Background
The field of interventional cardiology continues to evolve with the introduction of new techniques and technologies. From the first angioplasty to transcatheter aortic valve replacement (TAVR), dissemination of new treatments has generally required a training program of didactics and observation, culminating in a hands-on experience with a patient. Trainees and facilities starting new programs often require support from outside experts, traditionally known as “medical proctors,” whose costs are typically borne by the medical device industry.
While medical proctoring is a longstanding practice beneficial with respect to training, it may also be associated with some risks to the patient, operator, proctor and host institution. These risks include, but may not be limited to, lack of appropriate equipment at the host institution, scheduling of inappropriate patients for proctored cases, and unclear medico-legal indemnification of the proctor. These potential risks have not been previously documented or described in the medical literature. The aim of this position statement of SCAI is to educate all parties of the potential risks involved with medical proctoring and to recommend best practices to reduce the potential for adverse events, misunderstandings, conflicts of interest, and unexpected medico-legal liability. While this document focuses on industry-sponsored proctoring of approved uses of medical devices, some aspects may be applicable to investigational devices and off-label use.
SCAI is not structured to provide legal advice and the recommendations outlined herein should not be considered definitive or the “last word” on untested legal matters. SCAI and the authors of this paper assume no responsibility for any actions taken under any legal theory against a proctor based on the considerations outlined.
Development methodology
This statement has been developed according to SCAI Publications Committee policies for writing group composition, disclosure and management of relationships with industry (RWI), internal and external review, and organizational approval.1
The writing group has been organized to ensure diversity of perspectives and demographics, multi-stakeholder representation, and appropriate balance of RWI. Relevant author disclosures are included in Supplement 1. Before appointment, members of the writing group were asked to disclose financial and intellectual relationships from the 12 months prior to their nomination. A majority of the writing group disclosed no relevant, significant financial relationships. Disclosures were periodically reviewed during document development and updated as needed. SCAI policy requires that writing group members with a current, relevant financial interest are recused from participating in related discussions or voting on recommendations. Conflicts were identified and resolved as noted in the supplemental material. The work of the writing committee was supported exclusively by SCAI, a nonprofit medical specialty society, without commercial support. Writing group members contributed to this effort on a volunteer basis and did not receive payment or any form of compensation from SCAI.
Where applicable, literature searches were performed by group members designated to lead each section and initial section drafts were authored primarily by the section leads in collaboration with other members of the writing group. Recommendations were discussed by the full writing group until a majority of group members agreed on the text and qualifying remarks. All recommendations are supported by a short summary of the evidence or specific rationale.
Due to the legal issues addressed in this position statement, the writing committee included a lawyer with malpractice expertise. The draft document was also reviewed by a SCAI staff member with a law degree and separately by SCAI organizational counsel. Industry feedback was specifically sought through the public comment process.
The draft manuscript was peer reviewed in November 2021 and the document was revised to address pertinent comments. The writing group unanimously approved the final version of the document. The SCAI Publications Committee and Executive Committee endorsed the document as official society guidance in February 2022.
Definitions2,3
A preceptor (from Latin praeceptor “teacher, instructor”) provides the trainee with experience and training in a new skill. This is conducted in an environment where the preceptor has the primary responsibility for the care of the patient whether delivered by the preceptor themselves or the trainee. Attending physicians in accredited graduate medical education programs serve as preceptors for their trainees, but other less formal preceptorships can be appropriate for new technologies.
A proctor (from Middle English proctour “manager, steward”) as defined in American English is a person who monitors students during an examination of skills the students have already learned. The proctor differs from a preceptor in that the proctor functions as an independent observer to evaluate, not teach, the technical and cognitive skills of another physician, typically on behalf of the hospital or credentialing body. Proctors traditionally have no formal role for the patient’s care and outcome, these are solely the responsibility of the primary operator.
Different inpatient or outpatient practice settings, procedural specifications, case complexity, specific needs of student/institution, and hospital privilege requirements will determine the role of proctor for the type of procedure(s) being supervised. To date, the use of proctors during interventional cardiology procedures has represented a combination of the traditional roles of preceptor (teacher) and proctor (evaluator), particularly for new devices and techniques, often without clear delineation of the proctor’s responsibilities.
In order of increasing involvement and responsibility, the clinical proctor might be asked to:
-
1.
