Abstract
Background and Aims
It is unclear to what degree post-COVID-19 gastrointestinal (GI) symptoms are caused by the SARS-CoV-2 virus vs psychological factors related to the stress of the pandemic. To evaluate this, we compared rates of long-term GI and mental health symptoms in patients testing positive vs negative for SARS-CoV-2.
Methods
Adults presenting for SARS-CoV-2 testing from April to November 2020 were prospectively enrolled in a longitudinal cohort. Six to 12 months later, the presence and severity of current GI and mental health symptoms were assessed on a 5-point Likert scale. A multivariable logistic regression model was used to estimate the odds of a positive COVID test for predicting GI symptoms, stratified by sadness/anxiety.
Results
749 COVID-positive and 107 COVID-negative patients completed the survey. The prevalence of at least one GI symptom was higher in patients with COVID-19 (29 vs 18%, P = .01). However, after stratifying by sadness/anxiety, differences in GI symptoms according to COVID status were no longer significant. On multivariable analysis, the adjusted odds ratio for GI symptoms was 8.26 (95% CI 4.04–16.9) for positive COVID with sadness/anxiety, 8.74 (95% CI 2.63–29.0) for negative COVID with sadness/anxiety, and 1.16 (95% CI 0.57–2.39) for positive COVID without sadness/anxiety, compared to a reference group of negative COVID without sadness/anxiety.
Conclusion
After accounting for sadness and anxiety, there was no association between COVID-19 and the development of long-term GI symptoms. Post-COVID GI symptoms may be mediated bidirectionally through coexisting anxiety and depression, similar to disorders of gut-brain interaction.
Keywords: Irritable Bowel Syndrome, SARS-CoV-2, COVID-19, Post-Acute COVID-19 Syndrome, Disorders of Gut-Brain Interaction
Introduction
Six months after testing positive for SARS-CoV-2, up to 43% of people report persistent symptoms that they attribute to COVID-19.1,2 This phenomenon, sometimes referred to as “long COVID” or post-acute COVID-19 syndrome, remains incompletely understood but is often characterized by persistent dyspnea, fatigue, and cognitive dysfunction.3 Gastrointestinal (GI) symptoms such as nausea, abdominal pain, heartburn, diarrhea, and constipation are also common, with prevalence estimates ranging from 15% to 29%.4, 5, 6 As of January 1, 2023, there have been over 600 million cases of COVID-19 worldwide, a number which continues to increase. Thus, there are likely tens of millions of people also suffering from new or worsened GI symptoms post-COVID.2
The pathophysiology of post-COVID GI symptoms is unknown but the problem appears to share risk factors with disorders of gut-brain interaction (DGBI) such as irritable bowel syndrome (IBS). It is well-known that chronic GI symptoms can develop in response to infections of the digestive tract, a phenomenon known as postinfection IBS7, 8, 9, 10 and approximately 10% of patients meet criteria for IBS after a diagnosis of infectious enteritis.11 While COVID-19 is primarily a respiratory infection, the SARS-CoV-2 virus has been observed on intestinal biopsies and been shown to be associated with alterations in the gut microbiome.12,13 In cases of viral gastroenteritis, mucosal injury is typically acute and self-limited, suggesting that persistent GI symptoms may be more likely mediated by other pathways. There are multiple mechanisms in the pathogenesis of postinfection IBS, but alterations in serotonin signaling and the gut microbiome are likely key mediators.14,15
In a prior study, we observed a strong association between self-reported sadness or anxiety and new GI symptoms after COVID-19.4 This is not surprising, given that anxiety, depression, and somatization are established risk factors for the development of postinfection IBS.7,11 This prior study was limited by the lack of a control group who tested negative for SARS-CoV-2. Because the overall prevalence of DGBI increased during the COVID-19 pandemic,16, 17, 18 it is unclear to what degree the high prevalence of GI symptoms after COVID-19 is due to SARS-CoV-2 itself or a result of psychosocial distress exacerbated by the widespread uncertainty and stress of the pandemic.19 The goal of the current study was to evaluate the prevalence of GI symptoms in a cohort of patients tested for SARS-CoV-2 at the height of the pandemic, comparing those testing positive vs negative, and assess for interactions with mental health symptoms.
