Abstract
Patients with congenital heart disease now live well into adulthood because of advances in surgical techniques, improvements in medical management, and the development of novel therapeutic agents. As patients grow older into adults with congenital heart disease, many require catheter-based interventions for the treatment of residual defects, sequelae of their initial repair or palliation, or acquired heart disease. The past 3 decades have witnessed an exponential growth in both the type and number of transcatheter interventions in patients with congenital heart disease. With improvements in medical technology and device design, including the use of devices designed for the treatment of acquired valve stenosis or regurgitation, patients who previously would have required open-heart surgery for various conditions can now undergo percutaneous cardiac catheter-based procedures. Many of these procedures are complex and occur in complex patients who are best served by a multidisciplinary team. This review aims to highlight some of the currently available transcatheter interventional procedures for adults with congenital heart disease, the clinical outcomes of each intervention, and any special considerations so that the reader may better understand both the procedure and patients with adult congenital heart disease.
Keywords: adult congenital heart disease, congenital cardiac interventions, stenting, transcatheter interventions, transcatheter valve replacement
Central Illustration
Highlights
-
•
Many patients with adult congenital heart disease require transcatheter interventions.
-
•
Transcatheter interventions are broad and range from shunt closure to valve replacement.
-
•
Interventions in this population can be difficult because of anatomic and clinical complexities.
-
•
Multidisciplinary teams are necessary for success.
Introduction
The past 3 decades have witnessed an exponential growth in both the type and number of transcatheter interventions in patients with congenital heart disease (CHD). In 2000, the first transcatheter heart valve implanted in a human was designed for children with pulmonic valve disease1,2 and contributed to a revolution in the treatment of acquired aortic stenosis (AS) with transcatheter aortic valve replacement (TAVR).
Advances in surgical techniques, improvements in medical management, and the development of novel therapeutic agents now allow patients with CHD to live well into adulthood. However, many of these patients with adult congenital heart disease (ACHD) require catheter-based interventions for the treatment of residual defects, sequelae of their initial repair or palliation, or acquired heart disease.3,4
With improvements in medical technology and device design, including the use of devices designed for the treatment of acquired valve stenosis or regurgitation, patients who previously would have required open-heart surgery for various conditions can now undergo percutaneous cardiac catheter-based procedures. Many of these procedures are complex and occur in complex patients, who are best served by a multidisciplinary team. We aim to highlight some of the currently available transcatheter interventional procedures for adults with CHD, the clinical outcomes of each intervention, and any special considerations so that the reader may better understand both the procedure and patients with ACHD.
Special needs of patients with ACHD
Careful preprocedural planning is especially important for patients with ACHD because their complex anatomy and physiology, comorbid conditions, and previous experiences may lead to complications or unsuccessful procedures. The development of skills required for independent practice in ACHD interventions requires dedicated fellowship training and can be achieved by a combination of pathways, as described in a recent position statement by the Society for Cardiovascular Angiography and Interventions.5 Although the position statement by the Society for Cardiovascular Angiography and Interventions should be viewed as the absolute minimum training required, the nuances and complete toolbox of special skills can only truly be achieved with years of clinical experience.
A detailed history of patients’ previous procedures and operations, with a review of their original reports, is imperative, especially because it relates to vascular access and possible prior shunts. After childhood interventions (including central access catheters, cardiac catheterizations, or surgeries), iliofemoral vein and/or artery occlusion is commonplace. Because of collateral formation, these occlusions may not always be clinically apparent on physical examination or vascular ultrasound but can be noted based on previous procedural notes or dedicated cross-sectional imaging. It may be possible to perform hemodynamic catheterization via venous collaterals or the azygous vein or recanalization of an occluded iliofemoral vein with serial balloon dilations or stenting.6 Performing interventions using large-bore catheters may require additional consideration and thoughtfulness. Coronary artery disease is becoming increasingly common in the population with ACHD; percutaneous coronary interventions may be challenging because of anomalous origin or unusual orientation of the coronaries (particularly in patients with tetralogy of Fallot or transposition of the great arteries [TGA]) or after coronary reimplantation during infancy.
Most patients with moderate or complex ACHD have had many interactions with the health care system and undergone multiple procedures, and some of these experiences may have caused anxiety or trauma in the patient as well as their family. Vascular access sites may have increased sensitivity because of previous injuries, scarring, or fibrosis, and, therefore, higher doses of local anesthetic and conscious sedation are often needed. There is often higher anxiety associated with procedures in patients and their families because they may think of previous complications, worry about the risk of adverse outcomes, or worry whether the information obtained at the time of catheterization will lead to a surgery or prolonged hospital stay. In addition, young adults who are being treated for the first time in an adult catheterization laboratory often have different expectations from the procedural workflow, such as the use of preprocedural sedation, general anesthesia vs conscious sedation, the ability to have their parents present or involved, and the level of discomfort to expect. Discussion of the risks and benefits of the procedure, expectations, and questions is recommended during a clinic visit prior to the procedure, particularly for a new patient.
Multidisciplinary team discussion is also recommended prior to complex procedural planning, including topics such as anticoagulation management, intracardiac shunts, mechanical support, and backup surgical options. Some patients may have congenital comorbid conditions that may affect the airway (craniofacial abnormalities and cervical spinal instability in patients with Down syndrome). Acquired comorbidities, such as airway stenoses or hypoplasia, pulmonary hypertension, renal and hepatic dysfunction, prior sensitization to blood products, and polycythemia, with the risk of hyperviscosity, may also affect procedural planning and need discussion prior to the procedure. For example, patients with cyanosis, low muscle mass, or hepatic congestion due to right-heart disease or Fontan circulation have more renal and hepatic dysfunction than is apparent using standard laboratory evaluation and have a higher risk of bleeding.7
The American College of Cardiology National Cardiovascular Data Registry (IMproving Pediatric and Adult Congenital Treatments Registry) is a powerful quality improvement tool for pediatric and adult congenital cardiac catheterizations8; the other multicenter collaborations include the Congenital Cardiac Interventional Study Consortium9 and Congenital Cardiac Catheterization Project on Outcomes.10 Patient heterogeneity, variability in procedural selection and techniques, the need for extensive and detailed data entry, and rapid changes in available technologies have long been a significant challenge in registry and outcome research in ACHD; however, this has been somewhat mitigated by the electronic medical records and increased resources available for quality improvement and research.
Shunt-related interventions
Atrial-level shunts
Ostium secundum atrial septal defect closure
Atrial septal defect (ASD) is one of the more commonly encountered congenital heart malformations, accounting for approximately 10% to 15% of CHD in adults.11,12 Adult patients who have been diagnosed with an ASD usually present with progressive dyspnea on exertion, palpitations, and decreased exercise tolerance as a consequence of left-to-right shunting that results in right-heart overload, dilation, and dysfunction.13 Some patients may also develop pulmonary hypertension, tricuspid regurgitation (TR), and atrial arrhythmias. Patients who are asymptomatic early in life may develop symptoms as the degree of left-to-right shunting increases with age because of decreased left atrial and ventricular compliance as well as increased systemic arterial resistance.
The indications for ASD closure include symptoms; paradoxical embolism; significant shunting (pulmonary blood flow to systemic blood flow ratio, or Qp:Qs > 1.5:1) in the absence of symptoms; and/or the presence of right-heart enlargement, detected using echocardiography or magnetic resonance imaging (MRI) in the presence of normal or low pulmonary vascular resistance (less than one-third of systemic vascular resistance).4 Patients with severe pulmonary hypertension are usually not candidates for ASD closure; however, the use of pulmonary vasodilator therapy may reduce the pulmonary arterial pressure and resistance enough to permit ASD closure, with or without the use of a fenestrated device.14,15 Although the surgical outcomes of ASD closure are excellent, surgical closure of an ostium secundum ASD has a higher complication rate than transcatheter closure,16 and thus transcatheter closure has become the standard of care for most ostium secundum ASDs in adults. Preprocedural evaluation using transesophageal echocardiography (TEE) or cross-sectional imaging (cardiac computed tomography angiography [CTA] or MRI) is used to screen for associated defects, rule out partial anomalous pulmonary venous connections, and assess the rim size of ASDs.17, 18, 19 The procedure is usually performed under fluoroscopic and imaging guidance using either TEE or intracardiac echocardiography.20,21
Procedural success (complete closure with stable positioning of the device) has been reported in 94% to 98% of cases,22,23 with a low risk of embolization, thrombus formation, aortic root perforation or erosion,24,25 pericardial effusion, and arrhythmias; follow-up using echocardiography on postprocedural day 1, at 1 to 3 months, and then at 12 months is recommended. The most commonly used device in the United States is the Amplatzer septal occluder (Abbott). Amplatzer septal occluders (Figure 1) with sizes ranging from 4 to 38 mm have been approved by the United States Food and Drug Administration (FDA), with excellent long-term results.23,26 Of 1000 patients enrolled in a postapproval study, the Amplatzer device caused complications in only 0.65% (n = 6), with a risk of cardiac erosion of 0.3% over 2 years.23 The Gore Cardioform ASD occluder (Gore Medical; size, 27-48 mm) and the Gore Cardioform septal occluder (size, 20-30 mm) have also been approved by the FDA.23 Occlutech has an ASD and patent foramen ovale (PFO) occluder that is currently available in Canada but not in the United States. The device is being studied in active clinical trials (NCT04291898).
Figure 1.
Transcatheter occlusion of an ostium secundum atrial septal defect (ASD) using the Amplatzer septal occluder. (A) The top panel shows fluoroscopic images of a sizing balloon across the ASD to measure the waist of the defect, whereas the bottom panel shows the simultaneous transesophageal echocardiographic (TEE) images. (B) The device is now deployed across the ASD, as observed using fluoroscopy (top panel), and is seen to be in a stable position, as detected using TEE imaging (bottom panel). (C) After release of the device from the delivery cable, the septal occluder has aligned with the atrial septum in a more natural way (top panel) and is seen to be still in a stable position across the ASD while straddling the retroaortic rim using TEE imaging (bottom panel).
In older patients, the presence of left atrial and/or left ventricular diastolic dysfunction may result in increased left atrial pressure after device closure of ASD.27 Therefore, a careful hemodynamic assessment (this requires a second venous access) of the left atrial pressure at baseline and during temporary balloon occlusion of the ASD is important in such patients, and fenestrated ASD devices may be optimal.28
PFO closure
Patent foramen ovale is a common anatomic variant (present in ∼25% of the general population)29 that is a remnant of fetal circulation. The indications for PFO closure are symptoms such as cryptogenic stroke30, 31, 32 and paradoxical emboli; in select cases, platypnea-orthodeoxia,33 persistent hypoxia due to TR shunting blood across the PFO,34 and decompression illness may be considered.35 Transcatheter PFO closure was introduced in the early 2000s36 and has become more prevalent since 2017, when 3 trials showed a reduction in the risk of recurrent events after an index cryptogenic stroke in carefully selected patients (using clinical and anatomic features such as Risk of Paradoxical Embolism or RoPE score and PFO-Associated Stroke Causal Likelihood [PASCAL] Classification System)29,37 receiving antiplatelet therapy.30, 31, 32
Two devices, the Amplatzer PFO occluder and the Gore Cardioform septal occluder, that have been approved by the FDA for PFO closure. Although these devices have excellent procedural outcomes30, 31, 32 and helped reduce the absolute risk of stroke by 3.3% in 1 meta-analysis (with a number needed to treat of 30),38 an increased risk of new-onset atrial fibrillation (3.4%) was noted, with, however, a very low associated incidence of stroke and resolution of arrhythmia within the first 3 months.39 Other complications, such as pericardial effusion,24 device embolization, and device erosion, are extremely rare. A new device, NobleStitch,40 is being studied, and a clinical trial is underway in the United States (NCT04339699) to compare its effectiveness with that of the established Amplatzer device; the Occlutech Flex II device is being used in Europe and Canada and is under investigation in the United States (NCT05069558).
Transcatheter correction of superior sinus venosus atrial septal defects
Sinus venosus atrial septal defects (SVASDs) (superior or inferior type) are rare, comprising approximately 5% to 10% of all ASDs.41 Superior SVASD is a deficiency of the common wall between the superior vena cava (SVC) and right-sided pulmonary veins and is associated with an anomalous right upper pulmonary vein in up to 90% of cases. Inferior SVASD is a deficiency of the wall between the right atrium (RA) or inferior vena cava and the left atrium and can be associated with anomalous pulmonary venous drainage of the right lower pulmonary veins to the inferior vena cava or RA.42 Patients with SVASDs usually present with symptoms similar to patients with ostium secundum ASDs, although they may present earlier because of increased left-to-right shunting from both an atrial-level shunt and ≥1 anomalous right-sided pulmonary veins that drain into the SVC. Treatment is indicated to reverse right-heart enlargement and the following symptoms: dyspnea on exertion, decreased exercise tolerance, or right-heart failure.43 The standard of care is surgical repair4 using the Warden procedure or 2-patch repair. This repair can be technically challenging, and the operative complications include pulmonary vein stenosis (which may lead to dyspnea, pulmonary edema, or infarction), SVC stenosis, or sinus node dysfunction.41,44
However, over the last several years, transcatheter correction of superior SVASDs using covered stents (Figure 2) has become more established.43,45, 46, 47, 48 The procedure is technically challenging and is successful only in patients with suitable anatomy. Cardiac CTA is performed, and virtual or 3-dimensionally printed models are used to help with visualization of SVASDs, the anomalous pulmonary veins, and surrounding structures45 to assess whether the anatomy is favorable for the placement of a covered stent that will direct SVC blood to the RA, allowing for unobstructed anomalous pulmonary vein flow to then drain into the left atrium through the residual SVASD. The procedure is usually performed with the patient under general anesthesia given the need for intraprocedural imaging guidance using TEE (Figure 3).