Retrospectively review case(s) already completed (i.e., procedural indications, procedural records, angiography, periprocedural imaging) in order to recognize operators with successful case completion. This activity frequently occurs for procedures (i.e., percutaneous coronary intervention, closure of a patent foramen ovale) that are not new, where a well-trained provider merely requires peer review of a specific number of cases required for initial hospital privileging.
-
2.
Observe case(s) on site without being scrubbed in.
-
3.
Observe case(s) from within the procedure room, providing bedside cognitive advice to the operator.
-
4.
Scrub into and actively participate in the hands-on portions of the procedure.
Potential risks
By definition, the physician being proctored (referred in this document as the operator, host, or host physician), as well as the supporting catheterization lab staff, may have had little or no experience with a new procedure or new equipment. This initial experience is often described as being part of the “early learning curve,” highlighting the need for both quality of teaching as well as quantity of case volume. In an analysis of malpractice claims of surgical errors resulting in patient injury, inexperience or lack of technical competence was identified as the key contributing system factor in 41% of cases.4
Each party comes to the proctoring procedure with interests beyond the care of the individual patient; identifying and resolving these potential conflicts of interest can help mitigate some of the risks associated with the proctoring experience. The interventionalist being trained on the new device may be eager to get experience and may overlook the suitability of the patient or clinical situation for the procedure, or express bias when presenting the potential risks, benefits, and alternative treatments to patients. In their enthusiasm, there is a risk that they may not be completely transparent with their patient about their relative inexperience and the role of the proctor, which may lead to dissatisfaction with the care provided if there is an adverse outcome. Similarly, the device manufacturer has a strong financial incentive to have its product used and distributed. The proctor, on the other hand, is usually paid by the sponsor, does not have a therapeutic relationship with the patient, may be present for only part of the procedure, may not be involved in reviewing the appropriateness of the procedure, does not serve in a supervisory role, and may not always be sufficiently invested to ensure a successful outcome.
Despite the best intentions of all interested parties, the potential for inappropriate or unsafe use of new technologies exists. It is the position of this committee that patient safety is paramount, and we underscore that it is the patient’s right to have the appropriate procedure performed by adequately trained interventionalists who are proctored by expert physicians, and to be fully informed about the presence and intended extent of involvement of a proctor during their procedure in their consent.
Legal risks
With increasing involvement, the clinical proctor incurs increasing risk of liability for their participation in a procedure. The term “proctor” is not optimal for all circumstances, as it would be inappropriate for a proctor to assist a student or complete a test during an examination in any other context. However, given the conventional use of “proctor” to encompass a range of training activities and participation in medicine we will continue to use the term throughout this document.
The true risk of legal liability and potential exposure of the proctor to a charge of malpractice remains undefined. To our knowledge, no case law that sets a legal precedent for the activities of a clinical proctor has been reported. Laws governing medical practice differ by state. Accordingly, no specific statements or guidance can be made about how proctors can be fully protected from legal liability. The following observations, however, are generally applicable.
Legal analysis
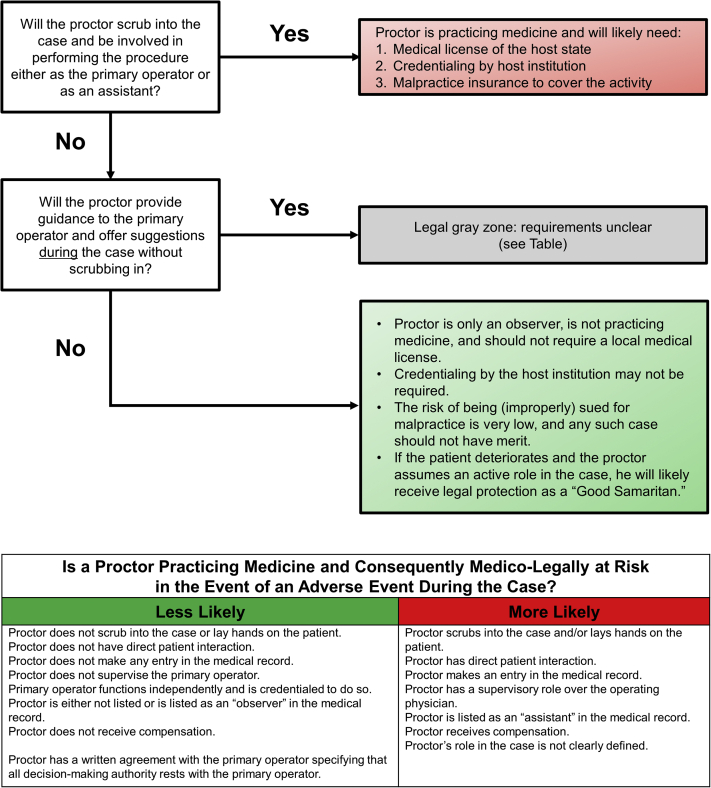
In most cases a doctor-patient relationship must be established in order for a physician to be held legally liable for any patient harm. A doctor-patient relationship is formed when a doctor has some type of professional contact with a patient. This contact may be direct or indirect. Direct contact occurs when a physician advises, examines, treats, or prescribes a medication for a patient, or performs a procedure, orders a test, or has a clinical discussion with a patient thereby creating a duty of care. A proctor who does any of these things may create a doctor- patient relationship and assumes the associated liability (Figure 1).
