Abstract
Background and Aims
Biliary tract cancer (BTC) consists of a group of hepatic and perihepatic tumors that are in close proximity but are anatomically different, including gallbladder cancer (GBC), cholangiocarcinoma (extrahepatic and intrahepatic [ICC]), and ampulla of Vater cancer (AVC). Most epidemiologic research has focused on 1 or more anatomic subtypes, or does not differentiate BTC from hepatocellular carcinoma or other primary liver cancers. Here, we provide a descriptive update on global incidence and mortality rates for BTC, overall and by anatomic subtypes.
Methods
Age-standardized rates (per 100,000 person-years) were derived from the International Agency for Research on Cancer, Cancer Incidence in Five Continents, Volume XI (2008–2012; 22 countries), and the World Health Organization Mortality Database (2006–2016; 38 countries).
Results
BTC incidence varied by country, with the highest in Chile (14.35) and the lowest in Vietnam (1.25). Mortality rates for BTC were highest for the Republic of Korea (11.64) and lowest for the Republic of Moldova (1.65). BTC mortality rates increased over time in 24 of 34 countries. Patients aged ≥75 years had 5–10 times higher mortality rates than the overall BTC rate in all countries. In most countries, incidence rates were highest for GBC, and mortality rates highest for ICC, while both were lowest for AVC. Females had and died from GBC more frequently than males. For ICC, extrahepatic cholangiocarcinoma, and AVC, males trended toward higher incidence and mortality rates.
Conclusion
The increasing incidence and mortality trends reported here indicate a need for improved prevention and treatment for all BTC subtypes.
Keywords: Biliary Tract Neoplasms, Epidemiological Studies, Patient-Focused Care, Public Health
Introduction
Biliary tract cancer (BTC) comprises a diverse group of epithelial hepatic and perihepatic malignancies that emerge from the biliary tree. The anatomic subtypes comprise gallbladder cancer (GBC), cholangiocarcinoma (both extrahepatic [ECC] and intrahepatic [ICC]), and ampulla of Vater cancer (AVC).1 Considered a rare cancer in most of the world, incidence of BTC and its subtypes varies greatly between geographic regions. It is diagnosed most frequently in East Asian and South American countries and is associated with risk factors such as liver fluke infection, chronic liver disease, inflammatory biliary tract diseases, obesity, and tobacco and alcohol use, depending on subtype.2, 3, 4, 5, 6 Due to the asymptomatic behavior of BTC, diagnosis most frequently occurs at advanced stages of disease, when treatment options are limited.7 The 5-year survival rate for the United States (US) and European patients with BTC is less than 20%.8,9
Few studies compare the epidemiology of BTC across all subtypes, and even fewer do so across multiple global regions. In studies that have reported data from multiple countries, the focus is often limited to only 1 or 2 anatomic subtypes, multiple BTC subtypes are grouped and reported as one, or BTC is not differentiated from hepatocellular carcinoma or other types of primary liver cancer.10, 11, 12, 13 Torre et al11 previously reported global trends in BTC mortality; however, data collection was limited to GBC and other and unspecified cancers of the biliary tract, notably excluding ICC. Results were reported for the combined BTC group, giving no insights into global mortality rates for specific disease subtypes.11 Similarly, Ouyang et al13 recently reported on global BTC burden but did not stratify the results by anatomic location, and ICC was excluded from the analyses. As classification coding systems have evolved to better define BTC subtypes, analysis of data coded with older versions may lead to misinterpretation of previous disease incidence rates.14 However, it is evident from existing literature that BTC subtypes have differing risk factors, prognoses, and outcomes after treatment and that epidemiologic trends should be discussed separately, as well as together, to facilitate comparisons with other studies.2,3,6 Thus, there is a need to define accurate epidemiologic trends that will allow specific risk factors to be identified, guiding experts in implementing policies to improve diagnosis and survival. Here, we performed a cross-sectional study of incidence and mortality data from 2 international databases to analyze trends in overall BTC and each anatomic subtype, by country, region, gender, and age. To our knowledge, this is the first report combining data on worldwide incidence and mortality of all BTC subtypes per the International Classification of Diseases, Tenth Revision.