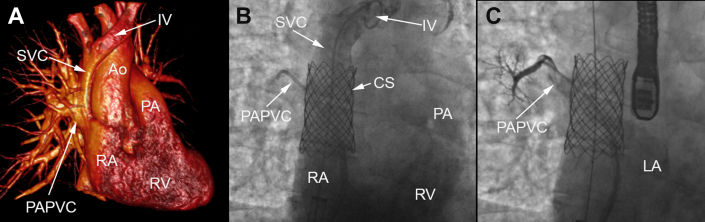
Figure 2.
Transcatheter stenting of a superior sinus venosus defect with associated partial anomalous pulmonary venous connection. (A) Three-dimensional rendering from a computed tomography angiogram demonstrating a superior sinus venosus defect with the associated partial anomalous pulmonary venous connection. (B) Deployment of a covered stent within the superior vena cava to exclude the sinus venosus defect, which allows systemic venous blood from the superior vena cava to drain into the right atrium while pulmonary venous blood can drain into the left atrium from the partial anomalous pulmonary venous connection. (C) Angiogram of the partial anomalous pulmonary venous connection demonstrating unobstructed blood flow back to the left atrium from behind the covered stent and through the sinus venosus defect. Ao, aorta; CS, covered stent; IV, innominate vein; LA, left atrium; PA, pulmonary artery; PAPVC, partial anomalous pulmonary venous connection; RA, right atrium; RV, right ventricle; SVC, superior vena cava.
Figure 3.
Transesophageal echocardiography during stenting of a superior sinus venosus defect. (A) Traditional bicaval view of the superior sinus venosus defect, with superior vena cava blood draining into both the left and right atriums. (B) Color Doppler imaging demonstrating blood flow with mainly left-to-right shunting across the superior sinus venosus defect. (C) Traditional bicaval view of the superior sinus venosus defect after deployment of a covered stent to direct superior vena cava blood to the right atrium while excluding the superior sinus venosus defect to prevent shunting of left atrial blood to the right atrium. (D) Color Doppler imaging demonstrating only a trace residual left-to-right shunt around the covered stent at the end of the case. CS, covered stent; LA, left atrium; RA, right atrium; SVC, superior vena cava. The asterisk indicates the superior sinus venosus defect.
A description of the largest cohort to date demonstrated that the procedure has good short-term outcomes, with no incidence of sinus node dysfunction.48 However, one of the drawbacks of this procedure is difficulty in using just 1 stent to adequately cover the SVC superiorly and the SVC-RA junction inferiorly while maintaining stent stability. Forty percent (n = 10) of the patients in the study required covered Cheatham-Platinum stents (B. Braun Inc) that were longer than 6 cm, which are currently not available commercially in the United States. Furthermore, 52% (n = 13) of the patients in the study required additional stents to secure the covered stent, and 24% of the patients (n = 6) had covered stents that either migrated or embolized during the procedure. If the stent is not long enough, there may still be residual shunting at the inferior margin of the stent, as noted in 44% (n = 11) of the patients in the study by Hansen et al.48 In fact, the last 9 patients in the study had custom-made 7- or 8-cm-long, covered 10-zig Cheatham-Platinum stents to increase the zone of apposition in the SVC to prevent migration or embolization and reduce the chance of residual leaks around the stent at the SVC-RA junction. Larger studies and more experience with this technique will lead to further iterations and improvements of the procedure; however, a select subset of patients with superior SVASDs may benefit from transcatheter correction of the defect, and this may help avoid a surgical option.
Ventricular septal defect closure
Ventricular septal defects (VSDs) comprise approximately 20% of all congenital heart lesions49,50 and can occur anywhere along the ventricular septum. Patients with unrepaired VSDs are usually asymptomatic if the shunt is small or may present with evidence of left-heart volume overload (pulmonary venous congestion, shortness of breath, or dyspnea on exertion) due to increased left-to-right shunting and pulmonary overcirculation. Adult patients with very large unrepaired VSDs usually present with irreversible pulmonary hypertension and right-to-left shunting, with cyanosis, or Eisenmenger syndrome.4,51 The indication for VSD repair is evidence of left ventricular volume overload and a hemodynamically significant shunt with a Qp:Qs ratio of ≥1.5:1 as long as the pulmonary pressure or pulmonary vascular resistance is normal or low.4 Patients with worsening aortic valve regurgitation or a history of infective endocarditis may also be candidates for VSD closure. Patients with irreversible pulmonary hypertension or Eisenmenger syndrome are not candidates for VSD closure.4
The types of VSDs that are amenable to transcatheter closure are primarily muscular or apical VSDs and some membranous VSDs,49,52,53 whereas defects in close proximity to valves or ventricular-free walls require surgical repair. The only device that has been approved by the FDA for transcatheter VSD closure is the Amplatzer muscular or PI (postmyocardial infarction) muscular VSD occluder (Abbott), and it has been demonstrated to be effective in the treatment of both congenital and acquired (postmyocardial infarction) muscular VSDs.54,55 Other devices, such as the Amplatzer ductal occluder I and Amplatzer ductal occluder II (Abbott), can be used in an off-label fashion to close certain types of VSDs that have favorable anatomy (such as a membranous VSD with a windsock-shaped aneurysmal sac).49 Closure of membranous VSDs is feasible; however, the development of conduction abnormalities is a major concern, with rates reported to be as high as 6% of cases,49,52,53 although membranous VSDs associated with aneurysms of the ventricular septum can usually be treated, with a low risk of conduction system abnormalities.56 The long-term results are favorable, with procedural success of >90% and complications such as heart block (up to 6%), hemolysis (1%-2%), and embolization (1%-2%) being relatively rare.49
Patent ductus arteriosus closure
The patent ductus arteriosus (PDA) is a vestige from fetal circulation that connects the descending aorta and pulmonary artery (PA).57, 58, 59 The incidence of an isolated PDA in term infants is approximately 2.9 per 10,000 live births,60 and it is more common in patients with genetic syndromes such as DiGeorge or CHARGE (coloboma, heart defects, choanal atresia, growth retardation, genital abnormalities, and ear abnormalities).61 Patients with a small PDA (Qp:Qs < 1.5) may have no symptoms, and its diagnosis may be incidental; however, some may have a continuous murmur on examination. Patients with a moderately sized PDA (Qp:Qs, 1.5:1-2.2:1) may have exercise intolerance, heart failure, and evidence of left-sided (left atrial and/or left ventricular) volume overload detected using echocardiography. Adult patients with a large PDA (Qp:Qs > 2.2:1) will most likely have evidence of irreversible pulmonary hypertension and differential cyanosis (normal oxygenation of the upper extremities, with cyanosis of the lower extremities due to the flow of deoxygenated blood from the PDA), consistent with the diagnosis of Eisenmenger syndrome. However, these patients may not have a murmur because of equalization of pressures on both the right and left sides of the heart.
Patent ductus arteriosus closure is indicated in patients with left-to-right shunting with symptoms or asymptomatic patients with left-heart enlargement. PDA closure should not be attempted in those with Eisenmenger syndrome.4 A history of infective endocarditis involving the PDA is rare and is an indication for PDA closure.62 In adults (and children) with PDA, the outcomes of closure have been very good, including in select patients with moderately elevated pulmonary arterial pressure and resistance.63 The closure of most PDAs can be accomplished via catheterization, with minimal morbidity and a high rate of success.58 In most patients, a single venous access alone is required to cross the PDA in an antegrade fashion and deploy a device. In patients with elevated pulmonary resistance, pulmonary bed vasoreactivity to pulmonary vasodilatory agents or reduction in pulmonary arterial pressure and resistance during test occlusion may predict a favorable outcome with device occlusion.58
The currently available devices include coils, which are used for closure of small-sized PDAs, and multiple occlusion devices, including the Amplatzer duct occluder (approved in the United States) and the Occlutech PDA device.58,64, 65, 66, 67 The risks of PDA closure are low and include device embolization, hemolytic anemia due to high-pressure residual shunting across the PDA, vascular access complications, and infection.58,67,68
Creation of shunts
Although many interventions in patients with ACHD are aimed at closing shunts, in some cases, palliation with transcatheter shunt creation can be considered. In patients with severe pulmonary arterial hypertension but low left atrial pressure, creation of an atrial-level shunt may help decompress the right ventricle (RV) (at the expense of lowering systemic saturation) and treat the following symptoms: end-stage right-heart failure and syncope.69 Transcatheter creation of a Potts shunt (from the descending aorta to the left PA) has also been described in select patients with severe pulmonary hypertension as a way to preserve oxygenated flow to the coronaries and cerebral circulation while allowing right-to-left shunting from the PA to the descending aorta.70 Creation of an atrial-level shunt was thought to be a promising therapy in patients with diastolic dysfunction and heart failure with preserved ejection fraction71; however, the results of a recent randomized control trial did not demonstrate a benefit with the creation of an atrial-level shunt over a sham intervention.72 In select patients, creation or enlargement of a VSD via a transcatheter or hybrid technique may be helpful in improving cardiac output, such as in patients with a double-outlet RV and restrictive VSDs.73,74
Valve interventions
Balloon angioplasty of native pulmonary valve stenosis
Pulmonary valve stenosis is fairly common, comprising approximately 7% of all congenital heart defects in some epidemiologic studies.75 Pulmonic stenosis can occur at 3 locations: at the valvular, subvalvular, or supravalvular level. Valvular stenosis is the most amenable to transcatheter intervention. Valvular pulmonic stenosis mainly occurs as an isolated lesion but can also be associated with certain conditions such as tetralogy of Fallot, congenital rubella syndrome, and Noonan syndrome. Patients with pulmonary valve stenosis present with exertional dyspnea and may show signs of right ventricular hypertrophy during transthoracic echocardiography. The indications for intervention for pulmonic stenosis include symptomatic patients with moderate-to-severe pulmonic stenosis (a peak Doppler velocity of >3 m/s and a peak gradient of ≥36 mm Hg during echocardiography) or asymptomatic patients with severe pulmonic stenosis (a peak Doppler velocity of ≥4 m/s, a peak gradient of ≥64 mm Hg, or a mean gradient of ≥35 mm Hg).4
Balloon valvuloplasty is the treatment of choice for isolated pulmonary valve stenosis if the valve is mobile and doming, with a lower chance of success in those with dysplastic (seen in 15% of cases) or calcified valves.76 Compared with surgical valvotomy, transcatheter balloon valvuloplasty results in lower mortality and morbidity and is now considered the standard of care, with surgery relegated to those in whom transcatheter interventions have failed or those with incompatible anatomy. The original catheter-based technique was described by Rubio and Limon-Lason77 in 1956, whereas the currently employed percutaneous static balloon valvuloplasty technique was first reported by Kan et al78 in 1982. The outcomes of the procedure are excellent, with a low risk of complications4,76,79; the use of a balloon-to-annulus ratio of 1.2:1.25 is preferred.80 Postprocedural mild pulmonary regurgitation (PR) is usually well tolerated, and there will be a low rate of restenosis over the next decade,81,82 although approximately 25% of patients at 20-year follow-up will require pulmonary valve replacement. The technique for balloon valvuloplasty has not changed significantly over the years. However, advancements in medical device technology have improved the delivery profile of the balloons used for the intervention and made vascular access site injury less likely.
Pulmonary valve replacement
Transcatheter pulmonary valve replacement (TPVR) is the most commonly performed transcatheter valve procedure in patients with ACHD for the treatment of dysfunction of a native pulmonary valve, a valve within a homograft or RV-PA conduit, or a prosthetic pulmonary valve.83 The largest group of patients with ACHD requiring TPVR are those with repaired tetralogy of Fallot83; patients with a pulmonary atresia-intact ventricular septum, pulmonary stenosis with surgical valvotomy, repaired truncus arteriosus with dysfunctional RV-PA conduits, congenital aortic valve stenosis due to the Ross procedure and subsequent homograft dysfunction, and double-outlet RV after Rastelli procedure can also require TPVR.
In patients with predominant PR, the 2018 American College of Cardiology or American Heart Association guidelines on the management of patients with ACHD recommend consideration of valve replacement in symptomatic patients (dyspnea, chest pain, and/or exercise intolerance referable to PR or otherwise unexplained); asymptomatic patients with evidence of RV or left ventricle (LV) systolic dysfunction, severe RV enlargement (an indexed RV end-diastolic volume of >160 mL/m2 or an indexed RV end-systolic volume of >80 mL/m2), and an RV systolic pressure of more than two-third of systemic pressure; or those with objective, progressive reduction in exercise capacity.4 Patients with sustained tachyarrhythmias and moderate-to-severe PR may also benefit from TPVR.4 In patients with prosthetic or homograft pulmonic stenosis, the guidelines recommend intervention in a fashion similar to that in patients with native pulmonic stenosis.4
In the current era of TPVR (Central Illustration), the most widely used valves in patients with ACHD in the United States are the Melody valve2 (Medtronic Inc) and Edwards Sapien valve84,85 (Edwards Lifesciences). The Melody valve is indicated for the treatment of pulmonary valve annuli with diameters ranging from 16 to 22 mm, whereas the Sapien valve is indicated for the treatment of valve annuli with diameters ranging from 20 to 29 mm. Both the valve platforms are associated with excellent short- and intermediate-term outcomes and are comparable with surgical valves in terms of longevity and the risk of reintervention.83,85, 86, 87
Central Illustration.
Timeline of the evolution of the transcatheter pulmonary valve technologies. CE, European Commission; FDA, Food and Drug Administration; HDE, humanitarian device exemption; TPV, transcatheter pulmonary valve; US, United States.
