheterization laboratory; CTO, chronic total occlusion; ECMO, extracorporeal membrane oxygenation; EF, ejection fraction; IABP, intra-aortic balloon pump; ICU, intensive care unit; IVL, intravascular lithotripsy; LVAD, left ventricular assist device; OR, operating room; pVAD, percutaneous ventricular assist device; PCI, percutaneous coronary intervention; RVAD, right ventricular assist device.
Table 3.
Operator experience.
| New interventional cardiologist | Experienced interventional cardiologist | Very experienced interventional cardiologist |
|---|---|---|
| <3 y of experience | 3-10 y of experience | >10 y of experience |
| Limited exposure to atherectomy devices | Competent to use atherectomy devices | Extensive complex PCI experience |
| Limited STEMI/shock experience | Intermediate experience in STEMI/shock | Significant STEMI/shock experience |
| Limited prior experience and judgment, familiar with guidelines only | Prior practice in cardiac surgery facility, is familiar with surgical perspective | |
| Should avoid ASCs, independent atherectomy cases, and have case selection reviewed by colleague and scrub in on higher risk cases | Should be able to independently practice all of IC in any setting with standard facility oversight | |
ASC, ambulatory surgery center; IC, interventional cardiology ; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction.
PCI in freestanding ambulatory locations
The United States has been leading the migration of PCI outside of the hospital, driven by market forces and reimbursement policies. PCI outside the hospital setting may be performed in a freestanding ASC or OBL. For simplification of discussion, PCI in both settings will be referred to as Ambulatory PCI (AMB-PCI). ASCs and OBLs can provide more convenient and timely care, are more local for patients, and reduce costs. ASCs must meet criteria outlined by Medicare at the federal level, as well as any additional state requirements.43,44 Many states also have certificate of need laws that must be met prior to beginning a PCI program. ASCs and OBLs should meet the same facility, equipment, supplies, and other common requirements for catheterization laboratories as noted above.
At present, only 0.9% of 2021 Medicare claims for coronary stenting Current Procedural Terminology (CPT) code 92928 occurred in ASCs, with the remaining 99% split evenly between inpatient and outpatient hospital procedures.45 In a commercial insurance claims database, 0.9% of ambulatory PCIs from 2007 to 2016 were done in ASCs and 99.1% were done in hospital outpatient departments.46 However, the Bain & Company Medtech Physician survey estimates that up to 33% of all cardiac procedures will move to the ambulatory setting in the coming years.47
As noted previously, judicious case selection is paramount for the safe performance of AMB-PCI. Most patients with acute coronary syndromes are admitted to hospitals and therefore are not considered for procedures in ASCs/OBLs. Patient comorbidities, particularly those that might require ancillary support, would favor the hospital setting:
-
1.
Decompensated heart failure/severe left ventricular dysfunction
-
2.
Respiratory compromise (hypoxia at rest)
-
3.
High risk of blood transfusion
-
4.
At risk for acute kidney injury
-
5.
History of severe contrast allergy
-
6.
Critical valvular heart disease
-
7.
Any condition likely to require overnight observation
Other scenarios not listed here may also favor the hospital setting; the guiding principle for the physician should be to avoid cases with a significant possibility of requiring support beyond what can be readily provided in the ambulatory setting.
Some lesion subsets carry higher risk and therefore should be approached with caution for AMB-PCI. Unprotected left main lesions, heavily calcified lesions, CTOs, and vein grafts should generally be considered for transfer to a setting with greater support. Yet even these lesions can be, and have been, done in ASC/OBL environments. It is incumbent on the physician to exercise good judgment and practice within the limits of both their own skillset and that of their team. Although ad hoc AMB-PCI is common practice, interventional cardiologists should strongly consider staged PCI for lesions with an increased risk of complications.48
The same standards for credentialing should apply across all places of service. This applies to both interventional cardiologists and staff. Early career interventional cardiologists and inexperienced staff should avoid the AMB-PCI environment. Independent practice within the ambulatory environment should be reserved for experienced interventional cardiologists with an established record of acceptable outcomes.
