Abstract
The potential for DNA vaccines encoding mutated versions of the same antigen to modulate immune responses in C3H/HeN mice was investigated. We created expression plasmids that encoded several versions of glycoprotein D (gD) from bovine herpesvirus 1, including authentic membrane-anchored glycoprotein (pSLRSV.AgD), a secreted glycoprotein (pSLRSV.SgD), and an intracellular protein (pSLRSV.CgD). Immunization of an inbred strain of mice with these plasmids resulted in highly efficacious and long-lasting humoral and cell-mediated immunity. We also demonstrated that the cell compartment in which plasmid-encoded gD was expressed caused a deviation in the serum immunoglobulin (Ig) isotype profile as well as the predominant cytokines secreted from the draining lymph node. Immunization of C3H/HeN mice with DNA vaccines encoding cell-associated forms of gD resulted in a predominance of serum IgG2a and gamma interferon-secreting cells within the spleens and draining lymph nodes. In contrast, mice immunized with a secreted form of this same antigen displayed immune responses characterized by greater levels of interleukin 4 in the draining lymph node and IgG1 as the predominant serum isotype. We also showed evidence of compartmentalization of distinct immune responses within different lymphoid organs.
Bovine herpesvirus 1 (BHV-1) is a member of the subfamily Alphaherpesvirinae and the causative agent of infectious bovine rhinotracheitis and infectious pustular vulvovaginitis (79). BHV-1 has also been shown to cause conjunctival infections, abortions, meningo-encephalitic diseases, and infectious balanoposthitis (30, 84). BHV-1 may be the primary viral agent involved in the development of secondary opportunistic bacterial infections leading to “shipping fever” in cattle (4, 70, 86). Of the 13 proteins associated with the viral lipid envelope, glycoproteins B (gB), C (gC), and D (gD) are consistently recognized by convalescent-phase sera from BHV-1-infected animals (15, 78). Immunization of cattle with each of these individual glycoproteins, formulated with a conventional adjuvant, leads to protective immune responses that include neutralizing serum antibodies and cell-mediated immunity (CMI) (3, 35, 71, 79). Immunization of cattle with gD typically results in the humoral responses of the greatest magnitude (3, 81). Also, gD has been demonstrated to efficiently induce CMI and humoral immune responses in C57BL/6 (H-2b) mice following immunization in a novel water-in-oil adjuvant (5). Finally, a DNA-based vaccine encoding the authentic, membrane-anchored versions of gB, gC, and gD induced humoral immune responses in BALB/c (H-2d) mice and cattle (gD only) (20).
DNA immunization, also termed polynucleotide, nucleic acid, or genetic immunization, represents an exciting, novel approach to eliciting protective immune responses in animals (24, 75, 76). These vaccines are typically composed of bacterially derived plasmid DNA carrying eukaryotic gene regulatory elements driving the expression of genes encoding antigens. Delivery of these DNA-based vaccines into animals can be carried out by a variety of methods, including direct injection of DNA vaccines solubilized in saline (32, 41, 68, 77, 83).
Somatic cell uptake of DNA, followed by expression of encoded antigen, is a fundamental feature of DNA-based immunization (16, 17, 59, 76). Antigen presentation by transiently transfected, professional antigen-presenting cells (APC) or indirect presentation following acquisition of antigen from nonprofessional APCs by professional APCs is a crucial component of the process (16, 17, 59, 76). A significant feature of DNA-based vaccines, unlike most conventional vaccines, is the unique ability to stimulate humoral, cell-mediated, and CD8+ cytotoxic responses in immunized animals. The ability to induce a potent Th1-type immune response is of considerable importance, because with many pathogens, cell-mediated immunity and not the presence of antibody is correlated with protection (28, 75). Recall responses of CD4+ Th1-type T cells typically result in the secretion of gamma interferon (IFN-γ), interleukin 2 (IL-2), and tumor necrosis factor alpha (48). It has been demonstrated that specific hypomethylated CpG motifs within bacterially derived DNA can exhibit a potent adjuvant effect that is, in part, responsible for induction of this Th1-type response that is a characteristic feature of DNA-based vaccines (56, 57).
Humoral immunity also plays a significant role in prevention of infection, and it has been shown that in many instances, immunoglobulin G2a (IgG2a) is the predominant isotype induced following immunization with DNA-based vaccines (75). Furthermore, it has been demonstrated that the Th1 cytokine IFN-γ is an important B-cell switch factor for the induction of antigen-specific IgG2a-secreting B cells and that many viral infections in mice induce a humoral response characterized by a predominance of IgG2a (18, 19, 67). Conversely, production of antigen-specific IgG1 and IgE antibody depends, at least in part, on the presence of the Th2 cytokine IL-4 (66). Of course, a variety of other factors, including mouse strain, dose of antigen, type of pathogen, route of infection or immunization, vaccine formulations, antigen form (soluble or modified), and early induction of IL-6 can all have an impact on the specific character of the developing immune repertoire (1, 2, 11, 14, 31, 53, 85).
In an effort to further characterize the immune repertoire to a DNA-based vaccine, we immunized C3H/HeN (H-2k) mice with plasmids encoding several deletion mutants of BHV-1 gD. Our data show that C3H/HeN mice receiving plasmids encoding cell-associated forms (cytosolic or plasma membrane anchored) developed serum isotype profiles that were predominantly IgG2a, with an almost exclusive production of the Th1 cytokine IFN-γ following antigen restimulation of splenocytes in vitro. In contrast, mice immunized with a plasmid encoding the secreted form of BHV-1 gD displayed a predominance of IgG1 in serum despite the presence of antigen-specific IFN-γ-secreting splenocytes. In an effort to understand the relationship between splenic Th1 profiles and a serum isotype that reflected the influence of IL-4, we determined the antigen-specific levels of IL-4, IFN-γ, and IgG isotype-secreting B cells from the iliac lymph node. This node is the primary draining node of the deep tissues of the quadriceps muscle mass. The data show that draining lymph node cytokine and antigen-specific antibody isotype profiles more accurately reflect the predominant serum isotype of C3H/HeN mice immunized with cell-associated or -secreted forms of the BHV-1 gD antigen. The relevance of these observations is discussed in the context of manipulating immune responses by controlling the intra- or extracellular location of the antigen when it is first perceived by the immune system.
MATERIALS AND METHODS
Plasmid construction.
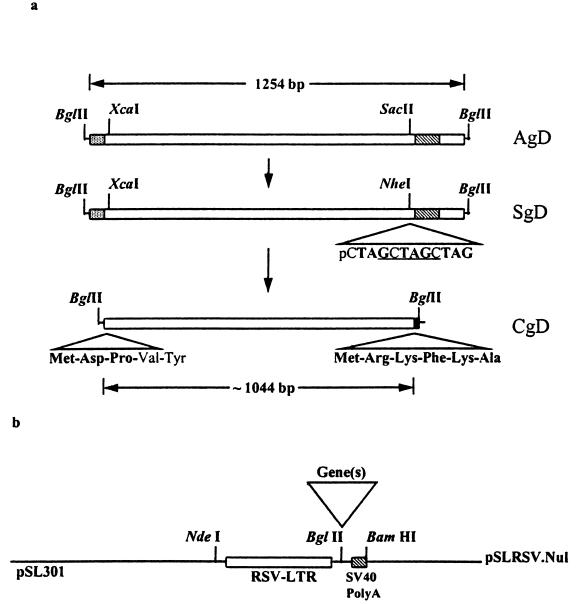
All restriction enzymes and DNA-modifying enzymes, as well as markers and plasmids, were purchased from Pharmacia Biotech (Quebec, Canada) or New England Biolabs Ltd. (Mississauga, Ontario, Canada) unless indicated otherwise. Vectors pSLRSV.AgD and pSLRSV.SgD were created by partially digesting pRSV1.3 and pRSV1.3tgIV (20) with NdeI-BamHI and ligating the 947-bp fragment containing the Rous sarcoma virus-long terminal repeat (RSV-LTR) promoter or enhancer, genes encoding the authentic or secreted forms of BHV-1 gD, and the simian virus 40 (SV40) polyadenylation signals into NdeI-BamHI-cut pSL301 (72, 74) (Fig. 1a).
FIG. 1.
Diagrammatic depiction of expression cassettes. Panel a depicts membrane-anchored (AgD), secreted (SgD), and cytosolic (CgD) forms of BHV-1 gD. The expression cassettes all utilized the high-copy-number pSL301 plasmid backbone. The evolution of secreted and cytosolic versions of BHV-1 gD is shown as are details regarding relevant changes in coding sequences, start codons (CgD), and termination codons. The NheI restriction sequence (underlined) and triple stop codons (boldface) are shown for gene SgD. Novel amino acids translated at the amino and carboxy termini of CgD are in boldface. Gene and construct designations are indicated to the immediate right of each diagram. All genes encoding full-length or truncated versions of BHV-1 gD were inserted at the BglII site (▿) shown in panel b. Panel b depicts the null vector (pSLRSV.Nul) with the RSV-LTR enhancer or promoter and the short polyadenylation sequence derived from SV40.