The acute procedural complications include injury to the tricuspid valve, valve embolization, injury to the PA, coronary compression, or delivery system fracture due to retained equipment in the patient.88 The use of a long sheath (>60 cm) can prevent damage to the tricuspid valve while crossing it using the valve delivery system.89 The long-term complications include valve dysfunction90 and infective endocarditis. Infective endocarditis is a serious concern and may have occurred in up to 10% of patients with dysfunctional homografts being considered for intervention; a history of endocarditis, immunocompromised state, and residual stenosis after TPVR are risk factors for endocarditis following TPVR.91, 92, 93, 94 Stent fracture has been noted to be a problem with the Melody valve, especially when prestenting is not performed within conduits or native right ventricular outflow tracts (RVOTs). Prestenting does not appear to be necessary for bioprosthetic valves for valve-in-valve procedures, given the presence of a metallic or plastic ring, which protects the stent platform from compressive forces.95 The cobalt chromium stent frame of the Sapien valve is significantly more durable, can withstand high compressive forces, and is not prone to fracture; therefore, prestenting prior to Sapien valve implantation does not appear necessary as long as there is no proximal or distal stenosis at the valve location and an adequate valve size for the patient’s body surface area can be placed.88,96,97 Evaluation for coronary arterial compression prior to valve implantation is a critical portion of the procedure because coronary artery compression can occur in up to 5% of patients, especially in those with abnormal coronary anatomy or reimplanted coronary arteries98 and those with stenotic homografts or conduits that will be dilated using a balloon and stented. Coronaries adjacent to bioprosthetic valves are usually at a low risk of compression unless valve fracture is required. Preprocedural computed tomography or MRI can be used to assess the coronary distance to the proposed landing zone of the device (which requires a collaborative review between interventionalists and radiologists) and identify high-risk (<3 mm) and low-risk (>15 mm) anatomies99; most patients are in an intermediate risk zone and require compression testing at the time of the procedure.
Patients with native RVOTs (such as patients with tetralogy of Fallot and those who have undergone transannular patch repair) have a dilated pulmonary valve annulus, and, thus, there is often no landing zone for a balloon-expandable transcatheter pulmonary valve. The treatment of large-diameter (>30 mm), native RVOTs is especially challenging given that the largest, commercially available, balloon-expandable TPVR platforms are the 29-mm Sapien 3 or Ultra valves. Hybrid surgical plication of the PA or the use of a PA band can be considered via sternotomy or thoracotomy in order to establish a “landing zone” for TPVR.100,101 The Venus P valve (Venus Medtech) and the Harmony valve (Medtronic) are self-expanding, covered, hourglass-shaped, RVOT reducer platforms, with the valve in the central waist,102 and the Harmony valve has now been approved by the FDA for use in patients with ACHD with severe PR (Figure 4). Edwards Lifesciences has developed the Alterra self-expanding RVOT reducer (Figure 5), in which the 29-mm Sapien S3 valve is subsequently implanted (during the same procedure or a separate procedure); this system also had excellent early results and has now been approved by the FDA for native or surgically repaired RVOTs with severe PR.103 A detailed analysis of the anatomy of RVOTs using gated cardiac CTA is necessary to establish candidacy and a target landing zone for these devices.
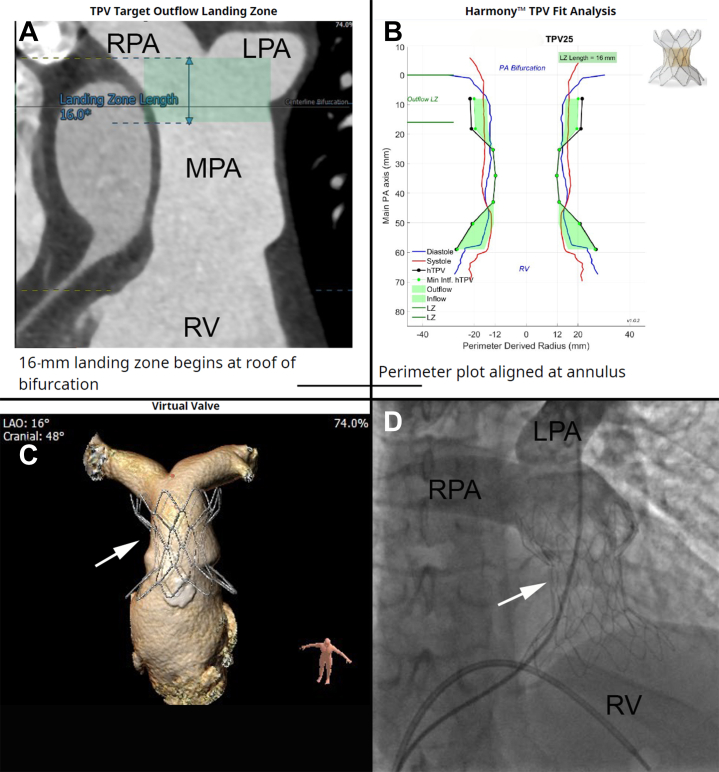
Figure 4.
Harmony valve implantation. (A) Using a preprocedural computed tomography angiogram of the chest, the operator receives an analysis of the patient’s pulmonary artery and right ventricular outflow tract, which can help predict the appropriate location for deployment of the valve. (B) Medtronic uses the computed tomography angiogram data to create a perimeter plot, which helps predicts the potential fit of a Harmony transcatheter pulmonary valve within the pulmonary artery. (C) Three-dimensional rendering of how the valve would fit within the pulmonary artery. (D) Fluoroscopic image of the Harmony valve after deployment. Angiography with a pigtail catheter demonstrates no residual pulmonary valve regurgitation. The arrow indicates the Harmony valve. LPA, left pulmonary artery; LZ, landing zone; MPA, main pulmonary artery; PA, pulmonary artery; RPA, right pulmonary artery; RV, right ventricle; TPV, transcatheter pulmonary valve.
Figure 5.
Alterra prestent and Sapien valve deployment. (A) A lateral projection of a right ventriculogram demonstrating a dilated pulmonary artery that is suitable for an Alterra prestent. (B) Three-dimensional rendering using computed tomography angiogram data simulating placement of the Alterra prestent within the dilated pulmonary artery. (C) A lateral projection of the deployed Alterra prestent in a stable position as well as the Edwards Sapien S3 valve within the Alterra prestent. (D) A computed tomography angiogram of the pulmonary artery in a simulated lateral projection after the prestent and valve have been deployed demonstrating stable position of both the valve within the prestent and the prestent within the pulmonary artery. MPA, main pulmonary artery; RV, right ventricle; S3, Edwards Sapien S3 valve.
Percutaneous repair of native atrioventricular valve regurgitation: Edge-to-edge repair
Severely regurgitant atrioventricular valves can develop in many patients with ACHD as sequelae of their underlying congenital anatomy or as residual from their surgeries. For example, patients with congenitally corrected TGA can develop significant systemic TR as they get older, and the systemic ventricle starts to dilate and fail.104 Patients with unbalanced atrioventricular septal defects and single-ventricle physiology also develop significant atrioventricular valve regurgitation over time.105 For patients with systemic atrioventricular valve regurgitation, the ACHD guidelines4 recommend extrapolation of the American College of Cardiology/American Heart Association guidelines for the management of valvular heart disease for mitral valve regurgitation, which include interventions for severe regurgitation in the presence of symptoms (heart failure, dyspnea, and decreased exercise tolerance), or in asymptomatic patients with evidence of left ventricular dysfunction.106
Although surgery for repairing or replacing severely regurgitant atrioventricular valves is an acceptable strategy, some patients may be at a prohibitively high surgical risk, and percutaneous transcatheter edge-to-edge repair has been successful in this patient population.106, 107, 108 The use of MitraClips (Abbott) has been established as an effective therapy in adult patients with primary and secondary mitral valve regurgitation107,108 but has also been demonstrated to be effective in a select group of patients with CHD, mainly those with systemic atrioventricular valves (dextro-TGA [D-TGA]) and atrial switch or those with congenitally corrected TGA with systemic TR) or a common atrioventricular valve.109,110 Experience with this technology is still limited in the congenital space, and the procedure can be technically challenging, especially given the differences in anatomy and the need for excellent transesophageal echocardiographic images to guide the intervention.111 There have been <25 cases reported in the literature so far.109,112,113 Nevertheless, it is a new technique that has the potential to help many patients with ACHD with severe valvular regurgitation, especially those who are at a prohibitively high risk of surgical repair or replacement. Early feasibility studies of new clip devices, such as the Edwards PASCAL system (Edwards Lifesciences), have also shown promising results.114
Prosthetic atrioventricular valve replacement: Valve-in-valve, valve-in-ring, or novel techniques for the treatment of regurgitant or stenotic valve
Tricuspid valve
Primary tricuspid valve dysfunction in patients with CHD is often associated with Ebstein anomaly or congenital dysplasia, which may result in chronic severe TR.115,116 Secondary TR occurs with progressive RV and tricuspid annular dilation, which is usually due to RV volume or pressure overload. The indications for intervention include regurgitation or stenosis with symptoms of right-heart failure as well as arrhythmias.4
Transcatheter tricuspid valve replacement (TTVR) is feasible, and there is growing evidence for the use of the Melody and Sapien valves in both surgical rings and failing tricuspid valve bioprostheses.116, 117, 118, 119 The procedural success rate is high, and short-term hemodynamic benefits are evident. A registry of 306 patients undergoing TTVR demonstrated a cumulative 3-year incidence of death of 17%, reintervention rate of 12%, and valve-related adverse outcomes (endocarditis, thrombosis, or dysfunction) in 8% of the patients. Eight patients (2.6%) developed valve thrombosis during study follow-up.118 Perivalvular regurgitation is common when TTVR is performed in surgical bands and rings but is mild in most cases and can be frequently managed with a variety of transcatheter occlusion devices.117
Several devices are being developed for transcatheter replacement of native tricuspid valves, such as the EVOQUE valve120 (Edwards Lifesciences). Clinical trials are ongoing to evaluate the efficacy of the device (NCT04482062) in the treatment of functional TR.
Mitral valve
Congenital mitral valve disease results in stenotic, regurgitant, or mixed disease. Stenotic lesions of the mitral valve can occur at the supra-annular, annular, valvular, or subvalvular level, such as in the cor triatriatum, supramitral ring, double-orifice mitral valve, parachute mitral valve, or hammock mitral valve.116 Regurgitant lesions are usually due to issues of the mitral valve apparatus, such as in partial or complete atrioventricular septal defects, myxomatous mitral valve leaflets, or flail or ruptured chordae. Secondary mitral valve regurgitation usually occurs in the setting of progressive annular dilation because of poor ventricular function or cardiomyopathy.
The field of transcatheter mitral valve replacement is still in its early phase. However, many devices in early clinical trials have shown encouraging results, especially in patients who are too high risk of surgery.121,122 Most procedures are performed in the valve-in-valve and valve-in-ring population123 and in those with calcified mitral annuli to allow for anchoring, most commonly using the Melody and Sapien valves.116,123 Preprocedural imaging is important for predicting and evaluating strategies to mitigate the risk of left ventricular outflow tract (LVOT) obstruction by the valve scaffold. The procedure can often be performed via the transseptal approach; TEE is required for procedural imaging guidance.123,124 Although technical procedural success is usually very high (95% of cases), the use of these valves in transcatheter mitral valve replacement has been limited to patients who are at a prohibitively high risk of surgery because the outcomes of transcatheter mitral valve replacement are relatively poor (with a 30-day mortality rate of 8.2%), mainly because of LVOT obstruction.125 Trials for the development of transcatheter valve delivery systems meant specifically for the mitral valve are currently ongoing for both transapical and transseptal approaches.122,124 As the technology improves, widespread adoption of this technology as an alternative to conventional surgery may be the future.
Aortic valve replacement
Aortic stenosis or aortic regurgitation (AR) occurs in many different congenital cardiac conditions and can be due to primary valvular lesions or secondary dysfunction. The spectrum of clinical disease of AS varies from the bicuspid aortic valve causing isolated disease to complex lesions, such as hypoplastic left-heart syndrome, which is associated with mitral valve hypoplasia or atresia in addition to aortic valve stenosis or atresia. The estimated prevalence of congenital AS is between 1.1 to 4.9 per 10,000 live births.12,60 AR can occur in conjunction with AS in patients with degenerative bicuspid aortic valves or in isolation because of secondary aortic root dilation. AR can also occur because of aortic valve prolapse in conjunction with membranous VSDs or subvalvular-aortic stenosis. In patients with a history of aortic valve surgery with an autograft (Ross procedure), homograft, or bioprosthetic valve, all of these valves can degenerate and develop AS and/or AR. Prosthetic aortic valves, especially in patients with ACHD, degenerate more rapidly in young adults than in older patients.126 When patients become symptomatic, develop decreased LV function or objectively decreased exercise tolerance, or have severe AR with increasing LV size, intervention is recommended.106
Transcatheter aortic valve replacement has become a widely used alternative to surgical aortic valve replacement predominantly in patients with calcific aortic valve stenosis, with overall good outcomes.127 The potential complications of TAVR include perivalvular leaks, conduction abnormalities, thromboembolism, stroke, valve embolization, aortic annular rupture, coronary occlusion, renal injury, bleeding, and vascular access injury.128 TAVR in patients with AS due to the bicuspid aortic valve has been shown to be feasible and effective, with favorable valve performance, even in low-risk patients,129 but does have an increased risk of stroke, pacemaker implantation, and perivalvular leaks,130, 131, 132 especially in patients with significantly calcified bicuspid aortic valves.131 For patients with primarily AR, TAVR is generally not recommended with the currently available devices because the lack of leaflet calcification makes valve anchoring difficult; the Jena valve is in clinical trials (NCT04415047).