Methods
Study Design and Data Sources
Incidence estimates of BTC were extracted on December 02, 2019, from the International Agency for Research on Cancer’s Cancer Incidence in Five Continents, Volume XI (IARC CI5-XI). IARC CI5-XI combines high-quality cancer incidence data for diagnoses made between 2008 and 2012 from 343 population-based cancer registries in 65 countries.12 Countries with incidence data obtained postmortem, or from patients with an unknown diagnosis year, were excluded. Data by ethnicity and race were not available from IARC CI5-XI. Data regarding the incidence of BTC in the US population from 2008 to 2012 were obtained from the US National Program of Cancer Registries, which collects cancer occurrence, treatments, and outcomes representing 97% of the US population.15
BTC mortality data were obtained from the World Health Organization (WHO) Mortality Database, which collects annual mortality data, including age, gender, and cause of death, from member states’ death registration systems.16 Cross-sectional mortality rates for 2006–2016 were compiled, and countries were excluded if there were <5 years of data on both patients with BTC and the general population and if they reported <85% death registration coverage in the most recent period. Quality of mortality data is shown in Table A1.
Patients diagnosed with any primary BTC (according to the International Classification of Diseases, Tenth Revision) in IARC CI5-XI or in the WHO Mortality Database were included in the analysis (Table A2). Patients with BTC diagnoses per the International Classification of Diseases for Oncology, Third Edition, were obtained from the US National Program of Cancer Registries (Table A2). As BTC is extremely rare in young adults and children,17 only patients aged ≥20 years were included. All data were collected, deidentified, and made publicly available by the respective database organizations; as such, institutional review board approval was not required for this study.
All authors had access to the study data and reviewed and approved the final manuscript.
Statistical Analysis
All analyses were performed in R 4.0.2 or Microsoft Excel. For both mortality and incidence, data were evaluated for all BTCs, and for each BTC anatomic subtype, by geography and gender. Mortality data were also evaluated by age. All rates were age-standardized and reported per 100,000 person-years. Incidence and mortality rates were adjusted to the 1960 Segi World Standard Population,12 consistent with recent mortality analyses in BTC.10,11 Rates based on <15 BTC cases or deaths were excluded. In IARC CI5-XI, the number of cases and corresponding person-years at risk are directly available for each age group for the BTC subtypes ICC and GBC and all other BTC types (all C24); however, to calculate the age-standardized rate for AVC, ECC, and “not otherwise specified,” we assumed the same age distribution of cases.
The relative mortality rate is the ratio of age-standardized mortality rate for patients aged 75+ years and those aged between 20 and 75 years. Temporal trends of BTC mortality rates were analyzed by estimating annual percent change using the weighted least squares method.
Results
Incidence Rates
BTC Overall
Twenty-two countries met the criteria for inclusion: diagnoses recorded prior to death, year of diagnoses known, and rates based on >15 BTC cases. BTC incidence rates (cases per 100,000 person-years) varied widely, ranging from 12.42 in Chile to 1.12 in Vietnam (Figure 1A). Countries in the Asia-Pacific region and South America had overall higher incidence rates (Asia-Pacific, 1.12–9.00; South America, 2.73–12.42) than European and North American countries (Europe, 2.00–3.59; North America, 2.33–2.35) (Figure 1A). Similarly, within the United States, the BTC incidence rate was 1.3-fold higher for Asian-Americans than that for the general US population (2.99 vs 2.33).
Figure 1.
Incidence of BTC (A) overall and (B) for subtypes, by country, from 2008 to 2012. ASR, age-standardized rate; AVC, ampulla of Vater Cancer; BTC, biliary tract cancer; ECC, extrahepatic cholangiocarcinoma; GBC, gallbladder cancer; ICC, intrahepatic cholangiocarcinoma; NOS, not otherwise specified.
ECC
The highest ECC incidence rate was in the Republic of Korea, with a rate of 2.71, and lowest in Vietnam and Argentina (0.10 and 0.11, respectively) (Figure 1B and Table A3). In Bulgaria, France, Germany, Hong Kong, Japan, the Republic of Korea, and Thailand, incidence of ECC in males was about twice higher than that in females (Table 1).