We created a third expression vector, designated pSLRSV.CgD, which encodes a cytosolic, or intracellular, version of BHV-1 gD that lacks both the signal sequence and transmembrane domain encoded within authentic, full-length gD (72) (Fig. 1a). This construct was generated by subcloning the XcaI-NheI subfragment of pSLRSV.SgD into XcaI-NheI-digested pSL301. This clone was digested with XcaI and EcoRV, and the gD fragment was blunt end ligated into SmaI-digested pAA505. The prokaryotic expression cassette pAA505 is a derivative of pGH433 with a multiple cloning site consisting of NcoI, BamHI, and SmaI downstream from a Tac promoter (69). The appropriate orientation of the ligation product created a novel start codon contributed by pAA505 followed by Asp and Pro prior to in-frame commencement of the gD amino acid sequence Tyr Val Asp Pro immediately downstream from the signal peptide sequence. Clone pAACgD was digested with DdeI and Eco47III, end repaired with the Klenow fragment of Escherichia coli DNA polymerase I, and blunt end ligated to a BglII linker containing in-frame stop codons (GTAGCTAGATCTG). This ligation product was then digested with BglII, purified with a Geneclean kit (BIO 101, Inc.), and ligated into BglII-digested pSLRSV.Nul. This construct displays six additional amino acids (Met-Arg-Lys-Phe-Lys-Ala), contributed by the NheI-EcoRV fragment of pSL301, at the carboxy terminus. A null vector was created by digesting the pSLRSV.AgD with BglII to excise the gene encoding BHV-1 gD and religation of the vector to create pSLRSV.Nul (Fig. 1b).
Bacterial hosts, mammalian cell lines, and tissue culture reagents.
JM105 (New England Biolabs, Inc.), HB101 (Invitrogen), and DH5α (Clontech Laboratories, Inc.) were the bacterial strains used to amplify plasmid DNA. Murine L929 connective tissue cell line was received from the American Type Culture Collection (ATCC; Rockville, Md.) (NCTC clone 929). The Madin-Darby bovine kidney (MDBK) cell line was obtained from ATCC (CCL 22). C3H/HeN mice were purchased from Charles River (St. Constante, Quebec, Canada). All media and media supplements were purchased from GIBCO/BRL (Burlington, Ontario, Canada) or Sigma Chemical Co. (St. Louis, Mo.), unless otherwise indicated. All tissue culture plasticware was purchased from Corning, Inc. (Corning, N.Y.) or Costar/Nucleopore Canada, Inc. (Toronto, Ontario, Canada) unless indicated otherwise.
Transient transfection of COS-7 cells.
COS-7 cells at 70 to 80% confluency in six-well tissue culture plates were transfected with pSLRSV.AgD, pSLRSV.SgD, pSLRSV.CgD, or pSLRSV.Nul. Lipofectamine (GIBCO-BRL) was utilized to facilitate transfection at a ratio of 5 μg of Lipofectamine to 1 μg of plasmid DNA. Plasmid DNA was purified with Qiagen columns (Qiagen Inc., Santa Clarita, Calif.) and combined with Lipofectamine as described in the protocol insert. Optimem containing DNA-lipid complexes was removed from cells after 5 h, and complete medium containing glucose (Dulbecco’s modified Eagle’s medium) and 15% fetal bovine serum (FBS) was added to the cells. After 12 h, transfected cell monolayers were washed with warm phosphate-buffered saline (PBS: 0.137 M NaCl, 0.003 M KCl, 0.008 M Na2HPO4, 0.001 M NaH2PO4), and 1.5 ml of methionine-free minimal essential medium containing 2% dialyzed FBS and 50 μCi of 35S-labelled l-methionine per ml (Tran35S-Label; ICN Pharmaceuticals, Inc., St. Laurent, Quebec, Canada) was added to each well. Cells were incubated for 24 h and harvested for immunoprecipitation. Cell-associated antigen or extracellular antigen was detected by immunoprecipitation of media or cell lysates as described previously (73).
All immunoprecipitated pellets were washed two times in 500 μl of cold radioimmunoprecipitation assay buffer containing protease inhibitors. Following the final wash, pellets were resuspended in 40 μl of a 1.5× reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer. Samples were mixed, boiled for 3 min, and chilled on ice for 5 min prior to loading on a 12 by 16-cm SDS-PAGE gel (4% stacking and 10% separating). Electrophoresis was carried out for 4 h at 180 V. Gels were fixed (40% methanol, 10% acetic acid) for 15 min and equilibrated for 30 min in a fluorographic agent (Amplify; Amersham, Oakville, Ontario, Canada). Gels were dried for 2 h at 80°C under vacuum and exposed to preflashed Kodak X-OMAT AR film for 12 to 24 h.
Mouse injections.
Inbred 7-week-old female C3H/HeNCrlBR mice were injected with column-purified (Qiagen, Inc.) plasmid DNA dissolved in normal saline. All injections of DNA were intramuscular (i.m.), while injections of purified recombinant truncated gD (rtgD) formulated in VSA3 (VSA3/rtgD) (BIOSTAR Inc., Saskatoon, Saskatchewan, Canada) were subcutaneous and carried out as described previously (3). All other mice received injections of 50 μg of plasmid DNA in the left and right quadriceps muscle mass with DNA at a concentration of 1.0 μg/μl. All mice were given booster injections at 14 days. Nonlethal tail bleeds were carried out every 2 weeks until the experiments were terminated.
Spleen and lymph node cell harvests.
Mouse splenocytes were prepared as described previously (5). Splenocytes were resuspended at a final concentration of 107 cells/ml in RPMI 1640 supplemented with 10% FBS (Sigma Chemical Co.), 100 U of penicillin per ml, and 100 μg of streptomycin per ml (Sigma Chemical Co.), 2 mM l-glutamine (GIBCO, Life Technologies), 1 mM sodium pyruvate (GIBCO, Life Technologies), 10 mM HEPES (GIBCO, Life Technologies), and 5 × 10−5 M 2-mercaptoethanol (Sigma Chemical Co.) (complete RPMI 1640). A modification of a method for the harvest of Peyer’s patch lymphocytes was utilized to prepare single-cell suspensions from lymph nodes (67). Briefly, iliac lymph nodes were collected into 15-ml tubes containing sterile chilled PBS. Pooled nodes were decanted into a sterile Petri dish, and excess PBSA was discarded. Nodes were diced with a sterile scalpel in a small volume of CMF solution (0.1× Ca2+ Mg2+-free Hanks balanced salt solution [HBSS], 10 mM HEPES [pH 7.2], 25 mM NaHCO3 [pH 7.2], 2% FBS) and then mixed with 10 ml of digestion buffer (1× HBSS, 10% FBS, 15 mM HEPES [pH 7.2]) containing 150 U of collagenase per ml (CLS 1; Worthington Biochemical Corporation) and 0.015 mg of DNase I per ml (Pharmacia Biotech, Inc., Baie d’Urfe, Quebec, Canada). Diced nodes in digestion buffer were transferred to silanized 25- to 50-ml Ehrlenmeyer flasks containing a sterile Teflon-coated stir bar and incubated at 37°C, with stirring, for 90 min. Samples were harvested into sterile 15-ml plastic tubes and allowed to sit for 10 min. Large pieces of undigested material were returned to the Erhlenmeyer flask and incubated for an additional 30 to 60 min with 5.0 ml of digestion buffer. This material was combined with the initial harvest, and cells were centrifuged (250 × g) to pellet. Digestion buffer was decanted, and the cells were resuspended in PBSA (GIBCO-BRL). Cells were counted with a hemocytometer and centrifuged (250 × g) one final time. Cells were resuspended to a final concentration of 107 cells/ml.
ELISA.
Immulon II microtiter plates (Dynatech Laboratories, Inc., Alexandria, Va.) were coated with purified recombinant truncated gD (Biostar, Inc.) at a concentration of 0.050 μg/well in enzyme-linked immunosorbent assay (ELISA) coating buffer (0.012 M Na2CO3, 0.038 M NaHCO3 [pH 9.6]), and coating was allowed to proceed overnight at 4°C. Plates were washed five times in PBS (0.137 M NaCl, 0.003 M KCl, 0.008 M Na2HPO4, 0.001 M NaH2PO4) with 0.05% Tween 20 (PBST) prior to addition of fourfold dilutions of mouse sera prepared in PBST with 0.5% gelatin (PBST-g) (Bio-Rad Laboratories, Ltd., Mississauga, Ontario, Canada). After a 2-h incubation, plates were washed in PBST, and an affinity-purified biotinylated goat anti-mouse Ig (Zymed Laboratories Inc., San Francisco, Calif.) diluted to 1/5,000 in PBST-g was added to each plate. After incubation of 60 min, plates were washed extensively, and streptavidin-alkaline phosphatase (Gibco, Life Technologies, Burlington, Ontario, Canada), diluted to 1/2,000 in PBST-g, was added to each plate. Following a 60-min incubation, plates were washed six times in PBST. Prior to addition of substrate, the plates were washed two additional times in PBS. Development of plates involved the addition of 0.01 M p-nitrophenyl phosphate (Sigma Chemical Co., St. Louis, Mo.) in substrate buffer (0.104 M diethanolamine [Sigma Chemical Co.], 0.5 mM MgCl2). Absorbances were read on a model 3550 Microplate Reader (Bio-Rad Laboratories, Ltd.) at 30 min and again at 60 min at 405 nm with a reference wavelength of 490 nm. Antibody isotyping ELISAs were carried out in a similar fashion, except that biotinylated goat anti-murine IgG1, IgG2a, IgG2b, and IgG3 (Caltag Laboratories, San Francisco, Calif.) were used at a dilution of 1/8,000. Biotinylated goat anti-murine IgM was used at 1/2,000. The incubation time with these antibodies was 60 min. Addition of streptavidin-alkaline phosphatase and development of plates were performed as described for total IgG ELISA.