Materials and Methods
Between April and November 2020, patients who sought polymerase chain reaction testing for SARS-CoV-2 at Columbia University Irving Medical Center were prospectively enrolled in a study to assess long-term symptoms. At this time, SARS-CoV-2 testing was not widely available and only those with symptoms suggestive of an upper respiratory infection (eg, fever, cough) met the criteria for testing; GI symptoms were not considered criteria for SARS-CoV-2 testing at this time.20 Patients who underwent testing were asked to enroll in the cohort before the results of their polymerase chain reaction tests were known; therefore, the longitudinal cohort included both those who tested positive for SARS-CoV-2 and were diagnosed with COVID-19 and controls who tested negative for SARS-CoV-2.
Between 6 and 12 months of follow-up, all cohort participants were asked to complete an electronic survey assessing the presence and severity of a broad range of symptoms. The survey instrument included questions related to GI symptoms that were similar to the validated National Institutes of Health Gastrointestinal Patient-Reported Outcomes Measurement Information System questions on reflux/heartburn, abdominal pain, nausea/vomiting, diarrhea, and constipation but omitted the domains of fecal incontinence, bloating, and disrupted swallowing.21 Specifically, participants were asked to indicate if any of these symptoms were present during the past 7 days and, if so, to rate them on a 5-point Likert scale (very mild, mild, moderate, severe, very severe). Two additional questions asked whether participants had sadness or anxiety during the past 7 days, which were rated on the same 5-point Likert scale. Patients were asked to indicate yes or no to whether a list of pre-existing conditions (prior to testing for COVID-19) were present, for example, ‘Do you have any history of psychosocial or mental health disability.’ In the main analysis, we classified GI symptoms and sadness/anxiety as present or absent. Two patients who initially tested negative for SARS-CoV-2 but subsequently tested positive prior to the time of the survey were excluded.
Continuous variables were compared in patients testing positive vs negative for SARS-CoV-2 using the Student’s t-test or Mann-Whitney U test for nonparametric data. Categorical variables including the prevalence of GI and mental health symptoms were compared using chi-square tests. A multivariable logistic regression model was also used to evaluate the interaction between mental health and COVID-19 status for predicting GI symptoms, adjusting for demographic characteristics. All statistical analyses were performed with Stata version 17 (College Station, Tx). Alpha 0.05 was considered statistically significant for all analyses.
Results
The survey response rate was 749 of 1810 (41%) among those who tested positive for SARS-CoV-2 and 109 of 363 (30%) among those who tested negative (P < .01). Two patients who initially tested negative subsequently tested positive for COVID and were excluded, leaving 107 COVID-negative patients in the analysis (Table 1). Among those with COVID-19, 15% were hospitalized and 1.7% required mechanical ventilation. None of the COVID-19-negative patients were hospitalized. The median age and percentage of female participants were similar in those with and without COVID-19, although a higher proportion of those with COVID-19 reported a pre-existing mental health problem (5% vs 1%, P = .05). Participants with COVID-19 were also more likely to be of Hispanic ethnicity.
Table 1.
Baseline Characteristics Stratified by SARS-CoV-2 Test Result
| Patient characteristics | COVID status |
P value | |
|---|---|---|---|
| Negative (n = 107) | Positive (n = 749) | ||
| Age (median, IQR) | 41 (35–54) | 43 (33–57) | .79 |
| Age category | .002 | ||
| Under 30 | 5 (4.7%) | 110 (14.7%) | |
| 30–39 | 42 (39.3%) | 204 (27.2%) | |
| 40–59 | 45 (42.1%) | 272 (36.3%) | |
| >=60 | 15 (14.0%) | 163 (21.8%) | |
| Sex | .78 | ||
| Male | 34 (31.8%) | 248 (33.1%) | |
| Female | 73 (68.2%) | 501 (66.9%) | |
| Race | .02 | ||
| White | 63 (58.9%) | 454 (60.6%) | |
| Black | 7 (6.5%) | 72 (9.6%) | |
| Asian | 4 (3.7%) | 73 (9.8%) | |
| American Indian | 1 (0.9%) | 8 (1.1%) | |
| Other | 11 (10.3%) | 70 (9.4%) | |
| Chose not to answer | 21 (19.6%) | 72 (9.6%) | |
| Ethnicity | <.001 | ||
| Hispanic | 13 (12.2%) | 208 (27.8%) | |
| Non-Hispanic | 71 (66.4%) | 524 (70.0%) | |
| Chose not to answer | 23 (21.5%) | 17 (2.3%) | |
| Psychosocial or mental health disability | .047 | ||
| No | 106 (99.1%) | 710 (94.8%) | |
| Yes | 1 (0.9%) | 39 (5.2%) | |
IQR, interquartile range.
