Abstract
Chronic venous disease is a common disease, the prevalence of which increases with age, and can cause debilitating symptoms that adversely affect the quality of life. The risk factors include family history, female sex, obesity, pregnancy, parity, and history of deep vein thrombosis. Moreover, it is associated with venous obstruction, reflux, or both, which, in turn, leads to ambulatory venous hypertension. Chronic venous disease is the leading cause of leg ulcers, which place a significant cost burden on the health care system. Compression therapy remains the cornerstone of treatment, particularly for more advanced disease. Superficial saphenous vein reflux can be associated with significant symptoms. Catheter techniques, both thermal and nonthermal, have demonstrated efficacy and safety in successful closure and symptom improvement. Deep vein obstruction can be broadly divided into thrombotic and nonthrombotic and can lead to symptomatic chronic venous disease. Recanalization using balloons and stents has been increasingly used and studied in such patients. It is critical to develop training opportunities and guidelines to improve evidence-based and appropriate care for cardiologists treating chronic venous disease.
Keywords: chronic venous disease, chronic venous insufficiency, May-Thurner syndrome, postthrombotic syndrome, saphenous vein ablation, varicose veins
Central Illustration
Highlights
-
•
Chronic venous disease is common and can cause debilitating symptoms.
-
•
While compression therapy remains the cornerstone treatment, catheter techniques have been safely used to occlude incompetent saphenous veins.
-
•
Deep vein obstruction can be recanalized using balloons and stents.
-
•
Development of training opportunities and guidelines are critical for cardiologists treating chronic venous disease.
Introduction
Lower-extremity venous disease is more prevalent than peripheral arterial disease1 and can be associated with progressive leg discomfort, heaviness, edema, discoloration, and ulceration.2, 3, 4, 5, 6 Its prevalence increases with age and can impose a significant burden on patients’ quality of life.7,8 In the United States (US), >25 million adults have chronic venous insufficiency (CVI).8
Studies on the prevalence of varicose veins have reported values as high as 57% in men and 73% in women.8,9 In addition to age, the risk factors for chronic venous disease include positive family history, female sex, obesity, pregnancy and parity, history of deep vein thrombosis (DVT), and prolonged standing.10, 11, 12 More severe manifestations of the disease, such as edema and ulcers, are more common in patients aged >65 years.3
The prevalence of venous leg ulcers can be as high as 2% of the population.13,14 Venous insufficiency and varicose veins are widespread worldwide and are common in Western countries.8,12,15 A US analysis of >20,000 individuals suggested that compared with Caucasians, African Americans present with more advanced venous disease and at a younger age.16
Chronic venous disease is the leading cause of leg ulcers.17 Individuals with CVI and skin changes appear to be at a greater risk of developing venous ulceration.18 Venous ulcers can frequently secrete exudate, be painful and malodorous, and take months to heal.8,19,20 They are typically found in the gaiter zone of the legs (particularly at the medial and lateral aspects of malleoli and pretibial regions). They are associated with depression and poor quality of life.21
An analysis of the United Kingdom National Health Service data between 2007 and 2017 put the cost of care of patients with venous leg ulcers at >£2 billion per annum, with home nurse visits being a major driver of the cost.22 In the US, an estimated 2.2% of Medicare beneficiaries have venous leg ulcers, with an annual payer burden of $14.9 billion.23
Over the past decade, there has been a dramatic rise in the number of endovascular venous procedures performed, with cardiology being one of the leading specialties providing care.24
Pathophysiology
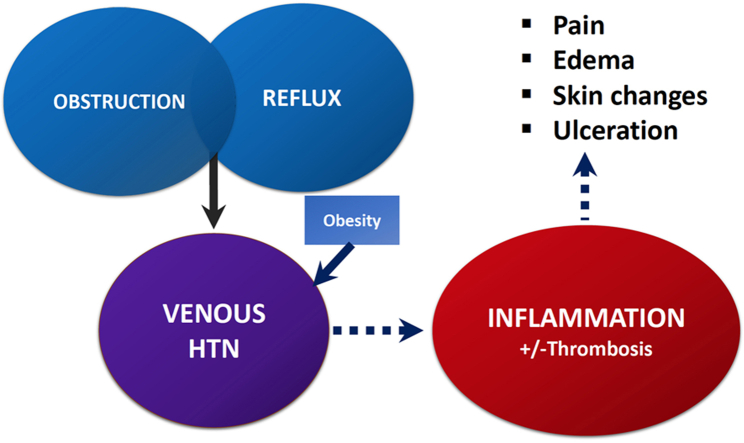
From the mechanical standpoint, chronic venous disease can be associated with venous obstruction, reflux, or both, which is thought to result in ambulatory venous hypertension (Central Illustration).25 This, in turn, can lead to inflammation.26,27 Veins possess thinner media than arteries, are more distensible, and have unidirectional valves to assist with antegrade flow. The calf muscles (particularly, the soleus muscle) can act as pumps to assist in venous return.28,29 Elevated venous pressures can lead to remodeling of the venous walls, leading to development of enlarged and tortuous veins (Figure 1).30 Animal studies have demonstrated that venous hypertension is associated with valve remodeling and leukocyte infiltration.31, 32, 33 Ambulatory venous hypertension is associated with greater damage and ulceration of leg skin.34,35
Central Illustration.
Schematic representation of the pathophysiology of chronic venous disease. HTN, hypertension.
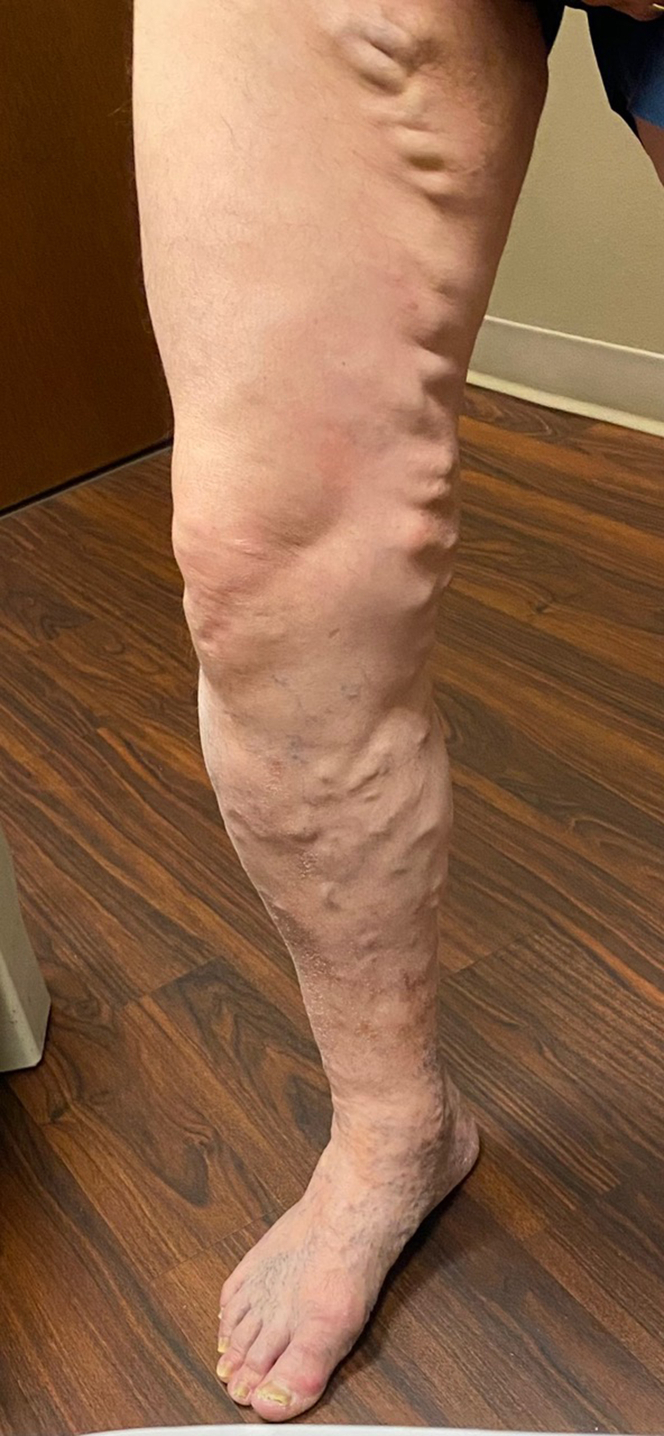
Figure 1.
Varicose veins in the anteromedial aspect of the thigh and calf extending into the ankle and foot.
A human study in which venous congestion was induced led to greater expression of endothelin-1, a mediator of inflammation.36 Alterations in the levels of matrix metalloproteinase may play a role in venous structure and function37; furthermore, in patients with lipodermatosclerosis, there is overexpression of matrix metalloproteinase38 and venous ulcers, with poor healing.39 There is greater dermal expression of mononuclear cells in patients with CVI,40 and mast cell activation may be the etiology of pruritus, which is experienced by many patients.41
The levels of type III collagen, elastin, and laminin, important for elasticity, are decreased in varicose veins.42, 43, 44 Vascular smooth muscles can lose their contractility.45,46 Saphenous veins in humans with venous insufficiency and varicose veins showed less contractile responses to norepinephrine and angiotensin II.47
Increased endothelial permeability leads to extravasation of red blood cells, which then break down in the interstitium into ferric iron and hemoglobin, potentially leading to hemosiderin deposition, inflammation, and hyperpigmentation.48,49
The levels of inflammatory markers, such as C-reactive protein, interleukin 6, and d-dimer, are increased in blood collected from varicose veins.50,51 Blood collected directly from limbs of patients with venous insufficiency has a lower white cell count than control blood samples, lending credence to the likelihood of white blood cell trapping.52 Additionally, there is increased expression of the adaptor protein insulin receptor substrate-4 in the varicose veins of individuals with chronic venous disease53; however, its precise physiologic role is unclear.
There are data to support the importance of adequate venous return for cardiac functioning. For example, as far back as 1970, it was known that ligation of the inferior vena cava could lead to exertional dyspnea.54 These patients could not adequately augment their cardiac index with exercise. It may be plausible that impaired venous return, such as that due to obstruction, can lead to exercise intolerance.55 A single-center retrospective analysis of 85 patients with varicose vein disease who had undergone echocardiography suggested that they had lower tricuspid and mitral inflow velocities in early diastole but higher late diastolic velocities.56 A prospective study (n = 129) reported similar findings, particularly with patients with more advanced venous disease.57 This might suggest an increased compensatory atrial ejection fraction in patients with venous insufficiency. However, there were many confounders in this study, and it is unclear how well the characteristics of the control group were otherwise matched.
Utilizing the German Gutenberg Health Study database of >12,000 participants from a large single-center cohort, multivariate regression models showed that more advanced classes of venous insufficiency were associated with a higher 10-year risk of incident cardiovascular disease. More advanced classes of venous insufficiency were also associated with a greater risk of all-cause mortality.58 The reason for this is unclear but may represent shared risk factors. Furthermore, the Framingham Heart Study noted an association between varicose veins and future atherosclerotic disease.1 A statistically significant risk of coronary artery disease was noted in women with varicose veins. However, women with varicose veins had higher blood pressures and were more obese and sedentary.
Classification and symptom scores
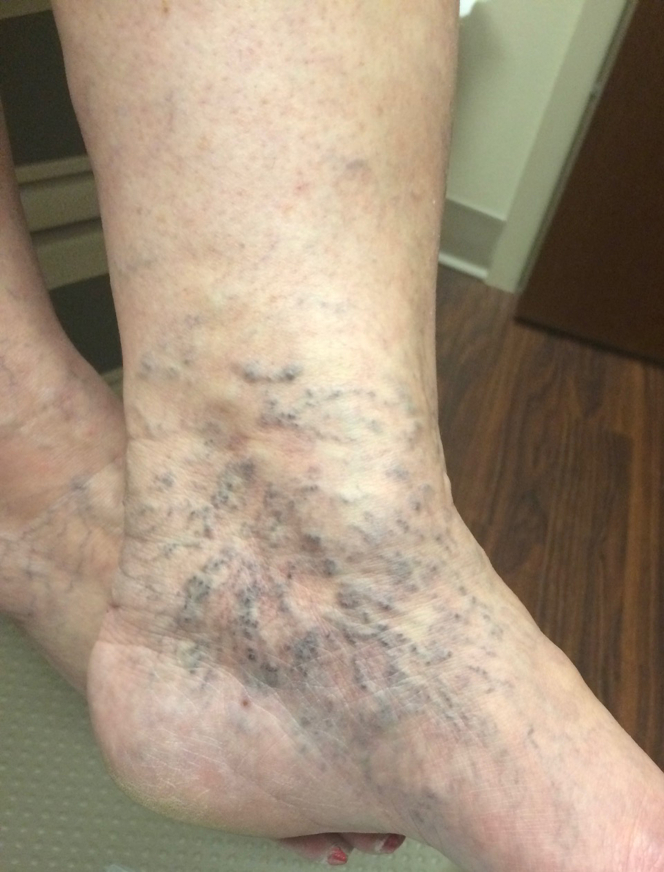
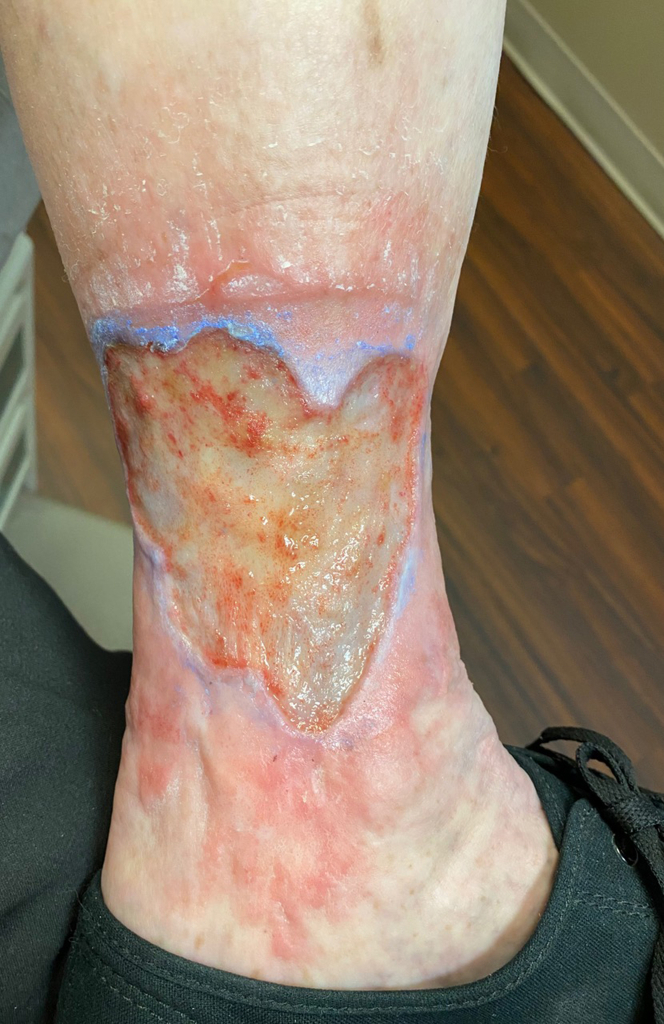
The clinical, etiology, anatomy, pathology classification of the description of venous disease was developed in 1993, and the most recent revision was published in 2020.59 The C (clinical) class, ranging from 1 to 6, is often utilized alone to describe the severity of the disease (Table 1). For example, corona phlebectatica, found in the ankle region (Figure 2), and hyperpigmentation (Figure 3) are included in the C4 class, which denotes advanced disease. Ulceration (C6 disease) is the most advanced class (Figure 4).
