Abstract
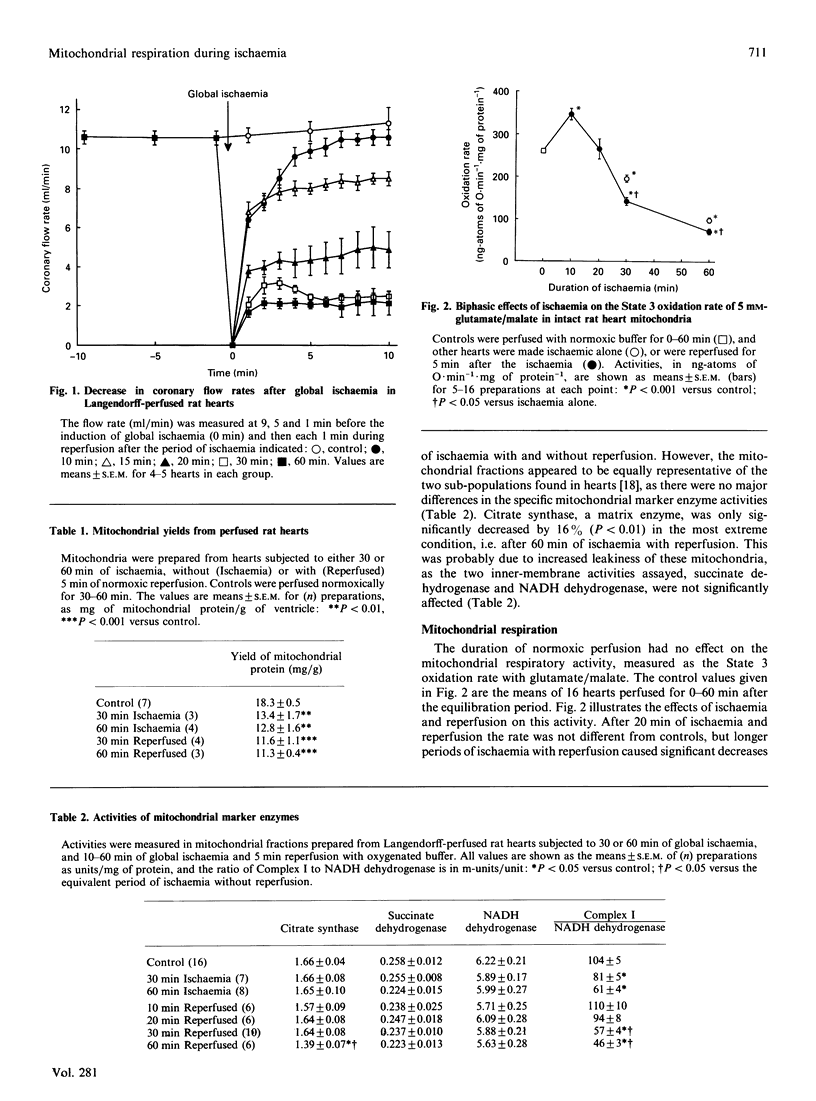
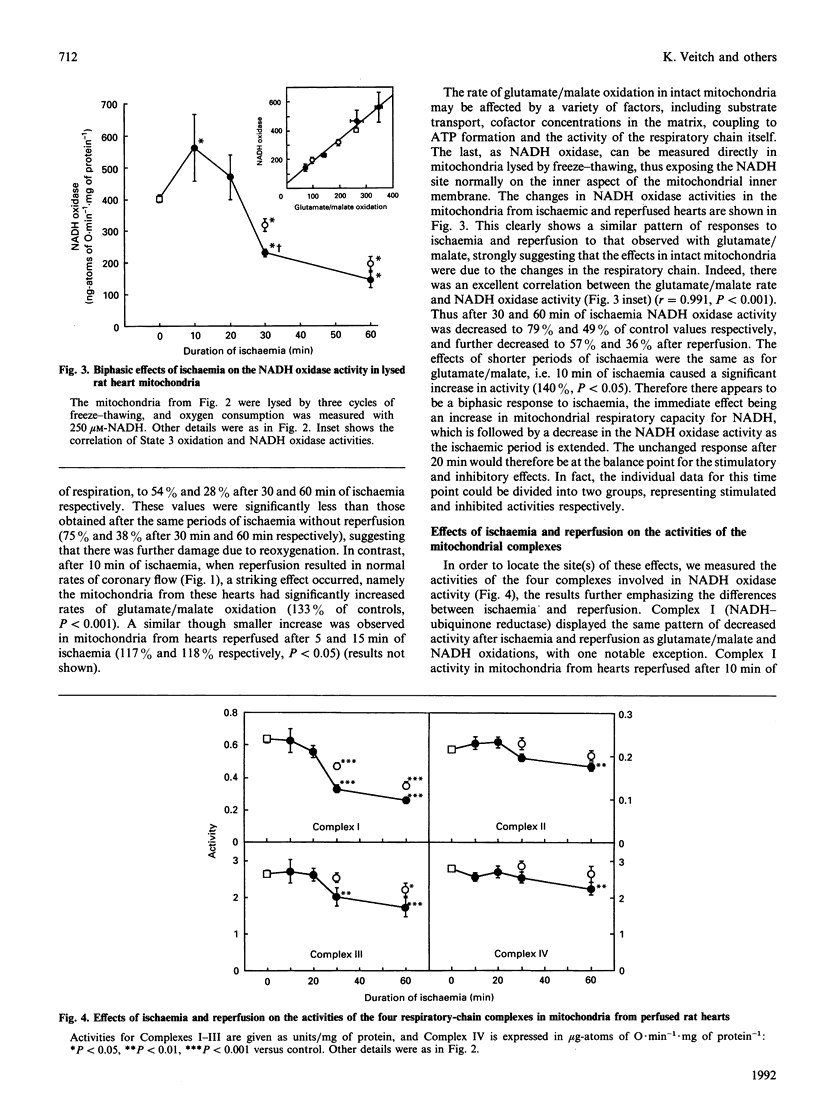
Studies of Langendorff-perfused rat hearts have revealed a biphasic response of the mitochondrial respiratory chain to global ischaemia. The initial effect is a 30-40% increase in the rate of glutamate/malate oxidation after 10 min of ischaemia, owing to an increase in the capacity for NADH oxidation. This effect is followed by a progressive decrease in these oxidative activities as the ischaemia is prolonged, apparently owing to damage to Complex I at a site subsequent to the NADH dehydrogenase component. This damage is exacerbated by reperfusion, which causes a further decrease in Complex I activity and also decreases the activities of the other complexes, most notably of Complex III. Perfusion for up to 1 h with anoxic buffer produced only the increase in NADH oxidase activity, and neither anoxia alone, nor anoxia and reperfusion, caused loss of Complex I activity. Perfusing for 3-10 min with anoxic buffer before 1 h of global ischaemia had a significant protective effect against the ischaemia-induced damage to Complex I.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cadenas E., Boveris A., Ragan C. I., Stoppani A. O. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys. 1977 Apr 30;180(2):248–257. doi: 10.1016/0003-9861(77)90035-2. [DOI] [PubMed] [Google Scholar]
- Fry M., Green D. E. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J Biol Chem. 1981 Feb 25;256(4):1874–1880. [PubMed] [Google Scholar]
- Fuller E. O., Goldberg D. I., Starnes J. W., Sacks L. M., Delivoria-Papadopoulos M. Mitochondrial respiration following acute hypoxia in the perfused rat heart. J Mol Cell Cardiol. 1985 Jan;17(1):71–81. doi: 10.1016/s0022-2828(85)80093-6. [DOI] [PubMed] [Google Scholar]
- Giocondi M. C., Le Grimellec C. Temperature dependence of plasma membrane physical state in living Madin-Darby canine kidney cells. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1004–1009. doi: 10.1016/0006-291x(89)90773-0. [DOI] [PubMed] [Google Scholar]