Currently, there are little published data on outcomes with AMB-PCI. Using a commercial insurance database from 2007 to 2016, 0.9% of PCIs (n = 849) performed in an ASC were less likely to undergo physiologic assessment and more likely to have bleeding complications compared with hospital outpatient procedures.46 Additional nonpublished data are available from National Cardiovascular Partners, which manages 20 catheterization laboratories and ASCs in 6 states with 135 interventional cardiologists performing PCI. Three-day and 30-day PCI outcomes have been collected on 10,581 patients from 2013 through 2021. The combined urgent and emergent transfer rate from the centers following PCI was 0.87% (n = 92), hospitalization rate within 72 hours after discharge home from the facility was 0.04% (n = 4), and cardiovascular death rate of 0.04% (n = 4) at 30 days from 2013 to 2021 (internal data-unpublished, personal communication courtesy of Kelly Bemis). A critical need in this space will be the publication of such data, reporting of registry results, and ultimately conducting prospective collaborative studies.
Reimbursement and economic considerations
The economics of insurance reimbursement in the United States strongly favors the outpatient migration of PCI. Most coronary interventions in no-SOS facilities involve hospital inpatients or outpatients, but increasingly, coronary interventions are performed in ASCs although the number remains small. Although physicians are often oblivious to financial considerations regarding the hospital, physicians who have a financial interest in an ambulatory place of service as investors, owners, or practitioners are significantly affected by costs and reimbursement.
Reimbursement for PCI services serves 2 purposes. The first is payment for the professional work performed by the physician. The second is reimbursement for the facility cost of providing PCI services. Physician work is generally described using CPT codes. CMS assigns relative value units (RVUs) to each CPT code. CMS payments to providers are based on the physician work RVU of a service multiplied by the conversion factor, which is set annually by CMS ($33.59 for 2022), with small modifications for local factors.49,50 Most private payers recognize CPT codes and reimburse for services based on the service’s RVU value multiplied by the payer’s conversion factor. Payment for physician effort often constitutes a small proportion of the total cost of a procedure (often <10%) and is generally agnostic to the practice setting (with the exception of a global fee for OBLs).
CMS reimbursement for the cost of providing PCI services is based on the facility expense, which varies according to the place of service. CMS recognizes 50 different places of service,51 with Table 4 including those relevant to this discussion.
Table 4.
Centers for Medicare & Medicaid Services designations of place of service and applicable reimbursement.43,44
| Place of service | CMS designation for place of service | Commercial payors | CMS covers | Facility CMS reimbursement | Provider CMS reimbursement |
|---|---|---|---|---|---|
| Inpatient hospital care | Place of service 21 | All PCI | All PCI | DRG + | CPT |
| Outpatient hospital procedures | Place of service 22 | All PCI (excluding CTO, STEMI) | All PCI (excluding CTO, STEMI) | APC | CPT |
| Ambulatory surgical centers | Place of service 24 | Similar to CMS coverage with some contractual exceptions | Ambulatory PCI excluding CTO, bypass grafts, atherectomy | ASC | CPT |
| Physician office-based laboratory | Place of service 11 | PCI in many states | Diagnostic heart cath only | CPT global payment | |
APC, ambulatory payment classifications; ASC, ambulatory surgery center; cath, catheterization; CMS, Centers for Medicare & Medicaid Services; CPT, Current Procedural Terminology; CTO, chronic total occlusion; DRG, diagnosis-related group; STEMI, ST-elevation myocardial infarction.