Cytokine ELISPOT.
A cytokine-specific enzyme-linked immunospot assay (ELISPOT) was used as described previously (5, 21). Briefly, single-cell suspensions isolated from spleens, lymph nodes, or bone marrow were stimulated in vitro for 20 h in the presence of authentic gD (0.4 μg/ml). After antigen stimulation, cells were washed twice in complete RPMI 1640 and diluted to a concentration of 107 cells/ml of complete RPMI 1640. Nitrocellulose plates (FILTAplate; 0.45-μm pore diameter; Polyfiltronics, Inc., Rockland, Mass.) were prepared by being coated overnight at 4°C with 2.5 μg of purified antimurine IL-4 (catalog no. 11B11) or 5 μg of purified antimurine IFN-γ (catalog no. R4-6A2) (Pharmingen, San Diego, Calif.) per μl diluted in carbonate-bicarbonate buffer (45.3 mM NaHCO3, 18.2 mM Na2CO3 [pH 9.6]). After the coating, unbound antibody was washed from the wells three times with sterile PBST followed by three washes with sterile PBS. Wells were blocked for 2 h at 37°C with complete RPMI 1640. Blocking medium was decanted, and 100 μl of antigen-stimulated cell suspensions was added to triplicate wells. After a 20-h incubation at 37°C and 5% CO2, plates were washed twice in tap water and five times in PBST to remove cells and nonspecifically bound cytokine. One hundred microliters of biotinylated antimurine IL-4 (catalog no. BVD6-24G2) and anti-IFN-γ (catalog no. XMG1.2) (Pharmingen Canada, Inc.) monoclonal antibodies (3 μg of each per ml in 1% FBS–PBS) was added to the appropriate wells, and the mixture was incubated for 4 to 6 h at ambient temperature on an oscillating mixer. Plates were washed six times in PBST, and 100 μl of a 1/1,000 dilution of streptavidin-alkaline phosphatase (Bio/Can Scientific, Mississauga, Ontario, Canada) in 1% FBS–PBS was added to each well. After a 2-h incubation at ambient temperature, the plates were washed as before with two final washes in PBS. Plates were developed by the addition of 50 μl of the substrates 5-bromo-4-3-indolyl phosphate (BCIP) and nitroblue tetrazolium NBT (Moss, Inc., Pasadena, Md.). Plates were allowed to develop at ambient temperature for 10 to 30 min, after which reactions were stopped by extensive washing in distilled water. After air drying, spots were counted under a dissecting microscope.
B-cell ELISPOT involved the use of nitrocellulose plates (Millipore Milliscreen-HA; Millipore, Bedford, Mass.) that were coated overnight at 4°C with purified recombinant truncated gD at 5.0 μg/ml in carbonate-bicarbonate buffer (pH 9.6). Coated plates were washed three times in sterile PBST, followed by three washes in sterile PBS. Nonspecific binding sites were then blocked with complete RPMI 1640 at 37°C for 1 to 2 h. Fresh, unstimulated cells from spleen or lymph nodes, prepared as described above, were added in a volume of 100 μl (107 cells/ml) to each well. Uncoated but blocked control wells also received cells from each test sample in triplicate. Plates were incubated at 37°C and 5% CO2 for 10 to 12 h. After the incubation period, all cells were washed out of the wells by two initial tap water washes followed by six PBST washes. Biotinylated antimurine IgG1 or IgG2a antibodies (1/8,000 dilution) were added in a 100-μl volume of PBST (with 1% FBS) and incubated with mixing for 3 to 4 h at ambient temperature. After being washed in PBST six times, streptavidin-alkaline phosphatase (1/1,000 dilution in PBS-T with 1% FBS) was added to each well (100 μl). After a 60-min incubation, plates were washed six times in PBST and two times in PBS. Plates were developed as described for the cytokine ELISPOT.
Serum neutralization assays.
Sera from mice immunized with DNA-based or subunit vaccines were assayed for virus neutralizing capacity by a BHV-1 plaque reduction assay as described previously (66). Briefly, 110 μl of two- or fourfold dilutions of heat-inactivated sera (in RPMI 1640 containing 2% FBS and a 1:40 dilution of guinea pig complement [GIBCO/BRL]) were mixed with 110 μl of live BHV-1 for 60 min. Following incubation, 100 μl of each dilution was placed on confluent MDBK cells, in duplicate, in a 96-well flat-bottom culture plate (Nunc, Inc., Naperville, Ill.). Plates were incubated for 48 h, and plaques were visualized by decanting the medium overlay and staining the cells with 1% crystal violet in 70% ethanol. Neutralizing titers are expressed as the reciprocal of the highest dilution of antibody that caused 50% reduction of plaques relative to the virus control wells.
Statistical analysis.
Data were entered into a database in the statistical analysis program Instat GraphPad. Differences in antibody titers among vaccine groups were investigated with the nonparametric Mann-Whitney U test.
RESULTS
In vitro transfection of COS-7.
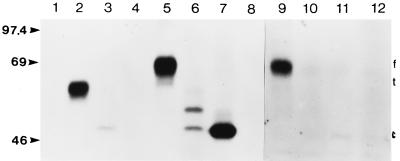
Transient transfection of COS-7 cells with expression plasmids demonstrated that the genes were functional. Second, these studies demonstrated that the cytosolic (pSLRSV.CgD), authentic (pSLRSV.AgD), and secreted (pSLRSV.SgD) forms of BHV-1 gD occurred within the predicted cell compartment. COS-7 cells transiently transfected with pSLRSV.AgD yielded labelled protein that occurred exclusively in the whole-cell lysate or plasma membrane fraction (Fig. 2, lanes 5 and 9, respectively). Transfection with pSLRSV.SgD yielded a slightly smaller protein that occurred almost exclusively within the extracellular fraction (Fig. 2, lane 2). Several smaller bands do occur in the whole-cell lysate following transfection with pSLRSV.SgD (Fig. 2, lane 6). These bands, in all likelihood, represent the secreted form of gD at various stages of glycosylation (34, 72, 73). We anticipated that the removal of gene sequences encoding the translocation and transmembrane domains from authentic gD would yield a construct (pSLRSV.CgD) encoding a protein that would occur primarily in the cytosolic fraction of cells harboring this plasmid. The presence of a single band within lane 7 (Fig. 2) demonstrates that this was indeed the case. Some of the cytosolic protein encoded by pSLRSV.CgD does occur in the extracellular space, as indicated by the faint band in lane 3 (Fig. 2); however, it is likely that this is simply a result of cell death during the transfection procedure and subsequent release of soluble cytosolic proteins into the media. The molecular weights and relative gel mobilities of each of the proteins encoded by these plasmids are consistent with previous studies (34, 72, 74) or as predicted based on cloned sequence data.
FIG. 2.
Autoradiograph of immunoprecipitated forms of membrane-anchored, secreted, and cytosolic BHV-1 gD. Preconfluent COS-7 cells transiently transfected with pSLRSV.AgD, pSLRSV.SgD, pSLRSV.CgD, or pSLRSV.Nul were grown for 48 h in the presence of 35S-labelled methionine and cysteine. Radioactively labelled gD was immunoprecipitated from medium and/or cell lysates by using an anti-gD monoclonal antibody pool (see Materials and Methods). SDS-PAGE of precipitates demonstrates the calculated molecular masses (kDa) of mutated gD and cellular localization as predicted. Immunoprecipitates of media collected from COS-7 cells transfected with the following are shown: 1, pSLRSV.AgD; 2, pSLRSV.SgD; 3, pSLRSV.CgD; and 4, pSLRSV.Nul. Immunoprecipitates of lysates of COS-7 cells transfected with the following are shown: 5, pSLRSV.AgD; 6, pSLRSV.SgD; 7, pSLRSV.CgD; and 8, pSLRSV.Nul. Immunoprecipitates of plasma membrane-associated gD are shown: 9, pSLRSV.AgD; 10, pSLRSV.SgD; 11, pSLRSV.CgD; and 12, pSLRSV.Nul. Molecular mass markers are indicated on the left. The positions of membrane-anchored (f), secreted (t), and cytosolic (c) versions of gD are indicated on the right.
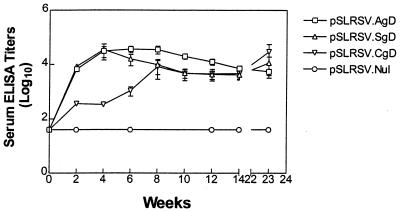
Serum IgG kinetics in DNA-immunized mice.