Table 1.
The C classes of the clinical, etiology, anatomy, pathology classification system for chronic venous disease.
| C class | Description |
|---|---|
| C0 | No visible or palpable signs of venous disease |
| C1 | Telangiectasias or reticular veins |
| C2 | Varicose veins |
| C2r | Recurrent varicose veins |
| C3 | Edema |
| C4 | Changes in skin or subcutaneous tissue secondary to chronic venous disease |
| C4a | Pigmentation or eczema |
| C4b | Lipodermatosclerosis or atrophie blanche |
| C4c | Corona phlebectatica |
| C5 | Healed |
| C6 | Active venous ulcer |
| C6r | Recurrent active venous ulcer |
Higher scores denote more severe forms of disease.
r, recurrent.
Figure 2.
Corona phlebectatica.
Figure 3.
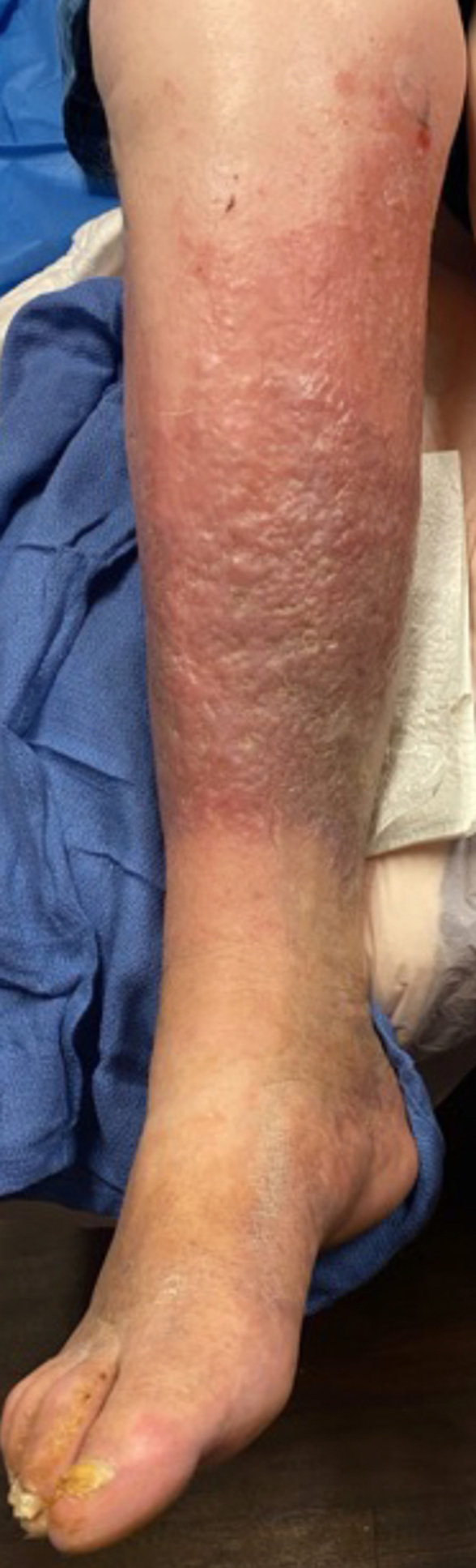
Pigmentation, inflammation, and edema (lipodermatosclerosis).
Figure 4.
Venous ulcer, with periulcer inflammation.
The E (etiology) class can be designated as primary, secondary, congenital, or as no cause identified. The A (anatomy) class refers to the site of pathology: deep, superficial, or perforator veins. The P (pathology) class includes reflux, obstruction, both, or none.
The Venous Clinical Severity Score (VCSS) is a validated symptom score60 to measure the severity of venous disease and response to treatment.61,62 It has been utilized in numerous venous intervention trials. In a prospective evaluation of patients with varicose veins, VCSS was strongly correlated with 2 other scores, the Aberdeen Varicose Vein Questionnaire and the Chronic Venous Insufficiency quality-of-life Questionnaire scores (r = 0.7, P < .0001).63 A number of other venous quality-of-life or symptom severity scores, including EuroQoL-5D64 and VEINES-QoL, have been utilized.65 The VVSym Q (HASTI) score incorporates 5 symptoms (heavy, aching, swelling, throbbing, and itching legs), with 5 possible responses ranging from all of the time (1) to none of the time (5). Therefore, higher scores indicate milder disease. Moreover, the score is correlated well with VEINES-QoL.66
The Villalta score has been most frequently used, to date, for diagnosing postthrombotic syndrome (PTS) and quantifying its severity.67, 68, 69 It incorporates 5 patient-reported symptoms and 6 physician-reported findings into a single value (5-9, mild; 10-14, moderate; ≥15 or presence of venous ulcer, severe).
Compression therapy
Compression therapy is the cornerstone of the treatment of chronic venous disease,70,71 particularly in its more advanced states.72 Conrad Jobst’s observation that hydrostatic pressures in a pool (which increase with depth) relieved the symptoms of venous insufficiency led to him developing compression stockings to emulate the same effect.73 Fundamentally, compression should be graduated, applying higher pressures at the ankle level than more cephalad. The increased lower-limb venous pressure in patients with CVI that accompanies standing can drive fluid into interstitial spaces.74,75 Although compression does not necessarily lower vein pressure, it can reduce interstitial pooling and, in turn, decrease inflammation.76 The levels of inflammatory cytokines within venous ulcers decrease with compression therapy.77
Compression garments for the legs can take the form of stockings, bandages, Velcro wrap devices, pumps, or a combination.78, 79, 80 Compression stockings can assist in improving venous return and reduce edema.81 Compression for lower severity of venous disease (C2-C3) can decrease discomfort and edema, even as stand-alone therapy.82 Compression, particularly at higher pressures (30-40 mm Hg), has been shown to improve venous ulcer healing and decrease recurrence.79,80 There is no strong evidence that compression therapy improves procedural success after vein ablation; however, it may lower postprocedural edema and discomfort.83
The grades of compression measured at the ankle can be divided into light (<20 mm Hg), class I (21-30 mm Hg), class II (31-40 mm Hg), and class III (>40 mm Hg), although other classification systems exist.80 Trials of varying quality have been performed to evaluate the potential role of compression therapy following saphenous vein thermal ablation and sclerotherapy. The main benefit appears to be to lessen postprocedural discomfort.83,84
Compression therapy improves venous ulcer healing and lowers recurrence.72 Stronger compression pressures appear to be more effective.85
There are conflicting data on the efficacy of compression therapy in the prevention of PTS after acute lower-limb DVT. Compression therapy has been utilized after DVT; however, there have been mixed findings on its efficacy in lowering the rates of PTS. A prospective control study conducted in China did demonstrate lower rates of PTS at 24 months with knee-high compression stockings with a pressure of 30 to 40 mm Hg.86 This is in contrast to the SOX trial, in which no benefit was observed. As a differentiator among studies, in the SOX trial, participants were mailed stockings 2 weeks following DVT and appeared to have lower compliance with compression.87
Chronic edema of the leg can be due to a number of causes, including lymphedema, venous obstruction or valvular reflux, congestive heart failure, or obesity. It is a known risk factor for recurrent cellulitis, and compression therapy was shown to significantly reduce recurrent cellulitis in a prospective randomized controlled trial (RCT).88
A proportion of patients with venous leg ulcers have concurrent peripheral arterial disease. There has been reluctance among some clinicians to recommend compression garments for so-called mixed arteriovenous ulcers. In a review of 10 studies of mixed ulcers, compression with stockings with a pressure of 20- to 30 mm Hg appeared to be both safe and beneficial for ulcer healing, with an ankle brachial pressure index (ABI) of ≥0.5.89 Patients with lower ABIs may be considered for arterial revascularization prior to initiation of compression. In a small retrospective study (n = 20), patients with venous ulcers and ABI between 0.5 and 0.75 who underwent arterial revascularization first healed (on an average of 8 weeks) faster.90 Similarly, a single-arm study suggested faster ulcer healing when arterial revascularization was performed, with 75% of ulcers healing by 10 weeks.91
A common challenge with compression therapy is noncompliance because of discomfort and inability to apply (don) and remove (doff).92,93 At least 15% of the elderly cannot apply compression wear at all.94 The presence of joint arthritis, frailty, and lack of flexibility may all contribute. In an analysis of 58 clinical studies, good compliance, defined as wearing compression >50% of the time, was reported in only two-thirds of patients (at a median of 12 months of follow-up).95 The compliance was lower with higher-pressure (>25 mm Hg) stockings. In addition, in the US, compression hosiery is typically not reimbursed by health insurance.
Superficial venous reflux
Varicose veins are more common in women than in men.1 Superficial valvular incompetence has been frequently found in individuals with CVI and venous ulcers.96
Various surgical techniques for the treatment of varicose veins have been used in the past century. A common approach has been flush ligation of the saphenofemoral junction, accompanied by stripping of the great saphenous vein (GSV) down to the knee level.97,98 Additionally, stab phlebectomy can be performed concurrently or subsequently. In addition, the small saphenous vein (SSV) can be stripped in a similar manner. The complications of surgery include infection (<6%), DVT (<5%), and, rarely, saphenous or sural nerve injury.
Surgical stripping of the saphenous veins has largely been replaced, particularly in Western countries, by ablation (Table 2).99, 100, 101, 102, 103, 104 Thermal ablation is a percutaneous ultrasound-guided technique. The GSV is easily identifiable using duplex ultrasound (Figure 5) and can be interrogated, with the patient preferably in the standing position, to look for reflux (Figure 6). Endovenous laser ablation (EVLA) and radiofrequency ablation (RFA) are percutaneous modes of thermal ablation. Intravenously, both devices employ a low-profile fiber, directly delivering heat energy to the venous endothelium, leading to injury, thrombosis, and eventual fibrosis and occlusion of the vein. Using ultrasound guidance, a sheath is inserted into the target vein, through which the ablation fiber or catheter is advanced, ensuring that its tip is at least 2 cm distal to the deep venous system. Tumescent anesthesia is percutaneously injected around the target vein under ultrasound guidance, before application of thermal ablation. Tumescent preparations typically contain lidocaine, epinephrine, bicarbonate, and saline. They act as anesthetics and heat sinks for thermal ablation and protect surrounding structures from thermal injury. Epinephrine can constrict the vein, allowing for better contact with the ablation device.
Table 2.
Venous ablation modalities.
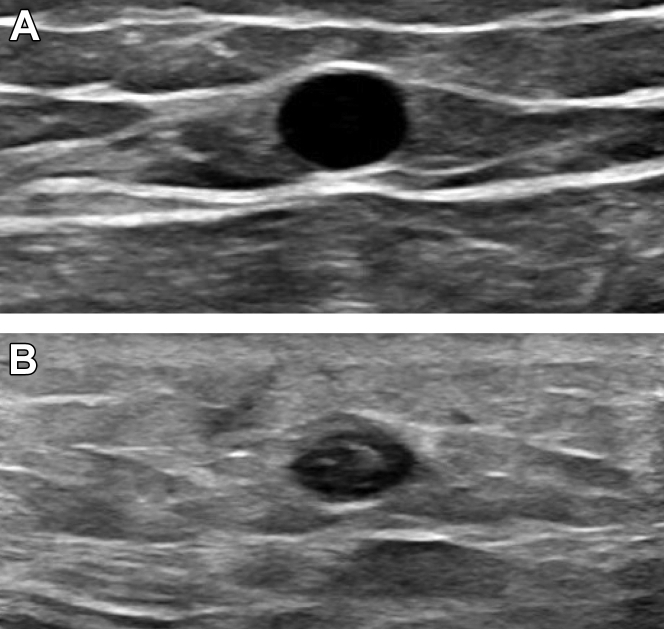
Figure 5.
(A) A transverse view of the great saphenous vein observed using duplex ultrasound. (B) The great saphenous vein 2 weeks after ablation, which was noncompressible and with hyperechoic content.
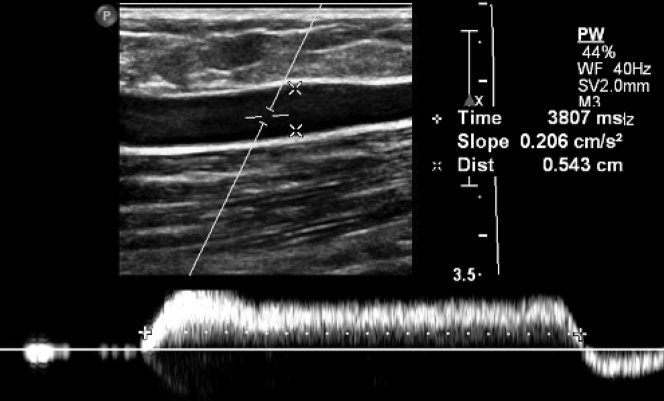
Figure 6.
Great saphenous vein reflux demonstrated using pulsed-wave Doppler. The reflux time in this case was 3807 milliseconds (>500 milliseconds is considered abnormal).