Table 1.
Incidence Rates of BTC Subtypes, by Country and Gender, 2008–2012
| Country | ECC |
ICC |
GBC |
AVC |
||||
|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | |
| Argentina | 0.12 | 0.10 | 0.68 | 0.55 | 1.69 | 2.36 | 0.16 | 0.19 |
| Australia | 0.67 | 0.44 | 0.74 | 0.55 | 0.52 | 0.94 | 0.43 | 0.25 |
| Brazil | 0.51 | 0.40 | 0.51 | 0.46 | 0.64 | 1.27 | 0.40 | 0.32 |
| Bulgaria | 0.42 | 0.24 | 0.42 | 0.24 | 0.69 | 1.39 | 0.32 | 0.18 |
| Canada | 0.83 | 0.58 | 0.51 | 0.45 | 0.54 | 0.89 | 0.43 | 0.28 |
| Chile | 0.38 | 0.45 | 0.28 | 0.24 | 5.05 | 13.75 | 0.92 | 0.72 |
| China | 1.01 | 0.85 | 0.70 | 0.47 | 1.07 | 1.63 | 0.27 | 0.19 |
| France | 0.80 | 0.42 | 1.32 | 0.76 | 0.45 | 0.64 | 0.52 | 0.34 |
| Germany | 0.98 | 0.56 | 0.80 | 0.59 | 0.48 | 0.85 | 0.53 | 0.31 |
| Hong Kong | 0.99 | 0.55 | 0.72 | 0.62 | 0.84 | 0.99 | 0.55 | 0.32 |
| India | 0.13 | 0.11 | 0.51 | 0.38 | 0.98 | 1.96 | 0.53 | 0.34 |
| Italy | 1.06 | 0.73 | 0.88 | 0.59 | 0.81 | 1.23 | 0.50 | 0.30 |
| Japan | 3.68 | 1.85 | 0.90 | 0.49 | 1.96 | 1.89 | 0.61 | 0.32 |
| Poland | 0.11 | 0.13 | 0.47 | 0.47 | 0.80 | 2.17 | 0.51 | 0.32 |
| Republic of Korea | 3.81 | 1.89 | 3.10 | 1.41 | 2.99 | 2.83 | 1.16 | 0.75 |
| Spain | 0.89 | 0.49 | 0.88 | 0.57 | 0.66 | 0.96 | 0.63 | 0.30 |
| Switzerland | 0.72 | 0.51 | 0.97 | 0.73 | 0.39 | 0.63 | 0.51 | 0.23 |
| Thailand | 1.50 | 0.72 | 2.26 | 1.28 | 0.71 | 1.03 | 0.49 | 0.39 |
| Turkey | 0.57 | 0.39 | 0.35 | 0.28 | 0.55 | 1.04 | 0.57 | 0.33 |
| United Kingdom | 0.37 | 0.31 | 0.76 | 0.69 | 0.34 | 0.68 | 0.42 | 0.28 |
| United States | 0.68 | 0.47 | 0.72 | 0.57 | 0.47 | 0.82 | 0.40 | 0.25 |
| Vietnam | 0.10 | 0.10 | 0.18 | 0.14 | 0.33 | 0.42 | 0.29 | 0.15 |
Rates are age-standardized and reported as cases per 100,000 person-years.
AVC, ampulla of Vater cancer; BTC, biliary tract cancer; ECC, extrahepatic cholangiocarcinoma; F, female; GBC, gallbladder cancer; ICC, intrahepatic cholangiocarcinoma; M, male.
ICC
ICC incidence rate was highest in the Republic of Korea (2.18) and lowest in Vietnam (0.16) (Figure 1B and Table A3). ICC incidence in males was approximately twice as high as that in females in Bulgaria, France, Japan, the Republic of Korea, and Thailand (Table 1).