Transcatheter aortic valve replacement has also been used in patients with D-TGA after arterial switch and valve sparing aortic root repair with recurrent AR116 and in those with existing bioprosthetic aortic valves.123 As technology improves, more and more patients undergoing TAVR are receiving moderate sedation and going home in 1 to 3 days, with >95% of all procedures being performed via the transfemoral approach.128 Nevertheless, given the various anatomic variations and considerations, such as aortic root size, coronary artery anomalies, and aortic valve or annular calcification, a nuanced heart team approach should be used for each congenital cardiac case with AS or AR to evaluate whether TAVR or surgical aortic valve replacement would be the best option for that patient at that point in their life, especially because most patients require several interventions over the course of their lifespan.132
A critical benefit of multidisciplinary team discussion involving ACHD interventionalists with experience in TAVR and surgeons includes surgical planning to increase the chances of successful valve-in-valve TAVR in the future (such as the size of implanted surgical prosthesis and coronary distance), which may also impact the feasibility of valve-in-valve TAVR vs surgical aortic valve replacement as the next procedure. Newer devices for TAVR are constantly under development with the aim to improve deliverability, durability, deployment, and positioning. This will hopefully allow TAVR technology to expand to congenital patients with predominant AR without calcific AS.116
Aortic interventions
Balloon angioplasty and stenting of coarctation of the aorta
Coarctation of the aorta (CoA) is a narrowing of the descending aorta, usually at the insertion point of the ductus arteriosus, just distal to the left subclavian artery. CoA has a prevalence of 2.7 to 4.4 per 10,000 children, accounting for 2% to 5% of all congenital heart defects.12,60 Most cases occur sporadically but are also associated with certain genetic conditions, such as Turner syndrome (in as many as 5%-15% of women with CoA),133 and are often seen in conjunction with other congenital heart defects, with the most common being the bicuspid aortic valve (in 30%-50% of patients with CoA).134 Up to 10% of adults with bicuspid aortic valve and CoA are found to have intracranial aneurysms.135
Depending on the severity of coarctation, patients can present at birth with heart failure and/or shock when the ductus arteriosus starts to close. If the coarctation is not as severe, then patients may be asymptomatic and can present later in childhood or even in adulthood. Careful history taking may reveal chest pain, cold extremities, and claudication with physical exertion. Adults with CoA most often present with hypertension. The natural history of untreated CoA is quite grim, with an average survival of 35 years and a mortality of 75% by 46 years of age.136 Surgical or transcatheter treatment of CoA is recommended if patients have hypertension and a significant gradient across the coarctation (an upper or lower extremity resting catheterization peak-to-peak gradient of >20 mm Hg, a mean echo Doppler gradient of >20 mm Hg, an upper or lower extremity resting peak-to-peak gradient of >10 mm Hg, a mean Doppler gradient of >10 mm Hg in the presence of decreased LV function, AR, or collateral flow).4 When CoA is treated early on prior to the development of significant complications, the long-term survival is excellent, reaching up to 90% at 20 years in a study of patients with childhood cardiac interventions.137
For adult patients with CoA, transcatheter treatment is usually the first line, although a surgical approach may be preferred for hypoplastic aortic arch, genetic conditions such as Turner syndrome and other connective tissue disorders, or other congenital cardiac lesions requiring treatment. Transcatheter balloon angioplasty of aortic coarctation was initially performed in 1982 by Singer et al138 and has been performed for the treatment of discrete native and recurrent narrowing of the descending aorta, with a high immediate success rate; however, aortic wall complications occurred in 10% of patients and reobstruction occurred in 32% of patients.139 Thus, the preferred method of transcatheter treatment of CoA is stent implantation (Figure 6), which was first reported by de Lezo et al140 in 1995. Open- and closed-cell-design stents as well as covered stent platforms are now widely used in preference to balloon angioplasty alone. The immediate results are excellent, with a low risk of complications (<5%) and a low risk of aortic wall injury (3.1%). The intermediate reobstruction rate is 15%; however, the majority of these were only mild restenosis.139 The intermediate results of 1 study demonstrated that 4% of patients with stenting of the CoA underwent an unplanned reintervention because of recurrent coarctation or pseudoaneurysm formation, with an average time to reintervention of 2.84 years, whereas another study found a cumulative aneurysm incidence of 6% at 5 years.9,139 The availability of covered stents (balloon-expandable or self-expanding) platforms may further improve the safety profile of stents, reduce catastrophic aortic wall injury, and allow the treatment of CoA-associated pseudoaneurysms or aneurysms.141,142
Figure 6.
Stenting of a coarctation of the aorta. (A) 3-dimensional rendering using a preprocedural computed tomography angiogram of the chest. There is a tight coarctation of the aorta that appears to be remote from the left subclavian artery. (B) Lateral fluoroscopic projection of an aortogram demonstrating a tortuous, discrete coarctation. (C) Placement of a covered stent across the coarctation with resolution of the waist. (D) Aortogram after stent deployment demonstrating no residual coarctation and no evidence of dissection, hematoma, or rupture of the aorta.
PA interventions
Balloon angioplasty and stenting of PA stenosis
Stenosis of the proximal or branch PAs may occur in isolation but is usually associated with other cardiac lesions such as tetralogy of Fallot, Alagille syndrome, Williams syndrome, Noonan syndrome, or congenital rubella syndrome.143,144 Branch PA stenosis often occurs following surgical shunt placement. The diagnosis of peripheral PA stenosis in adults is rare but is underrecognized as a cause of right ventricular hypertension, progressive dyspnea, and fatigue on exertion.144
The indication for the treatment of PA stenosis is when the lesion results in elevation of right ventricular systolic pressure or reduces perfusion to a lung segment distal to the stenosis.145 Surgical repair of PA stenosis is possible; however, the results are frequently suboptimal, particularly if the lesions are peripheral. Lock et al146 described a percutaneous static balloon angioplasty technique for the treatment of peripheral PA stenosis in 1983. An adequate result depends on the use of sufficiently large high-pressure balloons that tear the vascular intima and a part of the media, leaving a slim safety margin for this procedure.147 Elastic recoil of the PA segment undergoing balloon angioplasty is common, hence prompting the use of stents.145 The success rate of transcatheter intervention for peripheral PA stenosis is increased from 70% with balloon angioplasty alone to 90% with the use of stents.148 In general, the first and second arcade branches of the PAs are usually effectively treated with stents. Diffuse peripheral PA stenosis can be a very difficult problem, for which there is no surgical option (short of lung transplantation). Patients with multiple distal stenoses often require multiple balloon dilations.145 In some patients, thorough and aggressive dilation of these stenoses can lead to lasting and significant improvement of right ventricular pressure and function; however, care must be taken to avoid rupture of the distal PA vasculature. Proximal branch PA stenoses are also difficult to treat because of PA bifurcation. V-stenting or stenting the branch PA through a side strut of the first stent in a modified culotte technique are options for the treatment of the bifurcation.145
There are patients who have had stents placed in their PAs during childhood but have not had further dilation of the stents since and now present with stenosis at the site of stent implantation because of somatic growth. These stents either need to be dilated or intentionally fractured using high-pressure balloon inflation to reach an appropriate adult size.145 In cases of bilateral PA stenoses, lung perfusion scans or cardiac MRI imaging may be helpful to assess the distribution of flow.
Complications with the procedure are uncommon; however, major complications occurred in approximately 9% of all PA stenting procedures in the IMproving Pediatric and Adult Congenital Treatments registry. A patient weight of <4 kg, emergency procedures, and single-ventricle status were significantly associated with the risk of any adverse event, which included vessel rupture, stent embolization, and death.145 Another study reported a complication rate of 3%, with no deaths in their cohort of 183 patients.148
Special populations
Patients with single-ventricle physiology (Fontan procedure)
Univentricular heart defects are rare and account for 1 to 1.5 per 10,000 live births in some studies.12,60 Over the past 3 decades, advances in surgical techniques and refinements in the Fontan operation procedure have led to improved short- and medium-term survival of patients with single-ventricle physiology.149 The overall survival rates at 20 years after the Fontan procedure are as high as 87% in some cohorts. Nevertheless, freedom from late complications of the Fontan procedure is low, and in 1 study, it was observed that 50% of patients with Fontan circulation experienced a complication related to the Fontan circuit over a period of 20 years.149 In order to improve survival and quality of life, many of these adult patients with Fontan circulation undergo catheter-based interventions for the treatment of these complications. We will highlight a few of these interventions and the reasons for these interventions.
Patients with obstructions in the Fontan pathway can have significant symptoms because they lack a subpulmonary ventricle. Their pulmonary blood flow relies on a high systemic venous pressure driving flow through a low-resistance circuit, in addition to respirophasic fluctuations in pressure; any obstruction in this circuit, even with only a low (1-3 mm Hg) gradient, may be hemodynamically significant. Thus, interventions such as balloon angioplasty or stenting of the Fontan circuit, the branch PAs, or residual aortic obstructions such as CoA, are often performed for the treatment of heart failure or complications of a failing Fontan circuit, such as protein-losing enteropathy, plastic bronchitis, or Fontan-associated liver disease. These interventions have been previously described in earlier sections.
In certain patients with Fontan circulation, transseptal punctures are necessary in order to access the pulmonary venous atrium (eg, for an electrophysiologic study or creation of an atrial-level shunt).150,151 Although the techniques for transseptal punctures are varied, the use of TEE or intracardiac echocardiography is important to help with a safe puncture.21
The transcatheter creation of a Fontan fenestration was described as early as 1997 to aid in improving cardiac output and decreasing central venous pressure at the expense of systemic desaturation.152 The technique of using a transseptal puncture needle to cross into the pulmonary venous atrium and the use of serial balloon dilation over a wire to allow for a long sheath to cross for the delivery of a stent has been well established.153 However, successful variations of this technique, such as with the new atrial flow regulator (Occlutech), for the creation of a stable fenestration154 as well as creation of a fenestration from the PA to the pulmonary venous atrium in patients with unusually thick Fontan conduits155 have been described.
Many patients with Fontan circulation eventually develop heart failure and are referred for heart transplantation or even combined heart-liver transplantation. Preoperative hemodynamic catheterization includes the assessment of the Fontan pathway, as described above, evaluation and coiling or embolization of large aortopulmonary or venovenous collaterals that may lead to life-threatening intraoperative bleeding, and, in some cases, transjugular liver biopsy for risk stratification (in addition to noninvasive methods such as hepatic FibroScan). Agitated saline injections in the Fontan circuit and in the branch PAs, with transthoracic echocardiogram during the procedure, are useful for evaluating right-to-left shunts in patients with cyanotic Fontan circulation. The treatment of venovenous collaterals improves cyanosis156 and prevents perioperative bleeding at the time of transplantation but at the expense of increased systemic venous pressure and reduction in systemic ventricular preload. Some studies have shown an increase in long-term mortality after these procedures157; so, the coiling of venovenous collaterals needs to be well timed with the transplant listing. Aortopulmonary collaterals increase pulmonary blood flow but chronically volume load the single ventricle, which may trigger ventricular remodeling and increased end-diastolic pressure. This eventually leads to ventricular dysfunction and failure. Although the practice variation varies across institutions,158 coiling of aortopulmonary collaterals is a very common procedure to relieve overcirculation, treat hemoptysis, and prepare patients for transplantation by reducing their risk of perioperative bleeding.159,160
Percutaneous lymphatic embolization is a novel and specialized technique to identify pathways of lymphatic decompression and occlude these abnormal channels to treat protein-losing enteropathy and plastic bronchitis.161 Only a few centers are capable of specializing in these interventions because they require magnetic resonance lymphangiograms162; however, these procedures can be very effective.
D-TGA with atrial switch (as well as L-TGA with double switch)
There remains a large population of patients with D-TGA who have undergone an atrial-switch operation, with the creation of a baffle redirecting systemic venous return to the subpulmonary ventricle with the use of native atrial tissue (Senning procedure) or a prosthetic patch (Mustard procedure). There is also a population of young adult patients with congenitally corrected TGA who have undergone a double-switch procedure (arterial switch and atrial switch) during early childhood to allow the LV to be the systemic ventricle and prevent complications related to a failing systemic RV.163 Baffle leaks may occur and cause cyanosis or lead to paradoxical emboli due to right-to-left shunting. Baffle leak closure with cribriform septal defect or PFO closure devices is indicated in symptomatic patients or those with right-sided pacing wires, which increase the risk of paradoxical emboli.
Stenosis of the systemic venous baffle limbs is also seen because of progressive fibrosis or thrombosis; in some cases, it is associated with pacing wires. Symptoms may arise with low gradients in the venous circulation, making careful hemodynamic and angiographic assessments particularly important. Balloon dilation followed by stenting can be effective in relieving stenosis. In some cases, a hybrid procedure with removal of pacing wires, stenting, and replacement of the pacing wire is necessary.164 Pulmonary venous baffle obstructions are also seen to occur de novo or following compression due to stenting of a systemic venous baffle limb. Sometimes, stents are required for both systemic venous and pulmonary venous baffles to relieve obstructions in each pathway.165 Three-dimensionally printed models can be made to better understand the complex intracardiac anatomy of these patients and help with planning an intervention.166
Some patients with D-TGA treated with an atrial-switch procedure have LVOT obstructions or proximal PA stenosis. In most cases, a mild or moderate degree of obstruction is hemodynamically advantageous because the elevated subpulmonary left ventricular pressure maintains the interventricular septum in a midline position that leads to a favorable interventricular interaction, a reduced right ventricular size, and less TR. Acute systemic right ventricular dysfunction and worsening of TR after the treatment of LVOT obstructions have been described,167 and, thus, intervention for subpulmonic obstructions in these patients should be reserved for severe or critical stenosis or left ventricular dysfunction.
Pregnant patients
Patients with ACHD have more maternal and fetal morbidity and mortality than women without CHD168; during pregnancy, the cardiac output and intravascular volume increase, which can lead to heart failure as well as atrial and ventricular arrhythmias.169 Because pregnancy leads to increased cardiac output and circulating blood volume, the hemodynamics of existing lesions can change to the point where patients can become symptomatic. Although it is rare for a congenital patient to need a transcatheter intervention during pregnancy, there have been reports of patients needing coarctation stenting170 and ASD closure.171 Should there be a need for a transcatheter intervention during pregnancy, great care should be taken to shield the fetus from any radiation and minimize radiation exposure during the case.169 The use of imaging techniques, such as intracardiac echocardiography and TEE, may be helpful in reducing the amount of fluoroscopy needed for such interventions.
After device implantation, most patients require antiplatelet therapy or anticoagulation. Although aspirin is well tolerated during pregnancy, patients who require additional antiplatelet agents, such as clopidogrel, or anticoagulation may need to alter their medication regimen during pregnancy.172,173 The special considerations may include stopping clopidogrel 7 days prior to delivery to allow for the possibility of epidural or spinal catheter placement for analgesia delivery.173 There is wide practice variation among interventionalists while deciding on postprocedural antiplatelet and/or anticoagulation regimens after an intervention, and, thus, more studies are needed to shed light on this important decision.
