Abstract
Background and Aims
Effective acid suppression is a crucial component of Helicobacter pylori (H. pylori) eradication regimens. Approved treatments include dual, triple, and quadruple therapies composed of certain antibiotics in combination with proton pump inhibitors (PPIs). Vonoprazan, a potassium-competitive acid blocker, provides more potent and durable acid suppression than PPIs. We compared the efficacy of vonoprazan-based therapies vs approved standard regimens using new evidence from the phase 3 pHalconHP trial in North America and Europe.
Methods
Studies reporting first-line H. pylori eradication rates from empiric treatment with Food and Drug Administration–approved therapies and vonoprazan-containing therapies were identified via bibliographic searches of systematic literature reviews and a subsequent MEDLINE/Embase search using index terms for H. pylori and eradication. Randomized controlled trials comparing 2 or more relevant comparators were included in Bayesian network meta-analyses for grouped and distinct therapies.
Results
Twenty-three distinct regimens from 42 trials including 12,773 patients were identified. Vonoprazan-based triple therapy showed the highest relative efficacy (odds ratio: 2.73, 95% credible interval 2.11, 3.54) and 72.1% probability of being the best. North American, Western, and global scenarios were largely consistent. Vonoprazan-based therapies demonstrated higher odds of H. pylori eradication than each PPI-based triple therapy. Furthermore, vonoprazan-based triple therapy was superior to bismuth subcitrate quadruple therapy (odds ratio: 1.60, 95% credible interval: 1.07, 2.38).
Conclusion
Vonoprazan-based eradication regimens represent novel treatments for H. pylori infection on a global scale, offering efficacy that, in this analysis, is superior to PPI-based triple therapy and comparable or better than bismuth quadruple therapy.
Keywords: First-Line Regimens, Empiric Therapy, Network Meta-Analysis
Introduction
Helicobacter pylori (H. pylori) is the major cause of chronic gastritis and severe gastroduodenal pathologies, including peptic ulcer disease, gastric cancer, and mucosa-associated lymphoid tissue lymphoma.1, 2, 3 H. pylori gastritis is an infectious disease, recognized as a distinct nosological entity, included in the New International Classification of Disease (ICD 11).4 Current clinical guidelines recommend eradication therapy for any diagnosed H. pylori infection, even in the absence of clinical symptoms.5, 6, 7 Given the high prevalence of H. pylori infection and that treatment is required, careful selection of first-line eradication therapy to guarantee high efficacy is warranted to minimize the risk of antibiotic resistance development. Current Food and Drug Administration (FDA)-approved regimens for first-line treatment of H. pylori infection include proton pump inhibitor (PPI)-based triple therapy (ie, PPI [omeprazole, lansoprazole, esomeprazole, rabeprazole], clarithromycin, and amoxicillin or metronidazole), bismuth quadruple therapy (BiQT; ie, bismuth, PPI, tetracycline, and a nitroimidazole). In addition, in the United States, a rifabutin-containing triple therapy (omeprazole, amoxicillin, and rifabutin delayed release) has been added to first-line H. pylori eradication options. Outside the United States, rifabutin-containing regimens are typically used as rescue therapy.
Acid suppression is a fundamental component of H. pylori treatment regimens. Raising intragastric pH enables bacterial replication, rendering H. pylori more susceptible to antibiotics. Increased intragastric pH also increases the stability of selected antibiotics, including amoxicillin and clarithromycin.8,9 This is particularly true for amoxicillin, which is highly acid sensitive and therefore requires concomitant administration of acid suppressive therapy.
Vonoprazan is a potassium-competitive acid blocker providing more potent and durable acid suppression when compared with PPIs.10 This is advantageous when used in combination with amoxicillin (vonoprazan dual therapy) or amoxicillin plus clarithromycin (vonoprazan-based triple therapy) for H. pylori eradication. Vonoprazan-based triple therapy is approved for H. pylori eradication in Japan and a number of other Asian and non-Western countries. The efficacy of vonoprazan-based triple and dual eradication regimens in Western countries vs lansoprazole-based triple therapy was recently established in the phase 3 pHalconHP trial in Europe and North America, which supported the FDA approval of both vonoprazan-based regimens in the United States for the treatment of H. pylori infection in adults.11,12
Head-to-head trials are the gold standard for establishing comparative efficacy; however, in the absence of head-to-head trials, including relevant comparators for H. pylori eradication, network meta-analyses (NMAs) have been used to estimate the relative efficacy of vonoprazan-based eradication regimens. A recent meta-analysis by Rokkas et al13 found that vonoprazan-based triple therapy ranked best for first-line empiric therapy. Since then, new important data from the phase 3 European and North American study of vonoprazan have become available.11 In our present study, we conducted an NMA including recent data from the phase 3 pHalconHP trial to evaluate the comparative efficacy of eradication therapies for H. pylori.
Methods
Study Identification, Selection, and Data Collection
To identify prospective randomized controlled studies reporting H. pylori eradication rates achieved with approved therapies in treatment-naïve patients, we performed bibliographic searches of 3 contemporary systematic literature reviews by Rokkas et al, Xin et al, and Pharmaceutical Services Division13, 14, 15 to identify studies that met the population, intervention and comparators, outcomes measures, and study design (PICOS) criteria (Table 1). Because of the focus on exploring comparative efficacy in North America, we tailored the predetermined PICOS criteria to interventions that were approved by the FDA and had studies with treatment-naïve patients. In addition, we also included studies that included vonoprazan. Concomitant therapy was not included as they are not FDA approved, and there have been no randomized controlled trials assessing them in North America. Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of our research.
Table 1.
PICOS Criteria
| PICOS | Exclusion | |
|---|---|---|
| Patient population | H. pylori positive | |
| Intervention and comparators | FDA-approved regimens for eradication: | • Studies not including at least 2 of the interventions listed in the inclusion criteria |
| PPI-based triple therapies | • Studies evaluating different dosages, durations, and/or sequences (hybrid, sequential, and standard) | |
| • Omeprazole + clarithromycin + amoxicillin or metronidazole | • Non-FDA-approved treatments: Non-bismuth quadruple therapies, LOAD, ranitidine | |
| • Lansoprazole + clarithromycin + amoxicillin or metronidazole | • Non-standard antibiotics: Tinidazole, omidazole, levofloxacin, doxycycline, roxythromycin, moxifloxacin, furazolidone, which are not recommended for use by the ACG guidelines | |
| • Rabeprazole + clarithromycin + amoxicillin or metronidazole | ||
| • Esomeprazole + clarithromycin + amoxicillin or metronidazole | ||
| • Omeprazole + rifabutin + amoxicillin delayed release | ||
| Bismuth therapies | ||
| • Bismuth subcitrate potassium, metronidazole, and tetracycline hydrochloride | ||
| • Bismuth subsalicylate, metronidazole, tetracycline hydrochloride | ||
| Other | ||
| • High-dose amoxicillin dual | ||
| Outcomes measures | • First-line H. pylori eradication rate | • Studies not including the outcomes listed in the inclusion criteria |
| Study design | • Randomized clinical trials (including extension studies) | • Non-human/pre-clinical studies |
| • Systematic reviews and meta-analyses (for cross-checking only) | • Single arm and non-randomized or un-controlled studies | |
| • Non-interventional studies | ||
| • Retrospective studies | ||
| • Observational studies | ||
| • Case reports/series | ||
| Restrictions | • English language | • Non-English language studies |
| • Year limit: None | Abstract only | |
ACG, American College of Gastroenterology; FDA, Food and Drug Administration; PPI, proton pump inhibitor.