Endovenous laser ablation was approved in the US in 2001. The EVLA devices currently available in the US market include VenaCure (AngioDynamics) and Vari-Lase (Teleflex). The laser wavelength can target water or hemoglobin.105 The 1-year vein occlusion rates can surpass 90% with EVLA.101
Radiofrequency ablation was approved for vein ablations in 1999. The RFA devices currently available in the US are ClosureFast (Medtronic) and Venclose (Becton Dickinson). Five-year follow-up after RFA in patients with venous insufficiency revealed an occlusion rate of 92% and sustained symptom improvement.99 A 2016 meta-analysis revealed technical success rates of 89% for RFA and 85% for EVLA for the treatment of GSV incompetence.106 Compared with RFA, there is probably greater postprocedural pain and bruising after EVLA.107
Both EVLA and RFA procedures may be complicated by endothermal heat-induced thrombosis in <1% cases, wherein a thrombus may propagate into the deep system.108 However, the risk of pulmonary embolism as a result of endothermal heat-induced thrombosis is very low.109
A number of nonthermal ultrasound-guided methods have also been adopted to close the saphenous veins, including cyanoacrylate adhesive closure (CAC), mechanochemical ablation (MOCA), and foam sclerotherapy. These so-called nontumescent, nonthermal techniques have several advantages. They do not cause thermal injury such as burns or nerve damage. Without the need for tumescent application, they are typically less painful. The cyanoacrylate adhesive is delivered to the target vein percutaneously, where it rapidly polymerizes, leading to immediate closure and gradual fibrosis.
Sclerotherapy utilizes agents that once injected into a target vein, cause denaturation of surface proteins, luminal fibrosis, and obstruction.110 Sclerosants have been used for telangiectasis as well as reticular and varicose veins.111 Sclerotherapy improves the cosmetic appearance of varicose veins and, possibly, quality of life.112 In larger veins (eg, 3 mm), the sclerosing agent can be injected as a foam to displace more blood and enhance contact between the sclerosant and venous wall.113, 114, 115 Both air and CO2 have been utilized for foam formation. Both sodium tetradecyl sulfate and polidocanol have been approved for use as sclerotherapy agents in the US. Both are detergents. The potential complications with their use include hyperpigmentation and telangiectatic matting. There is little evidence to suggest clinically significant right-to-left shunting of sclerosants.116 There are reports of transient visual disturbance after sclerotherapy, although it is rare.117 DVT or ulceration is also rare. Intra-arterial injection can lead to tissue necrosis.118
There are few robust RCTs on the relative efficacy of the types of sclerosants or formulations.112,119 A proprietary formulation of 1% polidocanol named Varithena (Boston Scientific)103 was approved by the Food and Drug Administration (FDA) in 2013. The efficacy of Varithena in reducing the symptoms of venous reflux was demonstrated in VANISH-2.104 Proximal DVT occurred in 2.6% of patients.120 A small multicenter, prospective RCT (n = 77) reported extension of Varithena into the common femoral vein in 5.1%, tibial or peroneal vein DVT in 2.6%, isolated gastrocnemius or soleus vein DVT in 7.7% of the patients, with no pulmonary emboli.66
In MOCA (ClariVein; Merit Medical), a rotating metallic tip is used to scrape the venous endothelium at 3500 rpm while the operator simultaneously injects a sclerosant and slowly withdraws the rotating tip, leading to eventual endothelial fibrosis and vein occlusion.102 The device is advanced through a small sheath under ultrasound guidance. At 1 year of follow-up, MOCA demonstrated an 88% GSV occlusion and significant improvement of venous symptoms.102 However, MOCA can be complicated by hematoma, phlebitis and, rarely, DVT.121 An RCT comparing MOCA with thermal ablation found lower GSV saphenous occlusion rates with MOCA but equivalent symptom score improvements at 1 year.122
VenaSeal CAC (Medtronic) was FDA approved in 2015. Similar to other ablation techniques, using ultrasound, a sheath is advanced into the saphenous vein, through which cyanoacrylate is delivered and manual compression applied. In a head-to-head RCT, at 5 years, CAC demonstrated equivalent GSV occlusion rates and relief of symptoms compared with RFA.100 In both the arms of the study, ∼64% of participants received adjunctive sclerotherapy at 6 months (P = .77).123 A hypersensitivity (phlebitis-like) reaction was observed in up to 23% of CAC cases after the procedure.124,125
In a prospective, randomized, multicenter study of 798 patients treated for symptomatic varicose veins, the outcomes of EVLA, foam sclerotherapy, and surgery (high ligation and stripping) were compared.126 GSV or SSV reflux was required for inclusion. Compared with the scores at baseline, all the groups demonstrated improvement of the Aberdeen Varicose Vein Questionnaire quality-of-life score at 5 years. The EVLA and surgery groups experienced greater improvement than the sclerotherapy group. However, it must be noted that in 31% of patients in the EVLA group, sclerotherapy was also utilized at least once. EVLA was the most cost effective. Another prospective, randomized trial compared EVLA, RFA, sclerotherapy, and surgical stripping for symptomatic GSV reflux. There was greater postprocedural pain in the surgery and EVLA groups. At 1 year, in the sclerotherapy group, GSVs remained patent in 16.3% of patients, significantly higher than that for the other modalities.127 A 2021 Cochrane review of interventions for GSV reflux found equivalent technical success (GSV closure) up to 5 years and probably similar recurrence rates between RFA and EVLA.128 EVLA and high ligation with stripping were probably superior to (ultrasound-guided) foam sclerotherapy in terms of technical success.
The Early Venous Reflux Ablation (EVRA) trial compared compression therapy alone with compression therapy plus early endovenous ablation in patients with venous ulcers and superficial reflux. It demonstrated faster ulcer healing and lower ulcer recurrence rates in the compression plus early ablation group.129,130
Varicose veins can recur in ∼22% of cases after endovenous ablation (at 2 years of follow-up), with the most common underlying causes being recanalization of the GSV, followed by development of incompetence in the anterior accessory GSV.131 Additional factors can be SSV and perforator reflux.132 The potential contribution of pelvic vein disease has not been well studied.
There are limited quality data on the role of perforator ablation. Ablation of incompetent perforator veins may be considered in the setting of venous ulcer disease.133 It is typically reserved for cases in which wound care, compression, and ablation of the saphenous veins have already been attempted, without improvement, and the perforator(s) of interest are directed toward the affected area. RFA, EVLT, and sclerotherapy have demonstrated efficacy.134,135 Ultrasound-guided foam sclerotherapy appears to result in lower perforator closure rates than thermal ablation.135 The nerve injury rates and DVT rates are <1% with thermal perforator ablation.136 One study of ultrasound-guided perforator vein foam sclerotherapy reported calf DVT in 3% of patients.137 Moreover, great care must be taken to avoid intra-arterial foam injection, which can result in skin necrosis.
Deep venous reflux
Deep vein reflux can coexist with superficial reflux and appears to contribute to the severity of the symptoms of venous disease, including skin changes.138 One estimate placed the prevalence of deep vein reflux in patients with C4-6 disease at 10%; however, it may be higher.139,140 Deep vein reflux can also occur after DVT, which can contribute to PTS.141 Furthermore, thrombotic and nonthrombotic iliac vein obstructions may be associated with deep venous reflux.142,143
A number of surgical techniques have been attempted to restore deep valvular function, including transposition, transplantation, valvuloplasty, and neovalve formation, but are technically challenging, invasive, and rarely utilized.144, 145, 146, 147, 148 Early trials of BlueLeaf (InterVene), a catheter-based device to fashion venous valves from the vein wall, did not demonstrate efficacy in reducing deep vein reflux.149
There are some data to suggest that deep vein reflux improves in a subset of patients after their refluxing great saphenous veins undergo stripping or ablation.140,150 The improvement has been hypothesized to be related to the correction of “overflow” into the deep system using perforators.151,152
Nonthrombotic deep vein obstruction
In the classic form of May-Thurner syndrome, the left common iliac vein is compressed by the adjacent right common iliac artery against the lumbar vertebrae, although multiple other areas of potential compression can exist.153,154 Additional mechanisms of compression have been reported, such as secondary to iliac artery stents,155 tumors,156,157 and anterior lumbar disc migration,158 to name a few.
The prevalence of May-Thurner syndrome in the general population is variable, with rates of up to ∼25%159 and even higher among symptomatic patients.142,159 Although often clinically silent, it can lead to unilateral edema and even thrombosis of the lower limbs.160 Its diagnosis can be made using magnetic resonance, computed tomography venography, or invasive venography with intravascular ultrasound (IVUS). There are many areas that require further study, including the degree of stenosis that may be clinically significant. Values around the range of 50% to 60% have been proposed.161, 162, 163
Endovenous stenting of compressed or stenotic segments has become the invasive treatment of choice for symptomatic patients (Figures 7 and 8).164, 165, 166 A 2015 systematic review reported iliofemoral stent primary and secondary patency rates of 96% and 99%, respectively, at 1 year.167 Published reports of endovenous (caval) stenting began appearing in the 1980s and 1990s with the Gianturco stent.168, 169, 170 Unlike peripheral arterial stenoses, for which balloon interventions can suffice, iliocaval obstructions are best stented to overcome extrinsic compression or recoil and reobstruction.171 Venous stents must be flexible, possess radial strength, accommodate the relatively larger diameter of veins, and conform to the vessel’s curvature. The venous circulation is a low-pressure, slow-flow system. Undersizing of stent diameter can impede venous flow, may lower patency rates,172 and can even result in stent migration.173
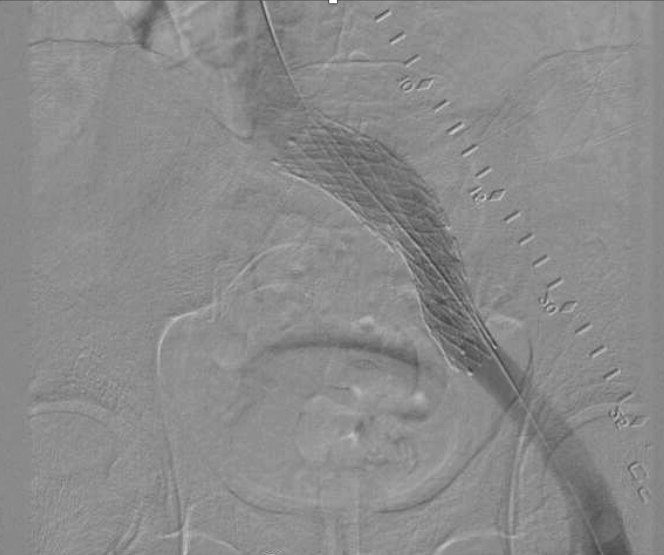
Figure 7.
Digital subtraction venogram of a left iliac vein stent after deployment. A radio-opaque ruler (in conjunction with intravenous ultrasound) can be used to identify the optimal stent landing zone.
Figure 8.
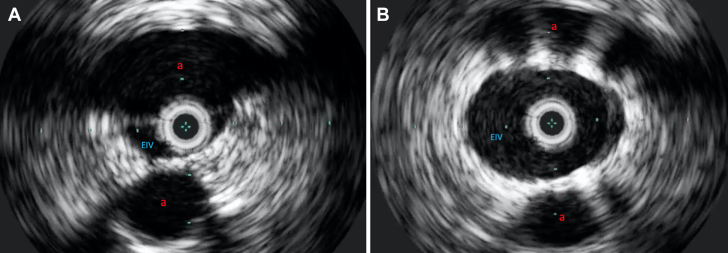
(A) An intravenous ultrasound image of the right external iliac vein compressed between the right internal and external iliac arteries. (B) Intravenous ultrasound after stent deployment within the right external iliac vein showed a markedly improved lumen area. a, artery.
With hip flexion, both the common femoral vein and iliac confluence become angulated.174 A study using computed tomography suggested that the common femoral vein can be compressed during hip flexion by the superior pubic ramus.175
A number of “dedicated” self-expanding venous stents have been approved by the FDA, including Venovo176 (Bard), Zilver Vena (Cook Medical), Abre177 (Medtronic), and Veniti Vici178 (Boston Scientific). All, except Vici, have an open cell design. None has demonstrated the significant foreshortening during deployment that characterizes Wallstents (Boston Scientific).
A number of techniques have been adopted for iliocaval bifurcation stenting, such as a double-barrel, fenestrated (similar to the coronary T-stent technique), or Z-stent.179 However, there are limited quality comparison data on their relative efficacy. The Gianturco Z-stent (Cook Medical) is rigid, with wide gaps between struts,180,181 allowing for deployment within the iliac confluence in conjunction with overlapping iliac stents.
There is lack of clarity on which patient subsets may benefit from venous stenting. The Venogram versus Intravascular ultrasound for Diagnosing and treating Iliofemoral Vein Obstruction (VIDIO) trial prospectively enrolled 100 patients with C4-C6 venous disease, of whom 68 underwent stenting based on imaging findings. The study demonstrated increased sensitivity of IVUS compared with that of venography for the detection of venous stenosis and >54% to be the optimal lumen area stenosis threshold using IVUS to intervene.182 However, the clinical outcomes of stenting were disappointing. At 6 months, only 41% of the patients had VCSS improvements of >4 points. In fact, 7.3% of the patients showed no change, and 13.2% showed worsening of VCSS.182
More recently, Jayaraj et al183 published a retrospective study on iliofemoral stenting. Their findings suggested that after stenting, patients with apparently less severe (<50% area) stenosis appear to improve just as much as those with >50% area stenosis. Moreover, when present, the ulcer healing rates were not significantly different between the groups. There clearly remains more to be learnt about identifying who needs and will benefit from iliac stents.