Hybrid procedures
Many patients with ACHD are at an increased surgical risk because of a history of multiple sternotomies, collateral formation, lung disease, kyphosis, or poor nutritional status. Because of the limitations of the currently available transcatheter technologies, a hybrid approach is sometimes needed to perform safe interventions in these patients. Hybrid approaches have long been a staple of congenital interventions in the pediatric world, mainly because of the small size of vascular structures in children, which limits the use of vascular access to deliver interventional equipment.174 Hybrid approaches, such as stenting of the ductus arteriosus, concomitant banding of the PAs, closure of VSDs via a right ventricular puncture, or transcatheter valve replacement via ventricular access, have been described.174 In adults with CHD, hybrid approaches have been used to perform transcatheter valve replacement of the pulmonary valve in patients with large RVOTs,175,176 transcatheter mitral valve replacements in those with difficult anatomy for a transseptal approach,177 or even for paravalvular leak occlusion of the mitral valve via transapical access.178,179 Although these procedures sometimes require sternotomy or a smaller chest incision, the use of cardiopulmonary bypass is minimized with hybrid procedures, which leads to reduced blood transfusion requirements compared with those for surgery.175 Nevertheless, hybrid procedures can lead to increased bleeding compared with just transcatheter procedures, and 1 series had a bleeding complication rate of 19% at 30 days.179 However, hybrid procedures can be a feasible option for certain high-risk patients with ACHD who need interventions, especially at centers with a strong working relationship between the interventional cardiologist and cardiothoracic surgeon.180
Conclusion
In summary, the field of transcatheter interventions for patients with ACHD has evolved rapidly over the past 60 years. Patients can now receive therapies for vascular obstructions, valve dysfunction, intracardiac shunts, and lymphatic problems via nonsurgical and transcatheter techniques. As these patients grow older and develop more sequelae of their residual defects, more patients will need transcatheter interventions, and the field will continue to grow with them. The risks and benefits of each procedure must be weighed in the context of the patient’s surgical journey, the likelihood of requiring future procedures, stage of life, and patient preference. Multidisciplinary team evaluation, including clinical ACHD, pediatric cardiology, anesthesia, surgery, and advanced cardiac imaging, is critical for preprocedural planning. The field has blossomed in partnership with colleagues in pediatric interventional cardiology on the one hand and adult structural heart disease interventions on the other. Building the unique expertise required to care for adults with CHD requires focused training, continuous research and quality improvement, and lifelong learning supported by a nationwide and international community of experts. Continued collaboration among adult congenital interventionalists, pediatric interventional cardiologists, and congenital heart surgeons is paramount for a successful ACHD program.
Acknowledgments
Declaration of competing interest
Dr Horlick reports a relationship with Abbott Cardiovascular Structural Heart Division that includes consulting or advisory, funding grants, and speaking and lecture fees; Medtronic that includes funding grants; and Edwards Lifesciences Corp that includes funding grants. Dr Aboulhosn reports a relationship with Edwards Lifesciences Corp that includes consulting or advisory, funding grants, speaking and lecture fees, and travel reimbursement; Medtronic that includes consulting or advisory, speaking and lecture fees, and travel reimbursement; and Abbott Cardiovascular Structural Heart Division that includes consulting or advisory, speaking and lecture fees, and travel reimbursement. He is an Editorial Board member of Adult Congenital Heart Association. Drs Tan and Schmidt reported no financial interests.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement
The research reported has adhered to the relevant ethical guidelines.
References
- 1.Bonhoeffer P., Boudjemline Y., Saliba Z., et al. Transcatheter implantation of a bovine valve in pulmonary position: a lamb study. Circulation. 2000;102(7):813–816. doi: 10.1161/01.cir.102.7.813. [DOI] [PubMed] [Google Scholar]
- 2.Bonhoeffer P., Boudjemline Y., Qureshi S.A., et al. Percutaneous insertion of the pulmonary valve. J Am Coll Cardiol. 2002;39(10):1664–1669. doi: 10.1016/S0735-1097(02)01822-3. [DOI] [PubMed] [Google Scholar]
- 3.Lui G.K., Saidi A., Bhatt A.B., et al. Diagnosis and management of noncardiac complications in adults with congenital heart disease: a scientific statement from the American Heart Association. Circulation. 2017;136(20):e348–e392. doi: 10.1161/CIR.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 4.Stout K.K., Daniels C.J., Aboulhosn J.A., et al. AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. J Am Coll Cardiol. 2019;73(12):e81–e192. doi: 10.1016/j.jacc.2018.08.1029. [DOI] [PubMed] [Google Scholar]
- 5.Aboulhosn J.A., Hijazi Z.M., Kavinsky C.J., et al. SCAI position statement on adult congenital cardiac interventional training, competencies and organizational recommendations. Catheter Cardiovasc Interv. 2020;96(3):643–650. doi: 10.1002/ccd.28885. http://www.ncbi.nlm.nih.gov/pubmed/32272495 [DOI] [PubMed] [Google Scholar]
- 6.Frazer J.R., Ing F.F. Stenting of stenotic or occluded iliofemoral veins, superior and inferior vena cavae in children with congenital heart disease: acute results and intermediate follow up. Catheter Cardiovasc Interv. 2009;73(2):181–188. doi: 10.1002/ccd.21790. [DOI] [PubMed] [Google Scholar]
- 7.Katz D.A., Lubert A.M., Gao Z., et al. Comparison of creatinine and cystatin C estimation of glomerular filtration rate in the Fontan circulation. Int J Cardiol Congenit Hear Dis. 2021;6:100286–100287. doi: 10.1016/j.ijcchd.2021.100286. [DOI] [Google Scholar]
- 8.Martin G.R., Beekman R.H., Ing F.F., et al. The IMPACT registryTM: improving pediatric and adult congenital treatments. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010;13(1):20–25. doi: 10.1053/j.pcsu.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Holzer R.J., Gauvreau K., McEnaney K., Watanabe H., Ringel R. Long-term outcomes of the coarctation of the aorta stent trials. Circ Cardiovasc Interv. 2021;14(6):e010308–e010309. doi: 10.1161/CIRCINTERVENTIONS.120.010308. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein B.H., Bergersen L., Armstrong A.K., et al. Adverse events, radiation exposure, and reinterventions following transcatheter pulmonary valve replacement. J Am Coll Cardiol. 2020;75(4):363–376. doi: 10.1016/j.jacc.2019.11.042. [DOI] [PubMed] [Google Scholar]
- 11.Van Der Linde D., Konings E.E., Slager M.A., et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(21):2241–2247. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Marelli A.J., Ionescu-Ittu R., Mackie A.S., Guo L., Dendukuri N., Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130(9):749–756. doi: 10.1161/CIRCULATIONAHA.113.008396. [DOI] [PubMed] [Google Scholar]
- 13.Rostad H., Sörland S. A trial septal defect of secundum type in patients under 40 years of age: a review of 481 operated cases. Symptoms, signs, treatment and early results. Scand J Thorac Cardiovasc Surg. 1979;13(2):123–127. doi: 10.3109/14017437909100977. [DOI] [PubMed] [Google Scholar]
- 14.Bradley E.A., Ammash N., Martinez S.C., et al. “Treat-to-close”: non-repairable ASD-PAH in the adult: results from the North American ASD-PAH (NAAP) Multicenter Registry. Int J Cardiol. 2019;291:127–133. doi: 10.1016/j.ijcard.2019.03.056. [DOI] [PubMed] [Google Scholar]
- 15.Yan C., Pan X., Wan L., et al. Combination of F-ASO and targeted medical therapy in patients with secundum ASD and severe PAH. JACC Cardiovasc Interv. 2020;13(17):2024–2034. doi: 10.1016/j.jcin.2020.04.027. [DOI] [PubMed] [Google Scholar]
- 16.Du Z.D., Hijazi Z.M., Kleinman C.S., Silverman N.H., Larntz K., Amplatzer Investigators Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol. 2002;39(11):1836–1844. doi: 10.1016/S0735-1097(02)01862-4. [DOI] [PubMed] [Google Scholar]
- 17.Silvestry F.E., Cohen M.S., Armsby L.B., et al. Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen ovale: from the American Society of Echocardiography and Society for Cardiac Angiography and Interventions. J Am Soc Echocardiogr. 2015;28(8):910–958. doi: 10.1016/j.echo.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Silvestry F.E., Kerber R.E., Brook M.M., et al. Echocardiography-guided interventions. J Am Soc Echocardiogr. 2009;22(3):213–231. doi: 10.1016/j.echo.2008.12.013. quiz 316-7. [DOI] [PubMed] [Google Scholar]
- 19.Hascoët S., Warin-Fresse K., Baruteau A.E., et al. Cardiac imaging of congenital heart diseases during interventional procedures continues to evolve: pros and cons of the main techniques. Arch Cardiovasc Dis. 2016;109(2):128–142. doi: 10.1016/j.acvd.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Rigatelli G. Expanding the use of intracardiac echocardiography in congenital heart disease catheter-based interventions. J Am Soc Echocardiogr. 2005;18(11):1230–1231. doi: 10.1016/j.echo.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Basman C., Parmar Y.J., Kronzon I. Intracardiac echocardiography for structural heart and electrophysiological interventions. Curr Cardiol Rep. 2017;19(10):102–103. doi: 10.1007/s11886-017-0902-6. [DOI] [PubMed] [Google Scholar]
- 22.Sommer R.J., Love B.A., Paolillo J.A., et al. ASSURED clinical study: new GORE® CARDIOFORM ASD occluder for transcatheter closure of atrial septal defect. Catheter Cardiovasc Interv. 2020;95(7):1285–1295. doi: 10.1002/ccd.28728. [DOI] [PubMed] [Google Scholar]
- 23.Turner D.R., Owada C.Y., Sang C.J., Khan M., Lim D.S. Closure of secundum atrial septal defects with the AMPLATZER septal occluder: a prospective, multicenter, post-approval study. Circ Cardiovasc Interv. 2017;10(8):1–7. doi: 10.1161/CIRCINTERVENTIONS.116.004212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar P., Orford J.L., Tobis J.M. Two cases of pericardial tamponade due to nitinol wire fracture of a gore septal occluder. Catheter Cardiovasc Interv. 2020;96(1):219–224. doi: 10.1002/ccd.28596. [DOI] [PubMed] [Google Scholar]
- 25.Amin Z., Hijazi Z.M., Bass J.L., Cheatham J.P., Hellenbrand W.E., Kleinman C.S. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: review of registry of complications and recommendations to minimize future risk. Catheter Cardiovasc Interv. 2004;63(4):496–502. doi: 10.1002/ccd.20211. [DOI] [PubMed] [Google Scholar]
- 26.Masura J., Gavora P., Podnar T. Long-term outcome of transcatheter secundum-type atrial septal defect closure using Amplatzer septal occluders. J Am Coll Cardiol. 2005;45(4):505–507. doi: 10.1016/j.jacc.2004.10.066. [DOI] [PubMed] [Google Scholar]
- 27.Ermis P., Franklin W., Mulukutla V., Parekh D., Ing F. Left ventricular hemodynamic changes and clinical outcomes after transcatheter atrial septal defect closure in adults. Congenit Heart Dis. 2015;10(2):E48–E53. doi: 10.1111/chd.12204. [DOI] [PubMed] [Google Scholar]
- 28.Abdelkarim A., Levi D.S., Tran B., Ghobrial J., Aboulhosn J. Fenestrated transcatheter ASD closure in adults with diastolic dysfunction and/or pulmonary hypertension: case series and review of the literature. Congenit Heart Dis. 2016;11(6):663–671. doi: 10.1111/chd.12367. [DOI] [PubMed] [Google Scholar]
- 29.Kent D.M., Ruthazer R., Weimar C., et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 2013;81(7):619–625. doi: 10.1212/WNL.0b013e3182a08d59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saver J.L., Carroll J.D., Thaler D.E., et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377(11):1022–1032. doi: 10.1056/NEJMoa1610057. [DOI] [PubMed] [Google Scholar]
- 31.Mas J.L., Derumeaux G., Guillon B., et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377(11):1011–1021. doi: 10.1056/NEJMoa1705915. [DOI] [PubMed] [Google Scholar]