Additional studies were identified via a ProQuest search of Embase and MEDLINE from database inception to search date of May 2021 with the following Medical Subject Heading terms: “H. Pylori” OR “H Pylori” or “Helicobacter Pylori” AND “eradication.” The complete search strategy is provided in Table A1. This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses extension statement.16 Eligibility was assessed by a single reviewer through an initial title-abstract screening, followed by a full-text screening according to the defined PICOS criteria. Studies were retained provided 2 study arms met the PICOS criteria. Nonstandard doses or interventions/comparators that did not meet the PICOS criteria were excluded. For all selected studies, available data, including study year, design, study location, intervention with dosage and duration, patient population, clarithromycin susceptibility information, and H. pylori eradication rates (intention-to-treat and per protocol), was extracted. A full listing of excluded studies including reasons for exclusion is provided in Table A2.
There were a large number of distinct regimens because studies used regimens that differed on the basis of the following:
-
1.
PPIs (lansoprazole, omeprazole, rabeprazole, esomeprazole)
-
2.
Antibiotic backbones (amoxicillin + clarithromycin, metronidazole + clarithromycin)
-
3.
Number of components (dual, triple, or quadruple therapy)
-
4.
Bismuth types (bismuth subcitrate, bismuth subsalicylate)
-
5.
Duration of treatment
To accommodate these variables, 2 treatment groupings were used (Table A3): distinct therapies, which did not assume similarity between PPIs; and grouped therapies, which assumed similarity between PPIs.
Statistical Analysis
For this NMA, we used the intention-to-treat H. pylori eradication rates of each trial. We conducted the analyses using 3 scenarios: all countries (global, base case), Western countries, and North American studies. The key base comparator was PPI-clarithromycin-based triple therapy. The classical assumptions of NMA (similarity, homogeneity, and consistency) were assessed by comparing the patient populations, interventions, comparators, outcomes, and study designs. Furthermore, we evaluated consistency using node splitting when closed loops were present, and heterogeneity was inspected using a funnel plot (Figure A1) and I2 based on a threshold of 70%, indicating significant heterogeneity for pairwise comparisons.
Frequentist meta-analysis was used to summarize H. pylori eradication rates by treatment type within each analysis scenario. Pooled proportions by treatment grouping were estimated with 95% confidence intervals based on a random effects model due to significant heterogeneity in H. pylori eradication rates.
The NMA was conducted using a Bayesian framework with a noninformative prior probability distribution. Model convergence was evaluated using Gelman-Rubin-Brooks plots and a potential scale reduction factor threshold of <1.05. The Markov-Chain Monte Carlo simulation was performed using the “gemtc” and “rjags” packages in R Studio with 8000 burn-in iterations and 50,000 actual iterations. Fixed effects and random effects models were constructed for each model scenario, and an optimal model was selected based on model fit statistics, I2, consistency, and convergence.
Reporting was conducted according to the International Society for Pharmacoeconomics and Outcomes Research ITC Good Research Practices Task Force guidance.17 Indirect odds ratios (ORs) were estimated with 95% credible intervals (CrI). Treatment rankings were summarized with Surface Under the Cumulative Ranking scores and estimated probability of being the best treatment. Significance was defined as P < .05 for all measures except heterogeneity.
Results
Based on the literature review, 23 distinct regimens were identified from 42 trials including empiric (ie, without knowledge of antibiotic susceptibility) therapy (Figure 1).18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59 A full listing of included studies is provided in Table A3. Studies were performed in East Asia (n = 23),18,23, 24, 25,28, 29, 30,32,33,36,38, 39, 40, 41, 42, 43, 44, 45, 46,48,52,53,58,59 South Asia (n = 3),19,49,55 Other (n = 1),51 and Western countries (n = 15),20,21,26,27,31,34,35,37,47,50,51,54, 55, 56, 57,60 among which 5 included North American study sites.26,31,47,56,57 Overall, identified studies included a total of 12,773 patients who received first-line eradication therapy.
Figure 1.
PRISMA diagram of studies included in the meta-analysis. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; SLR, systematic literature review.
Pooled Eradication Rates
The mean H. pylori eradication rates achieved with the interventions (grouped therapy and distinct therapies) are presented in Table 2. Based on the results of the all-countries scenario, vonoprazan-based triple therapy demonstrated the highest H. pylori eradication rates. Distinct PPI-based triple therapies (ie, omeprazole-based triple, esomeprazole-based triple, etc) did not differ significantly based on studies from all countries, which supports the validity of grouped therapy.
Table 2.
Pooled Eradication Rates From Selected Studies
| Grouped therapies | ||||||
|---|---|---|---|---|---|---|
| Treatment | All countries |
Western countries |
North America |
|||
| Na | Pooledb, % (95% CI) | Na | Pooledb, % (95% CI) | Na | Pooledb, % (95% CI) | |
| Vonoprazan-based triple therapy | 5 | 89.6 (84.1, 93.3) | 1 | 80.1 (76.2, 84.6) | 1 | 80.1 (76.2, 84.6) |
| Vonoprazan dual therapy | 2 | 80.3 (74.5, 85.1) | 1 | 77.2 (72.3, 81.4) | 1 | 77.2 (72.3, 81.4) |
| PPI-based triple therapy | 14 | 73.2 (64.5, 80.3) | 7 | 80 (68.9, 87.5) | 3 | 82.3 (67.2, 91.8) |
| PPI + high-dose amoxicillin | 3 | 65.6 (56.1, 74.0) | 2 | 65.7 (51.0, 77.8) | 2 | 65.7 (51.0, 77.8) |
| BiQT | 8 | 78.4 (68.1, 86.1) | 5 | 82 (74.1, 87.8) | 1 | 87.7 (81.1, 92.2) |
| RT-DR | 1 | 83.8 (78.4, 88.0) | 1 | 83.4 (78.4, 88.0) | 1 | 83.4 (78.4, 88.0) |
| Distinct therapies | ||||
|---|---|---|---|---|
| Treatment | All countries |
Western countries |
||
| Na | Pooledb, % (95% CI) | Na | Pooledb, % (95% CI) | |
| Vonoprazan-based triple therapy | 3 | 88.2 (81.4, 92.8) | 1 | 80.8 (76.2, 84.6) |
| Vonoprazan dual therapy | 2 | 80.3 (74.5, 85.1) | 1 | 77.2 (72.3, 81.4) |
| Esomeprazole triple | 12 | 83.3 (78.1, 87.5) | 5 | 87.8 (80.6, 92.6) |
| Omeprazole triple | 23 | 78.4 (74.6, 81.8) | 11 | 77.4 (71.1, 82.7) |
| Lansoprazole triple | 17 | 78.7 (71.8, 84.3) | 4 | 83.2 (78.4, 88.0) |
| Rabeprazole triple | 15 | 83.7 (79.3, 87.3) | 1 | 76.8 (69.8, 82.7) |
| PPI + high-dose amoxicillin | 3 | 65.6 (56.1, 74.0) | 2 | 65.7 (51.0, 77.8) |
| BiQT (subsalicylate) | 1 | 70.0 (61.2, 77.5) | N/A | |
| BiQT (subcitrate) | 7 | 79.6 (68.2, 87.6) | 5 | 82 (74.1, 87.8) |
| RT-DR | 1 | 83.8 (78.4, 88.0) | 1 | 83.7 (79.3, 87.3) |
BiQT, bismuth quadruple therapy with bismuth subcitrate or bismuth subsalicylate; CI, confidence interval; PPI, proton pump inhibitor; RT-DR, rifabutin-amoxicillin-omeprazole delayed-release triple therapy.