- Hardy L., Clark J. B., Darley-Usmar V. M., Smith D. R., Stone D. Reoxygenation-dependent decrease in mitochondrial NADH:CoQ reductase (Complex I) activity in the hypoxic/reoxygenated rat heart. Biochem J. 1991 Feb 15;274(Pt 1):133–137. doi: 10.1042/bj2740133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearse D. J. Reperfusion of the ischemic myocardium. J Mol Cell Cardiol. 1977 Aug;9(8):605–616. doi: 10.1016/s0022-2828(77)80357-x. [DOI] [PubMed] [Google Scholar]
- Hue L., Bontemps F., Hers H. The effects of glucose and of potassium ions on the interconversion of the two forms of glycogen phosphorylase and of glycogen synthetase in isolated rat liver preparations. Biochem J. 1975 Oct;152(1):105–114. doi: 10.1042/bj1520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hülsmann W. C., de Wit L. E., Schneydenberg C., Verkleij A. J. Loss of cardiac contractility and severe morphologic changes by acutely lowering the pH of the perfusion medium: protection by fatty acids. Biochim Biophys Acta. 1990 Feb 26;1033(2):214–218. doi: 10.1016/0304-4165(90)90016-p. [DOI] [PubMed] [Google Scholar]
- Jennings R. B. Early phase of myocardial ischemic injury and infarction. Am J Cardiol. 1969 Dec;24(6):753–765. doi: 10.1016/0002-9149(69)90464-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Watanabe H., Ozawa K., Hayashi H., Yamazaki N. Oxygen-derived free radicals related injury in the heart during ischemia and reperfusion. Jpn Circ J. 1989 Sep;53(9):1122–1131. doi: 10.1253/jcj.53.1122. [DOI] [PubMed] [Google Scholar]
- Mowbray J., Ottaway J. H. The flux of pyruvate in perfused rat heart. Eur J Biochem. 1973 Jul 16;36(2):362–368. doi: 10.1111/j.1432-1033.1973.tb02920.x. [DOI] [PubMed] [Google Scholar]
- Narabayashi H., Takeshige K., Minakami S. Alteration of inner-membrane components and damage to electron-transfer activities of bovine heart submitochondrial particles induced by NADPH-dependent lipid peroxidation. Biochem J. 1982 Jan 15;202(1):97–105. doi: 10.1042/bj2020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayler W. G., Ferrari R., Williams A. Protective effect of pretreatment with verapamil, nifedipine and propranolol on mitochondrial function in the ischemic and reperfused myocardium. Am J Cardiol. 