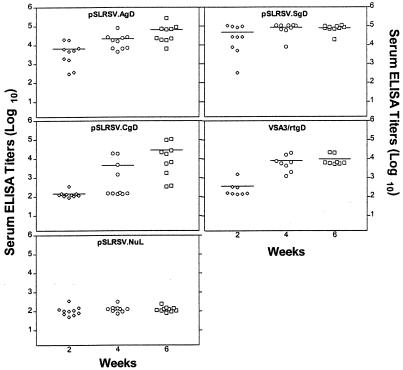
Immunization of C3H/HeN mice with a plasmid encoding the intracellular form of BHV-1 gD displayed antibody kinetics that differed from those in mice receiving cell surface or secreted forms of BHV-1 gD. Figure 3 shows that mice from all groups immunized with the plasmids encoding the authentic (pSLRSV.AgD) or secreted (pSLRSV.SgD) versions of BHV-1 were capable of seroconverting as early as 2 weeks following the initial immunization. Interestingly, mice immunized with plasmid encoding the intracellular version of gD (pSLRSV.CgD) showed a 2- to 4-week lag in serum titer development compared to that in mice immunized with constructs encoding antigen that would occur at (AgD constructs) or outside (SgD constructs) the cell surface. There was clear evidence in individual mice within all vaccine groups (excluding pSLRSV.Nul) that BHV-1 gD-specific serum IgG titers were maintained. Figure 4 demonstrates a representative experiment showing immune responses of individual mice. This experiment also shows a two-week delay in immune responses to the cytosolic gD, as does Fig. 3. Titers for mice immunized with a plasmid encoding the cytosolic version of gD are significantly (P < 0.05) lower than the 2- and 4-week titers in mice immunized with pSLRSV.AgD and significantly (P < 0.05) lower than the 2-, 4-, and 6-week titers of mice immunized with pSLRSV.SgD (Fig. 4). Figure 4 also demonstrates that all plasmid-immunized groups induce antibodies of similar magnitude to an authentic gD subunit protein formulated in the adjuvant VSA3.
FIG. 3.
Kinetics of serum anti-BHV-1 gD antibodies in C3H/HeN mice immunized with plasmids encoding cell-associated or secreted forms of BHV-1 gD. Each DNA-based vaccine group was comprised of five mice. Each mouse received 100 μg (2 μg/μl in normal saline) of plasmid DNA i.m. in the left quadriceps muscle mass on days 0 and 14. Serum ELISA titers for individual mice were determined by using the extrapolation function (Microsoft Excel) based on endpoint dilutions and with preimmune serum means (plus 3 standard deviations) as cutoffs. Endpoint titers are expressed as 1/log10. Serum antibody levels were determined for individual mice at each time point, except at 2 weeks, when blood samples for each group were pooled. Data are expressed as the geometric mean of four (pSLRSV.AgD) or two (pSLRSV.CgD) seropositive mice. Error bars show the standard error of the means. Only 23-week titers from mice that were seropositive at week 14 were determined.
FIG. 4.
Serum antibody levels after immunization of C3H/HeN mice with plasmids encoding cell-associated (AgD or CgD) or secreted (SgD) antigens. Experimental groups consisted of 10 mice and included a null plasmid control group and a subunit gD vaccine group. Mice 6 to 7 weeks of age were immunized in each quadriceps muscle mass with 50 μg of DNA (total of 100 μg) or subcutaneously with 400 ng of affinity-purified rtgD in 100 μl of VSA3 plus HBSS. All mice were given a booster injection at 2 weeks after initial immunization with the same dose. Serum ELISA titers were determined as described for Fig. 3. Plasmid construct or subunit gD designations are indicated in the top center of each graph. Each symbol represents a single mouse. The horizontal line in each column represents the mean antibody titer for the group.
Serum IgG isotype in DNA-immunized mice.
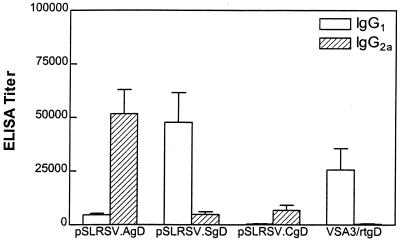
To further characterize the serum antibody responses, we determined the IgG isotype profiles in mice immunized with plasmids encoding each of the three different forms of BHV-1 gD and the subunit gD protein. Sera taken at 6 weeks following immunization were assessed for serum IgG isotypes by using an indirect ELISA. IgG1/IgG2a ratios clearly demonstrate that mice immunized with plasmids encoding cell-associated versions of BHV-1 gD (pSLRSV.AgD and pSLRSV.CgD) show a predominance of IgG2a, whereas mice immunized with a plasmid encoding the secreted version of gD (pSLRSV.SgD) show a predominance of IgG1 (Fig. 5). The mice immunized with the gD glycoprotein subunit vaccine developed a strong Th2-based response characterized by IgG1 without any IgG2a (Fig. 5).
FIG. 5.
Serum IgG isotype after immunization with DNA vaccines encoding cell-associated (AgD or CgD) or secreted (SgD) forms of BHV-1 gD or a gD subunit vaccine. BHV-1 gD-specific IgG1 or IgG2a isotypes were detected by ELISA with biotinylated secondary antibodies. Antibody titers were calculated as described for Fig. 3. Values are depicted as 1/log10 of antibody titer (determined by serial endpoint dilution analysis).
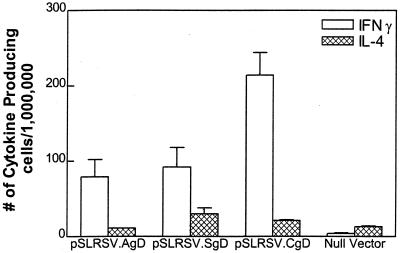
Splenic cytokine profiles reflect a Th1-like phenotype.
Following in vitro restimulation of pooled splenocytes from seropositive mice, we found a predominance of IFN-γ-secreting splenocytes in all groups of mice immunized with DNA encoding any form of BHV-1 gD (Fig. 6). We had anticipated a predominance of IFN-γ in mice immunized with cell-associated forms of gD largely because this cytokine has been shown to play an important role in Ig switching to IgG2a. However, the presence of a predominance of IFN-γ-secreting splenocytes harvested from mice immunized with pSLRSV.SgD and displaying a predominance of gD-specific IgG1 was somewhat unexpected.
FIG. 6.
Splenic cytokine profiles in mice 5 1/2 months postimmunization. Seropositive mice depicted in Fig. 3 were assessed for antigen-specific production of IFN-γ or IL-4. Spleens from seropositive mice were pooled and stimulated in vitro for ∼40 h with affinity-purified BHV-1 gD. Stimulated splenocytes were plated at 1.0 × 106 or 0.5 × 106 cells/well on Polyfiltronics plates. Cytokine-secreting splenocytes are depicted as the number of cytokine-producing cells per million plated cells. Mean values of triplicate wells are depicted.
Cytokine and AFC profiles in the iliac node.
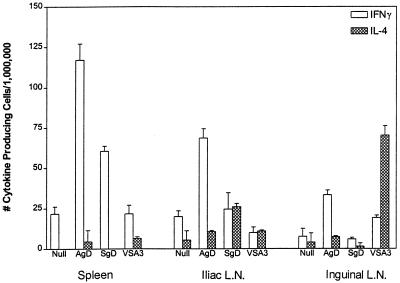
Since the cytokine profiles of the spleens did not correlate with the antibody isotypes in all instances, we tested the cytokine profiles of cells isolated from the draining lymph nodes. We determined that the iliac lymph node was the primary draining node for the deep tissues of the quadriceps muscle mass (data not shown). The draining lymph node cytokine and B-cell isotype ELISPOT profiles did correlate with the predominant serum isotype (Fig. 7).
FIG. 7.
Cytokine profiles in lymph nodes (L.N.) and spleen after immunization with DNA vaccines. Antigen-specific cytokine profiles were measured in several different lymphoid tissues of C3H/HeN mice immunized with plasmids encoding cell-associated or secreted versions of BHV-1 gD. Mice were euthanized 5 weeks after booster injection, and spleens and draining and nondraining lymph nodes were excised and pooled. Pooled cell populations were stimulated in vitro for 18 to 20 h with 400 ng of affinity-purified BHV-1 gD. Stimulated cells were harvested and plated on Polyfiltronics plates at 106 cells/well in triplicate. Pooled values are represented as mean values and as the number of cytokine-producing cells per million plated cells. Control wells for nonspecific binding of cytokine were set up for all groups, and mean values from control wells were subtracted from all groups.
The iliac lymph node showed a predominance of IFN-γ-secreting cells in mice immunized with the cell-associated pSLRSV.AgD and a significant increase in the level of IL-4 secretion in mice immunized with pSLRSV.SgD. The level of IL-4 production became significantly greater than the number of antigen-specific IFN-γ-secreting cells in the pSLRSV.SgD-vaccinated group when background levels of IFN-γ depicted in the null vector group were taken into consideration. There was a significant level of IL-4 production in restimulated inguinal lymph node cells taken from mice immunized subcutaneously with VSA3/rtgD. This is not surprising, considering immunization occurs at the dorsal thoracic midline and would be expected to drain to superficial nodes of the foreleg and neck and hind limb (inguinal node). Figure 7 further demonstrates that early splenic cytokine kinetics also show a predominance of IFN-γ-secreting cells. At first glance, there also appeared to be a predominance of IFN-γ-secreting splenocytes harvested from mice immunized with a subunit vaccine (VSA3/rtgD), however, this value became negligible when background cytokine production was subtracted (see the null vector group in Fig. 7). Although we had demonstrated that the iliac lymph node was the primary draining node for the deep tissues of the quadriceps muscle mass, additional PCR-based data suggested that DNA injected into this muscle group could access the more superficial inguinal lymph node (data not shown). We postulated that reflux of injected material along the needle tract and into the subcutaneous space could result in movement of injected plasmid DNA sequences into the superficial inguinal lymph node. Significant levels of IFN-γ-secreting cells in the pSLRSV.AgD group (Fig. 7, inguinal lymph node group) support this idea. However, the twofold-lower levels of IFN-γ-secreting cells within the pSLRSV.AgD-immunized mice and the absence of any IL-4 within the pSLRSV.SgD vaccine group (Fig. 7) (inguinal node group) suggest that the appearance of antigen-specific cytokine-secreting cells within the superficial inguinal lymph node following i.m. immunization with DNA vaccines may be an injection artifact.