Study arms.
Pooled (weighted) proportion meta-analysis was performed.
NMA Results
Grouped Therapy
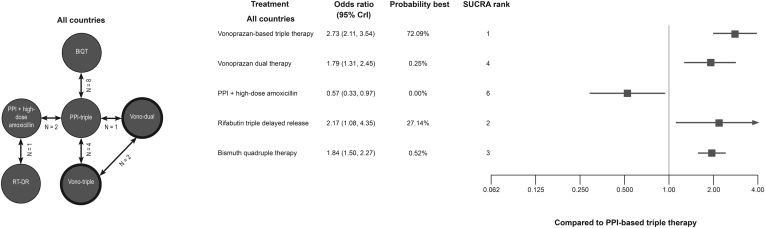
The literature review identified 16 studies with 33 treatment arms meeting the inclusion criteria for the grouped analysis.19,21,22,26,29,31,34, 35, 36,43,47,49,52,53,55 The resulting evidence network is presented in Figure 2.
Figure 2.
Evidence network and NMA results for the all-countries grouped scenario. BiQT, bismuth quadruple therapy (either bismuth subcitrate or bismuth subsalicylate + metronidazole + tetracycline + omeprazole/lansoprazole); CrI, credible interval; PPI, proton pump inhibitor; RT-DR, rifabutin triple therapy delayed released; SUCRA, surface under the cumulative ranking; Vono dual, vonoprazan dual therapy; Vono triple, vonoprazan-based triple therapy. Note: reference PPI-based triple therapy = 1.
The base comparator for the NMAs was PPI-clarithromycin triple therapy. The network forest plot in Figure 2 shows the ORs (95% CrIs) for all grouped interventions vs PPI-based triple therapy. According to the global scenario, vonoprazan-based triple therapy showed the highest relative efficacy (OR: 2.73; 95% CrI 2.11, 3.54). Vonoprazan-based triple therapy had a 72.1% probability of being the best. Rifabutin triple therapy delayed release (RT-DR; OR: 2.17, 95% CrI 1.08, 4.35) was second best, with a probability of being best of 27.1%. Vonoprazan-based triple therapy also showed higher efficacy relative to BiQT (OR: 1.48; 95% CrI 1.06, 2.07). Full pairwise comparisons are provided in Table A4.
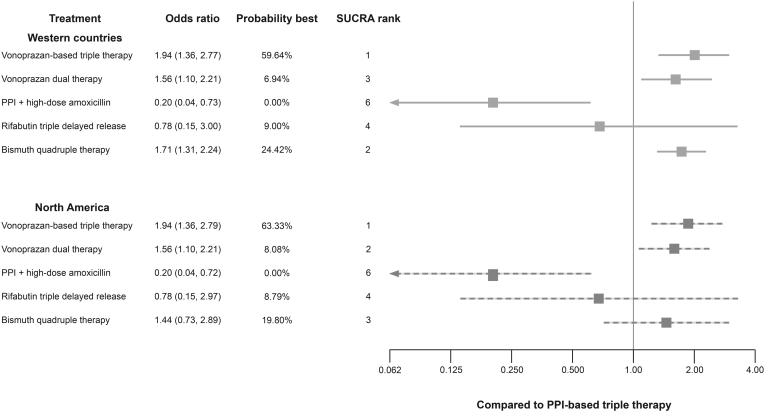
In addition to the base-case global scenario, a sensitivity analysis was undertaken by restricting the analysis to studies conducted in Western countries (Canada, Czech Republic, Germany, Greece, Hungary, Iceland, Ireland, Italy, the Netherlands, Poland, Spain, the United Kingdom, and the United States)20, 21, 22,26,27,31,34,35,37,47,54,56,57 and North America (the United States and Canada) as a distinct entity.31,47,56,57 The Western countries evidence network was composed of 17 treatment arms from 8 distinct studies (Figure A2).21,22,26,31,34,35,47 Vonoprazan-based triple therapy had the highest relative efficacy with a probability of being the best of 59.6% and BiQT was second with 24.4%. ORs for vonoprazan-based triple therapy vs BiQT and RT-DR were 1.13 (95% CrI 0.73, 1.77) and 2.50 (0.61, 13.34), respectively. Full pairwise comparisons are provided in Table A5.
For the North American scenario, the evidence network was composed of 9 treatment arms from 4 distinct studies (Figure A3).26,31,47 Among the included regimens, vonoprazan-based triple therapy had the highest relative efficacy. Compared with BiQT and RT-DR, vonoprazan-based triple therapy had an OR of 1.35 (95% CrI 0.62, 2.90) and 2.50 (0.62, 13.41), respectively. The probability of being the best for vonoprazan-based triple therapy was 63.3%, followed by BiQT (19.8%). Full pairwise comparisons for the North American scenario are provided in Table A6.
The results of both the Western countries and North American scenarios were largely consistent with the global scenario (Figure 3); however, the relative efficacy of RT-DR therapy was strongly influenced by the uncertainty of PPI + high-dose amoxicillin relative to PPI-based triple therapy. Overall, in both base-case and sensitivity scenarios, vonoprazan-based triple therapy was numerically favored vs all treatments and significantly better than grouped PPI-based triple therapy and BiQT.
Figure 3.
Grouped therapy NMA results for Western country and North American scenarios. PPI, proton pump inhibitor; SUCRA, surface under the cumulative ranking. Note: reference PPI-based triple therapy = 1.