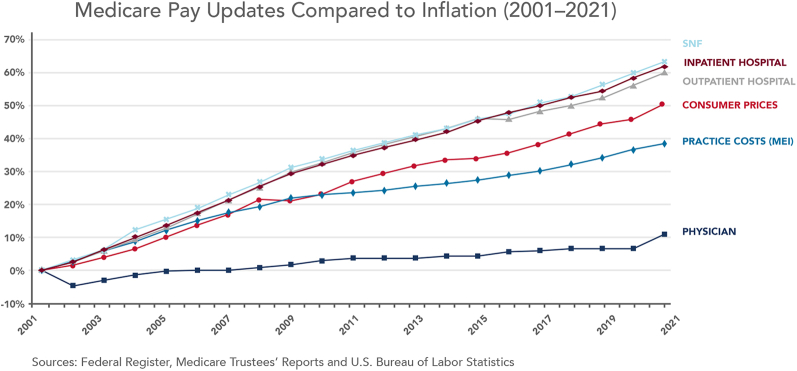
The facility reimbursement varies widely from one place of service to another, with reimbursements higher for hospital inpatients compared with hospital outpatients, which in turn is higher than ASCs and OBLs. For example, CMS payment for CPT code 92928 (coronary stenting) for 2022 is $5618 in the ASC setting compared with $10,258 in the hospital outpatient setting.49 Hospital costs are higher and reimbursed accordingly because of higher facility overhead costs, management costs, and compliance costs, along with cross-subsidizing of less profitable service lines and uncompensated care. Another major reason for higher costs based on site of service is that while physician reimbursement has only increased 7% from 2001 to 2021 (0.35% annually), facility fees have increased 60% (2.4% annually), outpacing inflation.52 Figure 2 and Table 5 demonstrate some examples of the resulting differences in total payments for a procedure based on location and type of insurance.53,54
Figure 2.
Medicare pay updates compared with inflation (2001-2021). According to data from Medicare & Medicaid Services (CMS), Medicare physician pay has increased just 11% over the last 2 decades, or 0.5% per year on average, compared with 60% for hospital fee updates and a 39% increase in practice expenses over the same period. MEI, Medicare Economic Index; SNF, skilled nursing facility.
Source: American Medical Association, Economic and Health Policy Research, October 2021.
Available from ama-assn.org/system/files/medicare-pay-chart-2021.pdf
Table 5.
Example reimbursement differences based on place of service and type of insurance.
| Place of service | Diagnostic catheterization facility fee | PCI facility fee, Single vessel DES | Physician professional fee |
|---|---|---|---|
| Hospital outpatient-commercial insurancea | $8100 | $29,426 | Contractual rates |
| Hospital outpatient-Medicareb | $2962 | $10,259 | $137-$436 for cath $628 one-vessel DES |
| ASC-Medicareb | $1321 | $6111 | $253-$650 depending on procedure |
| ASC commercial | Contractual rates | Contractual rates | Contractual rates |
| OBL Medicareb | $891-$1418 | Not covered | Global payment |
| OBL commercial | Contractual rates | Contractual rates in certain states | Global payment |
ASC, ambulatory surgery center; cath, catheterization; DES, drug-eluting stent; OBL, office-based laboratory; PCI, percutaneous coronary intervention.
Contractual average estimate based on Shields et al9 showing average commercial rate was 293% of Medicare rate
Based on US Medicare rates for 2022 published on CMS.gov
The above examples illustrate the large savings to payers that can accrue from a transition to AMB-PCI, and why. The responsible migration of PCI from the hospital outpatient setting to the ASC/OBL can provide value-based care and reduced costs for overburdened health care systems without incurring unnecessary risk.
Policy risks in the transition to AMB-PCI include inadvertently incentivizing higher cost or lower quality care. For instance, for hospital-based outpatient procedures, the patient copayment under Medicare is subject to a cap. Such cap does not apply to ASC-based procedures, thus making the patient copay potentially exceed that in the hospital outpatient department, even when such procedures are significantly less expensive to Medicare in the ASC. Deeply discounted reimbursement of PCI services at ASCs may force ASCs to affiliate with large systems and stifle competition. Lower profit margins for procedures in ASCs can potentially encourage unnecessary use, although this has not been demonstrated to date. The current absence of additional reimbursement for intracoronary imaging and hemodynamic assessment discourages the availability and use of these proven technologies, but this policy error is scheduled to be rectified according to the CMS proposed rule for 2023.