In the crude analysis (Table 2), participants with COVID-19 were more likely to report the presence of at least one persistent GI symptom at 6–12 months of follow-up compared to controls (29% vs 18%, respectively, P = .01). This comparison remained significant when only considering GI symptoms that were rated moderate or worse (11% vs 5%, P = .04). Among individual GI symptoms, participants with COVID-19 were significantly more likely to report heartburn/reflux and nausea/vomiting. There was a trend toward increased prevalence of abdominal pain and constipation in the COVID-19 group, but a similar prevalence of diarrhea in both groups.
Table 2.
Symptoms at Time of Survey 6–12 Months After COVID Testing, Stratified by COVID Status
| Patient reported symptoms | COVID status |
P value | |
|---|---|---|---|
| Negative (n = 107) | Positive (n = 749) | ||
| Gastrointestinal symptoms | |||
| Reflux/heartburn | 6 (5.6%) | 122 (16.3%) | .004 |
| Nausea/vomiting | 1 (0.9%) | 53 (7.1%) | .015 |
| Abdominal pain | 4 (3.7%) | 70 (9.4%) | .054 |
| Constipation | 6 (5.6%) | 83 (11.1%) | .083 |
| Diarrhea | 9 (8.4%) | 72 (9.6%) | .69 |
| Any gastrointestinal symptom | 19 (17.8%) | 220 (29.4%) | .012 |
| Any moderate gastrointestinal symptom | 5 (4.7%) | 83 (11.1%) | .041 |
| Mental health symptoms | .69 | ||
| Sadness | 10 (9.4%) | 199 (26.6%) | <.001 |
| Anxiety | 15 (14.0%) | 254 (33.9%) | <.001 |
| Sadness or anxiety | 16 (15.0%) | 280 (37.3%) | <.001 |
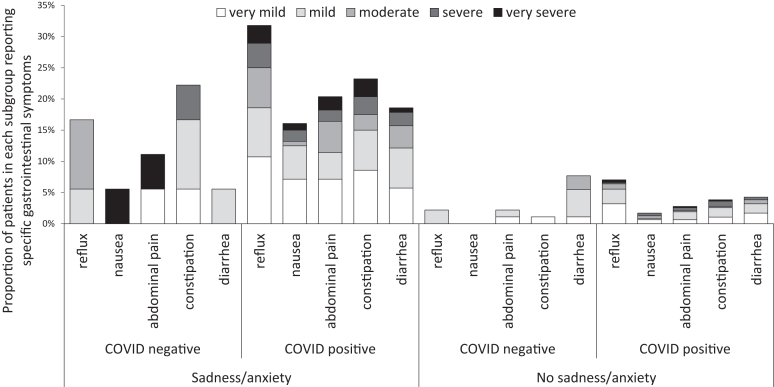
Next, we investigated how the presence of sadness and anxiety might interact with the relationship between persistent GI symptoms and COVID-19. Patients with COVID-19 were substantially more likely to report sadness (27 vs 9%, respectively, P < .001), anxiety (34 vs 14%, P < .001), or either symptom (37 vs 15%, P < .001) compared to COVID-negative controls at follow-up. Of note, given the pretesting reported prevalence of “psychosocial or mental health disability” of 5% among the COVID-19 positive group and 1% among the negative group, the majority of these symptoms appear to be new. When the prevalence of GI symptoms was stratified according to the presence of sadness/anxiety, the differences between COVID-19 positive and negative groups were no longer significant (Table 3) and these findings remained unchanged when symptom severity was incorporated (Figure).
Table 3.
Stratified Analysis Showing Effect Modification Between Sadness/Anxiety and Development of Long-Term Gastrointestinal Symptoms
| COVID-19 status | Persistent gastrointestinal symptoms |
||
|---|---|---|---|
| No (N, %) | Yes (N, %) | P value | |
| Among all patients | .012 | ||
| No COVID-19 | 88 (82%) | 19 (18%) | |
| COVID-19 | 529 (71%) | 220 (29%) | |
| Among patients with sadness or anxiety | .72 | ||
| No COVID-19 | 8 (50%) | 8 (50%) | |
| COVID-19 | 127 (45%) | 153 (55%) | |
| Among patients without sadness or anxiety | .58 | ||
| No COVID-19 | 80 (88%) | 11 (12%) | |
| COVID-19 | 402 (86%) | 67 (14%) | |
| Among patients with COVID-19 | <.01 | ||
| No sadness or anxiety | 402 (86%) | 67 (14%) | |
| Sadness or anxiety | 127 (45%) | 153 (55%) | |
Figure.