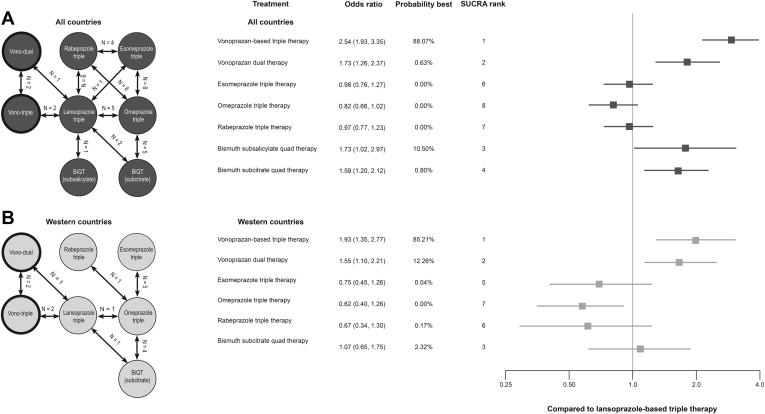
Distinct Therapies
The evidence network is composed of 78 treatment arms from 37 studies that met the inclusion criteria and were included in the NMA for distinct therapies (Figure 4 and Table A3).18,20,23,25,27,28,30,32,33,37, 38, 39, 40, 41, 42,44, 45, 46,48,50,51,54,56, 57, 58, 59 Of note, 2 treatment arms with BiQT (bismuth subcitrate potassium + metronidazole + tetracycline and bismuth subsalicylate + metronidazole + tetracycline hydrochloride) included lansoprazole, whereas the other study arms used omeprazole. Because of the limited number of treatment arms for these 2 BiQT regimens with lansoprazole, they were pooled with the majority of omeprazole treatment arms.
Figure 4.
Distinct therapies evidence networks and NMA results. (A) All countries scenario; (B) Western countries scenario. BiQT, bismuth quadruple therapy (either bismuth subcitrate or bismuth subsalicylate + metronidazole + tetracycline + omeprazole/lansoprazole); PPI, proton pump inhibitor; SUCRA, surface under the cumulative ranking; Vono dual, vonoprazan dual therapy; Vono triple, vonoprazan-based triple therapy. Note: reference lansoprazole-based triple therapy = 1.
Among PPI-based triple therapies, lansoprazole-based regimens demonstrated the highest relative efficacy; however, the differences between PPI-based triple therapies were not significant. Vonoprazan-based triple therapy was numerically favored to all treatments and significantly better than PPI-based triple therapy and bismuth subcitrate quadruple therapy (Figure 4). Vonoprazan-based therapies demonstrated higher odds of H. pylori eradication than each PPI-based triple therapy; vonoprazan-based triple and vonoprazan dual therapy had 2.5 (95% CrI: 1.93, 3.35) and 1.7 (1.26, 2.37) times higher odds of H. pylori eradication than lansoprazole-based triple therapy, respectively. Vonoprazan-based triple therapy was superior to bismuth subcitrate quadruple therapy (OR: 1.60, 95% CrI: 1.07, 2.38). Vonoprazan-based triple therapy had the highest relative efficacy and probability of being the best (88.1%); bismuth subsalicylate quadruple therapy ranked second (10.5%). Full pairwise comparisons are provided in Table A7.
An additional sensitivity analysis was performed using studies from Western countries only. The Western country evidence network was composed of 32 treatment arms from 13 distinct studies (Figure 4B).20, 21, 22,27,31,34,35,37,50,54,56,57 It demonstrated similar overall trends to the global analysis. According to this analysis, vonoprazan-based triple therapy had the highest relative efficacy and probability of being the best at 85.21% (Figure 4; full pairwise comparisons Table A8). Vonoprazan dual therapy ranked second with 12.26% probability of being best, followed by bismuth subcitrate quadruple therapy with 2.32%. Although we explored a North American scenario with the distinct therapies grouping, it was not possible due to limited available data.
Discussion
H. pylori poses a significant threat to human health globally,2,61 necessitating a highly effective first-line eradication regimen. This is especially important in the context of rising rates of resistance to key antibiotics, such as clarithromycin. Worldwide data demonstrate H. pylori eradication rates are declining5,6,62; however, there is a paucity of contemporary evidence regarding the efficacy of approved eradication regimens in North America specifically. Eradication rates with current regimens have yet to consistently exceed 90%; therefore, increased access to routine susceptibility testing is needed to support efforts to transition from empiric therapy to susceptibility-based therapeutic selection. Presently, such testing is little used in clinical practice, particularly when physicians use noninvasive H. pylori testing. As such, thorough characterization of the relative efficacy of available eradication regimens is critical to ensure optimal patient management while also considering the importance of antibiotic stewardship.
The results of the NMA support vonoprazan-based triple therapy as having a favorable efficacy profile to comparators used as first-line eradication regimens globally. These include PPI-based triple therapies and BiQT, as well as RT-DR, which is approved for first-line use in the United States only (rifabutin-containing regimens are restricted as rescue therapy in the rest of the world).
According to our NMA, vonoprazan was consistently superior to PPI-based triple therapy in all scenarios. Vonoprazan-based triple therapy ranked better than other regimens in the pairwise comparisons; however, the difference was not significant vs BiQT for the Western countries and North American grouped therapies scenarios and vs RT-DR grouped scenarios, for which there was no direct, extended network, which led to increased uncertainty.
As a potassium-competitive acid blocker, vonoprazan binds to both active and inactive H+, K+-ATPase in parietal cells and does not depend on acid for activation.10,63 Its antisecretory effect occurs within 2–3 hours of administration and is sustained over 24 hours with its Cmax and AUC unaffected by food.64,65 As such, vonoprazan exhibits enhanced and durable acid inhibition compared with traditional PPIs. Acid suppression, with particular emphasis also on nocturnal acid suppression, is required to achieve the intragastric pH range, ideally 6–8 when H. pylori is most susceptible to antibiotics, thereby ensuring the efficacy of all antibiotics, especially amoxicillin.8,9
Although there are no direct trials comparing vonoprazan-based therapy to BiQT, several analysis scenarios suggested vonoprazan-based triple therapy was superior to BiQT. Moreover, vonoprazan dual therapy was similar to BiQT. Although vonoprazan-based therapies were superior or comparable to BiQT in our analysis, as they are triple or dual regimens, they also may be easier for patient adherence compared with quadruple therapies. Patient nonadherence is an important factor that may lead to treatment failure.5, 6, 7 Because eradication therapy typically consists of complex multidrug regimens that are associated with side effects, patients often miss doses or discontinue treatment early.5,7,66 The simplicity of vonoprazan-based triple or dual therapy has the potential to achieve higher patient (and physician) acceptance and improved adherence, which may further contribute to eradication success. This is particularly important in routine clinical practice where health care providers are not able to monitor patients in the same manner as in clinical trials, and a sizable proportion of patients may be lost to follow-up.67, 68, 69
Unlike BiQT, RT-DR is generally reserved for salvage therapy worldwide. As rifabutin is an important antibiotic used to treat serious infections, including those caused by mycobacteria, concerns have been raised regarding its use as first-line therapy. However, as it is approved for first-line treatment of H. pylori infection in the United States and the ERADICATE-HP2 trial included only treatment-naïve patients,26 the trial met the inclusion criteria, and we included this regimen in the NMA.
