Abstract
Background and Aims
Current postpolypectomy surveillance guidelines are based primarily on data from non-Hispanic Whites (NHWs); thus, generalizability to non-Hispanic Blacks (NHBs) remains unknown. Hence, the primary objective of this study was to assess the validity of these guidelines for NHBs by comparing the prevalence of metachronous advanced colorectal neoplasia (ACN) between NHWs and NHBs undergoing surveillance colonoscopy.
Methods
This was a retrospective cross-sectional study of NHWs (N = 1500) and NHBs (N = 1260) aged 40–75 years who underwent surveillance colonoscopy at an academic safety net hospital between 2007 and 2017. The primary outcome measure was the prevalence of metachronous ACN, defined as an advanced adenoma, advanced sessile polyp, or invasive cancer. Multivariate logistic regression was used to measure associations between race/ethnicity and ACN prevalence after adjustment for potential confounding factors.
Results
Overall, the prevalence of metachronous ACN was similar for NHBs and NHWs (6.8% vs 7.4%, respectively; P = .60). The prevalence of metachronous cancers (0.2% vs 0.1%; P = .48), advanced adenomas (2.8% vs 3.8%; P = .14), advanced serrated polyps (3.5% vs 3.3%; P = .82), and large hyperplastic polyps ≥10 mm (0.2% vs 0.6%, P = .24) were also similar between the 2 groups. Moreover, race was not a determinant of metachronous ACN after adjustment for age, sex, education, type of insurance, indication (screen/surveillance) for baseline colonoscopy, surveillance interval, and findings at baseline colonoscopy (adjusted odds ratio, 0.96; 95% confidence interval, 0.70–1.30; P = .78).
Conclusion
Our study finds no significant difference in the prevalence of metachronous ACN between NHWs and NHBs undergoing appropriate postpolypectomy surveillance at an urban safety net hospital, suggesting that current guidelines are appropriate for both NHWs and NHBs.
Keywords: Colorectal Cancer, Colorectal Polyps, Surveillance, Healthcare Disparities
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer diagnosis among both men and women and second overall leading cause of cancer death in the US.1 Factors such as race/ethnicity, age, socioeconomic status, and tumor-related characteristics influence CRC outcomes.2 Although CRC incidence and mortality rates have markedly declined in recent years, non-Hispanic Black (NHB) individuals continue to experience a disproportionately higher burden of disease than other racial and ethnic groups.1
Endoscopic surveillance after the removal of precancerous adenomas has been shown to an effective strategy for reducing colorectal cancer incidence and mortality.3,4 To optimize effectiveness, current societal guidelines for postpolypectomy follow-up are based on associations between baseline colonoscopy findings and risk of metachronous advanced neoplasia.5 Colonoscopy every 3 years is recommended for those at increased risk after the finding of an advanced adenoma (defined as a tubular adenoma ≥ 10 mm in size or any adenoma with tubulovillous/villous histology or high-grade dysplasia) or multiple (≥3) nonadvanced adenomas; conversely, longer intervals are recommended for those with only low-risk findings (ie, 1–2 nonadvanced adenomas). Similar 3-year intervals are also recommended for those with advanced serrated lesions (including sessile serrated polyps ≥10 mm in size, any sessile serrated polyp with cytological dysplasia, or any traditional serrated adenoma) or multiple nonadvanced serrated lesions, depending on the size and location, vs 5–10 years for those with only 1–2 nonadvanced serrated polyps. Because of concerns of misdiagnosis of nondysplastic serrated polyps and unclear natural history, 3- to 5-year follow-up is now recommended for hyperplastic polyps ≥10 mm in size.
Importantly, current surveillance guidelines are based on data from predominantly non-Hispanic White (NHW) populations, and therefore, as acknowledged by the US Multi-Society Task Force on Colorectal Cancer, their generalizability to other racial and ethnic groups is less well defined.5 This concern is most relevant for NHBs who are more likely to be diagnosed with CRC, often a more advanced stage, and die of their disease than NHWs.1 Although these disparities have often been attributed to differences in access to screening, differential exposure to modifiable risk factors, and socioeconomic factors, differences in the molecular composition of tumors and anatomic distribution suggest that biologic and/or genetic factors may also play a role.1,6 Such factors might result in decreased polyp dwell time and accelerated progression to cancer in polyp-bearing patients. The recent finding of an increased incidence of interval cancers among NHBs compared with NHWs lends credence to this possibility.7 Thus, the primary aim of this study was to reevaluate the validity of current postpolypectomy guidelines for NHBs by comparing the prevalence of metachronous advanced colorectal neoplasia (ACN) between NHWs and NHBs undergoing surveillance colonoscopy at an urban safety net hospital.
Methods
Study Design and Subjects
This is a retrospective cross-sectional study of NHWs and NHBs aged 40–75 years who underwent surveillance colonoscopy at Boston Medical Center (BMC) between 2007 and 2017. Eligible patients were identified from BMC's electronic endoscopic reporting system (Provation® MD, Minneapolis, MN). To be eligible, patients had to be between the ages of 40 and 75 years, be asymptomatic, and have undergone their prior screening or surveillance examination at BMC. Patients with a history of precancerous polyps who underwent interval diagnostic colonoscopy because of alarm signs or symptoms were excluded. Patients with a history of invasive CRC requiring surgical resection or inflammatory bowel disease were excluded, as well as patients without a prior screening or surveillance colonoscopy in our system. Individuals with adenomatous polyps containing invasive cancer (malignant polyps) amenable to endoscopic resection alone and confirmation of complete eradication at 3–6 months were deemed eligible because such patients were typically managed similarly to those with advanced polyps.