Figure 1.
Understanding Medico-Legal Risk as a Proctor.
Even if a proctor has no direct patient interaction, they must still be careful to avoid forming a doctor-patient relationship by indirect contact. Indirect contact occurs when a physician assumes a formal role in the patient’s care. Examples include a radiologist who reads a chest x-ray (who is then liable for an errant interpretation even though that radiologist never saw the patient), an attending physician who oversees residents (who is liable for whatever residents do), a physician covering for a vacationing colleague (who is responsible for all of the colleague’s patients), and a physician who provides advice on a case after being formally consulted, even if they have not yet seen the patient.
Assuming that the proctor will avoid direct patient contact, the question then becomes: how much input and involvement can a proctor offer without indirectly creating a doctor – patient relationship and how should the proctor provide that input?
Because the nature of the proctoring relationship requires that the proctor review the patient’s records, discuss the case with the host physician and staff, make observations about the nature of the case and provide input on or approval of the planned strategic approach to the patient’s care, it is likely that the proctor will be brought into any litigation in the event of patient harm. It is therefore recommended that the proctor have malpractice coverage when assuming this role in the appropriate jurisdiction. Such coverage may be provided by the proctor’s own policy, by his or her employer, the host institution, the host physician, or the sponsoring industry partner. Adherence to the recommendations outlined in this document may support a defense that any actions taken by the proctor were for the safety and welfare of the patient in the context of expanding access to state-of-the-art procedures.
In terms of protecting the proctor, the following is recommended:
-
1.
The proctor should not have any supervisory role over the operating physician. The host physician must have completed sufficient didactic, hands-on, and preceptorship training to complete the procedure independently. The operating physician should be fully credentialed by their facility and permitted to do the procedure entirely on their own.
-
2.
Prior to the case, the proctor and the primary operating physician should both sign a document outlining the responsibilities of the proctor. This document should clearly state that all patient care decisions ultimately rest with the operating physician, that the proctor is not a supervisor but is acting as an advisor, and that any observations or suggestions made by the proctor are not necessarily recommendations upon which the operating physician must rely. This will serve to eliminate the possibility of the proctor becoming liable for supervision as occurs in the attending-resident situation.
-
3.
This document should review any expectations on the part of the physician being proctored, the patient, or anyone else for the proctor to assist in patient care in case of a complication or emergency, as the proctor who assumes a direct supervisory or “hands-on” role will have increased liability. However, although courts have not yet found a duty to rescue, failure to step in could result in liability as the proctor would be the senior physician with the most knowledge of how to address an emergency. The role of the proctor in the event of a life-threatening emergency should be discussed, documented, and clearly understood by both the proctor and host physician before the procedure begins.
-
4.
The patient should be educated on and sign an informed consent that clearly outlines the presence and limited role of the proctor for their procedure. Such a document can potentially include an explicit waiver of liability for the proctoring physician.
-
5.
To minimize the establishment of a doctor-patient relationship, the proctor should preferably avoid any patient contact or interaction. The proctor who observes, make notes, and provides a critique only after the case would likely be fully protected from liability, but would also be less effective in sharing their expertise.
-
6.
If direct patient contact and care are expected, the proctor should verify that they have malpractice insurance coverage for their activities and are credentialed by the host institution to provide those services.
-
7.
If the name of the proctor is included in the procedure note or medical record, they should be preferably listed as an “observer.”