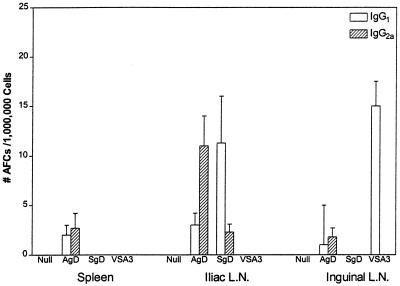
B-cell isotype ELISPOT data from mice immunized with DNA-based or a recombinant vaccine (VSA3/rtgD) are illustrated in Fig. 8. Splenic antibody-forming cells (AFCs) were either absent or below detection limits in all groups, except mice immunized with pSLRSV.AgD. The total number of AFCs in the spleens of mice immunized with pSLRSV.AgD amounts to approximately 200 to 250/spleen. Conversely, AFCs were easily detected in the iliac lymph nodes of mice immunized with DNA vaccines and displayed a predominance of IgG2a in mice immunized with pSLRSV.AgD and a predominance of IgG1 in mice immunized with pSLRSV.SgD. The B-cell ELISPOT data correlate well with the predominant cytokine (IFN-γ with IgG2a and IL-4 with IgG1) secreted from this node and with the predominant serum IgG isotype. The superficial inguinal lymph node showed a substantial number of IgG1-secreting AFCs taken from mice immunized with VSA3/rtgD, which is consistent with the serum isotype profile (Fig. 5) and the predominance of IL-4-secreting cells in these nodes (Fig. 7).
FIG. 8.
Number of AFCs secreting IgG1 or IgG2a after immunization with DNA vaccines. The data depicts antigen-specific IgG1 and IgG2a production in several different lymphoid tissues of C3H/HeN mice immunized with plasmids encoding cell-associated or secreted versions of BHV-1 gD. The mice depicted were euthanized 5 weeks after booster injection. Spleens and lymph nodes (L.N.) were excised and pooled. Pooled cells were plated in triplicate at 106 cells/well on Millipore ELISPOT plates. Data are presented as the mean number of Ig-secreting cells per million plated cells. Plate control wells for nonspecific binding of Ig isotypes were set up for all groups, and mean values were subtracted from all groups.
DNA vaccines elicit viral neutralizing antibodies.
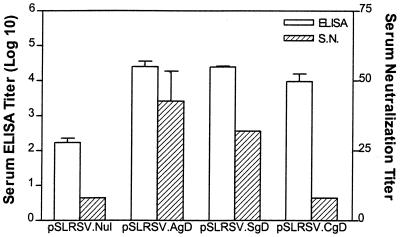
Serum antibodies taken from mice immunized with plasmids encoding the authentic or secreted versions of BHV-1 gD were able to neutralize BHV-1 in vitro. Individual immune sera from mice 7 weeks after an initial immunization (5 weeks post-booster injection) with 100-μg plasmids (pSLRSV.Nul, pSLRSV.AgD, pSLRSV.SgD, and pSLRSV.CgD) showed that only mice immunized with plasmids encoding the authentic and secreted versions of gD were able to effectively neutralize virulent BHV-1 (Fig. 9). In this assay, there was no significant advantage in neutralizing capacity noted between groups immunized with pSLRSV.AgD and those immunized with pSLRSV.SgD, but they were different from the null construct and those immunized with pSLRSV.CgD. Since neutralizing antibodies against gD are predominantly conformational (34), the lack of neutralizing antibody responses to pSLRSV.CgD is not surprising, since they would not be processed normally to produce the conformational epitopes.
FIG. 9.
Functional differences in serum antibodies after immunization with plasmids encoding different forms of BHV-1 gD. Serum antibody responses of mice following immunization and given a booster injection at 2 weeks with plasmids encoding cell membrane-anchored gD (pSLRSV.AgD), secreted gD (pSLRSV.SgD), cytoplasmic gD (pSLRSV.CgD), or null vector (pSLRSV.Nul) are shown. BHV-1 gD-specific ELISA titers are expressed as the reciprocal of the extrapolated (see Materials and Methods) dilution resulting in 3 standard deviations above the control value (prebleed sera). BHV-1 neutralizing antibody titers are expressed as the reciprocal of the highest dilution of antibody that caused a 50% reduction of plaques relative to the virus control. Results are expressed as geometric means, and bars indicate the standard error. S.N., serum neutralization.
DISCUSSION
DNA-based vaccines offer a number of potential advantages over conventional vaccines that include breadth of efficacy (single dosing, long-lasting immunity, CMI and humoral responses, appropriateness of responses), reduced downstream processing, and vaccine stability (3, 24, 76). Of these proposed advantages, duration and the protective efficacy of the immune responses are critical considerations when developing vaccines. In this article, we were able to demonstrate that immunization with plasmids encoding membrane-anchored (pSLRSV.AgD), secreted (pSLRSV.SgD), and cytosolic (pSLRSV.CgD) forms of BHV-1 gD induced potent humoral and cell-mediated immune responses. Furthermore, there was clear evidence of long-term maintenance of serum antibody levels in mice immunized with plasmids encoding the intracellular or secreted versions of BHV-1 gD at 23 weeks postimmunization (Fig. 3). Several studies support this observation (46, 59, 60); however, there is also recent evidence that the duration of in vivo expression, as well as humoral immunity following injection of plasmids encoding proteins (reporter proteins or antigens), can vary dramatically (45, 52).
Mice immunized with the intracellular version of gD (pSLRSV.CgD) displayed a lag in serum antibody appearance and lower overall serum titers (Fig. 3 and 4). This lag in serum antibody appearance may reflect the need for antigen-specific CD8* cytotoxic T cells to lyse in vivo-transfected host cells which subsequently release cytosolic antigens into the extracellular space. Recent evidence describing inflammatory reactions at the sites of DNA injection lend support to the contention that in vivo-transfected cells may become immunological targets as antigen-specific immunity develops (23). It also seems apparent that neutralizing serum titers raised against this cytosolic, nonglycosylated antigen are lower than those occurring in mice receiving plasmids encoding the secreted and membrane-anchored forms of gD which are posttranslationally modified (Fig. 2 and 9) (73). Indeed, it has been demonstrated previously that recombinant gD produced in E. coli generates excellent antibody titers when used to immunize cattle; however, serum neutralizing titers are nonexistent (79). The immunological significance of systemic antibody responses to antigens not normally occurring at the surface of pathogens (nonstructural proteins) is not well understood. There is recent evidence that humoral responses against nonstructural viral proteins of yellow fever, dengue, murine hepatitis, and rabies viruses can confer significant levels of protection to the respective viruses (42, 50, 61, 64).
In this study, mice immunized i.m. with plasmids encoding membrane-anchored and secreted forms of gD elicited immune responses of a similar magnitude and duration, which is consistent with the immune responses induced by plasmids encoding membrane-anchored and secreted forms of gD in cattle (80). However, following intradermal delivery, the plasmid encoding SgD elicited higher antibody titers and stronger antigen-specific proliferation than the plasmid encoding gD, both in C57BL/6 mice (10a) and in cattle (80). These higher humoral and cellular immune responses were correlated with enhanced protection of cattle from viral challenge. Thus, depending on the route of delivery, a mutation that leads to secretion of the expressed antigen may also have an effect on the magnitude of the immune responses induced.
Cytokine ELISPOT results from splenocytes harvested from several different experiments demonstrated an almost exclusive production of IFN-γ regardless of the cell compartment in which the DNA-encoded antigen was expressed (Fig. 6 and 7). These data are consistent with current published research demonstrating a Th1 type of immune response to many i.m. or intradermally administered DNA-based vaccines (24, 25, 76, 84). Recent evidence suggests that adjuvant effects of hypomethylated CpG motifs encoded within the plasmid DNA play a significant role in the skewing of immune responses toward a Th1-type immunity following immunization with these novel vaccines (56, 63). This tendency of DNA-based vaccines administered i.m. or intradermally to skew immunity toward a Th1 type of response is significant, because protective immune responses to many viral, bacterial, and parasitic diseases are characterized by a potent Th1 type of immunity (12, 19, 28, 29, 43, 49, 51, 55, 58, 62, 64). In this article, we demonstrated that the cell compartment in which the plasmid-encoded antigen DNA vaccine encoded the antigen had little impact on the overall splenic cytokine profile. However, it did appear that intracellular (pSLRSV.CgD) localization enhanced the magnitude of the Th1 response relative to membrane-anchored (pSLRSV.AgD) and secreted (pSLRSV.SgD) forms of antigen (Fig. 6). These data are consistent with research utilizing intracellular targeting technologies, such as ISCOMs or liposome-antigen formulations to enhance Th1-type immune responses (2, 32).