Patient demographic information was obtained from the electronic medical record used at BMC (EPIC). This included age, sex, race, ethnicity, education level, and type of insurance. Only patients who self-identified as “White” or “Black” and “non-Hispanic” when asked about race and ethnicity, respectively, were included in the primary analysis. BMC's Institutional Review Board approved the protocol for this study with waiver of informed consent on January 24, 2018.
Study Location
BMC is a private, not-for-profit, community-based, academic medical center affiliated with the Boston University School of Medicine. It is the largest safety net hospital in New England and provides care to a socioeconomically and racially/ethnically diverse patient population. It is also affiliated with a network of community health centers located in the greater Boston metropolitan area. Approximately 70% of BMC's patients are from racial and ethnic minority groups, including ∼32% NHBs, and more than 90% have some form of healthcare insurance.
Colonoscopy Findings and Histology
All colonoscopies were performed by board-certified attending gastroenterologists either alone or assisted by gastroenterology fellows. Although individual endoscopist adenoma detection rates (ADRs) were not available for much of the study period, the group's overall mean (standard deviation) ADR was 38% (11%) with a range of 18%–52% when measured as part of a quality improvement initiative. Endoscopic data, including the size (mm) and location of any polyps, depth of scope insertion (defined by colonic segment), and quality of the bowel preparation (excellent/good/fair/poor, adequate/inadequate or Boston Bowel Prep Scale [BBPS] score 0–9), were abstracted from the computerized endoscopic report generator database. All retrieved polypoid lesions were reviewed by board-certified pathologists with expertise in colorectal neoplasia and classified according to World Health Organization histologic criteria as conventional adenomas, serrated polyps, or invasive cancer.8 Conventional adenomas were subclassified as tubular, tubulovillous, or villous with or without high-grade dysplasia; conversely, serrated lesions were subclassified as hyperplastic polyps, sessile serrated adenomas/polyps with or without cytological dysplasia, and traditional serrated adenomas. For the purpose of this study, ACN was defined by the finding of an advanced adenoma, advanced sessile polyp, or invasive cancer. Multiplicity was defined as ≥ 3 nonadvanced adenomas or nonadvanced serrated polyps. Patients with nonneoplastic polyps (eg, hyperplastic polyps < 10 mm) or other findings (eg, carcinoid tumors) were categorized with normal examinations because neither are targets of postpolypectomy surveillance. Polyps located in the rectum, sigmoid, descending colon, or splenic flexure were classified as “distal,” whereas those located in the transverse colon, hepatic flexure, ascending colon, or cecum were classified as “proximal.”
Outcome Measures
The primary outcome measure was the prevalence of metachronous ACN among NHWs and NHBs undergoing surveillance colonoscopy at BMC after adjustment for age, sex, education, insurance, type of baseline examination (screening or surveillance), baseline findings, and surveillance interval. Secondary outcomes included (1) the prevalence of metachronous advanced adenomas and advanced serrated polyps among NHWs and NHBs after adjustment for the same confounders as for the primary outcome and (2) the association between baseline findings and risk of metachronous advanced neoplasia after stratification by surveillance interval. The rationale for examining the prevalence of advanced adenomas and advanced serrated polyps separately was to align our findings with current surveillance recommendations for the 2 histologic types of advanced lesions and because of limited data on racial differences in the prevalence of metachronous advanced serrated polyps. Patients with incomplete examinations due to failure to reach the cecum, inadequate bowel preparation (also defined as “poor” or BBPS score of 0–1 for any segment), or missing data due to unretrieved polyp specimens were ineligible and excluded from the analysis.
Statistical Analysis
NHBs and NHWs were compared on demographic characteristics, indication and findings from baseline colonoscopy, and surveillance interval (lag time) through chi-square tests. Findings from the index examination were compared between NHBs and NHWs through chi-square tests or the Fisher's exact test when expected cell frequencies were less than 5. Differences between NHBs and NHWs on the prevalence of metachronous ACN, by findings from the baseline colonoscopy and lag time, were described through odds ratios (ORs) and 95% confidence intervals (CIs) from multiple logistic regression models controlling for age and sex. Supplemental analyses examined differences between NHBs and NHWs on the prevalence of metachronous advanced adenomas and serrated polyps, by baseline colonoscopy and lag time, controlling for age and sex. Associations between metachronous ACN and demographic factors, indications and findings from baseline colonoscopy, and lag time were examined through univariate and multivariable logistic regression and described through ORs and 95% CIs.