- 32.Søndergaard L., Kasner S.E., Rhodes J.F., et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377(11):1033–1042. doi: 10.1056/NEJMoa1707404. [DOI] [PubMed] [Google Scholar]
- 33.Blanche C., Noble S., Roffi M., et al. Platypnea–orthodeoxia syndrome in the elderly treated by percutaneous patent foramen ovale closure: a case series and literature review. Eur J Intern Med. 2013;24(8):813–817. doi: 10.1016/j.ejim.2013.08.698. [DOI] [PubMed] [Google Scholar]
- 34.Zuberi S.A., Liu S., Tam J.W., Hussain F., Maguire D., Kass M. Partial PFO closure for persistent hypoxemia in a patient with Ebstein anomaly. Case Rep Cardiol. 2015;2015:531382–531383. doi: 10.1155/2015/531382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kavinsky C.J., Szerlip M., Goldsweig A.M., et al. SCAI guidelines for the management of patent foramen ovale. J Soc CardioVasc Angiogr Interv. 2022;1(4):100039–100040. [Google Scholar]
- 36.Furlan A.J. Brief history of patent foramen ovale and stroke. Stroke. 2015;46(2):e35–e37. doi: 10.1161/STROKEAHA.114.007772. [DOI] [PubMed] [Google Scholar]
- 37.Kent D.M., Saver J.L., Kasner S.E., et al. Heterogeneity of treatment effects in an analysis of pooled individual patient data from randomized trials of device closure of patent foramen ovale after stroke. JAMA. 2021;326(22):2277–2286. doi: 10.1001/jama.2021.20956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah R., Nayyar M., Jovin I.S., et al. Device closure versus medical therapy alone for patent foramen ovale in patients with cryptogenic stroke: a systematic review and meta-analysis. Ann Intern Med. 2018;168(5):335–342. doi: 10.7326/M17-2679. [DOI] [PubMed] [Google Scholar]
- 39.De Rosa S., Sievert H., Sabatino J., Polimeni A., Sorrentino S., Indolfi C. Percutaneous closure versus medical treatment in stroke patients with patent foramen ovale: a systematic review and meta-analysis. Ann Intern Med. 2018;168(5):343–350. doi: 10.7326/M17-3033. [DOI] [PubMed] [Google Scholar]
- 40.Chaudhry-Waterman N., Shapiro S., Thompson J. Use of the NobleStitchTM EL for the treatment of patients with residual right-to-left shunt following device closure of PFO. Clin Case Rep. 2021;9(4):1929–1932. doi: 10.1002/ccr3.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jost C.H., Connolly H.M., Danielson G.K., et al. Sinus venosus atrial septal defect: long-term postoperative outcome for 115 patients. Circulation. 2005;112(13):1953–1958. doi: 10.1161/CIRCULATIONAHA.104.493775. [DOI] [PubMed] [Google Scholar]
- 42.Li J., Al Zaghal A.M., Anderson R.H. The nature of the superior sinus venosus defect. Clin Anat. 1998;11(5):349–352. doi: 10.1002/(SICI)1098-2353(1998)11:5<349::AID-CA11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 43.Riahi M., Velasco Forte M.N., Byrne N., et al. Early experience of transcatheter correction of superior sinus venosus atrial septal defect with partial anomalous pulmonary venous drainage. EuroIntervention. 2018;14(8):868–876. doi: 10.4244/EIJ-D-18-00304. [DOI] [PubMed] [Google Scholar]
- 44.Stewart R.D., Bailliard F., Kelle A.M., Backer C.L., Young L., Mavroudis C. Evolving surgical strategy for sinus venosus atrial septal defect: effect on sinus node function and late venous obstruction. Ann Thorac Surg. 2007;84(5):1651–1655. doi: 10.1016/j.athoracsur.2007.04.130. [DOI] [PubMed] [Google Scholar]
- 45.Thakkar A.N., Chinnadurai P., Breinholt J.P., Lin C.H. Transcatheter closure of a sinus venosus atrial septal defect using 3D printing and image fusion guidance. Catheter Cardiovasc Interv. 2018;92(2):353–357. doi: 10.1002/ccd.27645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garg G., Tyagi H., Radha A.S. Transcatheter closure of sinus venosus atrial septal defect with anomalous drainage of right upper pulmonary vein into superior vena cava—an innovative technique. Catheter Cardiovasc Interv. 2014;84(3):473–477. doi: 10.1002/ccd.25502. [DOI] [PubMed] [Google Scholar]
- 47.Abdullah H.A., Alsalkhi H.A., Khalid K.A. Transcatheter closure of sinus venosus atrial septal defect with anomalous pulmonary venous drainage: innovative technique with long-term follow-up. Catheter Cardiovasc Interv. 2020;95(4):743–747. doi: 10.1002/ccd.28364. [DOI] [PubMed] [Google Scholar]
- 48.Hansen J.H., Duong P., Jivanji S.G., et al. Transcatheter correction of superior sinus venosus atrial septal defects as an alternative to surgical treatment. J Am Coll Cardiol. 2020;75(11):1266–1278. doi: 10.1016/j.jacc.2019.12.070. [DOI] [PubMed] [Google Scholar]
- 49.Morray B.H. Ventricular septal defect closure devices, techniques, and outcomes. Interv Cardiol Clin. 2019;8(1):1–10. doi: 10.1016/j.iccl.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Hoffman J.I.E., Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–1900. doi: 10.1016/S0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 51.Diller G.P., Alonso-Gonzalez R., Dimopoulos K., et al. Disease targeting therapies in patients with Eisenmenger syndrome: response to treatment and long-term efficiency. Int J Cardiol. 2013;167(3):840–847. doi: 10.1016/j.ijcard.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 52.Butera G., Carminati M., Chessa M., et al. Transcatheter closure of perimembranous ventricular septal defects: early and long-term results. J Am Coll Cardiol. 2007;50(12):1189–1195. doi: 10.1016/j.jacc.2007.03.068. [DOI] [PubMed] [Google Scholar]
- 53.Holzer R., de Giovanni J., Walsh K.P., et al. Transcatheter closure of perimembranous ventricular septal defects using the Amplatzer membranous VSD occluder: immediate and midterm results of an international registry. Catheter Cardiovasc Interv. 2006;68(4):620–628. doi: 10.1002/ccd.20659. [DOI] [PubMed] [Google Scholar]
- 54.Holzer R., Balzer D., Cao Q.L., Lock K., Hijazi Z.M. Amplatzer Muscular Ventricular Septal Defect Investigators. Catheter interventions for congenital disease device closure of muscular ventricular septal defects using the amplatzer muscular ventricular septal defect occluder. J Am Coll Cardiol. 2004;43(7):1257–1263. doi: 10.1016/j.jacc.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 55.Holzer R., Balzer D., Amin Z., et al. Transcatheter closure of postinfarction ventricular septal defects using the new amplatzer muscular VSD occluder: results of a U.S. registry. Catheter Cardiovasc Interv. 2004;61(2):196–201. doi: 10.1002/ccd.10784. [DOI] [PubMed] [Google Scholar]
- 56.Pedra C.A., Pedra S.R., Esteves C.A., et al. Percutaneous closure of perimembranous ventricular septal defects with the Amplatzer device: technical and morphological considerations. Catheter Cardiovasc Interv. 2004;61(3):403–410. doi: 10.1002/ccd.10797. [DOI] [PubMed] [Google Scholar]
- 57.Krichenko A., Benson L.N., Burrows P., Möes C.A., McLaughlin P., Freedom R.M. Angiographic classification of the isolated, persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am J Cardiol. 1989;63(12):877–880. doi: 10.1016/0002-9149(89)90064-7. [DOI] [PubMed] [Google Scholar]
- 58.Schneider D.J., Moore J.W. Patent ductus arteriosus. Circulation. 2006;114(17):1873–1882. doi: 10.1161/CIRCULATIONAHA.105.592063. [DOI] [PubMed] [Google Scholar]
- 59.Philip R., Waller B.R., Agrawal V., et al. Morphologic characterization of the patent ductus arteriosus in the premature infant and the choice of transcatheter occlusion device. Catheter Cardiovasc Interv. 2016;87(2):310–317. doi: 10.1002/ccd.26287. [DOI] [PubMed] [Google Scholar]
- 60.Reller M.D., Strickland M.J., Riehle-Colarusso T., Mahle W.T., Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998-2005. J Pediatr. 2008;153(6):807–813. doi: 10.1016/j.jpeds.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lewis T.R., Shelton E.L., Van Driest S.L., Kannankeril P.J., Reese J. Genetics of the patent ductus arteriosus (PDA) and pharmacogenetics of PDA treatment. Semin Fetal Neonatal Med. 2018;23(4):232–238. doi: 10.1016/j.siny.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thilén U., Aström-Olsson K. Does the risk of infective endarteritis justify routine patent ductus arteriosus closure? Eur Heart J. 1997;18(3):503–506. doi: 10.1093/oxfordjournals.eurheartj.a015272. [DOI] [PubMed] [Google Scholar]
- 63.Pas D., Missault L., Hollanders G., Suys B., De Wolf D. Persistent ductus arteriosus in the adult: clinical features and experience with percutaneous closure. Acta Cardiol. 2002;57(4):275–278. doi: 10.2143/AC.57.4.2005426. [DOI] [PubMed] [Google Scholar]
- 64.Pass R.H., Hijazi Z., Hsu D.T., Lewis V., Hellenbrand W.E. Multicenter USA Amplatzer patent ductus arteriosus occlusion device trial: initial and one-year results. J Am Coll Cardiol. 2004;44(3):513–519. doi: 10.1016/j.jacc.2004.03.074. [DOI] [PubMed] [Google Scholar]
- 65.Masura J., Gavora P., Podnar T. Transcatheter occlusion of patent ductus arteriosus using a new angled Amplatzer duct occluder: initial clinical experience. Catheter Cardiovasc Interv. 2003;58(2):261–267. doi: 10.1002/ccd.10413. [DOI] [PubMed] [Google Scholar]
- 66.Eicken A., Balling G., Gildein H.P., Genz T., Kaemmerer H., Hess J. Transcatheter closure of a non-restrictive patent ductus arteriosus with an Amplatzer muscular ventricular septal defect occluder. Int J Cardiol. 2007;117(1):e40–e42. doi: 10.1016/j.ijcard.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 67.Spies C., Ujivari F., Schräder R. Transcatheter closure of a 22 mm patent ductus arteriosus with an Amplatzer atrial septal occluder. Catheter Cardiovasc Interv. 2005;64(3):352–355. doi: 10.1002/ccd.20283. [DOI] [PubMed] [Google Scholar]
- 68.Wilson W.M., Shah A., Osten M.D., et al. Clinical outcomes after percutaneous patent ductus arteriosus closure in adults. Can J Cardiol. 2020;36(6):837–843. doi: 10.1016/j.cjca.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 69.Bauer A., Khalil M., Schmidt D., et al. Creation of a restrictive atrial communication in pulmonary arterial hypertension (PAH): effective palliation of syncope and end-stage heart failure. Pulm Circ. 2018;8(2) doi: 10.1177/2045894018776518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Esch J.J., Shah P.B., Cockrill B.A., et al. Transcatheter Potts shunt creation in patients with severe pulmonary arterial hypertension: initial clinical experience. J Heart Lung Transplant. 2013;32(4):381–387. doi: 10.1016/j.healun.2013.01.1049. [DOI] [PubMed] [Google Scholar]
- 71.Feldman T., Komtebedde J., Burkhoff D., et al. Transcatheter interatrial shunt device for the treatment of heart failure: rationale and Design of the Randomized Trial to REDUCE Elevated Left Atrial Pressure in Heart Failure (REDUCE LAP-HF I) Circ Heart Fail. 2016;9(7) doi: 10.1161/CIRCHEARTFAILURE.116.003025. [DOI] [PubMed] [Google Scholar]
- 72.Shah S.J., Borlaug B.A., Chung E.S., et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomised, multicentre, blinded, sham-controlled trial. Lancet. 2022;399(10330):1130–1140. doi: 10.1016/S0140-6736(22)00016-2. [DOI] [PubMed] [Google Scholar]
- 73.Lin C.H., Huddleston C., Balzer D.T. Transcatheter ventricular septal defect (VSD) creation for restrictive VSD in double-outlet right ventricle. Pediatr Cardiol. 2013;34(3):743–747. doi: 10.1007/s00246-012-0337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel N.D., Justino H., Ing F.F. Hybrid approach to ventricular septal defect enlargement. Catheter Cardiovasc Interv. 2019;94(5):732–737. doi: 10.1002/ccd.28227. [DOI] [PubMed] [Google Scholar]
- 75.Stephensen S.S., Sigfusson G., Eiriksson H., et al. Congenital cardiac malformations in Iceland from 1990 through 1999. Cardiol Young. 2004;14(4):396–401. doi: 10.1017/S1047951104004081. [DOI] [PubMed] [Google Scholar]
- 76.McCrindle B.W. Independent predictors of long-term results after balloon pulmonary valvuloplasty. Valvuloplasty and Angioplasty of Congenital Anomalies (VACA) Registry Investigators. Circulation. 1994;89(4):1751–1759. doi: 10.1161/01.cir.89.4.1751. [DOI] [PubMed] [Google Scholar]
- 77.Rubio V, Limon-Lason R. Treatment of pulmonary valvular stenosis and tricuspid stenosis using a modified catheter. Paper presented at: Second World Conference on Cardiology; 1954 September (Vol. 2, pp. 205-210); Washington, District of Columbia.