During knee extension, in ∼25% of individuals, the popliteal vein can become compressed by the gastrocnemius muscles.184 Although normally asymptomatic, occasionally, this can lead to symptoms and is referred to as popliteal vein entrapment syndrome.185,186 Patients with this syndrome can present with edema187 and, sometimes, DVT188 on the affected side. Its treatment includes compression stockings, with surgical decompression reserved for more severe cases.189
Thrombotic deep vein obstruction and PTS
Acute DVT of the lower extremities can lead to PTS, a chronic and, sometimes, debilitating condition, with limb venous hypertension and inflammation associated with chronic obstruction with or without reflux.143,190 Following DVT, the affected vein can be left with permanent luminal scarring and stenosis associated with synechiae. PTS is typically diagnosed 3 months after the original insult. The frequency of PTS (depending on the diagnostic methodology) has been estimated to range from 20% to >40%.191, 192, 193 It can lead to long-term pain, edema, discoloration, weeping, and ulceration, with diminished quality of life.194,195 The risk factors for PTS include more proximal location of DVT (the iliocaval or common femoral vein) and recurrent DVT.196,197 A number of symptom scales have been proposed, with the Villalta score being the most widely used.69
In 1960, Palma198 described a surgical technique to relieve postthrombotic unilateral obstruction by grafting the GSV between the 2 common femoral veins, allowing for diversion of venous outflow. Since then, a number of endovascular recanalization techniques have been described to relieve venous outflow obstruction.166 Recanalization can be combined with thrombolytic devices.199
Postthrombotic chronic total occlusions can be very challenging to cross because of the hard texture of the occluded lumen, ambiguous visualization of the true lumen, and large collaterals that are often formed. Supportive catheters and even sharp recanalization techniques have been used.200, 201, 202 The primary and secondary patency rates for postthrombotic iliac vein stents at 1 year have been shown to be ∼79% and 94%, respectively.167
At the time of writing of this article, there were no published RCTs to demonstrate the efficacy of deep vein interventions in relieving the symptoms of PTS, although there were predominantly retrospective data.166,203, 204, 205 Most of the published literature is on Wallstents. A 2020 systematic review of iliocaval stenting studies found no reports of periprocedural mortality or pulmonary embolism.204 The mean complication rate was 3%, and the complications included access-site hematoma, stent thrombosis, and bleeding. The primary and secondary patency rates (after a median of 33 months) were 64% and 85%, respectively. The ongoing National Institutes of Health-funded C-TRACT (Chronic venous Thrombosis: Relief with Adjunctive Catheter-Directed Therapy) trial (NCT03250247) seeks to evaluate the effect of iliac vein stenting, with or without superficial vein ablation, on the severity of PTS.
In most studies, patients undergoing stenting for postthrombotic disease were placed on anticoagulation therapy.204 There is currently a paucity of data on optimal anticoagulation or antiplatelet therapy after iliocaval stenting.206
Obesity and chronic venous disease
Obesity is a risk factor for chronic venous disease, and increased body mass index (BMI) appears to be correlated with the severity of its symptoms. Abdominal obesity may obstruct leg venous return and raise ambulatory venous pressures. In addition, high body mass is associated with poor ulcer healing.207 The predisposition to recurrent cellulitis may lead to lymphatic damage and lymphedema, an additional cause of edema. Patients with obesity can have difficulty reaching their feet to apply compression hosiery. A retrospective study found a positive correlation between BMI and the severity of CVI symptoms, including ulceration. This correlation appeared to be exclusive of the severity of venous reflux.208
Obesity is associated with increased intra-abdominal pressure, which, in turn, is associated with increased deep (femoral) vein pressure.209, 210, 211 Moreover, obesity is associated with lower venous wall shear stress,212 which, in turn, can be proinflammatory.213
Among 20 patients (39 limbs) with a BMI of >40 kg/m2 and clinical, etiology, anatomy, pathology class 4-6 venous disease, lower-extremity venous reflux was ruled out in 61% of the limbs using duplex ultrasound.214 However, the subjects were not screened for venous outflow stenosis.
In a nonrandomized study, patients with CVI and a BMI of ≥35 kg/m2 who were able to lose weight (with the mean BMI decreased from 50.1 to 32.9 kg/m2) with bariatric surgery demonstrated improvement of venous symptom scores.215
Pharmacologic therapy for chronic venous disease
A number of pharmaceutical agents, including rutosides, diosmin, hesperidin, pine bark extract (pycnogenol), horse chestnut extract (escin), and micronized purified flavonoid fraction, have been evaluated for symptoms of chronic venous disease.216,217 These agents, particularly micronized purified flavonoid fraction,218 may reduce some symptoms, including edema and leg cramping. However, their mechanisms of action are not clear.217,219 Rutosides may reduce capillary permeability.220 Flavonoids may lower venous inflammation and enhance venous tone.218 Pentoxifylline (400 mg 3 times daily) may have efficacy as an adjunct in venous ulcer therapy.221,222 Pentoxifylline decreases blood viscosity and thrombus formation and inhibits tumor necrosis factor A.223, 224, 225
Education and guideline development
According to a survey, ∼28% of venous procedures in the US are offered by medicine specialists, including the subspecialty of cardiology.226 As a group, cardiologists are second only to vascular surgeons in the volume of endovenous ablations performed in the US.227 The overall number of endovenous ablations performed have been rising.227 However, there is variation in clinical practice228 and, currently, lack of formal venous training in cardiovascular disease and interventional cardiology fellowships.
Although there has been a surge in published material on venous disease in both print and electronic media, its extent and scattered nature has made it more challenging for practitioners to consolidate and apply. A number of specialist societies, such as the American Venous Forum, Society for Vascular Surgery, American Vein and Lymphatic Society, Society of Interventional Radiology,71 European Venous Forum,229,230 Cardiovascular Interventional Radiological Society of Europe, and Canadian Interventional Radiology Association, have provided guidelines for the management of venous disease.231 The Society for Cardiovascular Angiography & Interventions (SCAI), in conjunction with other societies, published the criteria for appropriate use of peripheral artery intervention232 but not yet for venous disease at the time of writing of this article. However, efforts have begun to address this need.
There exist knowledge gaps and heterogeneity in the quality of venous studies published to date. High-quality data are scant compared with those on contemporary interventional cardiology. An SCAI guideline document will serve to assist cardiologists and interventional cardiologists who care for patients with venous disease to deliver safer, more efficacious, and evidence-based care.
Over the past 10 to 15 years, numerous medical specialties have entered the evaluation and management of CVI and, particularly, superficial venous disease. There have been no established standards for training, and because most procedures are performed in physicians’ offices, there is a low bar for entry into the CVI space. There is a wide variety of training and exposure for patients with CVI for each specialty, and this creates potential for vast disparities in care. Many venous operators only offer a single modality of therapy, and referrals for adjunctive venous therapies are inconsistent and fractionated in many communities. Expanding the number of competent providers can help address unmet needs in most communities; however, this must be balanced with avoiding overutilization and substandard training.
The Venous and Lymphatic Medicine (VLM) Work Group was formed in 2021 as a collaborative effort to address these disparities. The VLM Work Group is a multisociety, multispecialty collaborative that intends to define the training and certification requirements of physicians treating the venous and lymphatic systems in the future. The group includes executive leadership from the American Vein and Lymphatic Society, American Venous Forum, Society of Vascular Surgery, Society of Interventional Radiology, Society of Vascular Medicine, American Association of Dermatology, and SCAI. It also includes representatives from the American Board of Surgery, American Board of Radiology, Accreditation Council for Graduate Medical Education, and American Board of Medical Specialties. The mission of this group is to define VLM as a distinct specialty and in a manner that would allow physicians from various specialties to receive proper and comprehensive Accreditation Council for Graduate Medical Education-accredited training on the entire scope of venous and lymphatic diseases rather than the 1 particular aspect that is most germane to their primary specialty. Moving forward, this group plans to map out the optimal way for specialty recognition and certification that is inclusive of those who have already demonstrated competence and experience in the field of venous diseases.
Conclusions
Chronic venous disease of the lower extremities is common and can be associated with debilitating symptoms that adversely affect the quality of life. It is associated with venous obstruction, reflux, or both and often leads to chronic inflammation. In addition to compressive and medical therapies, a number of minimally invasive techniques have shown promise for deep vein recanalization, the closure of incompetent superficial veins, and the elimination of varicose veins. More quality clinical trials and training in comprehensive care for patients with venous diseases are critical to enhance patient care and advance the field.
Acknowledgments
Declaration of competing interest
Jeffrey G. Carr is a consultant for Becton Dickinson and on the advisory board for Medtronic. Robert R. Attaran reported no financial interests.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement
This work has adhered to relevant ethical guidelines.
References
- 1.Brand F.N., Dannenberg A.L., Abbott R.D., Kannel W.B. The epidemiology of varicose veins: the Framingham Study. Am J Prev Med. 1988;4(2):96–101. [PubMed] [Google Scholar]
- 2.Pannier F., Rabe E. Progression in venous pathology. Phlebology. 2015;30(suppl 1):95–97. doi: 10.1177/0268355514568847. [DOI] [PubMed] [Google Scholar]
- 3.Pappas P.J., Lakhanpal S., Nguyen K.Q., Vanjara R. The Center for Vein Restoration Study on presenting symptoms, treatment modalities, and outcomes in Medicare-eligible patients with chronic venous disorders. J Vasc Surg Venous Lymphat Disord. 2018;6(1):13–24. doi: 10.1016/j.jvsv.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Wrona M., Jockel K.H., Pannier F., Bock E., Hoffmann B., Rabe E. Association of venous disorders with leg symptoms: results from the Bonn Vein Study 1. Eur J Vasc Endovasc Surg. 2015;50(3):360–367. doi: 10.1016/j.ejvs.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Evans C.J., Fowkes F.G., Ruckley C.V., Lee A.J. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Community Health. 1999;53(3):149–153. doi: 10.1136/jech.53.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Criqui M.H., Jamosmos M., Fronek A., et al. Chronic venous disease in an ethnically diverse population: the San Diego Population Study. Am J Epidemiol. 2003;158(5):448–456. doi: 10.1093/aje/kwg166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carradice D., Mazari F.A., Samuel N., Allgar V., Hatfield J., Chetter I.C. Modelling the effect of venous disease on quality of life. Br J Surg. 2011;98(8):1089–1098. doi: 10.1002/bjs.7500. [DOI] [PubMed] [Google Scholar]
- 8.Beebe-Dimmer J.L., Pfeifer J.R., Engle J.S., Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol. 2005;15(3):175–184. doi: 10.1016/j.annepidem.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 9.da Silva A., Widmer L.K., Martin H., Mall T., Glaus L., Schneider M. Varicose veins and chronic venous insufficiency. Vasa. 1974;3(2):118–125. [PubMed] [Google Scholar]
- 10.Robertson L., Evans C., Fowkes F.G. Epidemiology of chronic venous disease. Phlebology. 2008;23(3):103–111. doi: 10.1258/phleb.2007.007061. [DOI] [PubMed] [Google Scholar]
- 11.Robertson L.A., Evans C.J., Lee A.J., Allan P.L., Ruckley C.V., Fowkes F.G. Incidence and risk factors for venous reflux in the general population: Edinburgh Vein Study. Eur J Vasc Endovasc Surg. 2014;48(2):208–214. doi: 10.1016/j.ejvs.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Salim S., Machin M., Patterson B.O., Onida S., Davies A.H. Global epidemiology of chronic venous disease: a systematic review with pooled prevalence analysis. Ann Surg. 2021;274(6):971–976. doi: 10.1097/SLA.0000000000004631. [DOI] [PubMed] [Google Scholar]
- 13.Eberhardt R.T., Raffetto J.D. Chronic venous insufficiency. Circulation. 2014;130(4):333–346. doi: 10.1161/CIRCULATIONAHA.113.006898. [DOI] [PubMed] [Google Scholar]
- 14.Chi Y.W., Raffetto J.D. Venous leg ulceration pathophysiology and evidence based treatment. Vasc Med. 2015;20(2):168–181. doi: 10.1177/1358863X14568677. [DOI] [PubMed] [Google Scholar]