Results
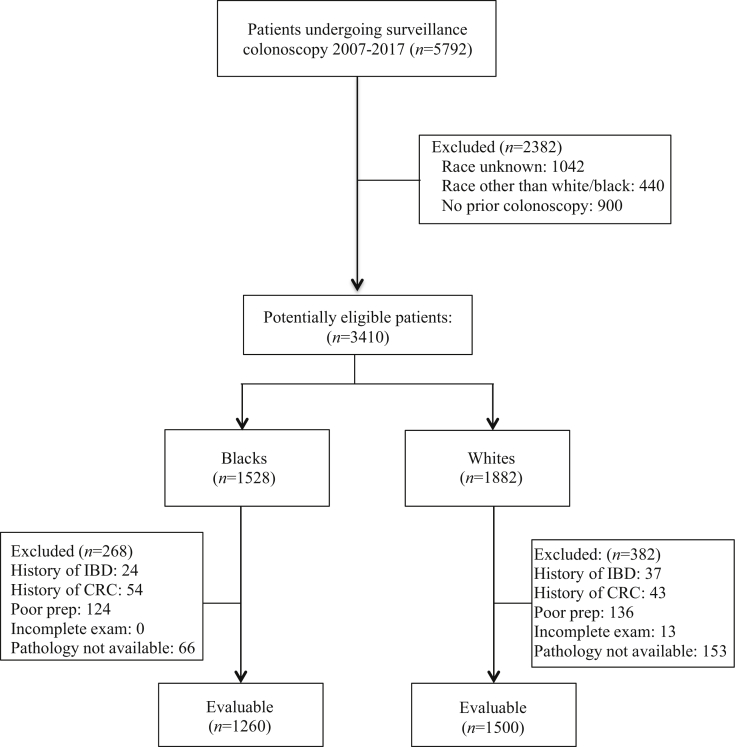
A total of 3410 self-identified NHB and NHW patients underwent postpolypectomy surveillance colonoscopy between January 2007 and December 2017, of which 1500 NHWs and 1260 NHBs met eligibility criteria (Figure). NHWs were more likely to be excluded because of incomplete bowel preparation or missing pathology due to failed polyp retrieval, whereas NHBs were more likely to be excluded because of prior invasive CRC requiring surgical resection. All unretrieved polyps for both groups were <5 mm in size. As shown in Table 1, significant differences were noted between the 2 groups with respect to many of the measured baseline characteristics, except for mean age and proportion of patients undergoing surveillance at ≤3 years, 4–5 years, and >5 years. The NHB cohort was predominantly female, less educated, and more likely to be covered by Medicaid or free care. NHBs were also more likely to have undergone prior surveillance rather than screening and more likely to have low-risk neoplastic polyps at baseline (P = .03); baseline findings were otherwise similar for the 2 groups (P > .05).
Figure.
Study flowchart. IBD, inflammatory bowel disease.
Table 1.
Patient Demographics by Race
| Characteristic | Non-Hispanic Blacks (n = 1260) | Non-Hispanic Whites (n = 1500) | P value |
|---|---|---|---|
| Age, mean ± SD | 62.0 ± 7.0 | 62.5 ±7.3 | .08 |
| Age, n (%) | .005 | ||
| 40–49 | 35 (2.8) | 57 (3.8) | |
| 50–59 | 481 (38.1) | 480 (32.0) | |
| 60–69 | 513 (40.7) | 658 (43.8) | |
| 70+ | 232 (18.4) | 307 (20.4) | |
| Sex, n (%) | <.001 | ||
| Female | 580 (46.0) | 567 (37.8) | |
| Male | 680 (54.0) | 932 (62.2) | |
| Missing | 0 | 1 | |
| Education, n (%) | <.001 | ||
| Less than high school | 493 (39.1) | 345 (23.0) | |
| High-school graduate | 446 (35.4) | 543 (36.2) | |
| More than high school | 274 (21.8) | 521 (34.7) | |
| Other/Unknown | 47 (3.7) | 91 (6.1) | |
| Insurance status, n (%) | <.001 | ||
| Commercial | 424 (33.6) | 684 (45.6) | |
| Free care | 59 (4.7) | 28 (1.9) | |
| Medicaid | 355 (28.2) | 251 (16.7) | |
| Medicare | 422 (33.5) | 537 (35.8) | |
| Indication for baseline colonoscopy, n (%) | <.001 | ||
| Screening | 652 (51.8) | 978 (65.2) | |
| Surveillance | 608 (48.2) | 522 (34.8) | |
| Findings at baseline colonoscopy, n (%) | .004 | ||
| Normal/nonneoplastic polyps/othera | 360 (28.6) | 523 (34.9) | |
| 1–2 Nonadvanced neoplastic polypsb | 563 (44.7) | 607 (40.5) | |
| Multiplicityc | 113 (9.0) | 108 (7.2) | |
| Hyperplastic polyps ≥ 10 mm | 3 (0.2) | 11 (0.7) | |
| Advanced adenomas and/or serrated polypsd | 206 (16.3) | 233 (15.5) | |
| Malignant polypse | 15 (1.2) | 18 (1.2) | |
| Surveillance interval, n (%) | .38 | ||
| ≤3 y | 304 (24.2) | 373 (25.0) | |
| 4–5 y | 349 (27.7) | 441 (29.5) | |
| >5 y | 606 (48.1) | 680 (45.5) |
SD, standard deviation.
Patients undergoing repeat surveillance because of prior adenomas or serrated polyps.
Includes both nonadvanced adenomas and serrated polyps.
Multiplicity defined by the presence of ≥ 3 nonadvanced adenomas only; no patient had ≥ 3 nonadvanced serrated polyps.
Advanced adenomas defined by size ≥ 10 mm or the presence of villous histology or high grade dysplasia; advanced serrated polyps defined by size ≥ 10 mm, the presence of cytological dysplasia or a traditional serrated adenoma of any size.
Malignant polyps defined as advanced adenomas containing invasive cancer amenable to endoscopic polypectomy alone and negative surveillance at 3–6 months.