GBC
GBC was the most common subtype of new BTC cases in 16 (73%) countries (Figure 1B and Table A3). GBC incidence was highest in Chile, with a rate of 9.68, which was over 3 times greater than the second highest GBC incidence rate (Republic of Korea, 2.90). Females trended toward higher incidence of GBC than males, with 10 countries having rates up to 2 times greater, and rates in Chile and Poland being around 3 times greater (Table 1).
AVC
AVC had the lowest incidence of all subtypes in 17 countries (77%) (Figure 1B and Table A3). AVC incidence rates were highest in the Republic of Korea (0.93) and lowest in Argentina (0.18). For AVC, a trend of higher incidence in males was observed in all countries except Argentina, where the incidence was similar between males and females (Table 1).
Mortality
BTC Overall
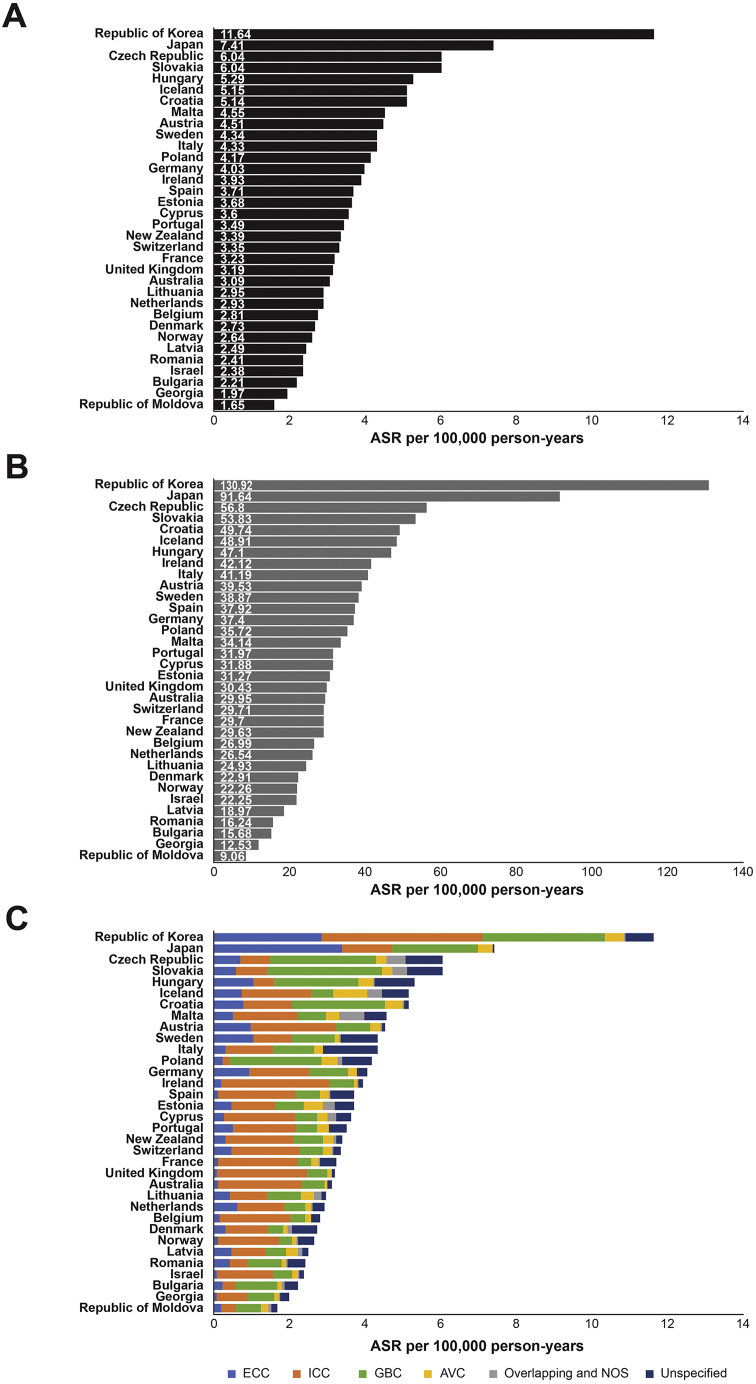
Thirty-four countries met the criteria for inclusion (≥5 years of data on both patients with BTC and the general population, and ≥85% death registration coverage), and all were in either the Asia-Pacific or European regions. The highest BTC mortality rate (deaths per 100,000 person-years) of 11.64 occurred in the Republic of Korea, and the lowest rate of 1.65 was in the Republic of Moldova (Figure 2A). In all countries, elderly patients (aged ≥75 years) had much higher BTC mortality rates than the overall population (Figure 2B and Table A4). The Republic of Korea had the highest (130.92) and the Republic of Moldova the lowest (9.06) BTC mortality rate for elderly patients. The relative mortality rate increase per 100,000 person-years for elderly patients, compared with all patients, was highest for Japan (20.33) and lowest in the Republic of Moldova (6.55) (Table A4). Elderly patients (aged ≥75 years) had strikingly high BTC mortality rates, compared with the overall population, in all countries (P < .001) (Figure 2B and Table A5). Higher-than-average mortality rates in multiple BTC subtypes were observed for Japan and the Republic of Korea.