Ambulatory surgery centers can be very profitable when performing procedures for patients with private insurance. For example, in 2020, the average profit margin for ASCs in Pennsylvania was 23.4%.55 Economically and clinically successful ASCs have low overhead costs, low costs of compliance with quality programs, careful selection of patients, and efficiencies because of the close involvement of physicians. Headwinds that may be faced by ASCs providing cardiac procedures are the costs of new CMS-mandated programs, the cost of maintaining equipment for emergencies (eg, balloon pumps, covered stents etc.), and migration of increasingly complex (and therefore expensive) cardiac procedures to the ASC setting.
Summary
PCI with no-SOS is as safe as PCI at centers with on-site surgery across randomized controlled trials, observational studies, and international experiences. Adequate operator experience, appropriate clinical judgment and case selection, and facility preparation are essential to a safe and successful PCI program with no-SOS. The economic benefits of PCI with no-SOS have driven and will continue to drive payers toward the migration of PCI to the ambulatory setting. This expert consensus statement summarizes the evidence supporting PCI with no-SOS and provides the community with the guidance necessary for this transition.
Declaration of competing interest
Cindy Grines, Rymer, Lyndon Box, Yazan Khatib, Theodore Schreiber, Stephen Waldo, and Alexis Matteau reported no financial interests. Arnold Seto has received speaker fees from Terumo and is a consultant for Medtronic. Poonam Velagapudi receives travel funding from Boston Scientific and Medtronic, and has received speaker fees from Medtronic. Jeffrey Carr holds equity in an ASC-OBL and is a consultant for Abbott. James Blankenship is a principal investigator for research on supersaturated oxygen infusion after anterior MI funded by Zoll.
J. Dawn Abbott is a consultant for Boston Scientific. Mamas A. Mamas has received speaker fees from Terumo and is a principal investigator for registry-based research on complex PCI outcomes funded by Abbott. Mladen I. Vidovich holds equity of intellectual property related to catheter technology and is a principal investigator for research on optical coherence tomography in left main funded by Boston Scientific. William Kent is a consultant for Medtronic and is an advisory to Abbott.
Nick Curzen is a chief investigator for the RIPCORD2 trial funded by Boston Scientific, FORECAST trial funded by HeartFlow, has received research grants from Boston Scientific and Beckmann Coulter, has received speaker fees from Abbott, Boston Scientific, and Edwards Lifesciences, has received travel funds from Biosensors, Medtronic, Edwards LifeSciences, and Boston Scientific, and is a consultant for Abbott, Boston Scientific, and Edwards LifeSciences.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Peer Review Statement
Given her role as associate editor, Cindy L. Grines had no involvement in the peer review of this article and has no access to information regarding its peer review.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at https://doi.org/10.1016/j.jscai.2022.100560.