It has been demonstrated that IFN-γ and IL-4 can facilitate the switching of Ig to IgG2a and IgG1, respectively (65, 66). However, despite the prevalence of IFN-γ production in spleens of mice immunized with the DNA-based vaccines, we found that the expected preponderance of serum IgG2a occurred only in mice immunized with plasmids encoding cell-associated gD (pSLRSV.AgD or pSLRSV.CgD) (Fig. 5). Surprisingly, mice immunized with a plasmid encoding the secreted form of gD consistently showed serum isotypes that were predominantly IgG1. It is becoming increasingly evident that the mechanistic routes to a specific Ig isotype are more complex than the singular presence or absence of either IFN-γ or IL-4 (14, 19, 40, 51). Certainly, the serum isotype profiles represent the sum of Ig secretion from plasma cells originating from the spleen and draining lymph nodes and residing in the bone marrow (64). Indeed, we found that cytokine and Ig isotype secretion from T and B cells harvested from the draining iliac node more accurately reflected the serum isotype character (Fig. 7 and 8). This apparent dichotomy between splenic cytokine profiles and those seen in draining lymph nodes underscores the importance of recognizing the contributions of each immune compartment (spleen, lymph nodes, bone marrow) to the serum profile at a given time point following immunization (10, 38, 47). Certainly, compartmentalization of immune responses is not a novel concept: mucosal B cells produce an abundance of IgA and exhibit relatively short-lived responses (22). Similarly, dendritic cells from different tissues have been demonstrated to facilitate Th1- or Th2-type immune responses (26).
There are at least three other factors that can influence the type of immune response that occurs following immunization with DNA-based vaccines. These factors include intrinsic, immunologically unique features of encoded antigens, the route and method of immunization, and the development of cytotoxic T lymphocytes (27, 36, 44, 54). Immunization of C57BL/6 mice i.m. with a plasmid encoding the secreted antigen Ag85 from Mycobacterium tuberculosis resulted in a predominance of serum IgG2b and IgG2a (36). However, immunization of the same inbred mouse strain with plasmids encoding the hepatitis B virus surface antigen results in the secretion of virus-like particles and the predominance of primarily IgG2a (44). Other researchers demonstrated that i.m. immunization of BALB/c mice with plasmids encoding either cell membrane-anchored or partially secreted forms of measles virus hemagglutinin elicited a predominance of serum IgG2a and IgG1, respectively (13). Similarly, the membrane-anchored form of bovine parainfluenza virus 3 hemagglutinin/neuraminidase (HN) elicited a Th1-based immune response, whereas the secreted form of HN induced a mixed to Th2-type response (82). Conversely, a plasmid encoding a secreted, amino-terminal portion of hepatitis C virus nucleocapsid failed to cause a deviation of the serum IgG isotype toward greater levels of IgG1 following i.m. injection into the tibialis anterior muscles of BALB/c mice (37).
The route or rather the method of immunization has also been demonstrated to have a major impact on the character of the ensuing immune repertoire (27, 54). Gene gun administration of DNA-based vaccines typically results in a predominance of serum IgG1. However, it has also been demonstrated that ballistic immunization of C3H/HeN mice with plasmid encoding the influenza virus nucleoprotein resulted in IgG2a as the predominant serum isotype (13). While it does seem that ballistic and i.m. deliveries of DNA-based vaccines represent opposite ends of the humoral isotypic spectrum, this is not, perhaps, surprising when one considers the vast difference in the immunological microenvironments these two anatomical sites represent (9, 33). Furthermore, the importance of the specific IgG isotype may be of questionable protective value, particularly if high neutralizing titers of IgG1 are in evidence (6–8). However, given that most protective immune responses to viral infections in mice are characterized by a predominance in IgG2a and that this isotype is clearly more efficient at complement fixation, the issue of Ig isotype contribution to protection against infectious disease and vaccine development strategies cannot be ignored (18, 19, 39, 51).
DNA-based vaccines represent a novel and simple method of eliciting potent humoral and cell-mediated immunity. Clearly the potential for these vaccines to induce potent Th1-type immune responses that are frequently in conjunction with high levels of IgG2a and cytolytic activity necessitates further exploration. In light of our demonstration that the cell compartment in which the plasmid encoded the antigen has a significant impact on the developing immunological phenotype, we might consider utilizing several different antigens from the same pathogen as the basis for generating a balanced, comprehensive immune response (25). Our data clearly suggest that Th1 and Th2 (as well as IgG2a and IgG1) responses are not mutually exclusive within the same animal and that early compartmentalization of B-cell and T-cell responses may allow the propagation of this apparent immunological paradigm. This apparent coexistence of Th1 and Th2 responses in mice vaccinated with DNA vaccines may be a transient phenomenon (63). For the moment, multivalent DNA-based vaccines encoding both cell surface (i.e., viral envelope glycoproteins) and intracellular (i.e., structural or nonstructural) antigens may indeed be a logical approach for the generation of appropriately protective immune responses. Ultimately, the potential for many DNA-based vaccines to elicit both CMI and antibody, in conjunction with longevity of these responses, will test theories regarding self-regulation of each or both arms of the immune repertoire.
ACKNOWLEDGMENTS
We thank Barry Carroll, Leona Boyer, Gail MacLeod, Norleen Caddy, and Jane Fitzpatrick for animal support. We thank Michelle Balaski for assistance in the preparation of the manuscript and P. Griebel, M. Baca-Estrada, D. Godson, M. Morsey, A. Vankessel, and G. Cox for helpful discussions. We also thank Donna Dent, Marlene Snider, S. Suradhat, and Z. Papp for additional assistance.
Financial support was provided by the Natural Sciences and Engineering Council of Canada and by the Medical Research Council of Canada.
REFERENCES
- 1.Aramaki Y, Suda H, Tsuchiya S. Interferon-inductive effect of liposomes as an immunoadjuvant. Vaccine. 1995;18:1809–1814. doi: 10.1016/0264-410x(95)00117-j. [DOI] [PubMed] [Google Scholar]
- 2.Audibert F M, Lise L D. Adjuvants: current status, clinical perspectives, and future prospects. Immunol Today. 1993;14:281–284. doi: 10.1016/0167-5699(93)90046-N. [DOI] [PubMed] [Google Scholar]
- 3.Babiuk L A, L’Italien J, van Drunen Littel-van den Hurk S, Zamb T, Lawman M J P, Hughes G, Gifford G A. Protection of cattle from bovine herpesvirus type I (BHV-1) infection by immunization with individual viral glycoproteins. Virology. 1987;159:57–66. doi: 10.1016/0042-6822(87)90347-3. [DOI] [PubMed] [Google Scholar]
- 4.Babiuk L A, Wardley R C, Rouse B T. Defense mechanisms against bovine herpesvirus: relationship of virus-host cell events to susceptibility to antibody-complement cell lysis. Infect Immun. 1975;12:958–963. doi: 10.1128/iai.12.5.958-963.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baca-Estrada M E, Snider M, Tikoo S K, Harland R, Babiuk L A, van Drunen Littel-van den Hurk S. Immunogenicity of bovine herpesvirus-1 glycoprotein D in mice: effect of antigen form on the induction of cellular and humoral immune responses. Viral Immunol. 1996;9:11–22. doi: 10.1089/vim.1996.9.11. [DOI] [PubMed] [Google Scholar]
- 6.Bachman M F, Kundig T M, Hengartner H, Zinkernagel R M. Regulation of IgG antibody titers by the amount persisting of immune-complexed antigen. Eur J Immunol. 1994;24:2567–2570. doi: 10.1002/eji.1830241046. [DOI] [PubMed] [Google Scholar]
- 7.Bachman M F, Odermatt B, Hengartner H, Zinkernagel R M. Induction of long-lived germinal centers associated with persisting antigen after viral infection. J Exp Med. 1996;183:2259–2269. doi: 10.1084/jem.183.5.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachman M F, Kalinke U, Althage A, Freer G, Burkhart C, Roost H-P, Aguet M, Hengartner H, Zinkernagel R M. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–2027. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- 9.Bos J D, Kapsenberg M L. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75–78. doi: 10.1016/0167-5699(93)90062-P. [DOI] [PubMed] [Google Scholar]
- 10.Boyle C M, Morin M, Webster R G, Robinson H L. Role of different lymphoid tissues in the initiation and maintenance of DNA-raised antibody responses to the influenza virus H1 glycoprotein. J Virol. 1996;70:9074–9078. doi: 10.1128/jvi.70.12.9074-9078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Braun R P, Babiuk L A, van Drunen Little-van den Hurk S. Enhanced immune responses to an intradermally delivered DNA vaccine expressing a secreted form of BHV-1 gD. Vaccine Res. 1997;6:151–164. [Google Scholar]