Adhering to these principles will serve to reduce, but not eliminate, the proctor’s exposure to liability. The fact that the proctoring physician is being compensated for their time, effort, and expertise will also likely be a determining factor on the part of any plaintiff’s attorney when deciding on whether to cite the proctor as a respondent in the case.
As previously discussed, these recommendations should not be relied upon by the proctor as the only source of information regarding potential legal liability. It is recommended that the proctor seek clarification from his or her malpractice carrier, personal attorney, the host institution and/or industry partner regarding such exposure. However, adherence to the principles outlined in this paper should provide strong evidence that the proctor’s primary concern is for patient safety and well-being even as they help disseminate new medical technologies and techniques.
Roles and responsibilities
Pre-procedure: Preparation
The host physician
As described in the definition section, proctoring is primarily for observation and evaluation of a host physician’s ability to perform a new procedure. A proctor may provide key guidance or helpful tips but should not be relied upon to provide direct teaching or step-by-step hands-on instruction as they would for an early beginner in a fellowship program or training course. If a greater degree of involvement is desired or expected of the proctor, then full medical privileging along with malpractice coverage may be needed. It is important that the host physician specify the role of the proctor that is desired (observing or hands-on involvement), as this will determine the type of privileging required.
The host physician should have already demonstrated sufficient baseline competency to be qualified to perform the procedure, which may be new to them, but should only be an incremental technical advancement that extends a well-established skill set. Specific requirements may be stipulated by the industry sponsor, governmental agencies, or professional societies, and it is incumbent upon the host physician that these are fulfilled prior to the proctoring event and documentation provided to the industry sponsor and proctor. First-time left atrial appendage occlusion implanters, for instance, must demonstrate adequate experience with transseptal interventional cardiac procedures.
The host physician should confirm that they have: 1) completed any training required by the industry sponsor and regulatory authorities, 2) reviewed the relevant literature, and 3) become thoroughly familiar with the procedural indications, risks, setup, procedural steps, and complication management. The physician should prepare for the proctored procedure as if it were a final examination. Not only is this expected for best patient care, but it also helps the host physician instill confidence in the cardiac team and in the proctoring relationship.
Patient selection should prioritize patient safety while also maximizing the overall experience. The host physician should identify patients who are medically appropriate based on criteria set forth by clinical guidelines or by professional societies. Each patient should be discussed with the proctor well in advance of the procedure to address clinical risk and potential technical challenges. Scheduling more than one proctored procedure makes the most of the presence of a proctor and may reinforce skills through repetition. Importantly, there should be sufficient time allotted for the cases and between cases to ensure proper team debriefing and feedback, and to account for potential delays associated with complications, equipment preparation, and staff training.
The host physician, industry specialist, and proctor should establish an equipment checklist for the procedure and potential complications. This list needs to be discussed well in advance of the procedure so that all the necessary equipment is available prior to the proctoring day.
Host institution and cath lab
The host physician should inform the host institution about the nature of the proctored procedure and the proctoring visit with sufficient notice (typically weeks in advance) to allow for privileging and legal arrangements. This physician should ensure that their own foundational training is adequate to perform the procedure, that appropriate patients are available, and that the cath lab staffing, equipment, and supplies are adequate for the new procedure.
The cath lab should establish protocols around the roles of proctors, industry clinical support, observers, and visiting physicians—what they will be allowed to do and how they are to identify themselves. Local staff members who are the best suited for involvement in the new procedure should be identified in advance to ensure their availability on the proctoring day. The cath lab schedule should be set to protect the time and staffing for the proctored procedure, including providing coverage for other urgent procedures.
The hosting institution should establish the local requirements for a proctor to participate in the case either as an active participant or passive observer. These could include: verify that the physician who is proctoring has equivalent privileges at another facility, querying the National Practitioner Data Bank, verifying medical license information, and obtaining evidence of his or her clinical competence.5 Temporary medical privileges would be necessary for any case where the proctor is expected to actively participate. Malpractice coverage or indemnification of the proctor for their activities during their visit should be extended in these cases. It is recommended that an institution’s legal counsel be involved in the development and implementation of proctoring guidelines. Additional requirements of clinical visitors including tuberculosis testing and vaccinations should be clarified with the compliance office of the host institution.