- 15.Moore H.M., Lane T.R., Thapar A., Franklin I.J., Davies A.H. The European burden of primary varicose veins. Phlebology. 2013;28(suppl 1):141–147. doi: 10.1177/0268355512475118. [DOI] [PubMed] [Google Scholar]
- 16.Dua A., Desai S.S., Heller J.A. The impact of race on advanced chronic venous insufficiency. Ann Vasc Surg. 2016;34:152–156. doi: 10.1016/j.avsg.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Nelzen O., Bergqvist D., Lindhagen A. Venous and non-venous leg ulcers: clinical history and appearance in a population study. Br J Surg. 1994;81(2):182–187. doi: 10.1002/bjs.1800810206. [DOI] [PubMed] [Google Scholar]
- 18.Lee A.J., Robertson L.A., Boghossian S.M., et al. Progression of varicose veins and chronic venous insufficiency in the general population in the Edinburgh Vein Study. J Vasc Surg Venous Lymphat Disord. 2015;3(1):18–26. doi: 10.1016/j.jvsv.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Margolis D.J., Bilker W., Santanna J., Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol. 2002;46(3):381–386. doi: 10.1067/mjd.2002.121739. [DOI] [PubMed] [Google Scholar]
- 20.Couzan S., Leizorovicz A., Laporte S., et al. A randomized double-blind trial of upward progressive versus degressive compressive stockings in patients with moderate to severe chronic venous insufficiency. J Vasc Surg. 2012;56(5) doi: 10.1016/j.jvs.2012.02.060. 1344-135. [DOI] [PubMed] [Google Scholar]
- 21.Maddox D. Effects of venous leg ulceration on patients’ quality of life. Nurs Stand. 2012;26(38):42–49. doi: 10.7748/ns2012.05.26.38.42.c9111. [DOI] [PubMed] [Google Scholar]
- 22.Phillips C.J., Humphreys I., Thayer D., et al. Cost of managing patients with venous leg ulcers. Int Wound J. 2020;17(4):1074–1082. doi: 10.1111/iwj.13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice J.B., Desai U., Cummings A.K., Birnbaum H.G., Skornicki M., Parsons N. Burden of venous leg ulcers in the United States. J Med Econ. 2014;17(5):347–356. doi: 10.3111/13696998.2014.903258. [DOI] [PubMed] [Google Scholar]
- 24.Prabhakar A.M., Misono A.S., Sheth R.A., et al. Changing medicare utilization of minimally invasive procedures for the treatment of chronic venous insufficiency. J Vasc Interv Radiol. 2017;28(6):818–824. doi: 10.1016/j.jvir.2017.02.034. [DOI] [PubMed] [Google Scholar]
- 25.Meissner M.H., Gloviczki P., Bergan J., et al. Primary chronic venous disorders. J Vasc Surg. 2007;46(6):54S–S67. doi: 10.1016/j.jvs.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 26.Pocock E.S., Alsaigh T., Mazor R., Schmid-Schonbein G.W. Cellular and molecular basis of venous insufficiency. Vasc Cell. 2014;6(1):24. doi: 10.1186/s13221-014-0024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansilha A., Sousa J. Pathophysiological mechanisms of chronic venous disease and implications for venoactive drug therapy. Int J Mol Sci. 2018;19(6):1669. doi: 10.3390/ijms19061669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uhl J.F., Gillot C. Anatomy of the foot venous pump: physiology and influence on chronic venous disease. Phlebology. 2012;27(5):219–230. doi: 10.1258/phleb.2012.012b01. [DOI] [PubMed] [Google Scholar]
- 29.Ludbrook J. The musculovenous pumps of the human lower limb. Am Heart J. 1966;71(5):635–641. doi: 10.1016/0002-8703(66)90313-9. [DOI] [PubMed] [Google Scholar]
- 30.Pfisterer L., Konig G., Hecker M., Korff T. Pathogenesis of varicose veins—lessons from biomechanics. Vasa. 2014;43(2):88–99. doi: 10.1024/0301-1526/a000335. [DOI] [PubMed] [Google Scholar]
- 31.Takase S., Pascarella L., Lerond L., Bergan J.J., Schmid-Schonbein G.W. Venous hypertension, inflammation and valve remodeling. Eur J Vasc Endovasc Surg. 2004;28(5):484–493. doi: 10.1016/j.ejvs.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Takase S., Pascarella L., Bergan J.J., Schmid-Schonbein G.W. Hypertension-induced venous valve remodeling. J Vasc Surg. 2004;39(6):1329–1334. doi: 10.1016/j.jvs.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 33.Takase S., Lerond L., Bergan J.J., Schmid-Schonbein G.W. The inflammatory reaction during venous hypertension in the rat. Microcirculation. 2000;7(1):41–52. [PubMed] [Google Scholar]
- 34.Payne S.P., London N.J., Newland C.J., Thrush A.J., Barrie W.W., Bell P.R. Ambulatory venous pressure: correlation with skin condition and role in identifying surgically correctible disease. Eur J Vasc Endovasc Surg. 1996;11(2):195–200. doi: 10.1016/s1078-5884(96)80051-7. [DOI] [PubMed] [Google Scholar]
- 35.Nicolaides A.N., Hussein M.K., Szendro G., Christopoulos D., Vasdekis S., Clarke H. The relation of venous ulceration with ambulatory venous pressure measurements. J Vasc Surg. 1993;17(2):414–419. doi: 10.1067/mva.1993.37694. [DOI] [PubMed] [Google Scholar]
- 36.Lin J., Chudasama N., Hayashi Y., et al. Peripheral venous congestion causes time- and dose-dependent release of endothelin-1 in humans. Physiol Rep. 2017;5(6) doi: 10.14814/phy2.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacColl E., Khalil R.A. Matrix metalloproteinases as regulators of vein structure and function: implications in chronic venous disease. J Pharmacol Exp Ther. 2015;355(3):410–428. doi: 10.1124/jpet.115.227330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herouy Y., Pornschlegel G., Stetter C., et al. Lipodermatosclerosis is characterized by elevated expression and activation of matrix metalloproteinases: implications for venous ulcer formation. J Invest Dermatol. 1998;111(5):822–827. doi: 10.1046/j.1523-1747.1998.00369.x. [DOI] [PubMed] [Google Scholar]
- 39.Meyer F.J., Burnand K.G., Abisi S., Tekoppele J.M., van Els B., Smith A. Effect of collagen turnover and matrix metalloproteinase activity on healing of venous leg ulcers. Br J Surg. 2008;95(3):319–325. doi: 10.1002/bjs.5946. [DOI] [PubMed] [Google Scholar]
- 40.Pappas P.J., Teehan E.P., Fallek S.R., et al. Diminished mononuclear cell function is associated with chronic venous insufficiency. J Vasc Surg. 1995;22(5):580–586. doi: 10.1016/s0741-5214(95)70042-0. [DOI] [PubMed] [Google Scholar]
- 41.Pappas P.J., Fallek S.R., Garcia A., et al. Role of leukocyte activation in patients with venous stasis ulcers. J Surg Res. 1995;59(5):553–559. doi: 10.1006/jsre.1995.1205. [DOI] [PubMed] [Google Scholar]
- 42.Sansilvestri-Morel P., Rupin A., Badier-Commander C., et al. Imbalance in the synthesis of collagen type I and collagen type III in smooth muscle cells derived from human varicose veins. J Vasc Res. 2001;38(6):560–568. doi: 10.1159/000051092. [DOI] [PubMed] [Google Scholar]
- 43.Sansilvestri-Morel P., Fioretti F., Rupin A., et al. Comparison of extracellular matrix in skin and saphenous veins from patients with varicose veins: does the skin reflect venous matrix changes? Clin Sci (Lond) 2007;112(4):229–239. doi: 10.1042/CS20060170. [DOI] [PubMed] [Google Scholar]
- 44.Kirsch D., Dienes H.P., Küchle R., et al. Changes in the extracellular matrix of the vein wall—the cause of primary varicosis? VASA. 2000;29(3):173–177. doi: 10.1024/0301-1526.29.3.173. [DOI] [PubMed] [Google Scholar]
- 45.Xiao Y., Huang Z., Yin H., Lin Y., Wang S. In vitro differences between smooth muscle cells derived from varicose veins and normal veins. J Vasc Surg. 2009;50(5):1149–1154. doi: 10.1016/j.jvs.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 46.Badier-Commander C., Couvelard A., Henin D., Verbeuren T., Michel J.B., Jacob M.P. Smooth muscle cell modulation and cytokine overproduction in varicose veins. An in situ study. J Pathol. 2001;193(3):398–407. doi: 10.1002/path.819. [DOI] [PubMed] [Google Scholar]
- 47.Rizzi A., Quaglio D., Vasquez G., et al. Effects of vasoactive agents in healthy and diseased human saphenous veins. J Vasc Surg. 1998;28(5):855–861. doi: 10.1016/s0741-5214(98)70061-8. [DOI] [PubMed] [Google Scholar]
- 48.Wlaschek M., Singh K., Sindrilaru A., Crisan D., Scharffetter-Kochanek K. Iron and iron-dependent reactive oxygen species in the regulation of macrophages and fibroblasts in non-healing chronic wounds. Free Radic Biol Med. 2019;133:262–275. doi: 10.1016/j.freeradbiomed.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 49.Caggiati A., Rosi C., Casini A., et al. Skin iron deposition characterises lipodermatosclerosis and leg ulcer. Eur J Vasc Endovasc Surg. 2010;40(6):777–782. doi: 10.1016/j.ejvs.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 50.Lattimer C.R., Kalodiki E., Geroulakos G., Hoppensteadt D., Fareed J. Are inflammatory biomarkers increased in varicose vein blood? Clin Appl Thromb Hemost. 2016;22(7):656–664. doi: 10.1177/1076029616645330. [DOI] [PubMed] [Google Scholar]
- 51.Lattimer C.R., Kalodiki E., Geroulakos G., Syed D., Hoppensteadt D., Fareed J. d-Dimer levels are significantly increased in blood taken from varicose veins compared with antecubital blood from the same patient. Angiology. 2015;66(9):882–888. doi: 10.1177/0003319714565168. [DOI] [PubMed] [Google Scholar]
- 52.Thomas P.R., Nash G.B., Dormandy J.A. Increased white cell trapping in the dependent legs of patients with chronic venous insufficiency. J Mal Vasc. 1991;16(1):35–37. [PubMed] [Google Scholar]
- 53.Ortega M.A., Fraile-Martinez O., Garcia-Montero C., et al. Chronic venous disease patients show increased IRS-4 expression in the great saphenous vein wall. J Int Med Res. 2021;49(9) doi: 10.1177/03000605211041275. 03000605211041275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varat M.A., Fowler N.O., Adolph R.J. Cardiac output response to exercise in patients with inferior vena caval ligation. Circulation. 1970;42(3):445–453. doi: 10.1161/01.cir.42.3.445. [DOI] [PubMed] [Google Scholar]
- 55.Segel M.J., Reuveny R., Luboshitz J., Shlomi D., Ben-Dov I. Chronic iliofemoral vein obstruction—an under-recognized cause of exercise limitation. Eur J Sport Sci. 2018;18(7):1022–1028. doi: 10.1080/17461391.2018.1461244. [DOI] [PubMed] [Google Scholar]
- 56.Rusinovich Y., Rusinovich V. Cardiac Doppler in patients with primary varicose veins of lower extremities. Phlebology. 2020;35(1):62–66. doi: 10.1177/0268355519848895. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J., Wu Z., Feng Q., Huang H., Ma Y. Cardiac Doppler parameters and progress in clinical manifestation of primary lower extremity varicose veins: a prospective study in China. Front Surg. 2022;9:791598. doi: 10.3389/fsurg.2022.791598. eCollection 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prochaska J.H., Arnold N., Falcke A., et al. Chronic venous insufficiency, cardiovascular disease, and mortality: a population study. Eur Heart J. 2021;42(40):4157–4165. doi: 10.1093/eurheartj/ehab495. [DOI] [PubMed] [Google Scholar]
- 59.Lurie F., Passman M., Meisner M., et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord. 2020;8(3):342–352. doi: 10.1016/j.jvsv.2019.12.075. [DOI] [PubMed] [Google Scholar]
- 60.Kakkos S.K., Rivera M.A., Matsagas M.I., et al. Validation of the new venous severity scoring system in varicose vein surgery. J Vasc Surg. 2003;38(2):224–228. doi: 10.1016/s0741-5214(03)00323-9. [DOI] [PubMed] [Google Scholar]
- 61.Rutherford R.B., Padberg F.T., Jr., Comerota A.J., Kistner R.L., Meissner M.H., Moneta G.L. Venous severity scoring: an adjunct to venous outcome assessment. J Vasc Surg. 2000;31(6):1307–1312. doi: 10.1067/mva.2000.107094. [DOI] [PubMed] [Google Scholar]
- 62.Meissner M.H., Natiello C., Nicholls S.C. Performance characteristics of the venous clinical severity score. J Vasc Surg. 2002;36(5):889–895. doi: 10.1067/mva.2002.128637. [DOI] [PubMed] [Google Scholar]
- 63.Kuet M.L., Lane T.R., Anwar M.A., Davies A.H. Comparison of disease-specific quality of life tools in patients with chronic venous disease. Phlebology. 2014;29(10):648–653. doi: 10.1177/0268355513501302. [DOI] [PubMed] [Google Scholar]
- 64.Iglesias C.P., Birks Y., Nelson E.A., Scanlon E., Cullum N.A. Quality of life of people with venous leg ulcers: a comparison of the discriminative and responsive characteristics of two generic and a disease specific instruments. Qual Life Res. 2005;14(7):1705–1718. doi: 10.1007/s11136-005-2751-9. [DOI] [PubMed] [Google Scholar]
- 65.Lamping D.L., Schroter S., Kurz X., Kahn S.R., Abenhaim L. Evaluation of outcomes in chronic venous disorders of the leg: development of a scientifically rigorous, patient-reported measure of symptoms and quality of life. J Vasc Surg. 2003;37(2):410–419. doi: 10.1067/mva.2003.152. [DOI] [PubMed] [Google Scholar]
- 66.Gibson K., Kabnick L. A multicenter, randomized, placebo-controlled study to evaluate the efficacy and safety of Varithena (polidocanol endovenous microfoam 1%) for symptomatic, visible varicose veins with saphenofemoral junction incompetence. Phlebology. 2017;32(3):185–193. doi: 10.1177/0268355516635386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lattimer C.R., Kalodiki E., Azzam M., Geroulakos G. Validation of the Villalta scale in assessing post-thrombotic syndrome using clinical, duplex, and hemodynamic comparators. J Vasc Surg Venous Lymphat Disord. 2013;1(1):104–105. doi: 10.1016/j.jvsv.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 68.Prandoni P. In: The Diagnostic and Therapeutic Management of Deep-Vein Thrombosis and its Sequelae. Prandoni P., editor. Editgraf; 1992. Symptomatic deep-vein thrombosis and the post-thrombotic syndrome; pp. 181–196. [Google Scholar]
- 69.Villalta S., Bagatella P., Piccioli A., Lensing A., Prins M.H., Prandoni P. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24(suppl 1):158a. [Google Scholar]