Table 2 summarizes the findings at index surveillance colonoscopy. Overall, the prevalence of metachronous ACN was similar for NHBs and NHWs (6.8% vs 7.4%, respectively; P = .60). The prevalence of interval cancers (0.2% vs 0.1%; P = .48) and metachronous polyps, including advanced adenomas (2.8% vs 3.8%; P = .14), advanced serrated polyps (3.5% vs 3.3%; P = .82), large hyperplastic polyps ≥ 10 mm (0.2% vs 0.6%, P = .24), and nonadvanced adenomas (42.7% vs 42.0%; P = .72), were also similar between the 2 groups. In contrast, metachronous nonadvanced sessile polyps were found in a higher proportion of NHWs (3.1% vs 0.6%, P < .001). With respect to anatomic location, there were no significant differences in the proportion of metachronous proximal ACN overall (71% vs 75%, P = .16), proximal advanced adenomas (63% vs 70%, P = .43), or proximal advanced serrated polyps (63% vs 73%, P = .27) between NHBs and NHWs.
Table 2.
Results of the Index Surveillance Examination by Race
| Most advanced finding, n (%) | Non-Hispanic Blacks (n = 1260) | Non-Hispanic Whites (n = 1500) | P value |
|---|---|---|---|
| Advanced neoplasia | 86 (6.8) | 111 (7.4) | .60 |
| Cancer | 5 (0.2) | 3 (0.1) | .48 |
| Advanced polyps | 81 (6.4) | 108 (7.2) | .42 |
| Advanced adenomas | 35 (2.8) | 57 (3.8) | .14 |
| Advanced serrated polyps | 44 (3.5) | 50 (3.3) | .82 |
| Both | 2 (0.3) | 1 (0.1) | .60 |
| Hyperplastic polyps ≥10 mm | 3 (0.2) | 9 (0.6) | .24 |
| Nonadvanced polyps | 544 (43.2) | 704 (46.9) | .048 |
| Nonadvanced adenomas | 529 (42.0) | 640 (42.7) | .72 |
| 1–2 (2, 3 only) | 410 (32.5) | 506 (33.7) | .51 |
| Multiplicity (≥3) | 119 (9.4) | 134 (8.9) | .64 |
| Nonadvanced serrated polyps | 7 (0.6) | 47 (3.1) | <.001 |
| 1–2 | 7 (0.6) | 47 (3.1) | <.001 |
| Multiplicity | 0 | 0 | – |
| Both | 8 (0.6) | 17 (1.1) | .17 |
| 1–2 | 8 (0.6) | 17 (1.1) | .17 |
| Multiplicity (≥3) | 0 | 0 | – |
| Normal/nonneoplasia polyps/other | 627 (49.8) | 676 (44.1) | .01 |
Table 3 shows the association between baseline findings and metachronous ACN after stratification by lag time between examinations. No significant differences were observed between NHBs and NHBs at the ≤3-year interval for patients with baseline ACN (11.0% vs 15.7%; P = .33), CRC (0% vs 6.2%; P = 1.00), advanced polyps (12.5% vs 17.1%; P = .38), hyperplastic polyps >10 mm (0% vs 0%), or multiplicity (3.8% vs 4.0%; P = .98). Similarly, no significant differences were observed for those with 1–2 nonadvanced polyps at the ≤3-yr (4.9% vs 9.1%; P = .28) and 4- to 5-year (4.9% vs 10.1%; P = .62) intervals. Lastly, no significant differences were observed at the ≥3-year (5.7% vs 6.4%, P = .81) or 4- to 5-year (7.9% vs 10.1%, P = .62) interval examinations among those with normal or non-non-neoplastic findings at a prior surveillance colonoscopy. Similar results were observed for both metachronous advanced adenomas and serrated polyps (Tables A1 and A2).
Table 3.
Association Between the Prevalence of Metachronous ACN and Baseline Findings Stratified by Interval Between Examinations (Lag Time)
| Lag time (y) | Prevalence of metachronous ACN, % (n/N) |
aOR (95% CI) (referent = White) | P value | |
|---|---|---|---|---|
| Non-Hispanic Blacks | Non-Hispanic Whites | |||
| ACN (malignant + advanced polyps) | ||||
| ≤3 | 11.0 (10/91) | 15.7 (19/121) | 0.66 (0.29–1.52) | .33 |
| 4–5 | 7.8 (6/77) | 9.1 (8/88) | 0.95 (0.30–2.97) | .82 |
| >5 | 11.3 (6/53) | 11.9 (5/42) | 1.01 (0.27–3.81) | .95 |
| Total | 10.0 (22/221) | 12.8 (32/251) | 0.77 (0.43–1.37) | .37 |
| Malignant polyps | ||||
| ≤3 | 0.0 (0/11) | 6.2 (1/16) | – | 1.00 |
| 4–5 | 0.0 (0/1) | 0.0 (0/1) | – | – |
| >5 | 0.0 (0/3) | 0.0 (0/1) | – | – |
| Total | 0.0 (0/15) | 5.6 (1/18) | – | 1.00 |
| Advanced polyps (adenomas + serrated polyps +both) | ||||
| ≤3 | 12.5 (10/80) | 17.1 (18/105) | 0.69 (0.30–1.63) | .38 |
| 4–5 | 7.9 (6/76) | 9.2 (8/87) | 0.95 (0.30–2.95) | .78 |
| >5 | 12.0 (6/50) | 12.2 (5/41) | 1.06 (0.28–3.98) | .98 |
| Total | 10.7 (22/206) | 13.3 (31/233) | 0.80 (0.44–1.43) | .40 |