In the all-countries grouped therapies scenario, vonoprazan-based triple therapy was 3 times as likely as RT-DR to be the best of all therapies evaluated. Comparison to RT-DR was challenging because the network required a 3-chain linkage, the ERADICATE-HP2 trial had small sample sizes, and the high-dose amoxicillin control arm demonstrated lower than expected efficacy. These factors limited our ability to draw meaningful conclusions regarding the comparative efficacy of RT-DR relative to vonoprazan-based triple and dual therapy as well as to PPI-based triple therapy.
Limitations
This NMA relies on data from 3 trials of vonoprazan-based regimens, including 2 that included a PPI as a comparator. Because of limited available data, we were unable to perform several subgroup and sensitivity analyses. An NMA of patients with clarithromycin-resistant strains was proposed but could not be performed because of the limited reporting of subgroup outcomes according to resistance rates. Although some trials provided susceptibility data, regimens were not adjusted based on that data; therefore, eradication rates and the resulting outcomes of our analysis constitute relative efficacy based on empiric therapy.
In addition, there was variability in the duration of treatment in identified studies, with many reporting 7 days vs the 10–14 days recommended by North American guidelines.5 We were not able to examine the impact of treatment duration in these meta-analyses due to limited data to form networks.
North American guidelines as well as the European H. pylori management consensus recommend against using PPI-based triple therapy containing clarithromycin as a first-line empiric treatment because of increasing resistance rates.5, 6, 7 Instead, clarithromycin-containing regimens are limited to those patients with individually documented antibiotic susceptibility or when the prevalence of clarithromycin resistance is known to be less than 15%.70 As a result, it has been argued that PPI-based triple therapy is not an appropriate comparator for inclusion in an NMA.71 However, we included PPI-based triple therapy because recent reports from the United States suggest that clarithromycin-based PPI-triple therapy remains the most commonly prescribed therapy.68,69,72,73 In addition, concomitant therapy, a quadruple clarithromycin-containing therapy has been shown to have acceptable efficacy in clarithromycin-resistant strains74,75; however, this regimen was not included, as it is not FDA-approved, and no randomized controlled trials have been conducted in North America.
There is some evidence that second-generation PPIs (ie, rabeprazole and esomeprazole) demonstrate slightly better effectiveness than first-generation PPIs (eg, lansoprazole), particularly in CYP2C19 extensive metabolizers.76 Although we did not observe this in the results of this NMA, the difference may potentially be because of the more frequent use of lansoprazole in studies in Asia. Of note, a recent study by Scarpignato et al, which explored Asian vs non-Asian race as a covariate for the pharmacokinetic profile of vonoprazan, demonstrated that race had a limited impact on exposure and that pharmacokinetic parameters were similar among individuals of non-Asian and Asian race.77 Further study of this issue is warranted.
Among all included studies, it is notable that only 11 were published in the last 10 years.19,26,29,33,34,36,43,45,46,52,53 Factors such as rising rates of antibiotic resistance may cause relative efficacy of treatments to change over time. The paucity of contemporary trials of PPI-based triple therapies may contribute to a lack of generalizability in the current treatment landscape. Residual confounding caused by effect modification in relative efficacy rates due to time and geographical effects may occur. NMAs incorporate anchored comparisons to mitigate these effects; however, confounding is still possible if the efficacy of PPI-based triple is changing relative to comparators such as PPI + high-dose amoxicillin. In addition, few studies were conducted in North America, and contemporary trials of PPI-based triple therapies have not been conducted in Western countries. As a result, the NMA relied on studies conducted in East Asia and historical studies in Western countries. Furthermore, studies were seldom conducted exclusively in North America; therefore, North American studies were defined as trials that included North American sites.
Strengths
This is the first NMA for vonoprazan to include data from North American and European patients; prior studies relied solely on evidence from Asia. The NMA included a number of different groupings, scenarios, and analyses to assess the relative efficacy of vonoprazan-based eradication regimens. Vonoprazan-based regimens consistently demonstrated the highest relative efficacy, probability of being best, and rank regardless of geography and treatment grouping. Furthermore, across comparisons, the efficacy of vonoprazan dual therapy did not vary significantly from BiQT.
Conclusion
According to the indirect treatment comparisons we conducted, vonoprazan-based triple therapy was numerically favored to all other therapies compared, had the highest probability of being the best therapy, and ranked first in each analysis scenario. Vonoprazan’s potent and durable acid suppression is reflected in greater efficacy of vonoprazan-based therapy than PPI-based therapies.
Vonoprazan-based eradication regimens provide a potential new treatment option for the treatment of H. pylori infection in Western countries and North America with efficacy that has been shown in this analysis to be superior to PPI-based triple therapy and comparable or better than BiQT. The results of this analysis strengthen the body of evidence supporting the favored position of vonoprazan-based therapies for empiric, first-line treatment of H. pylori infection.
Acknowledgments
Authors' Contributions:
Patrick Daniele, Corey Pelletier, Rinu Jacob, and Elizabeth Hubscher performed the research; Patrick Daniele and Corey Pelletier collected and analyzed the data; Elizabeth Hubscher, Patrick Daniele, Corey Pelletier, Peter Malfertheiner, Steven F. Moss, William D. Chey, Rinu Jacob, and Eckhard Leifke wrote and reviewed the paper; and Patrick Daniele, Gabriel Tremblay, Corey Pelletier, Peter Malfertheiner, Steven F. Moss, William D. Chey, Rinu Jacob, and Eckhard Leifke contributed to the conceptualization of the study. All authors approved the final version of the article, including the authorship list.
Footnotes
Conflicts of Interest: These authors disclose the following: P.M. has served as a speaker for Aboca, Bayer, Biocodex, Biohit, Malesci, Menarini, Luvos, and Mayoly-Spindler, a consultant for Aboca, Bayer, Danone, and an advisory board member for Bayer, Danone, Imevax, and Phathom. S.F.M. has been a consultant for Takeda, has served on advisory boards for RedHill Biopharma and Phathom Pharmaceuticals regarding novel H. pylori therapies, and has received research support from American Molecular Laboratories regarding molecular diagnostics for H. pylori. W.D.C. reported being a board member of the American College of Gastroenterology, GI on Demand, International Foundation of Functional GI Disorders, and the Rome Foundation; compensation as a consultant from AbbVie, Alfasigma, Allakos, Alnylam, Bayer, BioAmerica, Cosmo, Intrinsic Medicine, Ironwood Pharmaceuticals, QOL Medical, Nestle, Phathom Pharmaceuticals, RedHill Biopharma, Salix/Valeant, Takeda, Urovant, and Vibrant; grant/research support from BioAmerica, Commonwealth Diagnostics International, QOL Medical, Salix, and Vibrant; stock/stock options in GI on Demand, and Modify Health; and patents relating to methods and kits for identifying food sensitivities and intolerances, digital manometry, and a rectal expulsion device. C.P., R.J., and E.L. are employees of Phathom Pharmaceuticals. P.D., G.T., and E.H. are employees of Cytel, Inc, which served as a consultant on this project.
Funding: This study was funded in full by Phathom Pharmaceuticals.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: All data relevant to the study are included in the article or uploaded as supplementary information.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2022.06.009.