- 70.Lurie F., Lal B.K., Antignani P.L., et al. Compression therapy after invasive treatment of superficial veins of the lower extremities: clinical practice guidelines of the American Venous Forum, Society for Vascular Surgery, American College of Phlebology, Society for Vascular Medicine, and International Union of Phlebology. J Vasc Surg Venous Lymphat Disord. 2019;7(1):17–28. doi: 10.1016/j.jvsv.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 71.Masuda E., Ozsvath K., Vossler J., et al. The 2020 appropriate use criteria for chronic lower extremity venous disease of the American Venous Forum, the Society for Vascular Surgery, the American Vein and Lymphatic Society, and the Society of Interventional Radiology. J Vasc Surg Venous Lymphat Disord. 2020;8(4):505–525. doi: 10.1016/j.jvsv.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 72.O’Meara S., Cullum N., Nelson E.A., Dumville J.C. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2012;11(11):CD000265. doi: 10.1002/14651858.CD000265.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bergan J.J. In: Surgery of the Veins. Bergan J.J., Yao J.S., editors. Grune & Stratton; 1985. Conrad Jobst and the development of pressure gradient therapy for venous disease; pp. 529–540. [Google Scholar]
- 74.Beaconsfield P., Ginsburg J. Effect of changes in limb posture on peripheral blood flow. Circ Res. 1955;3(5):478–482. doi: 10.1161/01.res.3.5.478. [DOI] [PubMed] [Google Scholar]
- 75.Mellander S., ÖBerg B., Odelram H. Vascular adjustments to increased transmural pressure in cat and man with special reference to shifts in capillary fluid transfer. Acta Physiol Scand. 1964;61(1-2):34–48. doi: 10.1111/j.1748-1716.1964.tb02940.x. [DOI] [PubMed] [Google Scholar]
- 76.Abu-Own A., Shami S.K., Chittenden S.J., Farrah J., Scurr J.H., Smith P.D. Microangiopathy of the skin and the effect of leg compression in patients with chronic venous insufficiency. J Vasc Surg. 1994;19(6):1074–1083. doi: 10.1016/s0741-5214(94)70220-9. [DOI] [PubMed] [Google Scholar]
- 77.Beidler S.K., Douillet C.D., Berndt D.F., Keagy B.A., Rich P.B., Marston W.A. Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. J Vasc Surg. 2009;49(4):1013–1020. doi: 10.1016/j.jvs.2008.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stout N., Partsch H., Szolnoky G., et al. Chronic edema of the lower extremities: international consensus recommendations for compression therapy clinical research trials. Int Angiol. 2012;31(4):316–329. [PubMed] [Google Scholar]
- 79.Partsch H. Indications for compression therapy in venous and lymphatic disease consensus based on experimental data and scientific evidence under the auspices of the IUP. Int Angiol. 2008;27(3):193–219. [PubMed] [Google Scholar]
- 80.Attaran R.R., Ochoa Chaar C.I. Compression therapy for venous disease. Phlebology. 2017;32(2):81–88. doi: 10.1177/0268355516633382. [DOI] [PubMed] [Google Scholar]
- 81.Nehler M.R., Moneta G.L., Woodard D.M., et al. Perimalleolar subcutaneous tissue pressure effects of elastic compression stockings. J Vasc Surg. 1993;18(5):783–788. doi: 10.1067/mva.1993.48921. [DOI] [PubMed] [Google Scholar]
- 82.Motykie G.D., Caprini J.A., Arcelus J.I., Reyna J.J., Overom E., Mokhtee D. Evaluation of therapeutic compression stockings in the treatment of chronic venous insufficiency. Dermatol Surg. 1999;25(2):116–120. doi: 10.1046/j.1524-4725.1999.08095.x. [DOI] [PubMed] [Google Scholar]
- 83.Bakker N.A., Schieven L.W., Bruins R.M., van den Berg M., Hissink R.J. Compression stockings after endovenous laser ablation of the great saphenous vein: a prospective randomized controlled trial. Eur J Vasc Endovasc Surg. 2013;46(5):588–592. doi: 10.1016/j.ejvs.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 84.El-Sheikha J., Carradice D., Nandhra S., et al. Systematic review of compression following treatment for varicose veins. Br J Surg. 2015;102(7):719–725. doi: 10.1002/bjs.9788. [DOI] [PubMed] [Google Scholar]
- 85.Cullum N., Nelson E.A., Flemming K., Sheldon T. Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy. Health Technol Assess. 2001;5(9):1–221. doi: 10.3310/hta5090. [DOI] [PubMed] [Google Scholar]
- 86.Yang X., Zhang X., Yin M., Wang R., Lu X., Ye K. Elastic compression stockings to prevent post-thrombotic syndrome in proximal deep venous thrombosis patients without thrombus removal. J Vasc Surg Venous Lymphat Disord. 2022;10(2):293–299. doi: 10.1016/j.jvsv.2021.06.023. [DOI] [PubMed] [Google Scholar]
- 87.Kahn S.R., Shapiro S., Wells P.S., et al. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383(9920):880–888. doi: 10.1016/S0140-6736(13)61902-9. [DOI] [PubMed] [Google Scholar]
- 88.Webb E., Neeman T., Bowden F.J., Gaida J., Mumford V., Bissett B. Compression therapy to prevent recurrent cellulitis of the leg. N Engl J Med. 2020;383(7):630–639. doi: 10.1056/NEJMoa1917197. [DOI] [PubMed] [Google Scholar]
- 89.Lim S.L.X., Chung R.E., Holloway S., Harding K.G. Modified compression therapy in mixed arterial-venous leg ulcers: an integrative review. Int Wound J. 2021;18(6):822–842. doi: 10.1111/iwj.13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Georgopoulos S., Kouvelos G.N., Koutsoumpelis A., et al. The effect of revascularization procedures on healing of mixed arterial and venous leg ulcers. Int Angiol. 2013;32(4):368–374. [PubMed] [Google Scholar]
- 91.Lantis J.C., II, Boone D., Lee L., Mendes D., Benvenisty A., Todd G. The effect of percutaneous intervention on wound healing in patients with mixed arterial venous disease. Ann Vasc Surg. 2011;25(1):79–86. doi: 10.1016/j.avsg.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 92.Mayberry J.C., Moneta G.L., Taylor L.M., Jr., Porter J.M. Fifteen-year results of ambulatory compression therapy for chronic venous ulcers. Surgery. 1991;109(5):575–581. [PubMed] [Google Scholar]
- 93.Uhl J.F., Benigni J.P., Chahim M., Frederic D. Prospective randomized controlled study of patient compliance in using a compression stocking: importance of recommendations of the practitioner as a factor for better compliance. Phlebology. 2018;33(1):36–43. doi: 10.1177/0268355516682886. [DOI] [PubMed] [Google Scholar]
- 94.Franks P.J., Oldroyd M.I., Dickson D., Sharp E.J., Moffatt C.J. Risk factors for leg ulcer recurrence: a randomized trial of two types of compression stocking. Age Ageing. 1995;24(6):490–494. doi: 10.1093/ageing/24.6.490. [DOI] [PubMed] [Google Scholar]
- 95.Kankam H.K., Lim C.S., Fiorentino F., Davies A.H., Gohel M.S. A summation analysis of compliance and complications of compression hosiery for patients with chronic venous disease or post-thrombotic syndrome. Eur J Vasc Endovasc Surg. 2018;55(3):406–416. doi: 10.1016/j.ejvs.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 96.Labropoulos N., Leon M., Nicolaides A.N., Giannoukas A.D., Volteas N., Chan P. Superficial venous insufficiency: correlation of anatomic extent of reflux with clinical symptoms and signs. J Vasc Surg. 1994;20(6):953–958. doi: 10.1016/0741-5214(94)90233-x. [DOI] [PubMed] [Google Scholar]
- 97.Dwerryhouse S., Davies B., Harradine K., Earnshaw J.J. Stripping the long saphenous vein reduces the rate of reoperation for recurrent varicose veins: five-year results of a randomized trial. J Vasc Surg. 1999;29(4):589–592. doi: 10.1016/s0741-5214(99)70302-2. [DOI] [PubMed] [Google Scholar]
- 98.Keith L.M., Jr., Smead W.L. Saphenous vein stripping and its complications. Surg Clin North Am. 1983;63(6):1303–1312. doi: 10.1016/s0039-6109(16)43190-7. [DOI] [PubMed] [Google Scholar]
- 99.Proebstle T.M., Alm B.J., Gockeritz O., et al. Five-year results from the prospective European multicentre cohort study on radiofrequency segmental thermal ablation for incompetent great saphenous veins. Br J Surg. 2015;102(3):212–218. doi: 10.1002/bjs.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morrison N., Gibson K., Vasquez M., Weiss R., Jones A. Five-year extension study of patients from a randomized clinical trial (VeClose) comparing cyanoacrylate closure versus radiofrequency ablation for the treatment of incompetent great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2020;8(6):978–989. doi: 10.1016/j.jvsv.2019.12.080. [DOI] [PubMed] [Google Scholar]
- 101.Spreafico G., Kabnick L., Berland T.L., et al. Laser saphenous ablations in more than 1,000 limbs with long-term duplex examination follow-up. Ann Vasc Surg. 2011;25(1):71–78. doi: 10.1016/j.avsg.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 102.van Eekeren R.R., Boersma D., Holewijn S., Werson D.A., de Vries J.P., Reijnen M.M. Mechanochemical endovenous ablation for the treatment of great saphenous vein insufficiency. J Vasc Surg Venous Lymphat Disord. 2014;2(3):282–288. doi: 10.1016/j.jvsv.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 103.Todd K.L., III, Wright D.I., Gibson K., et al. Durability of treatment effect with polidocanol endovenous microfoam on varicose vein symptoms and appearance (VANISH-2) J Vasc Surg Venous Lymphat Disord. 2015;3(3):258–264. doi: 10.1016/j.jvsv.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 104.King J.T., O'Byrne M., Vasquez M., et al. Treatment of truncal incompetence and varicose veins with a single administration of a new polidocanol endovenous microfoam preparation improves symptoms and appearance. Eur J Vasc Endovasc Surg. 2015;50(6):784–793. doi: 10.1016/j.ejvs.2015.06.111. [DOI] [PubMed] [Google Scholar]
- 105.Kabnick L.S. Outcome of different endovenous laser wavelengths for great saphenous vein ablation. J Vasc Surg. 2006;43(1):88–93. doi: 10.1016/j.jvs.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 106.Balint R., Farics A., Parti K., et al. Which endovenous ablation method does offer a better long-term technical success in the treatment of the incompetent great saphenous vein? Review. Vascular. 2016;24(6):649–657. doi: 10.1177/1708538116648035. [DOI] [PubMed] [Google Scholar]
- 107.Goode S.D., Chowdhury A., Crockett M., et al. Laser and radiofrequency ablation study (LARA study): a randomised study comparing radiofrequency ablation and endovenous laser ablation (810 nm) Eur J Vasc Endovasc Surg. 2010;40(2):246–253. doi: 10.1016/j.ejvs.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 108.Dermody M., Schul M.W., O'Donnell T.F. Thromboembolic complications of endovenous thermal ablation and foam sclerotherapy in the treatment of great saphenous vein insufficiency. Phlebology. 2015;30(5):357–364. doi: 10.1177/0268355514529948. [DOI] [PubMed] [Google Scholar]
- 109.Kane K., Fisher T., Bennett M., et al. The incidence and outcome of endothermal heat-induced thrombosis after endovenous laser ablation. Ann Vasc Surg. 2014;28(7):1744–1750. doi: 10.1016/j.avsg.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 110.Bergan J., Cheng V. Foam sclerotherapy for the treatment of varicose veins. Vascular. 2007;15(5):269–272. doi: 10.2310/6670.2007.00066. [DOI] [PubMed] [Google Scholar]
- 111.Fegan W.G. Continuous compression technique of injecting varicose veins. Lancet. 1963;2(7299):109–112. doi: 10.1016/s0140-6736(63)92583-2. [DOI] [PubMed] [Google Scholar]
- 112.de Ávila Oliveira R., Riera R., Vasconcelos V., Baptista-Silva J.C. Injection sclerotherapy for varicose veins. Cochrane Database Syst Rev. 2021;12(12) doi: 10.1002/14651858.CD001732.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alder G., Lees T. Foam sclerotherapy. Phlebology. 2015;30(suppl 2):18–23. doi: 10.1177/0268355515589536. [DOI] [PubMed] [Google Scholar]
- 114.Orbach E.J. Clinical evaluation of a new technic in the sclerotherapy of varicose veins. J Int Coll Surg. 1948;11(4):396–402. [PubMed] [Google Scholar]
- 115.Tessari L., Cavezzi A., Frullini A. Preliminary experience with a new sclerosing foam in the treatment of varicose veins. Dermatol Surg. 2001;27(1):58–60. [PubMed] [Google Scholar]
- 116.Guex J.J., Allaert F.A., Gillet J.L., Chleir F. Immediate and midterm complications of sclerotherapy: report of a prospective multicenter registry of 12,173 sclerotherapy sessions. Dermatol Surg. 2005;31(2):123–128. doi: 10.1111/j.1524-4725.2005.31030. [DOI] [PubMed] [Google Scholar]
- 117.Willenberg T., Smith P.C., Shepherd A., Davies A.H. Visual disturbance following sclerotherapy for varicose veins, reticular veins and telangiectasias: a systematic literature review. Phlebology. 2013;28(3):123–131. doi: 10.1258/phleb.2012.012051. [DOI] [PubMed] [Google Scholar]
- 118.Hafner F., Froehlich H., Gary T., Brodmann M. Intra-arterial injection, a rare but serious complication of sclerotherapy. Phlebology. 2013;28(2):64–73. doi: 10.1258/phleb.2011.011155. [DOI] [PubMed] [Google Scholar]
- 119.Tisi P.V., Beverley C., Rees A. Injection sclerotherapy for varicose veins. Cochrane Database Syst Rev. 2006;4 doi: 10.1002/14651858.CD001732.pub2. CD001732. [DOI] [PubMed] [Google Scholar]
- 120.Todd K.L., III, Wright D.I., VANISH-2 Investor Group The VANISH-2 study: a randomized, blinded, multicenter study to evaluate the efficacy and safety of polidocanol endovenous microfoam 0.5% and 1.0% compared with placebo for the treatment of saphenofemoral junction incompetence. Phlebology. 2014;29(9):608–618. doi: 10.1177/0268355513497709. [DOI] [PubMed] [Google Scholar]
- 121.Deijen C.L., Schreve M.A., Bosma J., et al. Clarivein mechanochemical ablation of the great and small saphenous vein: early treatment outcomes of two hospitals. Phlebology. 2016;31(3):192–197. doi: 10.1177/0268355515600573. [DOI] [PubMed] [Google Scholar]
- 122.Vähäaho S., Mahmoud O., Halmesmäki K., et al. Randomized clinical trial of mechanochemical and endovenous thermal ablation of great saphenous varicose veins. Br J Surg. 2019;106(5):548–554. doi: 10.1002/bjs.11158. [DOI] [PubMed] [Google Scholar]
- 123.Morrison N., Kolluri R., Vasquez M., Madsen M., Jones A., Gibson K. Comparison of cyanoacrylate closure and radiofrequency ablation for the treatment of incompetent great saphenous veins: 36-month outcomes of the VeClose randomized controlled trial. Phlebology. 2019;34(6):380–390. doi: 10.1177/0268355518810259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Park I. Initial outcomes of cyanoacrylate closure, VenaSeal system, for the treatment of the incompetent great and small saphenous veins. Vasc Endovascular Surg. 2017;51(8):545–549. doi: 10.1177/1538574417729272. [DOI] [PubMed] [Google Scholar]