A pre-visit phone conference call between the host physician, proctor, and industry sponsor should be arranged to ensure that everything is in place and the expectations, roles, and responsibilities are clear. As they have the relevant expertise, the proctor and industry sponsor should ensure that the host physicians have received adequate pre-procedure training, the planned cases are appropriate, and the necessary equipment supplies are present. In particular, it is important to ensure that the hosting physician has been educated on the potential complications associated with the device or procedure and is familiar with bail-out strategies. Any outstanding questions should be resolved.
Day of procedure
Preparing the patient
Prior to the procedure day, the patient should be informed that they will be undergoing a procedure for which the operating physician will be proctored and industry clinical support will be involved in the case. The extent of the proctor’s involvement should be discussed with the patient in advanced. This information needs to be reinforced on the day of the procedure and incorporated into the consent discussion as noted previously. The patient needs to be comfortable knowing that the host physician may be doing a procedure or using a specific device for the first time or with minimal experience and must be given the freedom to choose to not proceed based on this information.
The key to providing informed consent while respecting patient autonomy is to genuinely acknowledge any patient concerns and work on establishing trust and rapport. If a patient feels that their physician has been sincere in explaining the risks and benefits of the procedure, is honest about their ability to use a new technology safely and are operating with the patient’s best interest as the priority, then the patient can consent to the novel device or procedure with confidence. Being well versed in the rates of success and complications from the pivotal randomized trials and post-market studies (if available) is extremely important in addressing patient concerns.
Often a technology or procedure is an incremental technology that builds upon a well-established skillset, which should provide reassurance to patients. If the technology or procedure is completely new with unknown and potentially significant risks, (e.g., a first-in-human study of an emerging technology) then this also needs to be explicitly stated in the consent discussion. The purpose of the consent is to have an honest discussion about what the patient will be undergoing and allow them to freely choose how to proceed.
The physician must be open to deferring the procedure at any time based on the patient’s desires and the physicians’ best clinical judgement, and not feel pressured to perform the procedure on that day merely because a proctor is available. Patient safety and best clinical care should always be paramount, and providers should feel comfortable canceling a procedure based on their clinical judgment.
Procedure day: Establish the ground rules
The proctor, host physician, the cath lab staff and industry clinical support should have time to be introduced and to review the technical details of the procedure/device, patient history, procedural flow, and equipment and procedural checklists. Ideally, responsible proctoring would include a didactic lecture by the proctor to the host physician and cath lab staff on the procedure, equipment, and complications. Only when the proctor feels that all concerns that would prevent a successful procedure have been addressed should the procedure be performed. The host physician should describe their experience and level of comfort with the procedure or similar techniques. It is critical to communicate the expectations of the host physician and proctor and establish the ground rules for what will happen during the procedure. Will the proctor be an observer offering evaluation and feedback, a consultant providing intraprocedural guidance, or an active participant allowed to scrub into the procedure? The roles of the cath lab staff members should also be established if necessary. As noted above, the host and proctor should discuss what the back-up plan will be in case of an emergency, the steps to manage the most common complications, and the kind of involvement is allowed and expected in these situations.
Creating a collaborative and effective environment
The proctor and primary operator may be performing a procedure together for the first time, with little or no prior contact. Each physician should recognize the interpersonal cues needed to understand each provider’s communication styles and gauge how each party interacts, follows directions, or accepts feedback. Some operators may be reticent to ask questions when necessary and may require more clear direction from the proctor. Similarly, some proctors may not feel comfortable offering verbal feedback until a problem is clearly present, which may be too late. Openly discussing the manner by which feedback will be provided and the critical portions of the procedure when this will occur will reduce the risk of any miscommunication or missed opportunities.
The host, proctor, and industry clinical specialist should clearly define the line of communication between the primary operator and proctor. The clinical specialist often acts as a liaison between the physicians and is frequently the conduit for communicating information. When multiple operators are present (as in a TAVR) it is sometimes helpful to establish a lead physician whose responsibility will be to interact with the proctor during a specific case.
Often when a new procedure is being introduced to a facility, several enthusiastic local physicians will want to participate in the procedure or observe. If there are too many physicians in the room, or worse yet preexisting conflicting internal team dynamics, then clear communication between the proctor and primary operator can break down. This can cause serious problems if there is a critical step in the procedure or a potential error about to occur, where the proctor needs to be able to alert the host physician of an imminent problem.