| Hyperplastic polyps ≥ 10 mm | ||||
| ≤3 | 0.0 (0/0) | 0.0 (0/3) | – | – |
| 4–5 | 100.0 (1/1) | 0.0 (0/4) | – | .20 |
| >5 | 0.0 (0/2) | 0.0 (0/4) | – | – |
| Total | 33.3 (1/3) | 0.0 (0/11) | – | .21 |
| Multiplicity (≥ 3 nonadvanced adenomas only) | ||||
| ≤3 | 3.8 (1/26) | 4.0 (1/25) | – | .98 |
| 4–5 | 7.7 (4/52) | 8.1 (5/62) | 1.05 (0.25–4.41) | .94 |
| >5 | 2.9 (1/35) | 28.6 (6/21) | 0.08 (0.01–0.74) | .01 |
| Total | 5.3 (6/113) | 11.1 (12/108) | 0.44 (016–1.23) | .12 |
| 1–2 Nonadvanced polyps (adenomas + serrated polyps + both) | ||||
| ≤3 | 4.9 (4/81) | 9.1 (9/99) | 0.51 (0.15–1.76) | .28 |
| 4–5 | 2.8 (4/143) | 6.4 (12/188) | 0.43 (0.13–1.41) | .13 |
| >5 | 7.7 (26/339) | 3.8 (12/320) | 2.14 (1.05–4.35) | .03 |
| Total | 6.0 (34/563) | 5.4 (33/607) | 1.12 (0.68–1.84) | .66 |
| Normal/nonneoplastic polyps/other | ||||
| ≤3 | 5.7 (6/106) | 6.4 (8/125) | 1.15 (0.36–3.66) | .81 |
| 4–5 | 7.9 (6/76) | 10.1 (10/99) | 0.75 (0.26–2.20) | .62 |
| >5 | 6.2 (11/177) | 5.1 (15/293) | 1.22 (0.55–2.73) | .62 |
| Total | 6.4 (23/360) | 6.5 (34/523) | 0.98 (0.57–1.70) | .95 |
As shown in Table 4, race was not a determinant of metachronous ACN in either our univariate (OR, 0.92; 95% CI, 0.69–1.24;) or multivariate analyses after adjustment for age, sex, education, type of insurance, indication (screen/surveillance) for baseline colonoscopy, surveillance interval, and findings at baseline colonoscopy (adjusted odds ratio [aOR], 0.96; 95% CI, 0.70–1.30; P = .78). The multivariate analyses identified ACN (aOR, 2.04; 95% CI, 1.34–3.09; P < .001) at baseline colonoscopy as the sole independent determinant of risk. Increasing age was also associated with risk in the univariate analyses (OR, 1.28; 95% CI, 1.04–1.57) but not after adjustment for the aforementioned covariates including race (aOR, 1.24; 95% CI, 0.98–1.57; P = .07). Similar analyses identified prior advanced adenomas (aOR, 3.84; 95% CI, 2.06–7.14; P < .001) and increasing age (aOR, 1.47; 95% CI, 1.04–2.06; P = .03) as independent determinants of risk for metachronous advanced adenomas and prior advanced serrated polyps (aOR, 4.04; 95% CI, 2.11–7.72; P < .001) as an independent determinant of risk for advanced serrated polyps.
Table 4.
Univariate and Multivariate Associations Between Patient Characteristics and Odds of Metachronous Advanced Colorectal Neoplasia
| Characteristic | Univariate OR (95% CI) | Multivariate aORa (95% CI) | P value |
|---|---|---|---|
| Race | |||
| Black vs White | 0.92 (0.69–1.24) | 0.96 (0.70–1.30) | .781 |
| Sex | |||
| Female vs male | 1.03 (0.77–1.39) | 1.10 (0.81–1.48) | .547 |
| Age | |||
| 10-y increase | 1.28 (1.04–1.57) | 1.24 (0.98–1.57) | .068 |
| Education | |||
| Less than high school | 1.03 (0.71–1.48) | 1.03 (0.71–1.49) | .891 |
| High-school graduate | Reference | Reference | – |
| Some college/college graduate | 1.21 (0.84–1.72) | 1.25 (0.87–1.79) | .237 |
| Insurance | |||
| Commercial | Reference | Reference | – |
| Free care | 0.70 (0.25–1.96) | 0.84 (0.29–2.37) | .737 |
| Medicaid | 1.08 (0.73–1.61) | 1.17 (0.77–1.77) | .464 |
| Medicare | 1.31 (0.94–1.82) | 1.18 (0.83–1.68) | .366 |
| Time since baseline examination | |||
| ≤3 y | 1.37 (0.97–1.95) | 1.10 (0.75–1.61) | .620 |
| 4–5 y | 1.12 (0.79–1.59) | 0.96 (0.67–1.39) | .842 |
| 5+ y | Reference | Reference | – |
| Indication for baseline examination | |||
| Surveillance vs screening | 1.27 (0.94–1.73) | 1.24 (0.90–1.72) | .190 |
| Findings from baseline examination | |||
| Normal/nonneoplastic polyps/other | Reference | Reference | – |
| Nonadvanced polyps | 0.89 (0.62–1.28) | 0.98 (0.67–1.44) | .935 |
| Multiplicity (3+ nonadvanced adenomas) | 1.30 (0.75–2.25) | 1.36 (0.76–2.41) | .298 |
| Hyperplastic polyps ≥10 mm | 1.12 (0.14–8.76) | 1.18 (0.15–9.26) | .874 |
| ACN (advanced/malignant polyps) | 1.89 (1.28–2.80) | 2.04 (1.34–3.09) | .001 |
Adjusted odds ratio controlling for race, sex, age, education, insurance, time since baseline examination (surveillance interval), indication for baseline examination, and findings at baseline examination.