- 125.Proebstle T.M., Alm J., Dimitri S., et al. The European multicenter cohort study on cyanoacrylate embolization of refluxing great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2015;3(1):2–7. doi: 10.1016/j.jvsv.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 126.Brittenden J., Cooper D., Dimitrova M., et al. Five-year outcomes of a randomized trial of treatments for varicose veins. N Engl J Med. 2019;381(10):912–922. doi: 10.1056/NEJMoa1805186. [DOI] [PubMed] [Google Scholar]
- 127.Rasmussen L.H., Lawaetz M., Bjoern L., Vennits B., Blemings A., Eklof B. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Br J Surg. 2011;98(8):1079–1087. doi: 10.1002/bjs.7555. [DOI] [PubMed] [Google Scholar]
- 128.Whing J., Nandhra S., Nesbitt C., Stansby G. Interventions for great saphenous vein incompetence. Cochrane Database Syst Rev. 2021;8(8) doi: 10.1002/14651858.CD005624.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gohel M.S., Heatley F., Liu X., et al. A randomized trial of early endovenous ablation in venous ulceration. N Engl J Med. 2018;378(22):2105–2114. doi: 10.1056/NEJMoa1801214. [DOI] [PubMed] [Google Scholar]
- 130.Gohel M.S., Mora M.J., Szigeti M., et al. Long-term clinical and cost-effectiveness of early endovenous ablation in venous ulceration: a randomized clinical trial. JAMA Surg. 2020;155(12):1113–1121. doi: 10.1001/jamasurg.2020.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.O'Donnell T.F., Balk E.M., Dermody M., Tangney E., Iafrati M.D. Recurrence of varicose veins after endovenous ablation of the great saphenous vein in randomized trials. J Vasc Surg Venous Lymphat Disord. 2016;4(1):97–105. doi: 10.1016/j.jvsv.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 132.Bush R.G., Bush P., Flanagan J., et al. Factors associated with recurrence of varicose veins after thermal ablation: results of the recurrent veins after thermal ablation study. Sci World J. 2014;2014:505843. doi: 10.1155/2014/505843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lawrence P.F., Hager E.S., Harlander-Locke M.P., et al. Treatment of superficial and perforator reflux and deep venous stenosis improves healing of chronic venous leg ulcers. J Vasc Surg Venous Lymphat Disord. 2020;8(4):601–609. doi: 10.1016/j.jvsv.2019.09.016. [DOI] [PubMed] [Google Scholar]
- 134.Reitz K.M., Salem K., Mohapatra A., et al. Complete venous ulceration healing after perforator ablation does not depend on treatment modality. Ann Vasc Surg. 2021;70:109–115. doi: 10.1016/j.avsg.2020.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hager E.S., Washington C., Steinmetz A., Wu T., Singh M., Dillavou E. Factors that influence perforator vein closure rates using radiofrequency ablation, laser ablation, or foam sclerotherapy. J Vasc Surg Venous Lymphat Disord. 2016;4(1):51–56. doi: 10.1016/j.jvsv.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 136.Marsh P., Price B.A., Holdstock J.M., Whiteley M.S. One-year outcomes of radiofrequency ablation of incompetent perforator veins using the radiofrequency stylet device. Phlebology. 2010;25(2):79–84. doi: 10.1258/phleb.2009.008084. [DOI] [PubMed] [Google Scholar]
- 137.Kiguchi M.M., Hager E.S., Winger D.G., Hirsch S.A., Chaer R.A., Dillavou E.D. Factors that influence perforator thrombosis and predict healing with perforator sclerotherapy for venous ulceration without axial reflux. J Vasc Surg. 2014;59(5):1368–1376. doi: 10.1016/j.jvs.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Danielsson G., Eklof B., Grandinetti A., Lurie F., Kistner R.L. Deep axial reflux, an important contributor to skin changes or ulcer in chronic venous disease. J Vasc Surg. 2003;38(6):1336–1341. doi: 10.1016/s0741-5214(03)00907-8. [DOI] [PubMed] [Google Scholar]
- 139.Kistner R.L., Ferris E.B., Randhawa G., Kamida C. A method of performing descending venography. J Vasc Surg. 1986;4(5):464–468. doi: 10.1067/mva.1986.avs0040464. [DOI] [PubMed] [Google Scholar]
- 140.Kim S.M., Jung I.M., Chung J.K. Improvements of deep vein reflux following radiofrequency ablation for saphenous vein incompetence. Phlebology. 2017;32(1):55–60. doi: 10.1177/0268355516629867. [DOI] [PubMed] [Google Scholar]
- 141.Kahn S.R., Comerota A.J., Cushman M., et al. The postthrombotic syndrome: evidence-based prevention, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2014;130(18):1636–1661. doi: 10.1161/CIR.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 142.Raju S., Neglen P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg. 2006;44(1):136–143. doi: 10.1016/j.jvs.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 143.Johnson B.F., Manzo R.A., Bergelin R.O., Strandness D.E., Jr. The site of residual abnormalities in the leg veins in long-term follow-up after deep vein thrombosis and their relationship to the development of the post-thrombotic syndrome. Int Angiol. 1996;15(1):14–19. [PubMed] [Google Scholar]
- 144.Queral L.A., Whitehouse W.M., Jr., Flinn W.R., Neiman H.L., Yao J.S., Bergan J.J. Surgical correction of chronic deep venous insufficiency by valvular transposition. Surgery. 1980;87(6):688–695. [PubMed] [Google Scholar]
- 145.Raju S., Neglen P., Doolittle J., Meydrech E.F. Axillary vein transfer in trabeculated postthrombotic veins. J Vasc Surg. 1999;29(6):1050–1062. doi: 10.1016/s0741-5214(99)70246-6. [DOI] [PubMed] [Google Scholar]
- 146.Taheri S.A., Lazar L., Elias S., Marchand P., Heffner R. Surgical treatment of postphlebitic syndrome with vein valve transplant. Am J Surg. 1982;144(2):221–224. doi: 10.1016/0002-9610(82)90512-8. [DOI] [PubMed] [Google Scholar]
- 147.Lugli M., Guerzoni S., Garofalo M., Smedile G., Maleti O. Neovalve construction in deep venous incompetence. J Vasc Surg. 2009;49(1):156–162. doi: 10.1016/j.jvs.2008.07.089. [DOI] [PubMed] [Google Scholar]
- 148.Camilli S., Guarnera G. External banding valvuloplasty of the superficial femoral vein in the treatment of primary deep valvular incompetence. Int Angiol. 1994;13(3):218–222. [PubMed] [Google Scholar]
- 149.Vasudevan T., Robinson D.A., Hill A.A., et al. Safety and feasibility report on nonimplantable endovenous valve formation for the treatment of deep vein reflux. J Vasc Surg Venous Lymphat Disord. 2021;9(5):1200–1208. doi: 10.1016/j.jvsv.2020.12.073. [DOI] [PubMed] [Google Scholar]
- 150.MacKenzie R.K., Allan P.L., Ruckley C.V., Bradbury A.W. The effect of long saphenous vein stripping on deep venous reflux. Eur J Vasc Endovasc Surg. 2004;28(1):104–107. doi: 10.1016/j.ejvs.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 151.Sales C.M., Bilof M.L., Petrillo K.A., Luka N.L. Correction of lower extremity deep venous incompetence by ablation of superficial venous reflux. Ann Vasc Surg. 1996;10(2):186–189. doi: 10.1007/BF02000764. [DOI] [PubMed] [Google Scholar]
- 152.Walsh J.C., Bergan J.J., Beeman S., Comer T.P. Femoral venous reflux abolished by greater saphenous vein stripping. Ann Vasc Surg. 1994;8(6):566–570. doi: 10.1007/BF02017413. [DOI] [PubMed] [Google Scholar]
- 153.May R., Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8(5):419–427. doi: 10.1177/000331975700800505. [DOI] [PubMed] [Google Scholar]
- 154.May R., Thurner J. A vascular spur in the vena iliaca communis sinistra as a cause of predominantly left-sided thrombosis of the pelvic veins. Z Kreislaufforsch. 1956;45(23-24):912–922. [PubMed] [Google Scholar]
- 155.Hermany P.L., Badheka A.O., Mena-Hurtado C.I., Attaran R.R. A unique case of may-thurner syndrome: extrinsic compression of the common iliac vein after iliac artery stenting. JACC Cardiovasc Interv. 2016;9(5):e39–e41. doi: 10.1016/j.jcin.2015.11.042. [DOI] [PubMed] [Google Scholar]
- 156.Hermus L., Tielliu I.F., Zeebregts C.J., Prins T.R., Van Den Dungen J.J. B-cell lymphoma related iliac vein occlusion treated by endovenous stent placement. Minerva Chir. 2012;67(3):277–282. [PubMed] [Google Scholar]
- 157.Liao T.Y., Hsu H.C., Wen M.S., Juan Y.H., Hung Y.H., Liaw C.C. Iliofemoral venous thrombosis mainly related to iliofemoral venous obstruction by external tumor compression in cancer patients. Case Rep Oncol. 2016;9(3):760–771. doi: 10.1159/000452943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xu F., Tian Z., Huang X., et al. A case report of May-Thurner syndrome induced by anterior lumbar disc herniation: novel treatment with radiofrequency thermocoagulation. Medicine (Baltimore) 2019;98(44) doi: 10.1097/MD.0000000000017706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kibbe M.R., Ujiki M., Goodwin A.L., Eskandari M., Yao J., Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39(5):937–943. doi: 10.1016/j.jvs.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 160.Negus D., Fletcher E.W., Cockett F.B., Thomas M.L. Compression and band formation at the mouth of the left common iliac vein. Br J Surg. 1968;55(5):369–374. doi: 10.1002/bjs.1800550510. [DOI] [PubMed] [Google Scholar]
- 161.Jayaraj A., Crim W., Knight A., Raju S. Characteristics and outcomes of stent occlusion after iliocaval stenting. J Vasc Surg Venous Lymphat Disord. 2019;7(1):56–64. doi: 10.1016/j.jvsv.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 162.Raju S., Kirk O., Davis M., Olivier J. Hemodynamics of “critical” venous stenosis and stent treatment. J Vasc Surg Venous Lymphat Disord. 2014;2(1):52–59. doi: 10.1016/j.jvsv.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 163.Raju S. Long-term outcomes of stent placement for symptomatic nonthrombotic iliac vein compression lesions in chronic venous disease. J Vasc Interv Radiol. 2012;23(4):502–503. doi: 10.1016/j.jvir.2012.01.068. [DOI] [PubMed] [Google Scholar]
- 164.Raju S., Darcey R., Neglen P. Unexpected major role for venous stenting in deep reflux disease. J Vasc Surg. 2010;51(2):401–408. doi: 10.1016/j.jvs.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 165.Raju S. Best management options for chronic iliac vein stenosis and occlusion. J Vasc Surg. 2013;57(4):1163–1169. doi: 10.1016/j.jvs.2012.11.084. [DOI] [PubMed] [Google Scholar]
- 166.Neglen P., Hollis K.C., Olivier J., Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46(5):979–990. doi: 10.1016/j.jvs.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 167.Razavi M.K., Jaff M.R., Miller L.E. Safety and effectiveness of stent placement for iliofemoral venous outflow obstruction: systematic review and meta-analysis. Circ Cardiovasc Interv. 2015;8(10) doi: 10.1161/CIRCINTERVENTIONS.115.002772. [DOI] [PubMed] [Google Scholar]
- 168.Charnsangavej C., Carrasco C.H., Wallace S., et al. Stenosis of the vena cava: preliminary assessment of treatment with expandable metallic stents. Radiology. 1986;161(2):295–298. doi: 10.1148/radiology.161.2.3763891. [DOI] [PubMed] [Google Scholar]
- 169.Carrasco C.H., Charnsangavej C., Wright K.C., Wallace S., Gianturco C. Use of the Gianturco self-expanding stent in stenoses of the superior and inferior venae cavae. J Vasc Interv Radiol. 1992;3(2):409–419. doi: 10.1016/s1051-0443(92)72054-5. [DOI] [PubMed] [Google Scholar]
- 170.Berger A., Jaffe J.W., York T.N. Iliac compression syndrome treated with stent placement. J Vasc Surg. 1995;21(3):510–514. doi: 10.1016/s0741-5214(95)70295-4. [DOI] [PubMed] [Google Scholar]
- 171.Nazarian G.K., Austin W.R., Wegryn S.A., et al. Venous recanalization by metallic stents after failure of balloon angioplasty or surgery: four-year experience. Cardiovasc Intervent Radiol. 1996;19(4):227–233. doi: 10.1007/BF02577640. [DOI] [PubMed] [Google Scholar]
- 172.Attaran R.R., Ozdemir D., Lin I.H., Mena-Hurtado C., Lansky A. Evaluation of anticoagulant and antiplatelet therapy after iliocaval stenting: factors associated with stent occlusion. J Vasc Surg Venous Lymphat Disord. 2019;7(4):527–534. doi: 10.1016/j.jvsv.2019.01.058. [DOI] [PubMed] [Google Scholar]
- 173.Sayed M.H., Salem M., Desai K.R., O'Sullivan G.J., Black S.A. A review of the incidence, outcome, and management of venous stent migration. J Vasc Surg Venous Lymphat Disord. 2022;10(2):482–490. doi: 10.1016/j.jvsv.2021.07.015. [DOI] [PubMed] [Google Scholar]
- 174.Maleti O., Perrin M. Reconstructive surgery for deep vein reflux in the lower limbs: techniques, results and indications. Eur J Vasc Endovasc Surg. 2011;41(6):837–848. doi: 10.1016/j.ejvs.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 175.Cheng C.P., Dua A., Suh G.Y., Shah R.P., Black S.A. The biomechanical impact of hip movement on iliofemoral venous anatomy and stenting for deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2020;8(6):953–960. doi: 10.1016/j.jvsv.2020.01.022. [DOI] [PubMed] [Google Scholar]
- 176.Dake M.D., O'Sullivan G., Shammas N.W., Lichtenberg M., Mwipatayi B.P., Settlage R.A. Three-year results from the venovo venous stent study for the treatment of iliac and femoral vein obstruction. Cardiovasc Intervent Radiol. 2021;44(12):1918–1929. doi: 10.1007/s00270-021-02975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Murphy E., Gibson K., Sapoval M., et al. Pivotal study evaluating the safety and effectiveness of the Abre venous self-expanding stent system in patients with symptomatic iliofemoral venous outflow obstruction. Circ Cardiovasc Interv. 2022;15(2) doi: 10.1161/CIRCINTERVENTIONS.121.010960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Razavi M., Marston W., Black S., Bentley D., Neglen P. The initial report on 1-year outcomes of the feasibility study of the VENITI VICI VENOUS STENT in symptomatic iliofemoral venous obstruction. J Vasc Surg Venous Lymphat Disord. 2018;6(2):192–200. doi: 10.1016/j.jvsv.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 179.Jayaraj A., Noel C., Kuykendall R., Raju S. Long-term outcomes following use of a composite Wallstent-Z stent approach to iliofemoral venous stenting. J Vasc Surg Venous Lymphat Disord. 2021;9(2):393–400.e2. doi: 10.1016/j.jvsv.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 180.Maeda M., Timmermans H.A., Uchida B.T., Uchida H., Keller F.S., Rosch J. In vitro comparison of the spiral Z stent and the Gianturco Z stent. J Vasc Interv Radiol. 1992;3(3):565–569. doi: 10.1016/s1051-0443(92)72016-8. [DOI] [PubMed] [Google Scholar]