Figure 2.
Mortality rates for BTC (A) overall, (B) overall in patients aged >75 years, and (C) for subtypes, by country, from 2006 to 2016. ASR, age-standardized rate; AVC, ampulla of Vater Cancer; BTC, biliary tract cancer; ECC, extrahepatic cholangiocarcinoma; GBC, gallbladder cancer; ICC, intrahepatic cholangiocarcinoma; NOS, not otherwise specified.
BTC mortality rates increased over time for 24 of the 34 countries (71%) (Table 2). Georgia had the greatest increase (16.84%), and Iceland the greatest decrease (−22.79%) in annual BTC mortality rate (Table 2).
Table 2.
BTC Mortality Rate Change for All Countries Included in the Analysis
| Country | Earliest year | ASR | Latest year | ASR | APC | |
|---|---|---|---|---|---|---|
| Australia | 2006 | 2.82 | 2016 | 3.43 | 2.20 | ▲ |
| Austria | 2006 | 4.27 | 2016 | 4.07 | 0.03 | – |
| Belgium | 2006 | 2.80 | 2016 | 2.94 | 1.06 | ▲ |
| Bulgaria | 2006 | 1.10 | 2015 | 2.08 | 5.92 | ▲ |
| Croatia | 2006 | 4.89 | 2016 | 4.97 | 0.22 | ▲ |
| Cyprus | 2006 | 2.45 | 2016 | 2.80 | 1.43 | ▲ |
| Czech Republic | 2006 | 7.06 | 2016 | 5.52 | −2.51 | ▼ |
| Denmark | 2006 | 2.55 | 2015 | 3.52 | 3.87 | ▲ |
| Estonia | 2006 | 0.44 | 2016 | 0.61 | 3.25 | ▲ |
| France | 2006 | 3.06 | 2014 | 3.38 | 1.55 | ▲ |
| Georgia | 2006 | 0.74 | 2015 | 2.05 | 16.84 | ▲ |
| Germany | 2006 | 4.08 | 2016 | 4.10 | 0.49 | ▲ |
| Hungary | 2006 | 5.37 | 2016 | 4.81 | −1.33 | ▼ |
| Iceland | 2006 | 3.77 | 2016 | 0.66 | −22.79 | ▼ |
| Ireland | 2007 | 3.75 | 2015 | 3.88 | 1.54 | ▲ |
| Israel | 2006 | 2.44 | 2016 | 2.21 | −0.29 | ▼ |
| Italy | 2006 | 4.28 | 2015 | 4.15 | −0.18 | ▼ |
| Japan | 2006 | 8.12 | 2016 | 6.59 | −2.01 | ▼ |
| Latvia | 2006 | 0.91 | 2015 | 2.83 | 15.10 | ▲ |
| Lithuania | 2006 | 2.38 | 2016 | 3.76 | 5.35 | ▲ |
| Malta | 2006 | 2.85 | 2015 | 3.62 | −0.63 | ▼ |
| Netherlands | 2006 | 2.35 | 2016 | 3.69 | 4.65 | ▲ |
| New Zealand | 2006 | 3.41 | 2013 | 3.53 | 0.59 | ▲ |
| Norway | 2006 | 2.22 | 2016 | 3.17 | 3.52 | ▲ |
| Poland | 2006 | 4.24 | 2016 | 3.97 | −0.99 | ▼ |
| Portugal | 2007 | 3.25 | 2016 | 4.42 | 3.78 | ▲ |
| Republic of Korea | 2006 | 3.62 | 2016 | 11.22 | 3.67 | ▲ |
| Republic of Moldova | 2006 | 0.89 | 2016 | 1.32 | 1.58 | ▲ |
| Romania | 2006 | 2.29 | 2016 | 2.53 | 1.41 | ▲ |
| Slovakia | 2006 | 3.21 | 2014 | 6.47 | 10.26 | ▲ |
| Spain | 2006 | 3.61 | 2015 | 3.96 | 1.12 | ▲ |