- 78.Kan J.S., White R.I., Mitchell S.E., Gardner T.J. Percutaneous balloon valvuloplasty: a new method for treating congenital pulmonary-valve stenosis. N Engl J Med. 1982;307(9):540–542. doi: 10.1056/NEJM198208263070907. [DOI] [PubMed] [Google Scholar]
- 79.Stanger P., Cassidy S.C., Girod D.A., Kan J.S., Lababidi Z., Shapiro S.R. Balloon pulmonary valvuloplasty: results of the valvuloplasty and angioplasty of Congenital Anomalies Registry. Am J Cardiol. 1990;65(11):775–783. doi: 10.1016/0002-9149(90)91387-l. [DOI] [PubMed] [Google Scholar]
- 80.Rao P.S. Percutaneous balloon pulmonary valvuloplasty: state of the art. Catheter Cardiovasc Interv. 2007;69(5):747–763. doi: 10.1002/ccd.20982. http://www.ncbi.nlm.nih.gov/pubmed/17330270 [DOI] [PubMed] [Google Scholar]
- 81.Chen C.R., Cheng T.O., Huang T., et al. Percutaneous balloon valvuloplasty for pulmonic stenosis in adolescents and adults. N Engl J Med. 1996;335(1):21–25. doi: 10.1056/NEJM199607043350104. [DOI] [PubMed] [Google Scholar]
- 82.Rao P.S., Galal O., Patnana M., Buck S.H., Wilson A.D. Results of three to 10 year follow up of balloon dilatation of the pulmonary valve. Heart. 1998;80(6):591–595. doi: 10.1136/hrt.80.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sinha S., Aboulhosn J., Asnes J., et al. Initial results from the off-label use of the SAPIEN S3 valve for percutaneous transcatheter pulmonary valve replacement: a multi-institutional experience. Catheter Cardiovasc Interv. 2019;93(3):455–463. doi: 10.1002/ccd.27973. [DOI] [PubMed] [Google Scholar]
- 84.Wilson W.M., Benson L.N., Osten M.D., Shah A., Horlick E.M. Transcatheter pulmonary valve replacement with the Edwards Sapien system: the Toronto experience. JACC Cardiovasc Intv. 2015;8(14):1819–1827. doi: 10.1016/j.jcin.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 85.Kenny D., Rhodes J.F., Fleming G.A., et al. 3-year outcomes of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position from the COMPASSION multicenter clinical trial. JACC Cardiovasc Intv. 2018;11(19):1920–1929. doi: 10.1016/j.jcin.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 86.Cheatham J.P., Hellenbrand W.E., Zahn E.M., et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131(22):1960–1970. doi: 10.1161/CIRCULATIONAHA.114.013588. [DOI] [PubMed] [Google Scholar]
- 87.McElhinney D.B., Zhang Y., Levi D.S., et al. Reintervention and survival after transcatheter pulmonary valve replacement. J Am Coll Cardiol. 2022;79(1):18–32. doi: 10.1016/j.jacc.2021.10.031. [DOI] [PubMed] [Google Scholar]
- 88.Shahanavaz S., Zahn E.M., Levi D.S., et al. Transcatheter pulmonary valve replacement with the Sapien prosthesis. J Am Coll Cardiol. 2020;76(24):2847–2858. doi: 10.1016/j.jacc.2020.10.041. [DOI] [PubMed] [Google Scholar]
- 89.Fukuda T., Tan W., Sadeghi S., et al. Utility of the long DrySeal sheath in facilitating transcatheter pulmonary valve implantation with the Edwards Sapien 3 valve. Catheter Cardiovasc Interv. 2020;96(6):E646–E652. doi: 10.1002/ccd.28776. [DOI] [PubMed] [Google Scholar]
- 90.Egbe A.C., Connolly H.M., Miranda W.R., Dearani J.A., Schaff H.V. Outcomes of bioprosthetic valves in the pulmonary position in adults with congenital heart disease. Ann Thorac Surg. 2019;108(5):1410–1415. doi: 10.1016/j.athoracsur.2019.05.068. [DOI] [PubMed] [Google Scholar]
- 91.Lluri G., Levi D.S., Miller E., et al. Incidence and outcome of infective endocarditis following percutaneous versus surgical pulmonary valve replacement. Catheter Cardiovasc Interv. 2018;91(2):277–284. doi: 10.1002/ccd.27312. [DOI] [PubMed] [Google Scholar]
- 92.McElhinney D.B., Benson L.N., Eicken A., Kreutzer J., Padera R.F., Zahn E.M. Infective endocarditis after transcatheter pulmonary valve replacement using the melody valve: combined results of 3 prospective North American and European studies. Circ Cardiovasc Interv. 2013;6(3):292–300. doi: 10.1161/CIRCINTERVENTIONS.112.000087. [DOI] [PubMed] [Google Scholar]
- 93.McElhinney D.B., Sondergaard L., Armstrong A.K., et al. Endocarditis after transcatheter pulmonary valve replacement. J Am Coll Cardiol. 2018;72(22):2717–2728. doi: 10.1016/j.jacc.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 94.Sadeghi S., Wadia S., Lluri G., et al. Risk factors for infective endocarditis following transcatheter pulmonary valve replacement in patients with congenital heart disease. Catheter Cardiovasc Interv. 2019;94(4):625–635. doi: 10.1002/ccd.28474. [DOI] [PubMed] [Google Scholar]
- 95.McElhinney D.B., Cheatham J.P., Jones T.K., et al. Stent fracture, valve dysfunction, and right ventricular outflow tract reintervention after transcatheter pulmonary valve implantation: patient-related and procedural risk factors in the US melody valve trial. Circ Cardiovasc Interv. 2011;4(6):602–614. doi: 10.1161/CIRCINTERVENTIONS.111.965616. [DOI] [PubMed] [Google Scholar]
- 96.Ghobrial J., Levi D.S., Aboulhosn J. Native right ventricular outflow tract transcatheter pulmonary valve replacement without pre-stenting. JACC Cardiovasc Intv. 2018;11(6):e41–e44. doi: 10.1016/j.jcin.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 97.Morgan G.J., Sadeghi S., Salem M.M., et al. SAPIEN valve for percutaneous transcatheter pulmonary valve replacement without “pre-stenting”: a multi-institutional experience. Catheter Cardiovasc Interv. 2019;93(2):324–329. doi: 10.1002/ccd.27932. [DOI] [PubMed] [Google Scholar]
- 98.Morray B.H., McElhinney D.B., Cheatham J.P., et al. Risk of coronary artery compression among patients referred for transcatheter pulmonary valve implantation: a multicenter experience. Circ Cardiovasc Interv. 2013;6(5):535–542. doi: 10.1161/CIRCINTERVENTIONS.113.000202. [DOI] [PubMed] [Google Scholar]
- 99.Rinaldi E., Sadeghi S., Rajpal S., et al. Utility of CT Angiography for the prediction of coronary artery compression in patients undergoing transcatheter pulmonary valve replacement. World J Pediatr Congenit Heart Surg. 2020;11(3):295–303. doi: 10.1177/2150135120905670. [DOI] [PubMed] [Google Scholar]
- 100.Suleiman T., Kavinsky C.J., Skerritt C., Kenny D., Ilbawi M.N., Caputo M. Recent development in pulmonary valve replacement after tetralogy of Fallot repair: the emergence of hybrid approaches. Front Surg. 2015;2:22–23. doi: 10.3389/fsurg.2015.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Travelli F.C., Herrington C.S., Ing F.F. A novel hybrid technique for transcatheter pulmonary valve implantation within a dilated native right ventricular outflow tract. J Thorac Cardiovasc Surg. 2014;148(2):e145–e146. doi: 10.1016/j.jtcvs.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 102.Benson L.N., Gillespie M.J., Bergersen L., et al. Three-year outcomes from the harmony native outflow tract early feasibility study. Circ Cardiovasc Interv. 2020;13(1):e008320–e008321. doi: 10.1161/CIRCINTERVENTIONS.119.008320. [DOI] [PubMed] [Google Scholar]
- 103.Shahanavaz S., Balzer D., Babaliaros V., et al. Alterra adaptive prestent and SAPIEN 3 THV for congenital pulmonic valve dysfunction: an early feasibility study. JACC Cardiovasc Intv. 2020;13(21):2510–2524. doi: 10.1016/j.jcin.2020.06.039. [DOI] [PubMed] [Google Scholar]
- 104.Franzen O., von Samson P., Dodge-Khatami A., Geffert G., Baldus S. Percutaneous edge-to-edge repair of tricuspid regurgitation in congenitally corrected transposition of the great arteries. Congenit Heart Dis. 2011;6(1):57–59. doi: 10.1111/j.1747-0803.2010.00428.x. [DOI] [PubMed] [Google Scholar]
- 105.Buratto E., Ye X.T., King G., et al. Long-term outcomes of single-ventricle palliation for unbalanced atrioventricular septal defects: Fontan survivors do better than previously thought. J Thorac Cardiovasc Surg. 2017;153(2):430–438. doi: 10.1016/j.jtcvs.2016.09.051. [DOI] [PubMed] [Google Scholar]
- 106.Otto C.M., Nishimura R.A., Bonow R.O., et al. ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77(4):450–500. doi: 10.1016/j.jacc.2020.11.035. [DOI] [PubMed] [Google Scholar]
- 107.Feldman T., Foster E., Glower D.D., et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364(15):1395–1406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 108.Stone G.W., Lindenfeld J.A., Abraham W.T., et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379(24):2307–2318. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- 109.Alshawabkeh L., Mahmud E., Reeves R. Percutaneous mitral valve repair in adults with congenital heart disease: report of the first case-series. Catheter Cardiovasc Interv. 2021;97(3):542–548. doi: 10.1002/ccd.29238. [DOI] [PubMed] [Google Scholar]
- 110.Tan W., Calfon Press M., Lluri G., Aboulhosn J. Percutaneous edge-to-edge repair for common atrioventricular valve regurgitation in a patient with heterotaxy syndrome, single ventricle physiology, and unbalanced atrioventricular septal defect. Catheter Cardiovasc Interv. 2020;96(2):384–388. doi: 10.1002/ccd.28782. [DOI] [PubMed] [Google Scholar]
- 111.Tan W., Aboulhosn J. Echocardiographic guidance of interventions in adults with congenital heart defects. Cardiovasc Diagn Ther. 2019;9(suppl 2):S346–S359. doi: 10.21037/cdt.2018.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Iriart X., Guérin P., Jalal Z., Thambo J.B. Edge to edge repair using a MitraClip for severe tricuspid valve regurgitation after a Mustard operation. Catheter Cardiovasc Interv. 2021;98(1):E108–E114. doi: 10.1002/ccd.29681. [DOI] [PubMed] [Google Scholar]
- 113.Guerin P., Jalal Z., Le Ruz R., et al. Percutaneous edge-to-edge repair for systemic atrioventricular valve regurgitation in patients with congenital heart disease: the first descriptive cohort. J Am Heart Assoc. 2022;11(10) doi: 10.1161/JAHA.122.025628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kodali S., Hahn R.T., Eleid M.F., et al. Feasibility study of the transcatheter valve repair system for severe tricuspid regurgitation. J Am Coll Cardiol. 2021;77(4):345–356. doi: 10.1016/j.jacc.2020.11.047. [DOI] [PubMed] [Google Scholar]
- 115.Burri M., Vogt M.O., Hörer J., et al. Durability of bioprostheses for the tricuspid valve in patients with congenital heart disease. Eur J Cardiothorac Surg. 2016;50(5):988–993. doi: 10.1093/ejcts/ezw094. [DOI] [PubMed] [Google Scholar]
- 116.Ghobrial J., Aboulhosn J. Transcatheter valve replacement in congenital heart disease: the present and the future. Heart. 2018;104(19):1629–1636. doi: 10.1136/heartjnl-2016-310898. [DOI] [PubMed] [Google Scholar]
- 117.Aboulhosn J., Cabalka A.K., Levi D.S., et al. Transcatheter valve-in-ring implantation for the treatment of residual or recurrent tricuspid valve dysfunction after prior surgical repair. JACC Cardiovasc Intv. 2017;10(1):53–63. doi: 10.1016/j.jcin.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 118.McElhinney D.B., Aboulhosn J.A., Dvir D., et al. Mid-term valve-related outcomes after transcatheter tricuspid valve-in-valve or valve-in-ring replacement. J Am Coll Cardiol. 2019;73(2):148–157. doi: 10.1016/j.jacc.2018.10.051. [DOI] [PubMed] [Google Scholar]
- 119.McElhinney D.B., Cabalka A.K., Aboulhosn J.A., et al. Transcatheter tricuspid valve-in-valve implantation for the treatment of dysfunctional surgical bioprosthetic valves: an international, multicenter registry study. Circulation. 2016;133(16):1582–1593. doi: 10.1161/CIRCULATIONAHA.115.019353. [DOI] [PubMed] [Google Scholar]
- 120.Fam N.P., von Bardeleben R.S., Hensey M., et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE system: a multicenter, observational, first-in-human experience. JACC Cardiovasc Intv. 2021;14(5):501–511. doi: 10.1016/j.jcin.2020.11.045. [DOI] [PubMed] [Google Scholar]
- 121.Bapat V., Rajagopal V., Meduri C., et al. Early experience with new transcatheter mitral valve replacement. J Am Coll Cardiol. 2018;71(1):12–21. doi: 10.1016/j.jacc.2017.10.061. [DOI] [PubMed] [Google Scholar]
- 122.Del Val D., Ferreira-Neto A.N., Wintzer-Wehekind J., et al. Early experience with transcatheter mitral valve replacement: a systematic review. J Am Heart Assoc. 2019;8(17):e013332–e013333. doi: 10.1161/JAHA.119.013332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Paradis J.M., Del Trigo M., Puri R., Rodés-Cabau J. Transcatheter valve-in-valve and valve-in-ring for treating aortic and mitral surgical prosthetic dysfunction. J Am Coll Cardiol. 2015;66(18):2019–2037. doi: 10.1016/j.jacc.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 124.Fiorilli P.N., Herrmann H.C., Szeto W.Y. Transcatheter mitral valve replacement: latest advances and future directions. Ann Cardiothorac Surg. 2021;10(1):85–95. doi: 10.21037/acs-2020-mv-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yoon S.H., Bleiziffer S., Latib A., et al. Predictors of left ventricular outflow tract obstruction after transcatheter mitral valve replacement. JACC Cardiovasc Intv. 2019;12(2):182–193. doi: 10.1016/j.jcin.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 126.Fuller S.M., Borisuk M.J., Sleeper L.A., et al. Mortality and reoperation risk after bioprosthetic aortic valve replacement in young adults with congenital heart disease. Semin Thorac Cardiovasc Surg. 2021;33(4):1081–1092. doi: 10.1053/j.semtcvs.2021.06.020. [DOI] [PubMed] [Google Scholar]
- 127.Mack M.J., Leon M.B., Thourani V.H., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 128.Carroll J.D., Mack M.J., Vemulapalli S., et al. STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76(21):2492–2516. doi: 10.1016/j.jacc.2020.09.595. [DOI] [PubMed] [Google Scholar]