Proportion of respondents who reported each gastrointestinal symptom, shaded according to severity and stratified by COVID-19 test result and reported sadness/anxiety. Subjects reported whether GI symptoms occurred during the preceding 7 days then rated each symptom on a 5-point Likert scale as shown.
The prevalence of GI symptoms at the time of the survey was significantly higher among patients with COVID-19 requiring hospitalization (51% vs 26%, P < .01), as was the prevalence of sadness or anxiety (54% vs 35%, P < .01). The association between sadness or anxiety and GI symptoms remained significant when stratifying by hospitalization status. Among nonhospitalized COVID-19 patients, 50% of patients with sadness or anxiety reported GI symptoms, compared to 13% of those without sadness or anxiety (P < .01). Among patients who required hospitalization for COVID-19, 73% of those with sadness or anxiety reported GI symptoms, compared to 25% of those without sadness or anxiety (P < .01).
In a multivariable logistic regression analysis, the interaction between COVID status and sadness/anxiety for predicting GI symptoms was assessed, adjusting for age, sex, race, ethnicity, and pre-existing psychosocial or mental health disability. In this analysis, the presence of sadness/anxiety was the key risk factor for predicting persistent GI symptoms. The adjusted odds ratio for the outcome of persistent GI symptoms was 8.26 (95% confidence interval [CI] 4.04–16.87) for those with COVID and with sadness/anxiety, 8.74 (95% CI 2.63–29.04) for those without COVID and with sadness/anxiety, and 1.16 (95% CI 0.57–2.39) for those with COVID and without sadness/anxiety (relative to those with a negative COVID test without sadness or anxiety) (Table 4).
Table 4.
Multivariable Model for Persistent Gastrointestinal Symptoms at 6–12 Months of Follow-Up Showing Effect Modification Between COVID Status and Sadness/Anxiety
| Adjusted odds ratio | 95% CI | |
|---|---|---|
| COVID status and reported sadness/anxiety | ||
| Negative COVID, negative sadness/anxiety | 1 (reference) | |
| Negative COVID, positive sadness/anxiety | 8.74 | 2.63–29.04 |
| Positive COVID, negative sadness/anxiety | 1.16 | 0.57–2.39 |
| Positive COVID, positive sadness/anxiety | 8.26 | 4.04–16.87 |
| Age | ||
| Under 30 | 1 (reference) | |
| 30–39 | 1.09 | 0.60–1.97 |
| 40–59 | 2.05 | 1.18–3.56 |
| >=60 | 1.87 | 1.00–3.49 |
| Sex | ||
| Male | 1 (reference) | |
| Female | 0.99 | 0.69–1.43 |
| Race | ||
| White | 1 (reference) | |
| Black | 1.03 | 0.57–1.86 |
| Asian | 2.19 | 1.20–4.00 |
| American Indian | 3.22 | 0.72–14.42 |
| Other | 1.54 | 0.81–2.93 |
| Chose not to answer | 1.38 | 0.72–2.68 |
| Ethnicity | ||
| Hispanic | 1 (reference) | |
| Non-Hispanic | 0.66 | 0.41–1.06 |
| Chose not to answer | 0.66 | 0.25–1.73 |
| Psychosocial or mental health disability | ||
| No | 1 (reference) | |
| Yes | 1.37 | 0.68–2.77 |
Discussion
GI symptoms after COVID-19 are commonly reported both during and after acute COVID-19 infection.22,23 In a previous study, we observed that 23% of patients hospitalized with COVID-19 reported diarrhea and 21% reported nausea/vomiting.6 At follow-up clinic appointment 3 months later, 16% reported at least one ongoing GI symptom that they perceived as new since COVID. The current study tested the hypothesis that these “long COVID” GI symptoms were related to the stress of the pandemic rather than infection with SARS-CoV-2 per se. Specifically, it utilized a unique cohort of patients who were tested for SARS-CoV-2 at the height of the pandemic, enrolled in a longitudinal cohort, and then received their SARS-CoV-2 test results. Because their COVID status was unknown at the time of enrollment, selection bias is unlikely and the cohort allows assessment of the SARS-CoV-2 test result (ie, the diagnosis of COVID) as an independent risk factor for the long-term development of GI symptoms. We found that COVID-19 was not independently associated with the development of GI symptoms because the relationship between COVID-19 and long-term GI symptoms depended on the concurrent development of self-reported sadness and anxiety, an epidemiologic phenomenon known as effect modification.