Supplementary Materials
References
- 1.Hooi J.K.Y., Lai W.Y., Ng W.K., et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 2.IARC Heliobacter pylori. 2018. https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono100B-15.pdf 385–435.
- 3.Marshall B., Warren J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;323:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 4.Sugano K., Tack J., Kuipers E.J., et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chey W.D., Leontiadis G.I., Howden C.W., et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 6.Malfertheiner P., Megraud F., O'Morain C.A., et al. Management of Helicobacter pylori infection—the Maastricht V/Florence consensus report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 7.Shah S.C., Iyer P.G., Moss S.F. AGA clinical practice update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology. 2021;160:1831–1841. doi: 10.1053/j.gastro.2020.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erah P. The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. J Antimicrob Chemother. 1997;39:5–12. doi: 10.1093/jac/39.1.5. [DOI] [PubMed] [Google Scholar]
- 9.Furuta T., Graham D.Y. Pharmacologic aspects of eradication therapy for Helicobacter pylori infection. Gastroenterol Clin North Am. 2010;39:465–480. doi: 10.1016/j.gtc.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Echizen H. The first-in-class potassium-competitive acid blocker, vonoprazan fumarate: pharmacokinetic and pharmacodynamic considerations. Clin Pharmacokinet. 2016;55:409–418. doi: 10.1007/s40262-015-0326-7. [DOI] [PubMed] [Google Scholar]
- 11.Chey W.D., Megraud F., Laine L., et al. S1382 vonoprazan dual and triple therapy for Helicobacter pylori eradication. Am J Gastroenterol. 2021;116:S634. [Google Scholar]
- 12.Phathom Pharmaceuticals, Inc VOQUEZNA TRIPLE PAK (vonoprazan tablets; amoxicillin capsules; clarithromycin tablets), co-packaged for oral use; VOQUEZNA DUAL PAK (vonoprazan tablets; amoxicillin capsules) co-packaged for oral use [prescribing information]. U.S. Food and Drug Administration website. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215152s000,215153s000lbl.pdf
- 13.Rokkas T., Gisbert J.P., Malfertheiner P., et al. Comparative effectiveness of multiple different first-line treatment regimens for Helicobacter pylori infection: a network meta-analysis. Gastroenterology. 2021;161:495–507.e4. doi: 10.1053/j.gastro.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Drug Assessment Working Group A systematic review of the comparative effectiveness of proton pump inhibitors for the treatment of adult patients with gastroesophageal reflux disease or peptic ulcer disease. 2016. https://www.ti.ubc.ca/2016/06/01/derp-ppi/
- 15.Xin Y., Manson J., Govan L., et al. Pharmacological regimens for eradication of Helicobacter pylori: an overview of systematic reviews and network meta-analysis. BMC Gastroenterol. 2016;16:80. doi: 10.1186/s12876-016-0491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutton B., Salanti G., Caldwell D.M., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 17.Hoaglin D.C., Hawkins N., Jansen J.P., et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR task force on indirect treatment comparisons good research practices: part 2. Value Health. 2011;14:429–437. doi: 10.1016/j.jval.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Adachi K., Hashimoto T., Ishihara S., et al. Comparison of five-day Helicobacter pylori eradication regimens: rabeprazole-based and omeprazole-based regimens with and without omeprazole pretreatment. Curr Ther Res Clin Exp. 2003;64:412–421. doi: 10.1016/S0011-393X(03)00120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alboraie M., Saad M., Al-Ali J., et al. Quadruple therapy versus standard triple therapy for eradication of Helicobacter pylori in Kuwait. Arab J Gastroenterol. 2015;16:131–135. doi: 10.1016/j.ajg.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Anagnostopoulos G.K., Tsiakos S., Margantinis G., et al. Esomeprazole versus omeprazole for the eradication of Helicobacter pylori infection: results of a randomized controlled study. J Clin Gastroenterol. 2004;38:503–506. doi: 10.1097/01.mcg.0000129061.54277.c6. [DOI] [PubMed] [Google Scholar]
- 21.Calvet X., Ducons J., Guardiola J., et al. One-week triple vs. quadruple therapy for Helicobacter pylori infection - a randomized trial. Aliment Pharmacol Ther. 2002;16:1261–1267. doi: 10.1046/j.1365-2036.2002.01278.x. [DOI] [PubMed] [Google Scholar]
- 22.Ching S.-S., Sabanathan S., Jenkinson L.-R. Treatment of Helicobacter pylori in surgical practice: a randomised trial of triple versus quadruple therapy in a rural district general hospital. World J Gastroenterol. 2008;14:3855–3860. doi: 10.3748/wjg.14.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi H.S., Park D.I., Hwang S.J., et al. Double-dose, new-generation proton pump inhibitors do not improve Helicobacter pylori eradication rate. Helicobacter. 2007;12:638–642. doi: 10.1111/j.1523-5378.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 24.Chu K.M., Choi H.K., Tuen H.H., et al. A prospective randomized trial comparing the use of omeprazole-based dual and triple therapy for eradication of Helicobacter pylori. Am J Gastroenterol. 1998;93:1436–1442. doi: 10.1111/j.1572-0241.1998.00458.x. [DOI] [PubMed] [Google Scholar]
- 25.Dojo M., Azuma T., Saito T., et al. Effects of CYP2C19 gene polymorphism on cure rates for Helicobacter pylori infection by triple therapy with proton pump inhibitor (omeprazole or rabeprazole), amoxycillin and clarithromycin in Japan. Dig Liver Dis. 2001;33:671–675. doi: 10.1016/s1590-8658(01)80043-8. [DOI] [PubMed] [Google Scholar]
- 26.Graham D.Y., Canaan Y., Maher J., et al. Rifabutin-based triple therapy (RHB-105) for Helicobacter pylori eradication: a double-blind, randomized, controlled trial. Ann Intern Med. 2020;172:795–802. doi: 10.7326/M19-3734. [DOI] [PubMed] [Google Scholar]
- 27.Hawkey C.J., Atherton J.C., Treichel H.C., et al. Safety and efficacy of 7-day rabeprazole- and omeprazole-based triple therapy regimens for the eradication of Helicobacter pylori in patients with documented peptic ulcer disease. Aliment Pharmacol Ther. 2003;17:1065–1074. doi: 10.1046/j.1365-2036.2003.01492.x. [DOI] [PubMed] [Google Scholar]