- 11.Bretscher P A, Wei G, Menon J N, Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science. 1992;257:539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 12.Brubaker J O, Thompson C M, Morrison L A, Knipe D M, Siber G R, Finberg R W. Th1-associated immune responses to beta-galactosidase expressed by a replication-defective herpes simplex virus. J Immunol. 1996;157:1598–1604. [PubMed] [Google Scholar]
- 13.Cardoso A I, Blixenkrone-Moller M, Fayolle J, Liu M, Buckland R, Wild T F. Immunization with plasmid DNA encoding for the measles virus haemagglutinin and nucleoprotein leads to humoral and cell-mediated immunity. Virology. 1996;225:293–299. doi: 10.1006/viro.1996.0603. [DOI] [PubMed] [Google Scholar]
- 14.Coffman R L, von der Weid T. Multiple pathways for the initiation of T helper 2 (Th2) responses. J Exp Med. 1997;185:373–375. doi: 10.1084/jem.185.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins J K, Butcher A C, Riegel C A. Immune response to bovine herpesvirus type 1 infections: virus-specific antibodies in sera from infected animals. J Clin Microbiol. 1985;21:546–552. doi: 10.1128/jcm.21.4.546-552.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Condon C, Watkins S C, Celluzi C M, Thompson K, Falo L D J. DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 17.Corr M, Lee D J, Carson V, Tighe H. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J Exp Med. 1996;184:1555–1560. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coutelier J-P, van der Logt J T M, Heessen F W A. IgG subclass distribution of primary and secondary immune responses concomitant with viral infection. J Immunol. 1991;147:1383–1386. [PubMed] [Google Scholar]
- 19.Coutelier J-P, van der Logt J T M, Heessen F W A, Vink A, van Snick J. Virally-induced modulation of murine IgG antibody subclasses. J Exp Med. 1988;168:2373–2378. doi: 10.1084/jem.168.6.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox G J M, Zamb T J, Babiuk L A. Bovine herpesvirus 1: immune responses in mice and cattle injected with plasmid DNA. J Virol. 1993;67:5664–5667. doi: 10.1128/jvi.67.9.5664-5667.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czerkinsky C, Andersson G, Ekre H P, Nilsson L A, Klareskog L, Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988;110:29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- 22.Czerkinsky C, Holmgren J. The mucosal immune system and prospects for anti-infectious and anti-inflammatory vaccines. Immunologist. 1995;3:97–103. [Google Scholar]
- 23.Davis H L, Brazolot-Millan C L, Watkins S C. Immune-mediated destruction of transfected muscle fibres after direct gene transfer with antigen-expressing plasmid DNA. Gene Ther. 1997;4:181–188. doi: 10.1038/sj.gt.3300380. [DOI] [PubMed] [Google Scholar]
- 24.Donnelly J J, Ulmer J B, Liu M A. Immunization with DNA. J Immunol Methods. 1994;176:145–152. doi: 10.1016/0022-1759(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 25.Doolan D L, Sedegah M, Hedstrom R C, Hobart P, Charoenvit Y, Hoffman S L. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ cell-, interferon gamma-, and nitric oxide-dependent immunity. J Exp Med. 1996;183:1739–1746. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Everson M P, McDuffie D S, Lemak D G, Koopman W J, McGhee J R, Beagley K W. Dendritic cells from different tissues induce production of different T cell cytokine profiles. J Leukoc Biol. 1996;59:494–498. doi: 10.1002/jlb.59.4.494. [DOI] [PubMed] [Google Scholar]
- 27.Feltquate D M, Heaney S, Webster R G, Robinson H L. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997;158:2278–2284. [PubMed] [Google Scholar]
- 28.Fresno M, Kopf M, Rivas L. Cytokines and infectious diseases. Immunol Today. 1997;18:56–58. doi: 10.1016/s0167-5699(96)30069-8. [DOI] [PubMed] [Google Scholar]
- 29.Gause W C, Halvorson M J, Lu P, Greenwald R, Linsley P, Urban J F, Finkelman F D. The function of co-stimulatory molecules and the development of IL-4-producing T cells. Immunol Today. 1997;18:115–120. doi: 10.1016/s0167-5699(97)01005-0. [DOI] [PubMed] [Google Scholar]
- 30.Gibbs E P J, Rweyemamu M M. Bovine herpesviruses, part 1. Vet Bull. 1977;47:317–343. [Google Scholar]
- 31.Guery J-C, Galbiati F, Smiroldo S, Adorini L. Selective development of T helper (Th)2 cells induced by continuous administration of low dose soluble proteins to normal and 2-microglobulin-deficient BALB/c mice. J Exp Med. 1996;183:485–497. doi: 10.1084/jem.183.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta R K, Relyveld E H, Lindblad E B, Bizzini B, Ben-Efraim S, Gupta C K. Adjuvants: a balance between toxicity and adjuvanticity. Vaccine. 1993;11:293–306. doi: 10.1016/0264-410x(93)90190-9. [DOI] [PubMed] [Google Scholar]
- 33.Hohlfeld R, Engel A G. The immunobiology of muscle. Immunol Today. 1994;15:269–274. doi: 10.1016/0167-5699(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 34.Hughes G, Babiuk L A, van Drunen Littel-van den Hurk S. Functional and topographical analysis of epitopes on bovine herpesvirus type 1 glycoprotein IV. Arch Virol. 1988;103:47–60. doi: 10.1007/BF01319808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hutchings D L, van Drunen Littel-van den Hurk S, Babiuk L A. Lymphocyte proliferative responses to separated bovine herpesvirus 1 proteins in immune cattle. J Virol. 1990;64:5114–5122. doi: 10.1128/jvi.64.10.5114-5122.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huygen K, Content J, Densi O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D’Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J-P, Lui M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 37.Inchauspe G, Vitvitski L, Major M E, Jung G, Spengler U, Maisonnas M, Trepo C. Plasmid DNA expressing a secreted or non-secreted form of hepatitis C virus nucleocapsid: comparative studies of antibody and T-helper responses following genetic immunization. DNA Cell Biol. 1997;16:185–195. doi: 10.1089/dna.1997.16.185. [DOI] [PubMed] [Google Scholar]
- 38.Justewicz D M, Morin M J, Robinson H L, Webster R G. Antibody-forming cell response to virus challenge in mice immunized with DNA encoding the influenza virus hemagglutinin. J Virol. 1995;69:7712–7717. doi: 10.1128/jvi.69.12.7712-7717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klaus G G B, Pepys M B, Kitajima K, Askonas B A. Activation of mouse complement by different classes of mouse antibody. Immunology. 1979;38:687–695. [PMC free article] [PubMed] [Google Scholar]
- 40.Kuhn R K, Rajewsky K, Muller W. Generation and analysis of interleukin-4 knockout mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 41.Li L, Hoffman R M. The feasibility of targeted selective gene therapy of the hair follicle. Nat Med. 1995;1:705–706. doi: 10.1038/nm0795-705. [DOI] [PubMed] [Google Scholar]
- 42.Lodmell D L, Esposito J J, Ewalt L C. Rabies virus antinucleoprotein antibody protects against rabies virus challenge in vivo and inhibits rabies virus replication in vitro. J Virol. 1993;67:6080–6086. doi: 10.1128/jvi.67.10.6080-6086.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahon B P, Katrak K, Nomoto A, Macadam A J, Minor P D, Mills K H G. Poliovirus-specific CD4+ Th1 clones with both cytotoxic and helper activity mediate protective humoral immunity against a lethal poliovirus infection in transgenic mice expressing the human poliovirus receptor. J Exp Med. 1995;181:1285–1292. doi: 10.1084/jem.181.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mancini M, Hadchouel M, Davis H L, Whalen R G, Tiollais P, Michel M-L. DNA-mediated immunization in a transgenic mouse model of the hepatitis B surface antigen chronic carrier state. Proc Natl Acad Sci USA. 1996;93:12496–12501. doi: 10.1073/pnas.93.22.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manthorpe M, Cornefert-Jemsen F, Hartikka J, Felgner J, Rundell A, Margalith M, Dwarki V. Gene therapy by intramuscular injection of plasmid DNA: studies on firefly luciferase gene expression in mice. Hum Gene Ther. 1993;4:419–431. doi: 10.1089/hum.1993.4.4-419. [DOI] [PubMed] [Google Scholar]
- 46.Michel M-L, Davis H L, Schleef M, Mancini M, Tiollais P, Whalen R G. DNA-mediated immunization to the hepatitis B surface antigen in mice: aspects of the humoral response mimic hepatitis B viral infection in humans. Proc Natl Acad Sci USA. 1995;92:5307–5311. doi: 10.1073/pnas.92.12.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mo X Y, Sarawar S R, Doherty P C. Induction of cytokines in mice with parainfluenza pneumonia. J Virol. 1995;69:1288–1291. doi: 10.1128/jvi.69.2.1288-1291.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mosmann T R, Cherwinski H, Bond M B, Giedlin M A, Coffman R L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 49.Mosmann T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 50.Nakanaga K, Yamanouchi K, Fujiwara K. Protective effect of monoclonal antibodies on lethal mouse hepatitis virus infection in mice. J Virol. 1986;59:168–171. doi: 10.1128/jvi.59.1.168-171.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen L, Knipe D M, Finberg R W. Mechanism of virus-induced Ig subclass shifts. J Immunol. 1994;152:478–484. [PubMed] [Google Scholar]