We observed that hospitalization for COVID-19 was strongly associated with a higher prevalence of both mental health and GI symptoms at follow-up, suggesting that severity of illness is a risk factor for long COVID symptoms. However, in both hospitalized and nonhospitalized COVID-19 patients, those with anxiety or sadness were more likely to report GI symptoms, suggesting that this interaction remains significant independent of illness severity.
In patients with and without COVID-19, DGBI such as IBS are common, with a global prevalence of IBS of 9.2% using the Rome III and 3.8% using the Rome IV criteria.24 The mechanisms of DGBI may include altered motility,25 visceral hypersensitivity,26 changes in the gut microbiome,27 and alterations in central nervous system processing.28 Psychosocial problems are common in IBS patients, with a prevalence of anxiety symptoms of 39.1% and depressive symptoms of 28.8%, approximately 3-fold higher than healthy subjects.29 Tricyclic antidepressants and cognitive behavioral therapy are recommended by the American Gastroenterological Association for the treatment of IBS as gut-brain neuromodulating therapy.30,31
The development of GI symptoms after an infection is not unique to COVID-19. Postinfection IBS has been observed after bacterial (E coli, Campylobacter, Shigella), viral (norovirus), and protozoal (giardia) enteritis.32, 33, 34, 35 It has been estimated that approximately 1 in 9 patients develop new symptoms of IBS after an episode of infectious enteritis, most commonly diarrhea predominant or mixed subtypes.11 Interestingly, the risk of postinfection IBS after viral enteritis, while comparable to bacterial or protozoal enteritis in the first year after infection, appears to decrease to that of the general population after 1 year. Psychological distress has been noted as an important risk factor for the development of postinfection IBS, with an odds ratio of 2.0 for patients with anxiety and 1.5 for patients with depression. Our study results suggest that it is reasonable to consider post-COVID GI symptoms in a similar intellectual framework.
Prior studies have suggested an important role of alterations in serotonin metabolism both for IBS in general and for COVID-19-related GI symptoms. Altered serum and mucosal serotonin compared to controls has been associated with IBS of all subtypes, and especially with postinfection IBS.36 In a small study of patients with COVID-19 who provided stool samples at the time of infection, there was decreased microbial tryptophan metabolism (the rate-limiting precursor of serotonin or 5-hydroxytryptamine) among patients who subsequently reported chronic GI symptoms.15 Gut microbiome L-tryptophan biosynthesis during acute COVID-19 was noted to be reduced among those who developed more severe GI symptoms as well as more severe mental health symptoms following COVID-19. Patients who reported the development of both GI and mental health symptoms had even more reduced 5-hydroxytryptamine levels at the time of COVID-19 infection. Other studies have also noted reductions in a tryptophan metabolism in patients with COVID-19.37, 38, 39 Although more research is necessary, these studies suggest alterations in serotonin signaling pathways may be a plausible biologic link between COVID-19 and the development of DGBI, although it may be more important in patients with more severe illness.
Other studies have investigated the association between post-COVID DGBI and psychiatric comorbidities. In an internet-based survey, among 164 patients with prior COVID-19 diagnosis and current GI symptoms, 66% met the criteria for a DGBI.23 Among this cohort, depression, but not anxiety, was significantly more prevalent among patients meeting criteria for a post-COVID DGBI. Not all prior studies agree with our results. In a prospective study of 883 hospitalized patients including 614 with and 289 without COVID-19, those with COVID were more likely to report acute GI symptoms at enrollment (59.3% vs 39.7%).40 At 12 months of follow-up, those with COVID-19 were more likely to meet Rome IV criteria for IBS (3.2% vs 0.5%). While there was a trend toward higher rates of anxiety and depression in the COVID-19 cohort, these differences were only statistically significant for depression at 6 months, suggesting against underlying psychiatric differences as the explanation for the higher rate of DGBI post-COVID. This study evaluated a different population (hospitalized vs primarily outpatient), which may account for these findings. Interestingly, this study noted that the use of proton pump inhibitors was a significant risk factor for the development of IBS post-COVID, and hypothesized that the effects of proton pump inhibitors may be mediated by alterations in the gut microbiome.