- 28.Inaba T., Mizuno M., Kawai K., et al. Randomized open trial for comparison of proton pump inhibitors in triple therapy for Helicobacter pylori infection in relation to CYP2C19 genotype. J Gastroenterol Hepatol. 2002;17:748–753. doi: 10.1046/j.1440-1746.2002.02790.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim S.Y., Jung S.W., Kim J.H., et al. Effectiveness of three times daily lansoprazole/amoxicillin dual therapy for Helicobacter pylori infection in Korea. Br J Clin Pharmacol. 2012;73:140–143. doi: 10.1111/j.1365-2125.2011.04048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kositchaiwat C., Ovartlarnporn B., Kachintorn U., et al. Low and high doses of rabeprazole vs. omeprazole for cure of Helicobacter pylori infection. Aliment Pharmacol Ther. 2003;18:1017–1021. doi: 10.1046/j.1365-2036.2003.01701.x. [DOI] [PubMed] [Google Scholar]
- 31.Laine L., Hunt R., El-Zimaity H., et al. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American trial. Am J Gastroenterol. 2003;98:562–567. doi: 10.1111/j.1572-0241.2003.t01-1-07288.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee V.W.Y., Chau T.S., Chan A.K.W., et al. Pharmacogenetics of esomeprazole or rabeprazole-based triple therapy in Helicobacter pylori eradication in Hong Kong non-ulcer dyspepsia Chinese subjects. J Clin Pharm Ther. 2010;35:343–350. doi: 10.1111/j.1365-2710.2009.01088.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu M.-K., Wu I.C., Lu C.-Y., et al. Randomized trial comparing rabeprazole- versus lansoprazole-based Helicobacter pylori eradication regimens. Kaohsiung J Med Sci. 2013;29:379–384. doi: 10.1016/j.kjms.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malfertheiner P., Bazzoli F., Delchier J.-C., et al. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet. 2011;377:905–913. doi: 10.1016/S0140-6736(11)60020-2. [DOI] [PubMed] [Google Scholar]
- 35.Mantzaris G.J., Petraki K., Archavlis E., et al. Omeprazole triple therapy versus omeprazole quadruple therapy for healing duodenal ulcer and eradication of Helicobacter pylori infection: a 24-month follow-up study. Eur J Gastroenterol Hepatol. 2002;14:1237–1243. doi: 10.1097/00042737-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Maruyama M., Tanaka N., Kubota D., et al. Vonoprazan-based regimen is more useful than PPI-based one as a first-line Helicobacter pylori eradication: a randomized controlled trial. Can J Gastroenterol Hepatol. 2017;2017 doi: 10.1155/2017/4385161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miehlke S., Schneider-Brachert W., Bästlein E., et al. Esomeprazole-based one-week triple therapy with clarithromycin and metronidazole is effective in eradicating Helicobacter pylori in the absence of antimicrobial resistance. Aliment Pharmacol Ther. 2003;18:799–804. doi: 10.1046/j.1365-2036.2003.01764.x. [DOI] [PubMed] [Google Scholar]
- 38.Miki I., Aoyama N., Sakai T., et al. Impact of clarithromycin resistance and CYP2C19 genetic polymorphism on treatment efficacy of Helicobacter pylori infection with lansoprazole- or rabeprazole-based triple therapy in Japan. Eur J Gastroenterol Hepatol. 2003;15:27–33. doi: 10.1097/00042737-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Miwa H., Nagahara A., Sato K., et al. Efficacy of 1 week omeprazole or lansoprazole-amoxycillin-clarithromycin therapy for Helicobacter pylori infection in the Japanese population. J Gastroenterol Hepatol. 1999;14:317–321. doi: 10.1046/j.1440-1746.1999.01867.x. [DOI] [PubMed] [Google Scholar]
- 40.Miwa H., Ohkura R., Murai T., et al. Impact of rabeprazole, a new proton pump inhibitor, in triple therapy for Helicobacter pylori infection-comparison with omeprazole and lansoprazole. Aliment Pharmacol Ther. 1999;13:741–746. doi: 10.1046/j.1365-2036.1999.00526.x. [DOI] [PubMed] [Google Scholar]
- 41.Miwa H., Yamada T., Sato K., et al. Efficacy of reduced dosage of rabeprazole in PPI/AC therapy for Helicobacter pylori infection: comparison of 20 and 40 mg rabeprazole with 60 mg lansoprazole. Dig Dis Sci. 2000;45:77–82. doi: 10.1023/a:1005409310412. [DOI] [PubMed] [Google Scholar]
- 42.Murakami K., Okimoto T., Kodama M., et al. Evaluation of three different proton pump inhibitors with amoxicillin and metronidazole in retreatment for Helicobacter pylori infection. J Clin Gastroenterol. 2008;42:139–142. doi: 10.1097/MCG.0b013e31802cbc1a. [DOI] [PubMed] [Google Scholar]
- 43.Murakami K., Sakurai Y., Shiino M., et al. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65:1439–1446. doi: 10.1136/gutjnl-2015-311304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murakami K., Sato R., Okimoto T., et al. Eradication rates of clarithromycin-resistant Helicobacter pylori using either rabeprazole or lansoprazole plus amoxicillin and clarithromycin. Aliment Pharmacol Ther. 2002;16:1933–1938. doi: 10.1046/j.1365-2036.2002.01368.x. [DOI] [PubMed] [Google Scholar]
- 45.Nishida T., Tsujii M., Tanimura H., et al. Comparative study of esomeprazole and lansoprazole in triple therapy for eradication of Helicobacter pylori in Japan. World J Gastroenterol. 2014;20:4362–4369. doi: 10.3748/wjg.v20.i15.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ozaki H., Harada S., Takeuchi T., et al. Vonoprazan, a novel potassium-competitive acid blocker, should be used for the Helicobacter pylori eradication therapy as first choice: a large sample study of vonoprazan in real world compared with our randomized control trial using second-generation proton pump inhibitors for Helicobacter pylori eradication therapy. Digestion. 2018;97:212–218. doi: 10.1159/000485097. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz H., Krause R., Sahba B., et al. Triple versus dual therapy for eradicating Helicobacter pylori and preventing ulcer recurrence: a randomized, double-blind, multicenter study of lansoprazole, clarithromycin, and/or amoxicillin in different dosing regimens. Am J Gastroenterol. 1998;93:584–590. doi: 10.1111/j.1572-0241.1998.169_b.x. [DOI] [PubMed] [Google Scholar]
- 48.Sheu B.S., Kao A.W., Cheng H.C., et al. Esomeprazole 40 mg twice daily in triple therapy and the efficacy of Helicobacter pylori eradication related to CYP2C19 metabolism. Aliment Pharmacol Ther. 2005;21:283–288. doi: 10.1111/j.1365-2036.2005.02281.x. [DOI] [PubMed] [Google Scholar]
- 49.Songür Y., Senol A., Balkarli A., et al. Triple or quadruple tetracycline-based therapies versus standard triple treatment for Helicobacter pylori treatment. Am J Med Sci. 2009;338:50–53. doi: 10.1097/MAJ.0b013e31819c7320. [DOI] [PubMed] [Google Scholar]
- 50.Spinzi G.C., Bierti L., Bortoli A., et al. Comparison of omeprazole and lansoprazole in short-term triple therapy for Helicobacter pylori infection. Aliment Pharmacol Ther. 1998;12:433–438. doi: 10.1046/j.1365-2036.1998.00319.x. [DOI] [PubMed] [Google Scholar]
- 51.Subei I.M., Cardona H.J., Bachelet E., et al. One week of esomeprazole triple therapy vs 1 week of omeprazole triple therapy plus 3 weeks of omeprazole for duodenal ulcer healding in Helicobacter pylori-positive patients. Dig Dis Sci. 2007;52:1505–1512. doi: 10.1007/s10620-006-9522-5. [DOI] [PubMed] [Google Scholar]