During the procedure
When the procedure starts, it is important to follow standard pre-procedure protocols, such as introducing team members, the proctor, and clinical specialist who will be in the room. The procedure checklist and team roles should be reviewed, and a time-out should be performed. The staff should verify that all necessary equipment is present and any equipment for performing a bailout or managing complications is in the room or readily available. The communication ground rules, chain of command, and lead physician should be re-identified for all team members. The role of the proctor as observer or active participant should be clearly stated out loud for all to hear as part of the pre-procedure time-out.
The proctor’s responsibilities include overseeing the procedure, providing timely instruction, answering questions, offering advice (solicited and unsolicited). Direct patient care by the proctor is only allowed with proper privileging of the proctor at the specific institution, unless an emergency situation develops.
Post-procedure
The proctor is strongly encouraged to remain on site until the procedure is fully completed. After conclusion of the procedure, the host physician should review any specific post-procedural care with the proctor, ask for feedback from the proctor on the case performance, and perform a debrief with the cath lab staff. Potential post-procedural issues and complications specific to the new device/procedure should be reviewed and the host physician should ensure that the staff managing the patient in the post-procedural period are comfortable taking over the care of the patient. The experienced proctor and clinical specialist should review any contingency plans for complications that might occur over the following 24-48 hours. There should be an available contact for questions and concerns that may arise during this period and beyond.
Medical documentation should identify the presence of the proctor and clearly state their role during the procedure, such as “observer,” or proctor, or physician operator. The specific name of an observer need not be included in the medical record, but a physician operator needs to be specifically named if they provided any form of medical care to the patient.
Finally, the proctor should have a final review of the case with all involved physicians upon completion of the procedure. A written report may be necessary to complete the review process, if requested by the trainees or institution (Table 1).
Table 1.
Checklist for proctor, host physician, host institution, industry
Checklist for Proctor
|
Industry sponsor
The industry sponsor should establish the required qualifications for new operators and proctors in conjunction with regulatory agencies. Some devices, such as orbital atherectomy and robotic-assisted PCI, require formal documentation of training as part of their approval by the Food and Drug Administration. Along with the host institution, industry sponsors should ensure that the host physician has completed sufficient didactic, hands-on, and preceptorship training to complete the procedure independently. The industry sponsor should ensure that the proctor has extensive knowledge of the procedure including knowledge of the indications, risks, alternative treatments, trouble-shooting the devices and management of complications from the procedure. Traditionally proctors have been identified by industry as highly-qualified leaders in the field, and we endorse this process. When applicable, proctor training should be formalized with continuous proctor evaluation and reviews of proctored cases, especially if a new device is involved.
The proctor and sponsor should prospectively agree to the roles, responsibilities, remuneration, and source of liability coverage in writing. The sponsor enters into a legal contract with the proctor and as such the proctor acts as an agent on behalf of the company. As a result, this writing group believes it is the responsibility of the sponsor to provide malpractice coverage if such coverage or indemnification is not provided by the host institution, even if this is not the current practice.
The industry sponsor should work with the host institution to determine the types of privileges necessary for the type of proctoring planned, facilitate acquisition of temporary privileges if needed, and determine responsibility for indemnification of the proctor for liability. The sponsor will also help guide the host physician to identify and screen potential patient candidates for the procedure. They should confirm that the cath lab has the necessary equipment and has adequately trained the staff who will be involved in the procedure.
Finally, the industry sponsor serves as the liaison between the host physician and proctor. They should establish a line of communication well in advance of the procedure to ensure the pre-procedural requirements are in place ahead of time and that any questions or concerns of the host physician are answered (Table 1).
On the day of the procedure, the industry clinical specialist should review the equipment and procedural checklist and provide any review of the procedural technique and procedural flow for the host physician and staff. The specialist is often the primary intermediary for the cath lab staff, host physician, and proctor and should help establish clear lines of communication with the staff, identify the chain of command and lead physician in the case.
The clinical specialists should also participate in the case debrief and provide feedback to both the host physician and proctor. Recommendations for post-procedural management and follow-up should also be reviewed.
Role of professional societies
Professional societies play an important role in the proctorship process, ideally establishing clear guidelines for which emerging procedures require proctoring. Professional societies should collaborate with industry in defining, endorsing, and certifying the qualification criteria for proctors and trainees. It is recommended that professional societies lead expert consensus efforts to develop protocols for patient selection, identification of metrics needed for physicians and institutions capable of performing the procedures and strategies for management of complications, as well as a standardized reporting structure. Societies can potentially provide a clearinghouse connecting industry proctoring programs with potential proctors and host physicians, or even assume responsibility for the proctoring process for selected procedures.