1980 Aug;46(2):242–248. doi: 10.1016/0002-9149(80)90064-8. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Grotyohann L. W. Role of glycolytic products in damage to ischemic myocardium. Dissociation of adenosine triphosphate levels and recovery of function of reperfused ischemic hearts. Circ Res. 1984 Dec;55(6):816–824. doi: 10.1161/01.res.55.6.816. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Rovetto M. J. Techniques for perfusing isolated rat hearts. Methods Enzymol. 1975;39:43–60. doi: 10.1016/s0076-6879(75)39008-3. [DOI] [PubMed] [Google Scholar]
- Otani H., Tanaka H., Inoue T., Umemoto M., Omoto K., Tanaka K., Sato T., Osako T., Masuda A., Nonoyama A. In vitro study on contribution of oxidative metabolism of isolated rabbit heart mitochondria to myocardial reperfusion injury. Circ Res. 1984 Aug;55(2):168–175. doi: 10.1161/01.res.55.2.168. [DOI] [PubMed] [Google Scholar]
- Palmer J. W., Tandler B., Hoppel C. L. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977 Dec 10;252(23):8731–8739. [PubMed] [Google Scholar]
- Pelikan P. C., Niemann J. T., Xia G. Z., Jagels G., Criley J. M. Enhancement of mitochondrial oxidative phosphorylation capability by hypoperfusion in isolated perfused rat heart. Circ Res. 1987 Dec;61(6):880–888. doi: 10.1161/01.res.61.6.880. [DOI] [PubMed] [Google Scholar]
- Peng C. F., Murphy M. L., Straub K. D. Effects of early reperfusion on the mechanical and biochemical characteristics of ischemic myocardium. J Surg Res. 1986 Nov;41(5):493–502. doi: 10.1016/0022-4804(86)90167-8. [DOI] [PubMed] [Google Scholar]
- Rao P. S., Cohen M. V., Mueller H. S. Production of free radicals and lipid peroxides in early experimental myocardial ischemia. J Mol Cell Cardiol. 1983 Oct;15(10):713–716. doi: 10.1016/0022-2828(83)90260-2. [DOI] [PubMed] [Google Scholar]
- Rasmussen U. F., Rasmussen H. N. The NADH oxidase system (external) of muscle mitochondria and its role in the oxidation of cytoplasmic NADH. Biochem J. 1985 Aug 1;229(3):631–641. doi: 10.1042/bj2290631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouslin W., Millard R. W. Canine myocardial ischemia: defect in mitochondrial electron transfer complex I. J Mol Cell Cardiol. 1980 Jun;12(6):639–645. doi: 10.1016/0022-2828(80)90021-8. [DOI] [PubMed] [Google Scholar]