- 181.Raju S., Ward M., Jr., Kirk O. A modification of iliac vein stent technique. Ann Vasc Surg. 2014;28(6):1485–1492. doi: 10.1016/j.avsg.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 182.Gagne P.J., Gasparis A., Black S., et al. Analysis of threshold stenosis by multiplanar venogram and intravascular ultrasound examination for predicting clinical improvement after iliofemoral vein stenting in the VIDIO trial. J Vasc Surg Venous Lymphat Disord. 2018;6(1):48–56. doi: 10.1016/j.jvsv.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 183.Jayaraj A., Powell T., Raju S. Utility of the 50% stenosis criterion for patients undergoing stenting for chronic iliofemoral venous obstruction. J Vasc Surg Venous Lymphat Disord. 2021;9(6):1408–1415. doi: 10.1016/j.jvsv.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 184.Leon M., Volteas N., Labropoulos N., et al. Popliteal vein entrapment in the normal population. Eur J Vasc Surg. 1992;6(6):623–627. doi: 10.1016/s0950-821x(05)80839-4. [DOI] [PubMed] [Google Scholar]
- 185.Raju S., Neglen P. Popliteal vein entrapment: a benign venographic feature or a pathologic entity? J Vasc Surg. 2000;31(4):631–641. doi: 10.1067/mva.2000.103786. [DOI] [PubMed] [Google Scholar]
- 186.Love J.W., Whelan T.J. Popliteal artery entrapment syndrome. Am J Surg. 1965;109(5):620–624. doi: 10.1016/s0002-9610(65)80016-2. [DOI] [PubMed] [Google Scholar]
- 187.Sinha S., Houghton J., Holt P.J., Thompson M.M., Loftus I.M., Hinchliffe R.J. Popliteal entrapment syndrome. J Vasc Surg. 2012;55(1):252–262. doi: 10.1016/j.jvs.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 188.White J.M., Comerota A.J. Venous compression syndromes. Vasc Endovascular Surg. 2017;51(3):155–168. doi: 10.1177/1538574417697208. [DOI] [PubMed] [Google Scholar]
- 189.Igari K., Sugano N., Kudo T., et al. Surgical treatment for popliteal artery entrapment syndrome. Ann Vasc Dis. 2014;7(1):28–33. doi: 10.3400/avd.oa.13-00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Johnson B.F., Manzo R.A., Bergelin R.O., Strandness D.E., Jr. Relationship between changes in the deep venous system and the development of the postthrombotic syndrome after an acute episode of lower limb deep vein thrombosis: a one- to six-year follow-up. J Vasc Surg. 1995;21(2):307–312. doi: 10.1016/s0741-5214(95)70271-7. [DOI] [PubMed] [Google Scholar]
- 191.Kahn S.R., Galanaud J.P., Vedantham S., Ginsberg J.S. Guidance for the prevention and treatment of the post-thrombotic syndrome. J Thromb Thrombolysis. 2016;41(1):144–153. doi: 10.1007/s11239-015-1312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kahn S.R., Shrier I., Julian J.A., et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149(10):698–707. doi: 10.7326/0003-4819-149-10-200811180-00004. [DOI] [PubMed] [Google Scholar]
- 193.Vedantham S., Goldhaber S.Z., Julian J.A., et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240–2252. doi: 10.1056/NEJMoa1615066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kahn S.R., Shbaklo H., Lamping D.L., et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6(7):1105–1112. doi: 10.1111/j.1538-7836.2008.03002.x. [DOI] [PubMed] [Google Scholar]
- 195.Kahn S.R., Hirsch A., Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med. 2002;162(10):1144–1148. doi: 10.1001/archinte.162.10.1144. [DOI] [PubMed] [Google Scholar]
- 196.Douketis J.D., Crowther M.A., Foster G.A., Ginsberg J.S. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med. 2001;110(7):515–519. doi: 10.1016/s0002-9343(01)00661-1. [DOI] [PubMed] [Google Scholar]
- 197.Tick L.W., Doggen C.J., Rosendaal F.R., et al. Predictors of the post-thrombotic syndrome with non-invasive venous examinations in patients 6 weeks after a first episode of deep vein thrombosis. J Thromb Haemost. 2010;8(12):2685–2692. doi: 10.1111/j.1538-7836.2010.04065.x. [DOI] [PubMed] [Google Scholar]
- 198.Palma E.C. Vein transplants and grafts in the surgical treatment of the postphlebitic syndrome. J Cardiovasc Surg (Torino) 1960;1:94–107. [PubMed] [Google Scholar]
- 199.Garcia M.J., Sterling K.M., Kahn S.R., et al. Ultrasound-accelerated thrombolysis and venoplasty for the treatment of the postthrombotic syndrome: results of the ACCESS PTS study. J Am Heart Assoc. 2020;9(3) doi: 10.1161/JAHA.119.013398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Raju S. Treatment of iliac-caval outflow obstruction. Semin Vasc Surg. 2015;28(1):47–53. doi: 10.1053/j.semvascsurg.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 201.Neglen P., Berry M.A., Raju S. Endovascular surgery in the treatment of chronic primary and post-thrombotic iliac vein obstruction. Eur J Vasc Endovasc Surg. 2000;20(6):560–571. doi: 10.1053/ejvs.2000.1251. [DOI] [PubMed] [Google Scholar]
- 202.McDevitt J.L., Srinivasa R.N., Gemmete J.J., et al. Approach, technical success, complications, and stent patency of sharp recanalization for the treatment of chronic venous occlusive disease: experience in 123 patients. Cardiovasc Intervent Radiol. 2019;42(2):205–212. doi: 10.1007/s00270-018-2090-1. [DOI] [PubMed] [Google Scholar]
- 203.Raju S., Neglen P. Percutaneous recanalization of total occlusions of the iliac vein. J Vasc Surg. 2009;50(2):360–368. doi: 10.1016/j.jvs.2009.01.061. [DOI] [PubMed] [Google Scholar]
- 204.Williams Z.F., Dillavou E.D. A systematic review of venous stents for iliac and venacaval occlusive disease. J Vasc Surg Venous Lymphat Disord. 2020;8(1):145–153. doi: 10.1016/j.jvsv.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 205.Yin M., Shi H., Ye K., et al. Clinical assessment of endovascular stenting compared with compression therapy alone in post-thrombotic patients with iliofemoral obstruction. Eur J Vasc Endovasc Surg. 2015;50(1):101–107. doi: 10.1016/j.ejvs.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 206.Notten P., Ten Cate H., ten Cate-Hoek A.J. Postinterventional antithrombotic management after venous stenting of the iliofemoral tract in acute and chronic thrombosis: a systematic review. J Thromb Haemost. 2021;19(3):753–796. doi: 10.1111/jth.15197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Alavi A., Sibbald R.G., Phillips T.J., et al. What’s new: management of venous leg ulcers: treating venous leg ulcers. J Am Acad Dermatol. 2016;74(4):643–664. doi: 10.1016/j.jaad.2015.03.059. [DOI] [PubMed] [Google Scholar]
- 208.Danielsson G., Eklof B., Grandinetti A., Kistner R.L. The influence of obesity on chronic venous disease. Vasc Endovascular Surg. 2002;36(4):271–276. doi: 10.1177/153857440203600404. [DOI] [PubMed] [Google Scholar]
- 209.Arfvidsson B., Eklof B., Balfour J. Iliofemoral venous pressure correlates with intraabdominal pressure in morbidly obese patients. Vasc Endovascular Surg. 2005;39(6):505–509. doi: 10.1177/153857440503900607. [DOI] [PubMed] [Google Scholar]
- 210.Willenberg T., Clemens R., Haegeli L.M., Amann-Vesti B., Baumgartner I., Husmann M. The influence of abdominal pressure on lower extremity venous pressure and hemodynamics: a human in-vivo model simulating the effect of abdominal obesity. Eur J Vasc Endovasc Surg. 2011;41(6):849–855. doi: 10.1016/j.ejvs.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 211.Willenberg T., Schumacher A., Amann-Vesti B., et al. Impact of obesity on venous hemodynamics of the lower limbs. J Vasc Surg. 2010;52(3):664–668. doi: 10.1016/j.jvs.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 212.Wiewiora M., Piecuch J., Gluck M., Slowinska-Lozynska L., Sosada K. Impact of weight loss due to sleeve gastrectomy on shear stress of the femoral vein in morbid obesity. Obes Surg. 2014;24(5):806–812. doi: 10.1007/s11695-013-1175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Passerini A.G., Milsted A., Rittgers S.E. Shear stress magnitude and directionality modulate growth factor gene expression in preconditioned vascular endothelial cells. J Vasc Surg. 2003;37(1):182–190. doi: 10.1067/mva.2003.66. [DOI] [PubMed] [Google Scholar]
- 214.Padberg F., Jr., Cerveira J.J., Lal B.K., Pappas P.J., Varma S., Hobson R.W., II Does severe venous insufficiency have a different etiology in the morbidly obese? Is it venous? J Vasc Surg. 2003;37(1):79–85. doi: 10.1067/mva.2003.61. [DOI] [PubMed] [Google Scholar]
- 215.Shaalan W., El Emam A., Lotfy H., Naga A. Clinical and hemodynamic outcome of morbidly obese patients with severe chronic venous insufficiency with and without bariatric surgery. J Vasc Surg Venous Lymphat Disord. 2021;9(5):1248–1256. doi: 10.1016/j.jvsv.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 216.Carpentier P., van Bellen B., Karetova D., et al. Clinical efficacy and safety of a new 1000-mg suspension versus twice-daily 500-mg tablets of MPFF in patients with symptomatic chronic venous disorders: a randomized controlled trial. Int Angiol. 2017;36(5):402–409. doi: 10.23736/S0392-9590.17.03801-9. [DOI] [PubMed] [Google Scholar]
- 217.Martinez-Zapata M.J., Cosp X.B., Moreno R.M., Vargas E., Capellà D. Phlebotonics for venous insufficiency. Cochrane Database Syst Rev. 2005;4(4):CD003229. doi: 10.1002/14651858.CD003229.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kakkos S.K., Nicolaides A.N. Efficacy of micronized purified flavonoid fraction (Daflon) on improving individual symptoms, signs and quality of life in patients with chronic venous disease: a systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Int Angiol. 2018;37(2):143–154. doi: 10.23736/S0392-9590.18.03975-5. [DOI] [PubMed] [Google Scholar]
- 219.Rabinovich A., Kahn S.R. How I treat the postthrombotic syndrome. Blood. 2018;131(20):2215–2222. doi: 10.1182/blood-2018-01-785956. [DOI] [PubMed] [Google Scholar]
- 220.de Jongste A.B., Jonker J.J., Huisman M.V., ten Cate J.W., Azar A.J. A double blind three center clinical trial on the short-term efficacy of 0-(beta-hydroxyethyl)-rutosides in patients with post-thrombotic syndrome. Thromb Haemost. 1989;62(3):826–829. [PubMed] [Google Scholar]
- 221.Varatharajan L., Thapar A., Lane T., Munster A.B., Davies A.H. Pharmacological adjuncts for chronic venous ulcer healing: a systematic review. Phlebology. 2016;31(5):356–365. doi: 10.1177/0268355515587194. [DOI] [PubMed] [Google Scholar]
- 222.Jull A.B., Arroll B., Parag V., Waters J. Pentoxifylline for treating venous leg ulcers. Cochrane Database Syst Rev. 2007;(3):CD001733. doi: 10.1002/14651858.CD001733.pub3. [DOI] [PubMed] [Google Scholar]
- 223.Colgan M.P., Dormandy J.A., Jones P.W., Schraibman I.G., Shanik D.G., Young R.A. Oxpentifylline treatment of venous ulcers of the leg. BMJ. 1990;300(6730):972–975. doi: 10.1136/bmj.300.6730.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Brenner M.A. Nonhealing venous stasis ulcers. Pentoxifylline as adjunctive therapy. J Am Podiatr Med Assoc. 1987;77(11):586–588. doi: 10.7547/87507315-77-11-586. [DOI] [PubMed] [Google Scholar]
- 225.Stellin G.P., Waxman K. Current and potential therapeutic effects of pentoxifylline. Compr Ther. 1989;15(5):11–13. [PubMed] [Google Scholar]
- 226.Gabel J., O'Dell T., Masuda E., et al. Who is treating venous disease in America today? J Vasc Surg Venous Lymphat Disord. 2019;7(4):610–614. doi: 10.1016/j.jvsv.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 227.Crawford J.M., Gasparis A., Almeida J., et al. A review of United States endovenous ablation practice trends from the Medicare Data Utilization and Payment Database. J Vasc Surg Venous Lymphat Disord. 2019;7(4):471–479. doi: 10.1016/j.jvsv.2019.01.066. [DOI] [PubMed] [Google Scholar]
- 228.Baber J.T., Jr., Mao J., Sedrakyan A., Connolly P.H., Meltzer A.J. Impact of provider characteristics on use of endovenous ablation procedures in Medicare beneficiaries. J Vasc Surg Venous Lymphat Disord. 2019;7(2):203–209. doi: 10.1016/j.jvsv.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 229.Nicolaides A., Kakkos S., Baekgaard N., et al. Management of chronic venous disorders of the lower limbs. Guidelines according to scientific evidence. Part I. Int Angiol. 2018;37(3):181–254. doi: 10.23736/S0392-9590.18.03999-8. [DOI] [PubMed] [Google Scholar]
- 230.Nicolaides A., Kakkos S., Baekgaard N., et al. Management of chronic venous disorders of the lower limbs. Guidelines according to scientific evidence. Part II. Int Angiol. 2020;39(3):175–240. doi: 10.23736/S0392-9590.20.04388-6. [DOI] [PubMed] [Google Scholar]
- 231.Kundu S., Grassi C.J., Khilnani N.M., et al. Multi-disciplinary quality improvement guidelines for the treatment of lower extremity superficial venous insufficiency with ambulatory phlebectomy from the Society of Interventional Radiology, Cardiovascular Interventional Radiological Society of Europe, American College of Phlebology and Canadian Interventional Radiology Association. J Vasc Interv Radiol. 2010;21(1):1–13. doi: 10.1016/j.jvir.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 232.Bailey S.R., Beckman J.A., Dao T.D., et al. ACC/AHA/SCAI/SIR/SVM 2018 appropriate use criteria for peripheral artery intervention: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Heart Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, and Society for Vascular Medicine. J Am Coll Cardiol. 2019;73(2):214–237. doi: 10.1016/j.jacc.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]