Proctoring in perspective with other forms of physician training
Peer-to-peer training has traditionally been central to physician education and is included in the Hippocratic oath. Nevertheless, this approach is resource intensive, requires a dedicated one-on-one interface, and thus has limited feasibility for large-scale adoption. The primary subspecialty training in procedures comes during formal graduate medical education fellowships, but additional training is necessary to introduce any new device iterations and techniques to practicing physicians. Tailored training programs including workshops, short-term fellowships or preceptorships, and remote proctoring can efficiently provide training in new technologies and techniques to a large group of practitioners:
-
a.
Fellowship training: Dedicated subspecialized interventional cardiology fellowship training programs (e.g., CHIP, structural, peripheral) beyond current ACGME programs have successfully launched in academic centers globally over the past decades. However, there is marked variability in case volumes, hands-on experience, didactic teaching, and disease mix between different programs, leaving learners at varying levels ranging from novice to full independence.
-
b.
Workshops: Workshops provide didactic and hands-on instruction on procedures and equipment in non-clinical settings (e.g., hands-on machine simulation, animal work). This approach is highly scalable in terms of the number of trainees per session. Some industries utilize workshops to meet regulatory requirements for device use (e.g., TAVR); others do not offer credentialing. This modality is often used as the initial basic training prior to in-person proctoring.
-
c.
Proctoring by Industry Clinical Specialists: Many companies utilize their own trained industry clinical specialists to be educators during procedures. Often, the initial cases for a new procedure (or device) will be proctored by a physician up to a requisite threshold number of cases, before being transitioned to support solely by clinical specialists. Depending on procedural complexity and regulatory requirements, as well as procedural performance, physician proctoring may continue to be required or tailored. Examples of procedures supported by clinical specialists include atherectomy, left atrial appendage closure, transcatheter edge-to-edge repair (TEER), and TAVR.
-
d.
Short-term Fellowship or Preceptorship: Increasingly, high-volume centers are offering short-term preceptorships, either with observational or full hands-on capacity, depending on the center’s or country’s credentialing laws. Many European centers have launched programs that include didactics, machine simulations, and hands-on experience, where trainees can perform procedures under the guidance of an expert preceptor. These programs are of variable lengths and are typically expensive both from tuition costs and loss of salary during the training period. Financial support by industry may be available in some cases.
-
e.
Remote Proctoring: With the COVID-19 pandemic, peer-to-peer interactions have increasingly become virtual. Several vendors can support remote proctoring by a physician directing the on-site trainee with audible and visual instructions (e.g., RCS, Proximie, Intouch, Avail, Odyssey, ExplORer, AIS). These platforms can be used for a variety of interventional procedures, and will have important roles for clinical proctoring in the future.
Conclusions
Clinical proctoring provides focused training for practitioners and plays an essential role in the safe dissemination of new technologies. Responsible proctoring practices including adequate preparation, patient selection, clear communication of roles, informed consent, and resolution of medico-legal issues can reduce the risks inherent to the introduction of a new procedure to a facility, and ensure patient safety.
Supplementary material
References
- 1.Szerlip M., Feldman D.N., Aronow H.D., et al. SCAI publications committee manual of standard operating procedures. Catheter Cardiovasc Interv. 2020;96(1):145–155. doi: 10.1002/ccd.28754. [DOI] [PubMed] [Google Scholar]
- 2.Proctoring versus precepting. Medical Staff Leader Insider, October 25, 2006. HCPro, a division of Simplify Compliance. http://www.hcpro.com/content.cfm?dp=MSL&content_id=63391&publication=871&
- 3.Guiding Principles for Privileging of Innovative Procedures in Gynecologic Surgery. American College of Obstetricians and Gynecologists Committee Opinion Number 674, September 2016. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2016/09/guiding-principles-for-privileging-of-innovative-procedures-in-gynecologic-surgery
- 4.Rogers S.O., Jr., Gawande A.A., Kwaan M., et al. Analysis of surgical errors in closed malpractice claims at 4 liability insurers. Surgery. 2006;140(1):25–33. doi: 10.1016/j.surg.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Proctoring conundrums: How to credential an OR observer. Credentialing Resource Center Insider, August 16, 2007. HCPro, a division of Simplify Compliance. http://www.hcpro.com/content.cfm?content_id=75377
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.