- 52.Norman J A, Hobart P, Manthorpe M, Felgner P, Wheeler C. Development of improved vectors for DNA-based immunization and other gene therapy applications. Vaccine. 1997;15:801–803. doi: 10.1016/s0264-410x(96)00247-2. [DOI] [PubMed] [Google Scholar]
- 53.Parish C R, Liew F Y. Immune response to chemically modified flagellin. III. Enhanced cell-mediated immunity during high and low zone antibody tolerance to flagellin. J Exp Med. 1971;135:298–311. doi: 10.1084/jem.135.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pertmer T M, Roberts T R, Haynes J R. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 1996;70:6119–6125. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peterson J D, Waltenbaugh C, Miller S D. IgG subclass responses to Theiler’s murine encephalomyelitis virus infection and immunization suggest a dominant role for Th1 cells in susceptible mouse strains. Immunology. 1992;75:652–658. [PMC free article] [PubMed] [Google Scholar]
- 56.Pisetsky D. Immune activation by bacterial DNA: a new genetic code. Immunity. 1996;5:303–310. doi: 10.1016/s1074-7613(00)80256-3. [DOI] [PubMed] [Google Scholar]
- 57.Pisetsky D S. DNA and the immune system. Ann Intern Med. 1997;126:169–171. doi: 10.7326/0003-4819-126-2-199701150-00015. [DOI] [PubMed] [Google Scholar]
- 58.Pope M, Chung S W, Mosmann T, Leibowitz J L, Gorczynski R M, Levy G A. Resistance of naive mice to murine hepatitis virus strain 3 requires development of a Th1, but not a Th2 response, whereas pre-existing antibody partially protects against primary infection. J Immunol. 1996;156:3342–3349. [PubMed] [Google Scholar]
- 59.Raz E, Carson D A, Parker S E, Parr T B, Abai A M, Aichinger G, Gromkowski S H, Singh M, Lew D, Yankauckas M, Baird S M, Rhodes G H. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci USA. 1994;91:9519–9523. doi: 10.1073/pnas.91.20.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rhodes G H, Dwarki V J, Abai A M, Felgner J, Felgner P L, Gromkowski S H, Parker S E. Injection of expression vectors containing viral genes induces cellular, humoral, and protective immunity. Vaccines 93. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993. [Google Scholar]
- 61.Schlesinger J J, Brandriss M W, Cropp C B, Monath T P. Protection against yellow fever in monkeys by immunization with yellow fever virus nonstructural protein NS1. J Virol. 1986;60:1153–1155. doi: 10.1128/jvi.60.3.1153-1155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scott P. IL-12 initiation cytokine for cell-mediated immunity. Science. 1993;260:496–497. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- 63.Shearer G M, Clerici M. Vaccine strategies: selective elicitation of cellular or humoral immunity? Trends Biotechnol. 1997;15:106–109. doi: 10.1016/S0167-7799(97)01011-1. [DOI] [PubMed] [Google Scholar]
- 64.Slifka M K, Ahmed R. Long-term humoral immunity against viruses: revisiting the issue of plasma cell longevity. Trends Microbiol. 1996;4:394–400. doi: 10.1016/0966-842X(96)10059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Snapper C M, Mond J J. Towards a comprehensive view of immunoglobulin class switching. Immunol Today. 1993;14:15–17. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- 66.Snapper C M, Paul W E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 67.Spalding D M, Koopman W J, Eldridge J H, McGhee J R, Steinman R M. Accessory cells in murine Peyer’s patches. J Exp Med. 1983;157:1646–1659. doi: 10.1084/jem.157.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stribling R, Brunette E, Liggitt D, Gaensler K, Debs R. Aerosol gene delivery in vivo. Proc Natl Acad Sci USA. 1992;89:11277–11281. doi: 10.1073/pnas.89.23.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Theisen M, Potter A A. Cloning, sequencing, expression, and functional studies of a 15,000-molecular-weight Haemophilus somnus antigen similar to Escherichia coli ribosomal protein S9. J Bacteriol. 1992;174:17–23. doi: 10.1128/jb.174.1.17-23.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tikoo S K, Campos M, Babiuk L A. Bovine herpesvirus 1 (BHV-1): biology, pathogenesis, and control. Adv Virus Res. 1995;45:191–223. doi: 10.1016/s0065-3527(08)60061-5. [DOI] [PubMed] [Google Scholar]
- 71.Tikoo S K, Campos M, Popowych Y I, van Drunen Littel-van den Hurk S, Babiuk L A. Lymphocyte proliferative responses to recombinant bovine herpesvirus type 1 (BHV-1) glycoprotein gD (gIV) in immune cattle: identification of a T cell epitope. Viral Immunol. 1995;8:19–25. doi: 10.1089/vim.1995.8.19. [DOI] [PubMed] [Google Scholar]
- 72.Tikoo S K, Fitzpatrick D R, Babiuk L A, Zamb T J. Molecular cloning, sequencing, and expression of functional bovine herpesvirus 1 glycoprotein gIV in transfected bovine cells. J Virol. 1990;64:5132–5142. doi: 10.1128/jvi.64.10.5132-5142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tikoo S K, Parker M D, van den Hurk J V, Kowalski J, Zamb T J, Babiuk L A. Role of N-linked glycans in antigenicity, processing, and cell surface expression of bovine herpesvirus 1 glycoprotein gIV. J Virol. 1993;67:726–733. doi: 10.1128/jvi.67.2.726-733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tikoo S K, Zamb T J, Babiuk L A. Analysis of bovine herpesvirus 1 glycoprotein gIV truncations and deletions expressed by recombinant vaccinia viruses. J Virol. 1993;67:2103–2109. doi: 10.1128/jvi.67.4.2103-2109.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ulmer J B, Deck R R, Dewitt C M, Donnelly J J, Liu M A. Generation of MHC class I-restricted cytotoxic T lymphocytes by expression of a viral protein in muscle cells: antigen presentation by non-muscle cells. Immunology. 1996;89:59–67. doi: 10.1046/j.1365-2567.1996.d01-718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ulmer J B, Donnelly J J, Liu M A. DNA vaccines promising: a new approach to inducing protective immunity. ASM News. 1996;62:476–479. [Google Scholar]
- 77.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, Hawe L A, Leander K R, Martinez D, Perry H C, Shiver J W, Montgomery D L, Liu M. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 78.van Drunen Littel-van den Hurk S, Babiuk L A. Polypeptide specificity of the antibody response after primary and recurrent infection with bovine herpesvirus 1. J Clin Microbiol. 1986;23:274–282. doi: 10.1128/jcm.23.2.274-282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Drunen Littel-van den Hurk S, Parker M D, Massie B, van den Hurk J V, Harland R, Babiuk L A, Zamb T. Protection of cattle from BHV-1 infection by immunization with recombinant glycoprotein gIV. Vaccine. 1993;11:25–35. doi: 10.1016/0264-410x(93)90336-v. [DOI] [PubMed] [Google Scholar]
- 80.van Drunen Littel-van den Hurk S, Braun R, Lewis P J, Karvonen B C, Baca-Estrada M, McCartney D, Watts T, Babiuk L A. Intradermal immunization with a bovine herpesvirus-1 DNA vaccine induces protective immunity in cattle. J Gen Virol. 1998;79:831–839. doi: 10.1099/0022-1317-79-4-831. [DOI] [PubMed] [Google Scholar]
- 81.van Drunen Littel-van den Hurk S, Tikoo S K, Liang X, Babiuk L A. Bovine herpesvirus-1 vaccines. Immunol Cell Biol. 1993;71:405–420. doi: 10.1038/icb.1993.47. [DOI] [PubMed] [Google Scholar]
- 82.van Drunen Littel-van den Hurk S, Braun R P, Karvonen B C, King T, Yoo D, Babiuk L A. Immune responses and protection induced by DNA vaccines encoding bovine parainfluenzavirus type 3 (BPIV-3) glycoproteins. Virology. 1999;260:35–46. doi: 10.1006/viro.1999.9793. [DOI] [PubMed] [Google Scholar]
- 83.Wheeler C J, Felgner P L, Tsai Y J, Marshall J, Sukhu L, Doh S G, Hartikka J, Nietupski J, Manthorpe M, Nichols M, Plewe M, Liang X, Norman J, Cheng S H. A novel cationic lipid greatly enhances plasmid DNA delivery and expression in mouse lung. Proc Natl Acad Sci USA. 1996;93:11454–11459. doi: 10.1073/pnas.93.21.11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wyler R, Engels M, Schwyzer M. Infectious bovine rhinotracheitis/vulvovaginitis (BHV-1) In: Wittmann G, editor. Herpesvirus diseases of cattle, horses and pigs. Developments in veterinary virology. Boston, Mass: Kluwer Academic Publishers; 1989. pp. 1–172. [Google Scholar]
- 85.Yang X, Gieni R, Mosmann T R, Hayglass K T. Chemically modified antigen preferentially elicits induction of Th1-like cytokine synthesis patterns in vivo. J Exp Med. 1993;178:349–353. doi: 10.1084/jem.178.1.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yates W D G. A review of infectious rhinotracheitis, shipping-fever pneumonia, and viral-bacterial synergism in respiratory diseases of cattle. Can J Comp Med. 1982;46:225–263. [PMC free article] [PubMed] [Google Scholar]