- 52.Sue S., Ogushi M., Arima I., et al. Vonoprazan- vs proton-pump inhibitor-based first-line 7-day triple therapy for clarithromycin-susceptible Helicobacter pylori: a multicenter, prospective, randomized trial. Helicobacter. 2018;23 doi: 10.1111/hel.12456. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki S., Gotoda T., Kusano C., et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut. 2020;69:1019–1026. doi: 10.1136/gutjnl-2019-319954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tulassay Z., Kryszewski A., Dite P., et al. One week of treatment with esomeprazole-based triple therapy eradicates Helicobacter pylori and heals patients with duodenal ulcer disease. Eur J Gastroenterol Hepatol. 2001;13:1457–1465. doi: 10.1097/00042737-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Uygun A., Kadayifci A., Safali M., et al. The efficacy of bismuth containing quadruple therapy as a first-line treatment option for Helicobacter pylori. J Dig Dis. 2007;8:211–215. doi: 10.1111/j.1751-2980.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- 56.Veldhuyzen Van Zanten S., Lauritsen K., Delchier J.C., et al. One-week triple therapy with esomeprazole provides effective eradication of Helicobacter pylori in duodenal ulcer disease. Aliment Pharmacol Ther. 2000;14:1605–1611. doi: 10.1046/j.1365-2036.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- 57.Veldhuyzen Van Zanten S., Machado S., Lee J. One-week triple therapy with esomeprazole, clarithromycin and metronidazole provides effective eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 2003;17:1381–1387. doi: 10.1046/j.1365-2036.2003.01554.x. [DOI] [PubMed] [Google Scholar]
- 58.Wu I.C., Wu D.-C., Hsu P.-I., et al. Rabeprazole- versus esomeprazole-based eradication regimens for H. pylori infection. Helicobacter. 2007;12:633–637. doi: 10.1111/j.1523-5378.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L., Mei Q., Li Q.S., et al. The effect of cytochrome P2C19 and interleukin-1 polymorphisms on H. pylori eradication rate of 1-week triple therapy with omeprazole or rabeprazole, amoxycillin and clarithromycin in Chinese people. J Clin Pharm Ther. 2010;35:713–722. doi: 10.1111/j.1365-2710.2009.01140.x. [DOI] [PubMed] [Google Scholar]
- 60.Pieramico O., Zanetti M.V., Innerhofer M., et al. Omeprazole-based dual and triple therapy for the treatment of Helicobacter pylori infection in peptic ulcer disease: a randomized trial. Helicobacter. 1997;2:92–97. doi: 10.1111/j.1523-5378.1997.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 61.Herrero R., Park J.Y., Forman D. The fight against gastric cancer - the IARC Working Group report. Best Pract Res Clin Gastroenterol. 2014;28:1107–1114. doi: 10.1016/j.bpg.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 62.Savoldi A., Carrara E., Graham D.Y., et al. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology. 2018;155:1372–1382.e17. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hori Y., Matsukawa J., Takeuchi T., et al. A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther. 2011;337:797–804. doi: 10.1124/jpet.111.179556. [DOI] [PubMed] [Google Scholar]
- 64.Mulford D.J., Leifke E., Hibberd M., et al. The effect of food on the pharmacokinetics of the potassium-competitive acid blocker vonoprazan. Clin Pharmacol Drug Dev. 2021;11:278–284. doi: 10.1002/cpdd.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kagami T., Furuta T. Letter: probing the consequences of potent acid inhibition by vonoprazan - authors' reply. Aliment Pharmacol Ther. 2016;44:305. doi: 10.1111/apt.13684. [DOI] [PubMed] [Google Scholar]
- 66.Fischbach L.A., van Zanten S., Dickason J. Meta-analysis: the efficacy, adverse events, and adherence related to first-line anti-Helicobacter pylori quadruple therapies. Aliment Pharmacol Ther. 2004;20:1071–1082. doi: 10.1111/j.1365-2036.2004.02248.x. [DOI] [PubMed] [Google Scholar]
- 67.Dong E., Chang J.I., Chen Q., et al. Su1268 – Helicobacter pylori infection: practice patterns in a community-based US healthcare system. Gastroenterology. 2019;156:S-524. [Google Scholar]
- 68.Kumar S., Sangitha R., Nachamkin I., et al. Resistance patterns of refractory Helicobacter pylori infection in a referral centre in the Delaware Valley. GastroHep. 2020;2:6–12. doi: 10.1002/ygh2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vakil N., Michalopoulos S., Zajichek A., et al. Su1266 – Helicobacter pylori testing and treatment in a large, integrated healthcare delivery system in the United States. Gastroenterology. 2019;156:S-524. [Google Scholar]
- 70.Fallone C.A., Moss S.F., Malfertheiner P. Reconciliation of recent Helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology. 2019;157:44–53. doi: 10.1053/j.gastro.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 71.Graham D.Y., Hernaez R., Rokkas T. Cross-roads for meta-analysis and network meta-analysis of H. pylori therapy. Gut. 2022;71:643–650. doi: 10.1136/gutjnl-2021-326170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mertz A. Helicobacter pylori treatment & eradication rates in Department of Defense patients from 2016-2018 presented at: ACG. 2020. https://www.eventscribe.com/2020/ACG/fsPopup.asp?Mode=presInfo&PresentationID=766539 [DOI] [PubMed]
- 73.Argueta E.A., Alsamman M.A., Moss S.F., et al. Impact of antimicrobial resistance rates on eradication of Helicobacter pylori in a US population. Gastroenterology. 2021;160:2181–2183. doi: 10.1053/j.gastro.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Molina-Infante J., Pazos-Pacheco C., Vinagre-Rodriguez G., et al. Nonbismuth quadruple (concomitant) therapy: empirical and tailored efficacy versus standard triple therapy for clarithromycin-susceptible Helicobacter pylori and versus sequential therapy for clarithromycin-resistant strains. Helicobacter. 2012;17:269–276. doi: 10.1111/j.1523-5378.2012.00947.x. [DOI] [PubMed] [Google Scholar]
- 75.Wu D.C., Hsu P.I., Wu J.Y., et al. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clin Gastroenterol Hepatol. 2010;8:36–41.e1. doi: 10.1016/j.cgh.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McNicholl A.G., Linares P.M., Nyssen O.P., et al. Meta-analysis: esomeprazole or rabeprazole vs. first-generation pump inhibitors in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 2012;36:414–425. doi: 10.1111/j.1365-2036.2012.05211.x. [DOI] [PubMed] [Google Scholar]
- 77.Scarpignato C., Leifke E., Smith N., et al. A population pharmacokinetic model of vonoprazan: evaluating the effects of race, disease status, and other covariates on exposure. J Clin Pharmacol. 2022;62:801–811. doi: 10.1002/jcph.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.