Our study has some strengths. It utilized a unique cohort including both COVID positive and negative patients and had standard assessments for GI symptoms that resembled the validated NIH PROMIS instruments. It also has limitations. Due to low test availability at the time of study recruitment, testing was primarily performed on symptomatic patients with a higher pretest probability for COVID-19, limiting the number of COVID-negative controls available for inclusion. The use of antibiotics (which may have effects on the gut microbiome and therefore GI symptoms) in patients was not recorded. Most notably, a baseline assessment of GI symptoms was not performed. Ideally, GI and mental health symptoms would have been comprehensively assessed at baseline and again 6–12 months later using the same validated instruments. Without a careful assessment of baseline sadness/anxiety, it is unclear whether COVID is more likely to increase the prevalence of GI symptoms in patients with pre-existing mental health disorders or whether COVID is associated with an increased prevalence of both GI and mental health disorders. The question of within-individual change in GI symptoms may ultimately require new prospective cohorts which serially assess symptoms over time using the same instruments.
In sum, this prospective cohort study is consistent with prior literature showing an increased prevalence of GI symptoms after COVID-19. However, there was effect modification of the relationship between COVID-19 and long-term GI symptoms based on self-reported sadness and anxiety. Once sadness and anxiety were accounted for, those who tested positive for COVID-19 were not necessarily more likely to develop subsequent persistent GI symptoms compared to controls who entered the cohort at the same time but tested negative for COVID-19. Put another way, patients with COVID-19 were more likely to have persistent GI symptoms than those who tested negative, but also more likely to report ongoing sadness/anxiety, and after accounting for these mental health symptoms the association between COVID and GI symptoms in our cohort was no longer significant. These results provide essential context for understanding post-COVID GI symptoms. They suggest that, if COVID-19 does cause persistent GI symptoms, it may be mediated through coexisting sadness/anxiety. This interpretation of our data would recognize that a strong (and perhaps bidirectional) relationship exists between mental health and post-COVID GI symptoms. Such a relationship would place post-COVID GI symptoms firmly within the framework of other DGBI such as IBS, which is strongly associated with psychological factors including depression and anxiety.16,41 This study also highlights the critical importance, not only of including a control group when assessing the prevalence of potential long COVID symptoms, but adjusting for the presence of mental health symptoms, which are often closely intertwined with GI symptoms.42
Conclusion
To conclude, this prospective COVID-19 cohort including COVID-negative controls found that coexisting mental health symptoms explained the association between testing positive for COVID-19 at the height of the pandemic and development of post-COVID GI symptoms. These findings may be useful in designing and interpreting results from longitudinal COVID-19 cohort studies.
Acknowledgments
Authors' Contributions:
John W. Blackett: Investigation, Analysis, Writing - original draft, review and editing. Mitchell S. V. Elkind: Conceptualization, Analysis, review and editing. Sheila O’Byrne: Investigation, review and editing. Milton Wainberg: Analysis, review and editing. Lin Chang: Analysis, review and editing. Daniel E. Freedberg: Conceptualization, Investigation, Analysis, Methodology, Writing - original draft, review and editing.
Footnotes
Conflicts of Interest: The authors disclose no conflicts.
Funding: Biospecimens and/or data utilized for this research were obtained from the Columbia University Biobank (CUB), which is supported by the Irving Institute for Clinical and Translational Research, home to Columbia University’s Clinical and Translational Science Award (CTSA) funded through Grant Number UL1TR001873. DEF was funded in part by the Department of Defense Peer-Reviewed Medical Research Program (PR181960).
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: Study data are available upon reasonable request to the corresponding author.
Reporting Guidelines: STROBE.