| Sweden | 2006 | 4.43 | 2016 | 4.16 | −0.30 | ▼ |
| Switzerland | 2006 | 3.27 | 2013 | 3.42 | −0.09 | ▼ |
| United Kingdom | 2006 | 2.59 | 2016 | 3.83 | 4.01 | ▲ |
▲, Mortality rate increased in the specified time period; ▼, mortality rate decreased in the specified time period; –, little to no change in the mortality rate in the specified time period (<±0.1 APC).
APC, annual percent change; ASR, age-standardized rate; BTC, biliary tract cancer.
ECC
The ECC mortality rate in Japan was the highest of all 34 countries studied and was 6 times greater than the average rate of all countries (3.37 vs 0.57). Males had higher ECC mortality than females in 28 countries (82%) (Table 3).
Table 3.
Mortality Rates of BTC Subtypes, by Country and Gender, 2006–2016
| Country | ECC |
ICC |
GBC |
AVC |
||||
|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | |
| Australia | 0.16 | 0.11 | 2.65 | 2.14 | 0.44 | 0.83 | 0.12 | 0.06 |
| Austria | 1.23 | 0.91 | 2.95 | 1.94 | 0.70 | 1.27 | 0.34 | 0.23 |
| Belgium | 0.20 | 0.11 | 2.35 | 1.77 | 0.33 | 0.52 | 0.19 | 0.14 |
| Bulgaria | 0.23 | 0.27 | 0.35 | 0.33 | 0.96 | 1.32 | 0.23 | 0.05 |
| Croatia | 1.15 | 0.68 | 1.69 | 1.13 | 2.02 | 3.14 | 0.65 | 0.42 |
| Cyprus | 0.38 | 0.22 | 2.59 | 1.52 | 0.51 | 0.70 | 0.29 | 0.34 |
| Czech Republic | 0.87 | 0.64 | 0.97 | 0.72 | 2.14 | 3.75 | 0.44 | 0.23 |
| Denmark | 0.28 | 0.36 | 1.23 | 1.20 | 0.33 | 0.47 | 0.17 | 0.13 |
| Estonia | 0.70 | 0.32 | 1.51 | 0.96 | 0.40 | 1.07 | 0.61 | 0.43 |
| France | 0.15 | 0.09 | 2.87 | 1.73 | 0.32 | 0.49 | 0.28 | 0.16 |
| Georgia | 0.06 | 0.07 | 1.34 | 0.55 | 0.60 | 0.80 | 0.17 | 0.10 |
| Germany | 1.18 | 0.89 | 2.03 | 1.42 | 0.74 | 1.39 | 0.31 | 0.19 |
| Hungary | 1.27 | 0.98 | 0.72 | 0.48 | 1.67 | 2.92 | 0.56 | 0.34 |
| Iceland | 0.83 | 0.59 | 1.59 | 2.17 | 0.86 | 0.53 | 1.34 | 0.34 |
| Ireland | 0.20 | 0.19 | 3.26 | 2.92 | 0.45 | 0.97 | 0.13 | 0.08 |
| Israel | 0.10 | 0.09 | 1.67 | 1.54 | 0.34 | 0.74 | 0.20 | 0.15 |
| Italy | 0.42 | 0.26 | 1.65 | 1.09 | 0.89 | 1.43 | 0.34 | 0.20 |
| Japan | 5.35 | 2.69 | 1.98 | 1.05 | 2.49 | 2.57 | 0.53 | 0.31 |
| Latvia | 0.67 | 0.39 | 1.05 | 0.87 | 0.39 | 0.69 | 0.44 | 0.26 |
| Lithuania | 0.53 | 0.38 | 1.17 | 0.95 | 0.64 | 1.22 | 0.47 | 0.31 |
| Malta | 0.52 | 0.67 | 2.50 | 1.18 | 0.90 | 0.63 | 0.44 | 0.40 |
| Netherlands | 0.71 | 0.59 | 1.58 | 1.18 | 0.43 | 0.77 | 0.21 | 0.16 |
| New Zealand | 0.43 | 0.27 | 2.07 | 1.75 | 0.53 | 1.10 | 0.34 | 0.33 |