Discussion
The findings of this study both reaffirm and strengthen the results of our prior study.9 As previously reported, this study finds no significant difference in the overall prevalence of metachronous ACN between NHWs and NHBs undergoing postpolypectomy surveillance in at an urban, safety net hospital. This study also finds no significant differences in the prevalence of metachronous advanced adenomas, advanced serrated polyps, or interval cancers at the recommended surveillance intervals after stratification by baseline findings. Moreover, race was not an independent determinant of metachronous ACN after adjustment for age, sex, education, type of insurance, indication (screen/surveillance) for baseline colonoscopy, surveillance interval, and findings at baseline colonoscopy. Together, these observations provide new evidence suggesting that the rates of adenoma and serrated polyp progression are similar for both NHWs and NHBs, and, given the low rate of interval cancers in both groups, further validate current guidelines for postpolypectomy surveillance for both racial groups.
Few studies have examined whether race is an independent determinant of metachronous ACN. We previously reported that the overall prevalence of ACN undergoing first-time surveillance colonoscopy between 2001 and 2010 was similar among NHBs and NHWs (11.3% vs 9.8%; aOR, 1.3; 95% CI, 0.69–2.4) after a median follow-up of 4.3 years.9 We also observed that while NHBs and NHWs with nonadvanced neoplasia had similar rates of ACN at the 1- to 3-, 4- to 5-, and 5-year follow-up intervals, NHBs with ACN or multiplicity at baseline had higher rates of ACN at the 1- to 3-year intervals, but the difference was nonsignificant. The major limitations of the study were its relatively small sample size (ie, NHWs, n = 246; NHBs, n = 203), thus raising the possibility of a type II statistical error, lack of data on patients undergoing repeat surveillance colonoscopy, and a small number of patients with serrated polyps at both screening colonoscopy and surveillance, thus precluding subgroup analyses exploring associations between race and advanced adenomas and advanced serrated polyps separately. In a secondary analysis of data from the Polyp Prevention Trial,10 Laiyemo et al. also found that NHBs had a similar risk of metachronous advanced adenomas as NHWs (8.5% vs 6.4%; risk ratio, 1.18; 95% CI, 0.68–2.05) over a mean follow-up of 8.3 years (range, 4.9–12.4). This study's major limitations were the small number of NHB participants compared with NHWs (ie, n = 126 vs n = 1668, respectively), failure to include nonadvanced and advanced serrated polyps in their analyses, the inability to control for surveillance intervals, and the fact that the study population represented a subset of participants in a randomized chemoprevention trial, thus raising concerns about generalizability. In a pooled analysis of 8 prospective surveillance studies,11 Martinez et al. also found no association between race and metachronous advanced adenomas (aOR, 1.08; 95% CI, 0.79–1.47) but again included a number of participants from various randomized prevention trials and failed to include serrated polyps in their analyses. The current study not only corroborates the results of these studies but also addresses their limitations including the inclusion of a more representative study population, adjustments for surveillance intervals, and a more in-depth analysis of the association between race and metachronous serrated polyps.
Our study also corroborates an extensive body of literature demonstrating the significance of baseline findings as predictors of metachronous ACN.11, 12, 13, 14, 15, 16, 17, 18 We observed that individuals with ACN at baseline, regardless of race, were significantly more likely to have metachronous ACN than those with 1–2 nonadvanced polyps at a 3-year follow-up examination, thus supporting the current US Multi-Society Task Force on Colorectal Cancer recommendation for earlier surveillance.5 We also observed that both NHBs and NHWs with 3 or more nonadvanced adenomas <10 mm were at an increased risk of ACN overall but not at the 3-year follow-up examination, thus supporting the revised guideline to extend the surveillance interval from 3 years, as previously recommended,19 to 3–5 years in the 2020 revised recommendations based on the polyp number.5 We did not find an association between the presence of hyperplastic polyps ≥ 10 mm and metachronous advanced neoplasia at any follow-up interval, thus arguing against the recommendation for more aggressive follow-up than those with normal findings in settings with pathological expertise in diagnosing more significant serrated lesions.5 We did not examine whether the risk of metachronous ACN was different for those with multiple diminutive (≤ 5 mm) vs small (6–9 mm) adenomas or synchronous nonadvanced adenomas and sessile serrated polyps on baseline examination, as suggested by others,12,20, 21, 22, 23 because neither the 2012 nor 2020 USMSTF guidelines tailored their recommendations based on these features5,19 and because of insufficient statistical power. Similarly, we also did not examine whether the risk was different for those with baseline proximal vs distal nonadvanced adenomas, also as suggested by others,11,18 for the same reasons.
Our study has several noteworthy strengths that lend credence to our findings. First, our study is the largest to date with respect to the number of NHB patients, thus increasing its power to identify significant differences in our primary outcome had one been observed. More specifically, our study had >80% of detecting a potentially clinically significant 3% difference in prevalence rates at the P < .05 level. Second, as previously noted, our study addresses several limitations of previous studies comparing the prevalence of metachronous ACN between NHWs and NHBs, including a more representative patient population than those that included participants in various chemoprevention trials, adjustments for surveillance intervals, and a more in-depth analysis of the association between race and metachronous serrated polyps. Third, the safety net healthcare setting provided a unique opportunity to assess the prevalence of ACN among a patient population devoid of financial and many other structural barriers to access CRC surveillance present in other healthcare settings. Eligible patients were offered surveillance examinations regardless of their ability to pay. Lastly, we restricted our analyses to patients with complete examinations, adequate bowel preparations, and complete retrieval of all polyp specimens to minimize misclassification.