Supplementary material
References
- 1.Richardson S.G., Morton P., Murtagh J.G., O’Keeffe D.B., Murphy P., Scott M.E. Management of acute coronary occlusion during percutaneous transluminal coronary angioplasty: experience of complications in a hospital without on site facilities for cardiac surgery. BMJ. 1990;300(6721):355–358. doi: 10.1136/bmj.300.6721.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dehmer G.J., Blankenship J., Wharton T.P., et al. The current status and future direction of percutaneous coronary intervention without on-site surgical backup: an expert consensus document from the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2007;69(4):471–478. doi: 10.1002/ccd.21097. [DOI] [PubMed] [Google Scholar]
- 3.Dehmer G.J., Blankenship J.C., Cilingiroglu M., et al. SCAI/ACC/AHA expert consensus document: 2014 update on percutaneous coronary intervention without on-site surgical backup. Catheter Cardiovasc Interv. 2014;84(2):169–187. doi: 10.1002/ccd.25371. [DOI] [PubMed] [Google Scholar]
- 4.Bradley S.M., Kaltenbach L.A., Xiang K., et al. Trends in use and outcomes of same-day discharge following elective percutaneous coronary intervention. JACC Cardiovasc Interv. 2021;14(15):1655–1666. doi: 10.1016/j.jcin.2021.05.043. [DOI] [PubMed] [Google Scholar]
- 5.Box L.C., Blankenship J.C., Henry T.D., et al. SCAI position statement on the performance of percutaneous coronary intervention in ambulatory surgical centers. Catheter Cardiovasc Interv. 2020;96(4):862–870. doi: 10.1002/ccd.28991. [DOI] [PubMed] [Google Scholar]
- 6.Alkhouli M., Alqahtani F., Kalra A., et al. Trends in characteristics and outcomes of patients undergoing coronary revascularization in the United States, 2003-2016. JAMA Netw Open. 2020;3(2) doi: 10.1001/jamanetworkopen.2019.21326. [DOI] [PubMed] [Google Scholar]
- 7.Waldo S.W., Gokhale M., O’Donnell C.I., et al. Temporal trends in coronary angiography and percutaneous coronary intervention: insights from the VA clinical assessment, reporting, and tracking program. JACC Cardiovasc Interv. 2018;11(9):879–888. doi: 10.1016/j.jcin.2018.02.035. [DOI] [PubMed] [Google Scholar]
- 8.Valle J.A., Tamez H., Abbott J.D., et al. Contemporary use and trends in unprotected left main coronary artery percutaneous coronary intervention in the United States: an analysis of the National Cardiovascular Data Registry research to practice initiative. JAMA Cardiol. 2019;4(2):100–109. doi: 10.1001/jamacardio.2018.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shields M.C., Ouellette M., Kiefer N., et al. Characteristics and outcomes of surgically ineligible patients with multivessel disease treated with percutaneous coronary intervention. Catheter Cardiovasc Interv. 2021;98(7):1223–1229. doi: 10.1002/ccd.29508. [DOI] [PubMed] [Google Scholar]
- 10.Nairooz R., Parzynski C.S., Curtis J.P., et al. Contemporary trends, predictors and outcomes of perforation during percutaneous coronary intervention (from the NCDR cath PCI registry) Am J Cardiol. 2020;130:37–45. doi: 10.1016/j.amjcard.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Masoudi F.A., Ponirakis A., de Lemos J.A., et al. Executive summary: trends in U.S. Cardiovascular care: 2016 report from 4 ACC National Cardiovascular Data Registries. J Am Coll Cardiol. 2017;69(11):1424–1426. doi: 10.1016/j.jacc.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Young M.N., Kolte D., Cadigan M.E., et al. Multidisciplinary heart team approach for complex coronary artery disease: single center clinical presentation. J Am Heart Assoc. 