References
- 1.Chen C., Haupert S.R., Zimmermann L., et al. Global prevalence of post COVID-19 condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593–1607. doi: 10.1093/infdis/jiac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2023. WHO coronavirus dashboard: world health organization.https://covid19.who.int/ [Google Scholar]
- 3.Subramanian A., Nirantharakumar K., Hughes S., et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28:1706–1714. doi: 10.1038/s41591-022-01909-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackett J.W., Wainberg M., Elkind M.S.V., et al. Potential long Coronavirus disease 2019 gastrointestinal symptoms 6 Months after Coronavirus infection are associated with mental health symptoms. Gastroenterology. 2022;162:648–650.e2. doi: 10.1053/j.gastro.2021.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yusuf F., Fahriani M., Mamada S.S., et al. Global prevalence of prolonged gastrointestinal symptoms in COVID-19 survivors and potential pathogenesis: a systematic review and meta-analysis. F1000Res. 2021;10:301. doi: 10.12688/f1000research.52216.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackett J.W., Li J., Jodorkovsky D., et al. Prevalence and risk factors for gastrointestinal symptoms after recovery from COVID-19. Neurogastroenterol Motil. 2022;34 doi: 10.1111/nmo.14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Card T., Enck P., Barbara G., et al. Post-infectious IBS: defining its clinical features and prognosis using an internet-based survey. United European Gastroenterol J. 2018;6:1245–1253. doi: 10.1177/2050640618779923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiller R. Postinfectious functional dyspepsia and postinfectious irritable bowel syndrome: different symptoms but similar risk factors. Gastroenterology. 2010;138:1660–1663. doi: 10.1053/j.gastro.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Wouters M.M., Van Wanrooy S., Nguyen A., et al. Psychological comorbidity increases the risk for postinfectious IBS partly by enhanced susceptibility to develop infectious gastroenteritis. Gut. 2016;65:1279–1288. doi: 10.1136/gutjnl-2015-309460. [DOI] [PubMed] [Google Scholar]
- 10.Dunlop S.P., Jenkins D., Spiller R.C. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol. 2003;98:1578–1583. doi: 10.1111/j.1572-0241.2003.07542.x. [DOI] [PubMed] [Google Scholar]
- 11.Klem F., Wadhwa A., Prokop L.J., et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology. 2017;152:1042–1054.e1. doi: 10.1053/j.gastro.2016.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian Q., Fan L., Liu W., et al. Direct evidence of active SARS-CoV-2 replication in the intestine. Clin Infect Dis. 2021;73:361–366. doi: 10.1093/cid/ciaa925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Q., Mak J.W.Y., Su Q., et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut. 2022;71:544–552. doi: 10.1136/gutjnl-2021-325989. [DOI] [PubMed] [Google Scholar]
- 14.Lee Y.Y., Annamalai C., Rao S.S.C. Post-infectious irritable bowel syndrome. Curr Gastroenterol Rep. 2017;19:56. doi: 10.1007/s11894-017-0595-4. [DOI] [PubMed] [Google Scholar]
- 15.Blackett J.W., Sun Y., Purpura L., et al. Decreased gut microbiome tryptophan metabolism and serotonergic signaling in patients with persistent mental health and gastrointestinal symptoms after COVID-19. Clin Transl Gastroenterol. 2022;13 doi: 10.14309/ctg.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almario C.V., Makaroff K., Alvarez G., et al. S496 examining the impact of the COVID-19 pandemic on the prevalence of Rome IV functional gastrointestinal disorders. J Am Coll Gastroenterol. 2021;116:S220–S221. [Google Scholar]
- 17.Nakov R., Dimitrova-Yurukova D., Snegarova V., et al. Increased prevalence of gastrointestinal symptoms and disorders of gut-brain interaction during the COVID-19 pandemic: an internet-based survey. Neurogastroenterol Motil. 2022;34 doi: 10.1111/nmo.14197. [DOI] [PubMed] [Google Scholar]
- 18.Gubatan J., Zikos T., Spear Bishop E., et al. Gastrointestinal symptoms and healthcare utilization have increased among patients with functional gastrointestinal and motility disorders during the COVID-19 pandemic. Neurogastroenterol Motil. 2022;34 doi: 10.1111/nmo.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie Y., Xu E., Al-Aly Z. Risks of mental health outcomes in people with covid-19: cohort study. BMJ. 2022;376 doi: 10.1136/bmj-2021-068993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nobel Y.R., Phipps M., Zucker J., et al. Gastrointestinal symptoms and Coronavirus disease 2019: a case-control study from the United States. Gastroenterology. 2020;159:373–375.e2. doi: 10.1053/j.gastro.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiegel B.M., Hays R.D., Bolus R., et al. Development of the NIH patient-reported outcomes measurement information system (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804–1814. doi: 10.1038/ajg.2014.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golla R., Vuyyuru S., Kante B., et al. Long-term gastrointestinal sequelae following COVID-19: a prospective follow-up cohort study. Clin Gastroenterol Hepatol. 2023;21:789–796.e1. doi: 10.1016/j.cgh.2022.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebrahim Nakhli R., Shanker A., Sarosiek I., et al. Gastrointestinal symptoms and the severity of COVID-19: disorders of gut-brain interaction are an outcome. Neurogastroenterol Motil. 2022;34 doi: 10.1111/nmo.14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oka P., Parr H., Barberio B., et al. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:908–917. doi: 10.1016/S2468-1253(20)30217-X. [DOI] [PubMed] [Google Scholar]