- Rouslin W. Mitochondrial complexes I, II, III, IV, and V in myocardial ischemia and autolysis. Am J Physiol. 1983 Jun;244(6):H743–H748. doi: 10.1152/ajpheart.1983.244.6.H743. [DOI] [PubMed] [Google Scholar]
- Rouslin W. Persistence of mitochondrial competence during myocardial autolysis. Am J Physiol. 1987 May;252(5 Pt 2):H985–H989. doi: 10.1152/ajpheart.1987.252.5.H985. [DOI] [PubMed] [Google Scholar]
- Schneider C. A., Taegtmeyer H. Fasting in vivo delays myocardial cell damage after brief periods of ischemia in the isolated working rat heart. Circ Res. 1991 Apr;68(4):1045–1050. doi: 10.1161/01.res.68.4.1045. [DOI] [PubMed] [Google Scholar]
- Shlafer M., Myers C. L., Adkins S. Mitochondrial hydrogen peroxide generation and activities of glutathione peroxidase and superoxide dismutase following global ischemia. J Mol Cell Cardiol. 1987 Dec;19(12):1195–1206. doi: 10.1016/s0022-2828(87)80530-8. [DOI] [PubMed] [Google Scholar]
- Taegtmeyer H., Roberts A. F., Raine A. E. Energy metabolism in reperfused heart muscle: metabolic correlates to return of function. J Am Coll Cardiol. 1985 Oct;6(4):864–870. doi: 10.1016/s0735-1097(85)80496-4. [DOI] [PubMed] [Google Scholar]
- Ueta H., Ogura R., Sugiyama M., Kagiyama A., Shin G. O2-. spin trapping on cardiac submitochondrial particles isolated from ischemic and non-ischemic myocardium. J Mol Cell Cardiol. 1990 Aug;22(8):893–899. doi: 10.1016/0022-2828(90)90120-q. [DOI] [PubMed] [Google Scholar]
- Vandeplassche G., Hermans C., Thoné F., Borgers M. Mitochondrial hydrogen peroxide generation by NADH-oxidase activity following regional myocardial ischemia in the dog. J Mol Cell Cardiol. 1989 Apr;21(4):383–392. doi: 10.1016/0022-2828(89)90649-4. [DOI] [PubMed] [Google Scholar]
- Vanneste W. H. Molecular proportion of the fixed cytochrome components of the respiratory chain of Keilin-Hartree particles and beef heart mitochondria. Biochim Biophys Acta. 1966 Jan 11;113(1):175–178. doi: 10.1016/s0926-6593(66)80132-7. [DOI] [PubMed] [Google Scholar]