2020;9(8) doi: 10.1161/JAHA.119.014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doll J.A., Plomondon M.E., Waldo S.W. Characteristics of the quality improvement content of cardiac catheterization peer reviews in the Veterans Affairs clinical assessment, reporting, and tracking program. JAMA Netw Open. 2019;2(8) doi: 10.1001/jamanetworkopen.2019.8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dehmer G.J., Badhwar V., Bermudez E.A., et al. 2020 AHA/ACC key data elements and definitions for coronary revascularization: a report of the American College of Cardiology/American Heart Association task force on clinical data standards (writing committee to develop clinical data standards for coronary revascularization) J Am Coll Cardiol. 2020;75(16):1975–2088. doi: 10.1016/j.jacc.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs A.K., Normand S.L., Massaro J.M., et al. Nonemergency PCI at hospitals with or without on-site cardiac surgery. N Engl J Med. 2013;368(16):1498–1508. doi: 10.1056/NEJMoa1300610. [DOI] [PubMed] [Google Scholar]
- 16.Garg S., Anderson S.G., Oldroyd K., et al. Outcomes of percutaneous coronary intervention performed at offsite versus onsite surgical centers in the United Kingdom. J Am Coll Cardiol. 2015;66(4):363–372. doi: 10.1016/j.jacc.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 17.Afana M., Koenig G.C., Seth M., et al. Trends and outcomes of non-primary PCI at sites without cardiac surgery on-site: the early Michigan experience. PLOS ONE. 2020;15(8) doi: 10.1371/journal.pone.0238048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J.M., Hwang D., Park J., Kim K.J., Ahn C., Koo B.K. Percutaneous coronary intervention at centers with and without on-site surgical backup: an updated meta-analysis of 23 studies. Circulation. 2015;132(5):388–401. doi: 10.1161/CIRCULATIONAHA.115.016137. [DOI] [PubMed] [Google Scholar]
- 19.Waldo S.W., Hebbe A., Grunwald G.K., Doll J.A., Schofield R. Clinical and anatomic complexity of patients undergoing coronary intervention with and without on-site surgical capabilities: insights from the Veterans Affairs Clinical Assessment, Reporting and Tracking (CART) Program. Circ Cardiovasc Interv. 2021;14(1) doi: 10.1161/CIRCINTERVENTIONS.120.009697. [DOI] [PubMed] [Google Scholar]
- 20.Ezad S., Williams T.D., Condon J., Boyle A.J., Collins N.J. Common themes in patients requiring urgent cardiothoracic surgery after percutaneous coronary interventions: case series and review of the literature. Cardiovasc Revasc Med. 2018;19(8):976–979. doi: 10.1016/j.carrev.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Pancholy S.B., Patel G.A., Patel N.R., et al. Trends, outcomes, and predictive score for emergency coronary artery bypass graft surgery after elective percutaneous coronary intervention (from a nationwide dataset) Am J Cardiol. 2021;144:46–51. doi: 10.1016/j.amjcard.2020.12.060. [DOI] [PubMed] [Google Scholar]
- 22.Verevkin A., von Aspern K., Leontyev S., Lehmann S., Borger M.A., Davierwala P.M. Early and long-term outcomes in patients undergoing cardiac surgery following iatrogenic injuries during percutaneous coronary intervention. J Am Heart Assoc. 2019;8(1) doi: 10.1161/JAHA.118.010940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiraide T., Sawano M., Shiraishi Y., et al. Impact of catheter-induced iatrogenic coronary artery dissection with or without postprocedural flow impairment: a report from a Japanese multicenter percutaneous coronary intervention registry. PLOS ONE. 2018;13(9) doi: 10.1371/journal.pone.0204333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slottosch I., Liakopoulos O., Kuhn E., et al. Outcome after coronary bypass grafting for coronary complications following coronary angiography. J Surg Res. 2017;210:69–77. doi: 10.1016/j.jss.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Thielmann M., Wendt D., Slottosch I., et al. Coronary artery bypass graft surgery in patients with acute coronary syndromes after primary percutaneous coronary intervention: a current report from the North-Rhine Westphalia surgical myocardial infarction registry. J Am Heart Assoc. 2021;10(18) doi: 10.1161/JAHA.121.021182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwok C.S., Sirker A., Nolan J., et al. A national evaluation of emergency cardiac surgery after percutaneous coronary intervention and postsurgical patient outcomes. Am J Cardiol. 2020;130:24–29. doi: 10.1016/j.amjcard.2020.05.041. [DOI] [PubMed] [Google Scholar]