Our study also had several important limitations. First, it was conducted at a single, urban, academic center, and so our findings may not be generalizable to other healthcare settings; however, as a safety net healthcare center, BMC provides care for a racially, ethnically, and socioeconomically diverse patient population. Second, the use of a convenience sample also raises concern about potential selection bias; however, this is offset by the fact that NHB and NHW patients had equal access to surveillance colonoscopy. Third, patients excluded because of failed polyp retrieval could have also resulted in selection bias, even though the retrieval rate was >90% for both groups and thus acceptable according to current guidelines.24 Importantly, all such polyps were <5 mm and thus unlikely to be classified as advanced; consequently, inclusion would not have a significant impact on final results (data not shown). Fourth, as previously noted, we lacked sufficient statistical power for several of our subgroup analyses, particularly whether the risk of metachronous ACN among NHWs and NHBs was different for combinations of nonadvanced adenomas and serrated lesions and whether proximal nonadvanced adenomas at baseline increase the risk of metachronous advanced adenomas. Fifth, similar to prior studies,9, 10, 11 we were unable to control for endoscopist ADR because of unavailable data for many of the years examined. Although ADR has been shown to be inversely associated with metachronous ACN,20 BMC uses an open-access system for both screening and surveillance in which patients are randomly assigned to all endoscopists, regardless of race or ethnicity, thus minimizing the impact of confounding due to ADR. Moreover, the group's mean ADR of 38% when measured was well above the recommended threshold of 25%.25 Sixth, our study design also precluded an accurate assessment of interval cancers, which have been reported in ∼0.6% of postpolypectomy patients,26 because individuals with a history of precancerous polyps who underwent diagnostic colonoscopy because of alarm signs or symptoms of CRC were excluded. However, we suspect that the number of symptomatic interval cancers was quite small for both groups given the relatively high ADR of the participating endoscopists.27 Seventh, the retrospective design also precludes a detailed analysis of the extent to which differential exposure to risk factors for polyp recurrence other than age, sex, and socioeconomic status may have influenced our results.28,29 Lastly, we relied on the subjective judgment of multiple endoscopists to provide data about polyp size, thereby raising the possibility of misclassification for ACN defined by size alone.
In conclusion, our study finds no significant differences in the prevalence of metachronous ACN between NHWs and NHBs after adjustment for baseline findings, surveillance interval, type of baseline examination, and select demographic characteristics. These observations suggest that differences in tumor biology resulting in diminished polyp dwell time and accelerated progression from benign polyp to cancer is an unlikely contributing factor to the higher incidence and mortality observed in NHBs. In the aggregate, our findings provide new evidence further validating current surveillance guidelines for both NHWs and NHBs.
Acknowledgments:
The authors thank Linda Rosen, MSEE, for her technical support with data curation and Remington Kim for assistance with data collection.
Authors' Contributions:
Dionne Rebello: Conceptualization: Equal; Data curation: Lead; Investigation: Equal; Formal analysis: Supporting; Writing – original draft: Supporting. Paul C. Schroy III: Conceptualization: Lead; Methodology: Lead; Formal analysis: Equal; Writing – original draft: Lead: Supervision: Lead. Anna Leszcynski: Conceptualization: Equal; Data curation: Equal; Writing – review and editing: Supporting. Alessandro Colletta: Data curation: Supporting; Writing – review and editing; Supporting. Elliott Rebello: Data curation: Supporting; Writing – review and editing: Supporting. Justin Mills: Data curation: Supporting; Writing – review and editing: Supporting. Timothy Heeren: Data curation: Supporting; Formal analysis: Lead; Supporting: Writing – original draft: Supporting. Hemant Roy: Conceptualization: Supporting; Writing – review and editing: Supporting.
Footnotes
Conflicts of interest: The authors disclose no conflicts.
Funding: The authors report no funding.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: Study data and analytic methods are available from the corresponding author upon request.
Writing Assistance: None.
Material associated with this article can be found in the online version at doi:10.1016/j.gastha.2021.09.001.