- 129.Forrest J.K., Ramlawi B., Deeb G.M., et al. Transcatheter aortic valve replacement in low-risk patients with bicuspid aortic valve stenosis. JAMA Cardiol. 2021;6(1):50–57. doi: 10.1001/jamacardio.2020.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Makkar R.R., Yoon S.H., Leon M.B., et al. Association between transcatheter aortic valve replacement for bicuspid vs tricuspid aortic stenosis and mortality or stroke. JAMA. 2019;321(22):2193–2202. doi: 10.1001/jama.2019.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yoon S.H., Kim W.K., Dhoble A., et al. Bicuspid aortic valve morphology and outcomes after transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76(9):1018–1030. doi: 10.1016/j.jacc.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 132.Vincent F., Ternacle J., Denimal T., et al. Transcatheter aortic valve replacement in bicuspid aortic valve stenosis. Circulation. 2021;143(10):1043–1061. doi: 10.1161/CIRCULATIONAHA.120.048048. [DOI] [PubMed] [Google Scholar]
- 133.Wong S.C., Burgess T., Cheung M., Zacharin M. The prevalence of turner syndrome in girls presenting with coarctation of the aorta. J Pediatr. 2014;164(2):259–263. doi: 10.1016/j.jpeds.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 134.Teo L.L., Cannell T., Babu-Narayan S.V., Hughes M., Mohiaddin R.H. Prevalence of associated cardiovascular abnormalities in 500 patients with aortic coarctation referred for cardiovascular magnetic resonance imaging to a tertiary center. Pediatr Cardiol. 2011;32(8):1120–1127. doi: 10.1007/s00246-011-9981-0. [DOI] [PubMed] [Google Scholar]
- 135.Curtis S.L., Bradley M., Wilde P., et al. Results of screening for intracranial aneurysms in patients with coarctation of the aorta. AJNR Am J Neuroradiol. 2012;33(6):1182–1186. doi: 10.3174/ajnr.A2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jenkins N.P., Ward C. Coarctation of the aorta: natural history and outcome after surgical treatment. Q J Med. 1999;92(7):365–371. doi: 10.1093/qjmed/92.7.365. [DOI] [PubMed] [Google Scholar]
- 137.Tennant P.W., Pearce M.S., Bythell M., Rankin J. 20-year survival of children born with congenital anomalies: a population-based study. Lancet. 2010;375(9715):649–656. doi: 10.1016/S0140-6736(09)61922-X. [DOI] [PubMed] [Google Scholar]
- 138.Singer M.I., Rowen M., Dorsey T.J. Transluminal aortic balloon angioplasty for coarctation of the aorta in the newborn. Am Heart J. 1982;103(1):131–132. doi: 10.1016/0002-8703(82)90539-7. [DOI] [PubMed] [Google Scholar]
- 139.Forbes T.J., Kim D.W., Du W., et al. Comparison of surgical, stent, and balloon angioplasty treatment of native coarctation of the aorta: an observational study by the CCISC (Congenital Cardiovascular Interventional Study Consortium) J Am Coll Cardiol. 2011;58(25):2664–2674. doi: 10.1016/j.jacc.2011.08.053. [DOI] [PubMed] [Google Scholar]
- 140.de Lezo J.S., Pan M., Romero M., et al. Balloon-expandable stent repair of severe coarctation of aorta. Am Heart J. 1995;129(5):1002–1008. doi: 10.1016/0002-8703(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 141.Taggart N.W., Minahan M., Cabalka A.K., et al. Immediate outcomes of covered stent placement for treatment or prevention of aortic wall injury associated with coarctation of the aorta (COAST II) JACC Cardiovasc Intv. 2016;9(5):484–493. doi: 10.1016/j.jcin.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 142.Sohrabi B., Jamshidi P., Yaghoubi A., et al. Comparison between covered and bare Cheatham-platinum stents for endovascular treatment of patients with native post-ductal aortic coarctation: immediate and intermediate-term results. JACC Cardiovasc Intv. 2014;7(4):416–423. doi: 10.1016/j.jcin.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 143.Ngo M.L., Aggarwal A., Knudson J.D. Peripheral pulmonary artery stenosis: an unusual case and discussion of genetic associations. Congenit Heart Dis. 2014;9(5):448–452. doi: 10.1111/chd.12198. [DOI] [PubMed] [Google Scholar]
- 144.Tonelli A.R., Ahmed M., Hamed F., Prieto L.R. Peripheral pulmonary artery stenosis as a cause of pulmonary hypertension in adults. Pulm Circ. 2015;5(1):204–210. doi: 10.1086/679727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zablah J.E., Morgan G.J. Pulmonary artery stenting. Interv Cardiol Clin. 2019;8(1):33–46. doi: 10.1016/j.iccl.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 146.Lock J.E., Castaneda-Zuniga W.R., Fuhrman B.P., Bass J.L. Balloon dilation angioplasty of hypoplastic and stenotic pulmonary arteries. Circulation. 1983;67(5):962–967. doi: 10.1161/01.cir.67.5.962. [DOI] [PubMed] [Google Scholar]
- 147.Nakanishi T., Tobita K., Sasaki M., et al. Intravascular ultrasound imaging before and after balloon angioplasty for pulmonary artery stenosis. Catheter Cardiovasc Interv. 1999;46(1):68–78. doi: 10.1002/(SICI)1522-726X(199901)46:1<68::AID-CCD18>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 148.Nakanishi T. Balloon dilatation and stent implantation for vascular stenosis. Pediatr Int. 2001;43(5):548–552. doi: 10.1046/j.1442-200x.2001.01462.x. [DOI] [PubMed] [Google Scholar]
- 149.Kotani Y., Chetan D., Zhu J., et al. Fontan failure and death in contemporary Fontan circulation: analysis from the last two decades. Ann Thorac Surg. 2018;105(4):1240–1247. doi: 10.1016/j.athoracsur.2017.10.047. [DOI] [PubMed] [Google Scholar]
- 150.Moore J.P., Hendrickson B., Brunengraber D.Z., Shannon K.M. Transcaval puncture for access to the pulmonary venous atrium after the extracardiac total cavopulmonary connection operation. Circ Arrhythm Electrophysiol. 2015;8(4):824–828. doi: 10.1161/CIRCEP.115.002969. [DOI] [PubMed] [Google Scholar]
- 151.Aldoss O., Divekar A. Modified technique to create diabolo stent configuration. Pediatr Cardiol. 2016;37(4):728–733. doi: 10.1007/s00246-015-1339-6. [DOI] [PubMed] [Google Scholar]
- 152.Kreutzer J., Lock J.E., Jonas R.A., Keane J.F. Transcatheter fenestration dilation and/or creation in postoperative Fontan patients. Am J Cardiol. 1997;79(2):228–232. doi: 10.1016/s0002-9149(96)00723-0. [DOI] [PubMed] [Google Scholar]
- 153.Rupp S., Schieke C., Kerst G., et al. Creation of a transcatheter fenestration in children with failure of Fontan circulation: focus on extracardiac conduit connection. Catheter Cardiovasc Interv. 2015;86(7):1189–1194. doi: 10.1002/ccd.26042. [DOI] [PubMed] [Google Scholar]
- 154.Lehner A., Schulze-Neick I., Haas N.A. Creation of a defined and stable Fontan fenestration with the new Occlutech Atrial Flow Regulator (AFR®) Cardiol Young. 2018;28(8):1062–1066. doi: 10.1017/S1047951118000720. [DOI] [PubMed] [Google Scholar]
- 155.Mehta C., Jones T., De Giovanni J.V. Percutaneous transcatheter communication between the pulmonary artery and atrium following an extra-cardiac Fontan: an alternative approach to fenestration avoiding conduit perforation. Catheter Cardiovasc Interv. 2008;71(7):936–939. doi: 10.1002/ccd.21453. [DOI] [PubMed] [Google Scholar]
- 156.Lluri G., Levi D.S., Aboulhosn J. Systemic to pulmonary venous collaterals in adults with single ventricle physiology after cavopulmonary palliation. Int J Cardiol. 2015;189(1):159–163. doi: 10.1016/j.ijcard.2015.04.065. [DOI] [PubMed] [Google Scholar]
- 157.Poterucha J.T., Johnson J.N., Taggart N.W., et al. Embolization of veno-venous collaterals after the Fontan operation is associated with decreased survival. Congenit Heart Dis. 2015;10(5):E230–E236. doi: 10.1111/chd.12276. [DOI] [PubMed] [Google Scholar]
- 158.Banka P., Sleeper L.A., Atz A.M., et al. Practice variability and outcomes of coil embolization of aortopulmonary collaterals before Fontan completion: a report from the Pediatric Heart Network Fontan Cross-Sectional Study. Am Heart J. 2011;162(1):125–130. doi: 10.1016/j.ahj.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Reardon L.C., Lin J.P., VanArsdell G.S., et al. Orthotopic heart and combined heart liver transplantation: the ultimate treatment option for failing Fontan physiology. Curr Transplant Rep. 2021;8(1):9–20. doi: 10.1007/s40472-021-00315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tan W., Reardon L., Lin J., et al. Occlusion of aortopulmonary and venovenous collaterals prior to heart or combined heart-liver transplantation in Fontan patients: a single-center experience. Int J Cardiol Congenit Hear Dis. 2021;6:100260–100261. doi: 10.1016/j.ijcchd.2021.100260. [DOI] [Google Scholar]
- 161.Dori Y., Keller M.S., Rome J.J., et al. Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation. 2016;133(12):1160–1170. doi: 10.1161/CIRCULATIONAHA.115.019710. [DOI] [PubMed] [Google Scholar]
- 162.Biko D.M., Smith C.L., Otero H.J., et al. Intrahepatic dynamic contrast MR lymphangiography: initial experience with a new technique for the assessment of liver lymphatics. Eur Radiol. 2019;29(10):5190–5196. doi: 10.1007/s00330-019-06112-z. [DOI] [PubMed] [Google Scholar]
- 163.Ly M., Belli E., Leobon B., et al. Results of the double switch operation for congenitally corrected transposition of the great arteries. Eur J Cardiothorac Surg. 2009;35(5):879–884. doi: 10.1016/j.ejcts.2009.01.051. [DOI] [PubMed] [Google Scholar]
- 164.Bradley E.A., Cai A., Cheatham S.L., et al. Mustard baffle obstruction and leak – How successful are percutaneous interventions in adults? Prog Pediatr Cardiol. 2015;39(2):157–163. doi: 10.1016/j.ppedcard.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Parekh D.R., Cabrera M.S., Ing F.F. Simultaneous transcatheter implantation of systemic and pulmonary venous baffle stents after mustard operation for d-transposition of the great arteries. Catheter Cardiovasc Interv. 2015;86(4):708–713. doi: 10.1002/ccd.25951. [DOI] [PubMed] [Google Scholar]
- 166.Yoo S.J., Thabit O., Kim E.K., et al. 3D printing in medicine of congenital heart diseases. 3D Print Med. 2015;2(1):1–12. doi: 10.1186/s41205-016-0004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Buber J., McElhinney D.B., Valente A.M., Marshall A.C., Landzberg M.J. Tricuspid valve regurgitation in congenitally corrected transposition of the great arteries and a left ventricle to pulmonary artery conduit. Ann Thorac Surg. 2015;99(4):1348–1356. doi: 10.1016/j.athoracsur.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 168.Ramage K., Grabowska K., Silversides C., Quan H., Metcalfe A. Association of adult congenital heart disease with pregnancy, maternal, and neonatal outcomes. JAMA Netw Open. 2019;2(5):e193667–e193668. doi: 10.1001/jamanetworkopen.2019.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Canobbio M.M., Warnes C.A., Aboulhosn J., et al. Management of pregnancy in patients with complex congenital heart disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2017;135(8):e50–e87. doi: 10.1161/CIR.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 170.Ciresi C.M., Patel P.R., Asdell S.M., Hopkins K.A., Hoyer M.H., Kay W.A. Management of severe coarctation of the aorta during pregnancy. JACC Case Rep. 2020;2(1):116–119. doi: 10.1016/j.jaccas.2019.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bredy C., Mongeon F.P., Leduc L., Dore A., Khairy P. Pregnancy in adults with repaired/unrepaired atrial septal defect. J Thorac Dis. 2018;10(suppl 24):S2945–S2952. doi: 10.21037/jtd.2017.10.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Alshawabkeh L., Economy K.E., Valente A.M. Anticoagulation during pregnancy: evolving strategies with a focus on mechanical valves. J Am Coll Cardiol. 2016;68(16):1804–1813. doi: 10.1016/j.jacc.2016.06.076. [DOI] [PubMed] [Google Scholar]
- 173.Yarrington C.D., Valente A.M., Economy K.E. Cardiovascular management in pregnancy: Antithrombotic Agents and Antiplatelet Agents. Circulation. 2015;132(14):1354–1364. doi: 10.1161/CIRCULATIONAHA.114.003902. [DOI] [PubMed] [Google Scholar]
- 174.Holoshitz N., Kenny D., Hijazi Z.M. Hybrid interventional procedures in congenital heart disease. Methodist Debakey CardioVasc J. 2014;10(2):93–98. doi: 10.14797/mdcj-10-2-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sosnowski C., Matella T., Fogg L., et al. Hybrid pulmonary artery plication followed by transcatheter pulmonary valve replacement: comparison with surgical PVR. Catheter Cardiovasc Interv. 2016;88(5):804–810. doi: 10.1002/ccd.26620. [DOI] [PubMed] [Google Scholar]
- 176.Porras D., Gurvitz M., Marshall A.C., Emani S.M. Hybrid approach for off-pump pulmonary valve replacement in patients with a dilated right ventricular outflow tract. Ann Thorac Surg. 2015;100(5):e99–e101. doi: 10.1016/j.athoracsur.2015.02.124. [DOI] [PubMed] [Google Scholar]
- 177.Harloff M.T., Papoy A.R., Aghayev A., Kaneko T. Hybrid valve-in-valve mitral valve replacement. JTCVS Tech. 2020;3:154–156. doi: 10.1016/j.xjtc.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lang N., Kozlik-Feldmann R., Dalla Pozza R., et al. Hybrid occlusion of a paravalvular leak with an Amplatzer Septal Occluder after mechanical aortic and mitral valve replacement. J Thorac Cardiovasc Surg. 2010;139(1):221–222. doi: 10.1016/j.jtcvs.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 179.Nijenhuis V.J., Swaans M.J., Post M.C., Heijmen R.H., de Kroon T.L., Ten Berg J.M. Open transapical approach to transcatheter paravalvular leakage closure: a preliminary experience. Circ Cardiovasc Interv. 2014;7(4):611–620. doi: 10.1161/CIRCINTERVENTIONS.113.001171. [DOI] [PubMed] [Google Scholar]
- 180.Holmes D.R., Rich J.B., Zoghbi W.A., Mack M.J. The heart team of cardiovascular care. J Am Coll Cardiol. 2013;61(9):903–907. doi: 10.1016/j.jacc.2012.08.1034. [DOI] [PubMed] [Google Scholar]