- 27.Aversano T., Lemmon C.C., Liu L., Atlantic CPORT Investigators Outcomes of PCI at hospitals with or without on-site cardiac surgery. N Engl J Med. 2012;366(19):1792–1802. doi: 10.1056/NEJMoa1114540. [DOI] [PubMed] [Google Scholar]
- 28.Afana M., Gurm H.S., Seth M., Frazier K.M., Fielding S., Koenig G.C. Primary percutaneous coronary intervention at centers with and without on-site surgical support: insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) Am Heart J. 2018;195:99–107. doi: 10.1016/j.ahj.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Hannan E.L., Zhong Y., Wu Y., et al. Treatment of coronary artery disease and acute myocardial infarction in hospitals with and without on-site coronary artery bypass graft surgery. Circ Cardiovasc Interv. 2019;12(1) doi: 10.1161/CIRCINTERVENTIONS.118.007097. [DOI] [PubMed] [Google Scholar]
- 30.Goel K., Gupta T., Kolte D., et al. Outcomes and temporal trends of inpatient percutaneous coronary intervention at centers with and without on-site cardiac surgery in the United States. JAMA Cardiol. 2017;2(1):25–33. doi: 10.1001/jamacardio.2016.4188. [DOI] [PubMed] [Google Scholar]
- 31.Koolen K.H., Mol K.A., Rahel B.M., et al. Off-site primary percutaneous coronary intervention in a new centre is safe: comparing clinical outcomes with a hospital with surgical backup. Neth Heart J. 2016;24(10):581–588. doi: 10.1007/s12471-016-0872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akasaka T., Hokimoto S., Sueta D., et al. Clinical outcomes of percutaneous coronary intervention for acute coronary syndrome between hospitals with and without onsite cardiac surgery backup. J Cardiol. 2017;69(1):103–109. doi: 10.1016/j.jjcc.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Noaman S., Vogrin S., Dinh D., et al. Percutaneous coronary intervention volume and cardiac surgery availability effect on acute coronary syndrome-related cardiogenic shock. JACC Cardiovasc Interv. 2022;15(8):876–886. doi: 10.1016/j.jcin.2022.01.283. [DOI] [PubMed] [Google Scholar]
- 34.Canadian Institute for Health Information. Cardiac Care Quality Indicators Report: Data Tables. 2020. https://www.cihi.ca/sites/default/files/document/ccqi-data-tables-2020-en.xlsx Accessed July 31, 2022.
- 35.Rocha R.V., Wang X., Fremes S.E., et al. Variations in coronary revascularization practices and their effect on long-term outcomes. J Am Heart Assoc. 2022;11(5) doi: 10.1161/JAHA.121.022770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dziewierz A., Brener S.J., Siudak Z., et al. Impact of on-site surgical backup on periprocedural outcomes of primary percutaneous interventions in patients presenting with ST-segment elevation myocardial infarction (from the ORPKI Polish National Registry) Am J Cardiol. 2018;122(6):929–935. doi: 10.1016/j.amjcard.2018.05.047. [DOI] [PubMed] [Google Scholar]
- 37.Hanson L., Vogrin S., Noaman S., et al. Long-term outcomes of unprotected left main percutaneous coronary intervention in centers without onsite cardiac surgery. Am J Cardiol. 2022;168:39–46. doi: 10.1016/j.amjcard.2021.12.051. [DOI] [PubMed] [Google Scholar]
- 38.Rashid M., Zaman M., Ludman P., et al. Left main stem percutaneous coronary intervention: does on-site surgical cover make a difference? Circ Cardiovasc Interv. 2022;15(10) doi: 10.1161/CIRCINTERVENTIONS.122.012037. [DOI] [PubMed] [Google Scholar]
- 39.Rao L.G., Rao A.M., Rao S.P., et al. Outcomes after coronary orbital atherectomy at centers without on-site surgical backup: diabetics versus non-diabetics and impact of access site. Cardiovasc Revasc Med. 2021;30:20–25. doi: 10.1016/j.carrev.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 40.Malhotra G., Stewart P. Outcomes of rotational atherectomy in three Large Queensland centres without onsite cardiac surgical backup in a contemporary patient cohort – a 9-year experience. Heart Lung Circ. 2022;31(suppl 3):S346. doi: 10.1016/j.hlc.2022.06.609. [DOI] [Google Scholar]