Supplementary Materials
References
- 1.Siegel R.L., Miller K.D., Goding Sauer A., et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 2.Ashktorab H., Kupfer S.S., Brim H., et al. Racial disparity in gastrointestinal cancer risk. Gastroenterology. 2017;153:910–923. doi: 10.1053/j.gastro.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winawer S.J., Zauber A.G., Ho M.N., et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 4.Zauber A.G., Winawer S.J., O'Brien M.J., et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S., Lieberman D., Anderson J.C., et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US Multi-Society Task Force on colorectal cancer. Am J Gastroenterol. 2020;115:415–434. doi: 10.14309/ajg.0000000000000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carethers J.M., Doubeni C.A. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354–367. doi: 10.1053/j.gastro.2019.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fedewa S.A., Flanders W.D., Ward K.C., et al. Racial and ethnic disparities in interval colorectal cancer incidence: a population-based cohort study. Ann Intern Med. 2017;166:857–866. doi: 10.7326/M16-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snover D., Ahnen D.J., Burt R.W., et al. Springer-Verlag; Berlin: 2010. Serrated polyps of the colon and rectum and serrated (“hyperplastic”) polyposis. [Google Scholar]
- 9.Kwah J., Schroy P.C., 3rd, Jacobson B.C., et al. Whites and blacks have similar risk of metachronous advanced colorectal neoplasia. Dig Dis Sci. 2014;59:2264–2271. doi: 10.1007/s10620-014-3132-4. [DOI] [PubMed] [Google Scholar]
- 10.Laiyemo A.O., Doubeni C., Brim H., et al. Short- and long-term risk of colorectal adenoma recurrence among whites and blacks. Gastrointest Endosc. 2013;77:447–454. doi: 10.1016/j.gie.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martínez M.E., Baron J.A., Lieberman D.A., et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832–841. doi: 10.1053/j.gastro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson J.C., Butterly L.F., Robinson C.M., et al. Risk of metachronous high-risk adenomas and large serrated polyps in individuals with serrated polyps on index colonoscopy: data from the New Hampshire colonoscopy registry. Gastroenterology. 2018;154:117–127.e2. doi: 10.1053/j.gastro.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubé C., Yakubu M., McCurdy B.R., et al. Risk of advanced adenoma, colorectal cancer, and colorectal cancer mortality in people with low-risk adenomas at baseline colonoscopy: a systematic review and meta-analysis. Am J Gastroenterol. 2017;112:1790–1801. doi: 10.1038/ajg.2017.360. [DOI] [PubMed] [Google Scholar]
- 14.Lieberman D.A., Weiss D.G., Harford W.V., et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077–1085. doi: 10.1053/j.gastro.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Pinsky P.F., Schoen R.E., Weissfeld J.L., et al. The yield of surveillance colonoscopy by adenoma history and time to examination. Clin Gastroenterol Hepatol. 2009;7:86–92. doi: 10.1016/j.cgh.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Saini S.D., Kim H.M., Schoenfeld P. Incidence of advanced adenomas at surveillance colonoscopy in patients with a personal history of colon adenomas: a meta-analysis and systematic review. Gastrointest Endosc. 2006;64:614–626. doi: 10.1016/j.gie.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 17.Schreiner M.A., Weiss D.G., Lieberman D.A. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology. 2010;139:1497–1502. doi: 10.1053/j.gastro.2010.06.074. [DOI] [PubMed] [Google Scholar]
- 18.van Heijningen E.M., Lansdorp-Vogelaar I., Kuipers E.J., et al. Features of adenoma and colonoscopy associated with recurrent colorectal neoplasia based on a large community-based study. Gastroenterology. 2013;144:1410–1418. doi: 10.1053/j.gastro.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Lieberman D.A., Rex D.K., Winawer S.J., et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Kim J.Y., Kim T.J., Baek S.Y., et al. Risk of metachronous advanced neoplasia in patients with multiple diminutive adenomas. Am J Gastroenterol. 2018;113:1855–1861. doi: 10.1038/s41395-018-0210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melson J., Ma K., Arshad S., et al. Presence of small sessile serrated polyps increases rate of advanced neoplasia upon surveillance compared with isolated low-risk tubular adenomas. Gastrointest Endosc. 2016;84:307–314. doi: 10.1016/j.gie.2016.01.064. [DOI] [PubMed] [Google Scholar]
- 22.Moon C.M., Jung S.A., Eun C.S., et al. The effect of small or diminutive adenomas at baseline colonoscopy on the risk of developing metachronous advanced colorectal neoplasia: KASID multicenter study. Dig Liver Dis. 2018;50:847–852. doi: 10.1016/j.dld.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Sneh Arbib O., Zemser V., Leibovici Weissman Y., et al. Risk of advanced lesions at the first follow-up colonoscopy after polypectomy of diminutive versus small adenomatous polyps of low-grade dysplasia. Gastrointest Endosc. 2017;86:713–721.e2. doi: 10.1016/j.gie.2017.02.034. [DOI] [PubMed] [Google Scholar]
- 24.Rembacken B., Hassan C., Riemann J.F., et al. Quality in screening colonoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE) Endoscopy. 2012;44:957–968. doi: 10.1055/s-0032-1325686. [DOI] [PubMed] [Google Scholar]
- 25.Rex D.K., Schoenfeld P.S., Cohen J., et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31–53. doi: 10.1016/j.gie.2014.07.058. [DOI] [PubMed] [Google Scholar]
- 26.Robertson D.J., Lieberman D.A., Winawer S.J., et al. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut. 2014;63:949–956. doi: 10.1136/gutjnl-2012-303796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wieszczy P., Waldmann E., Loberg M., et al. Colonoscopist performance and colorectal cancer risk after adenoma removal to stratify surveillance: two nationwide observational studies. Gastroenterology. 2021;160:1067–1074.e6. doi: 10.1053/j.gastro.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Lieberman D., Sullivan B.A., Hauser E.R., et al. Baseline colonoscopy findings associated with 10-year outcomes in a screening cohort undergoing colonoscopy surveillance. Gastroenterology. 2020;158:862–874.e8. doi: 10.1053/j.gastro.2019.07.052. [DOI] [PubMed] [Google Scholar]
- 29.Carot L., Navarro G., Naranjo-Hans D., et al. Predictors of metachronous risk polyps after index colonoscopy. Clin Transl Gastroenterol. 2021;12:e00304. doi: 10.14309/ctg.0000000000000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.