- 25.Chey W.Y., Jin H.O., Lee M.H., et al. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol. 2001;96:1499–1506. doi: 10.1111/j.1572-0241.2001.03804.x. [DOI] [PubMed] [Google Scholar]
- 26.Keszthelyi D., Troost F.J., Masclee A.A. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Methods to assess visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G141–G154. doi: 10.1152/ajpgi.00060.2012. [DOI] [PubMed] [Google Scholar]
- 27.Agnello M., Carroll L.N., Imam N., et al. Gut microbiome composition and risk factors in a large cross-sectional IBS cohort. BMJ Open Gastroenterol. 2020;7 doi: 10.1136/bmjgast-2019-000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Midenfjord I., Grinsvall C., Koj P., et al. Central sensitization and severity of gastrointestinal symptoms in irritable bowel syndrome, chronic pain syndromes, and inflammatory bowel disease. Neurogastroenterol Motil. 2021;33 doi: 10.1111/nmo.14156. [DOI] [PubMed] [Google Scholar]
- 29.Zamani M., Alizadeh-Tabari S., Zamani V. Systematic review with meta-analysis: the prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2019;50:132–143. doi: 10.1111/apt.15325. [DOI] [PubMed] [Google Scholar]
- 30.Drossman D.A., Tack J., Ford A.C., et al. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): a Rome Foundation Working team report. Gastroenterology. 2018;154:1140–1171.e1. doi: 10.1053/j.gastro.2017.11.279. [DOI] [PubMed] [Google Scholar]
- 31.Chang L., Sultan S., Lembo A., et al. AGA clinical practice guideline on the pharmacological management of irritable bowel syndrome with constipation. Gastroenterology. 2022;163:118–136. doi: 10.1053/j.gastro.2022.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Thabane M., Kottachchi D.T., Marshall J.K. Systematic review and meta-analysis: the incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:535–544. doi: 10.1111/j.1365-2036.2007.03399.x. [DOI] [PubMed] [Google Scholar]
- 33.Porter C.K., Faix D.J., Shiau D., et al. Postinfectious gastrointestinal disorders following norovirus outbreaks. Clin Infect Dis. 2012;55:915–922. doi: 10.1093/cid/cis576. [DOI] [PubMed] [Google Scholar]
- 34.Zanini B., Ricci C., Bandera F., et al. Incidence of post-infectious irritable bowel syndrome and functional intestinal disorders following a water-borne viral gastroenteritis outbreak. Am J Gastroenterol. 2012;107:891–899. doi: 10.1038/ajg.2012.102. [DOI] [PubMed] [Google Scholar]
- 35.Hanevik K., Wensaas K.A., Rortveit G., et al. Irritable bowel syndrome and chronic fatigue 6 years after giardia infection: a controlled prospective cohort study. Clin Infect Dis. 2014;59:1394–1400. doi: 10.1093/cid/ciu629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spiller R., Lam C. An update on post-infectious irritable bowel syndrome: role of genetics, immune activation, serotonin and altered microbiome. J Neurogastroenterol Motil. 2012;18:258–268. doi: 10.5056/jnm.2012.18.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas T., Stefanoni D., Reisz J.A., et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. 2020;5:e140327. doi: 10.1172/jci.insight.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lionetto L., Ulivieri M., Capi M., et al. Increased kynurenine-to-tryptophan ratio in the serum of patients infected with SARS-CoV2: an observational cohort study. Biochim Biophys Acta Mol Basis Dis. 2021;1867 doi: 10.1016/j.bbadis.2020.166042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ansone L., Briviba M., Silamikelis I., et al. Amino acid metabolism is significantly altered at the time of admission in hospital for severe COVID-19 patients: findings from longitudinal targeted metabolomics analysis. Microbiol Spectr. 2021;9 doi: 10.1128/spectrum.00338-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marasco G., Cremon C., Barbaro M.R., et al. Post COVID-19 irritable bowel syndrome. Gut. 2022 doi: 10.1136/gutjnl-2022-328483. [DOI] [PubMed] [Google Scholar]
- 41.Zia J.K., Lenhart A., Yang P.L., et al. Risk factors for abdominal pain-related disorders of gut-brain interaction in adults and Children: a systematic review. Gastroenterology. 2022;163:995–1023.e3. doi: 10.1053/j.gastro.2022.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amin-Chowdhury Z., Ladhani S.N. Causation or confounding: why controls are critical for characterizing long COVID. Nat Med. 2021;27:1129–1130. doi: 10.1038/s41591-021-01402-w. [DOI] [PubMed] [Google Scholar]