- 41.Akinseye O.A., Haji S.A., Koshy S.K.G., Ibebuogu U.N., Khouzam R.N., Garg N. Outcomes of percutaneous antegrade intraluminal coronary intervention of chronic total occlusion with remote surgical backup. Curr Probl Cardiol. 2019;44(12) doi: 10.1016/j.cpcardiol.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Alaour B., Onwordi E., Khan A., et al. Outcome of left main stem percutaneous coronary intervention in a UK nonsurgical center: a 5-year clinical experience. Catheter Cardiovasc Interv. 2022;99(3):601–606. doi: 10.1002/ccd.29530. [DOI] [PubMed] [Google Scholar]
- 43.Code of Federal Regulations, Title 42, Chapter IV, part 416.30. Ambulatory Surgical Centers. Government Publishing Office February 6, 2020. https://www.ecfr.gov/current/title-42/chapter-IV/subchapter-B/part-416/subpart-B/section-416.30
- 44.Jain K.M. 1st ed. Elsevier; 2020. Office-Based Endovascular Centers. [Google Scholar]
- 45.American Medical Association Relative Value Update database. Physician Payment Policy and Systems, American Medical Association, Chicago, IL Accessed July 31, 2022. https://commerce.ama-assn.org/store/ui/catalog/productDetail?product_id=prod280002&navAction=push.
- 46.Li K., Kalwani N.M., Heidenreich P.A., Fearon W.F. Elective percutaneous coronary intervention in ambulatory surgery centers. JACC Cardiovasc Interv. 2021;14(3):292–300. doi: 10.1016/j.jcin.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 47.van Biesen T., Johnson T., Bain & Company Ambulatory surgery center growth accelerates: is Medtech ready? https://www.bain.com/insights/ambulatory-surgery-center-growth-accelerates-is-medtech-ready/
- 48.Blankenship J.C., Moussa I.D., Chambers C.C., et al. Staging of multivessel percutaneous coronary interventions: an expert consensus statement from the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2012;79(7):1138–1152. doi: 10.1002/ccd.23353. [DOI] [PubMed] [Google Scholar]
- 49.2021 Cardiovascular Reimbursement Update. Medtronic. https://www.medtronic.com/content/dam/medtronic-com/us-en/hcp/reimbursement/documents/hepp-coronary-apv-reimbursement-update.pdf
- 50.CY 2022 Medicare Hospital Outpatient Prospective Payment System and Ambulatory Surgical Center Payment System Final Rule (CMS-1753FC). Centers for Medicare & Medicaid Services. https://www.cms.gov/newsroom/fact-sheets/cy-2022-medicare-hospital-outpatient-prospective-payment-system-and-ambulatory-surgical-center-0 Accessed February 14 2022.
- 51.Place of Service Codes for Professional Claims. Centers for Medicare & Medicaid Services. https://www.cms.gov/Medicare/Coding/place-of-service-codes/Place_of_Service_Code_Set
- 52.Medicare updates compared to inflation (2001-2021). American Medical Association, Economic and Health Policy Research, October 2021. https://www.ama-assn.org/system/files/medicare-pay-chart-2021.pdf
- 53.White C., Whaley C.M. Prices Paid to Hospitals by Private Health Plans Are High Relative to Medicare and Vary Widely - Findings from an Employer-Led Transparency Initiative. Rand Corporation. Document Number: RR-3033-RWJ. 2019. doi:10.7249/RR3033. https://www.rand.org/pubs/research_reports/RR3033.html [PMC free article] [PubMed]
- 54.Ambulatory Surgical Center (ASC) Payment. Centers for Medicare & Medicaid Services. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ASCPayment
- 55.George J. Philadelphia Business Journal. The most profitable Philadelphia-area ambulatory surgical centers in fiscal 2020. https://www.bizjournals.com/philadelphia/news/2021/11/17/phc4-ambulatory-surgery-centers-pennsylvania-2020.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.