| Norway | 0.16 | 0.11 | 1.74 | 1.63 | 0.28 | 0.49 | 0.16 | 0.11 |
| Poland | 0.25 | 0.22 | 0.26 | 0.22 | 1.31 | 3.45 | 0.64 | 0.38 |
| Portugal | 0.69 | 0.43 | 2.47 | 1.30 | 0.50 | 0.64 | 0.47 | 0.23 |
| Republic of Korea | 4.60 | 2.27 | 6.37 | 3.39 | 3.76 | 3.51 | 0.75 | 0.49 |
| Republic of Moldova | 0.22 | 0.15 | 0.49 | 0.37 | 0.51 | 0.82 | 0.25 | 0.18 |
| Romania | 0.50 | 0.37 | 0.65 | 0.38 | 0.99 | 0.95 | 0.15 | 0.10 |
| Slovakia | 0.57 | 0.66 | 1.11 | 0.65 | 2.17 | 4.03 | 0.43 | 0.23 |
| Spain | 0.15 | 0.08 | 2.80 | 1.70 | 0.59 | 0.90 | 0.37 | 0.18 |
| Sweden | 1.06 | 1.19 | 1.12 | 1.01 | 0.72 | 1.74 | 0.16 | 0.11 |
| Switzerland | 0.53 | 0.49 | 2.17 | 1.72 | 0.43 | 0.89 | 0.31 | 0.18 |
| United Kingdom | 0.11 | 0.08 | 2.67 | 2.49 | 0.38 | 0.79 | 0.16 | 0.11 |
Rates are age-standardized and reported as cases per 100,000 person-years.
AVC, ampulla of Vater cancer; BTC, biliary tract cancer; ECC, extrahepatic cholangiocarcinoma; F, female; GBC, gallbladder cancer; ICC, intrahepatic cholangiocarcinoma; M, male.
ICC
Of the BTC subtypes, ICC had the highest mortality rate in 25 of the 34 countries (74%), with 7 of those exhibiting rates of ≥2 (Figure 2C). The Republic of Korea had the highest ICC mortality rate (4.24) (Figure 2C). ICC mortality was higher in males, than in females, in 33 countries (97%) (Table 3).
GBC
In the remaining 9 countries for which ICC mortality was not the highest, the GBC mortality rate was highest in all (26%), with 7 of the countries exhibiting mortality rates of ≥2 (Figure 2C). The Republic of Korea had the highest GBC mortality rate (3.24) (Figure 2C). GBC mortality rates were higher in males in 4 countries (12%) (Table 3).
AVC
AVC had the lowest mortality rate in 21 countries (62%) (Table A5) and was higher for males in 33 countries (97%) (Table 3). In Bulgaria and Iceland, the AVC mortality rate was about 4 times greater for males than for females.
Discussion
This analysis combines incidence and mortality of overall BTC, and its anatomic subtypes, into one report for the first time. Overall, our analysis is consistent with past studies showing variation of BTC incidence and mortality across countries and regions, with generally higher rates in Asia and South America than in Europe and North America.2,5,10,11 Across most countries, GBC accounted for the greatest number of new cases, whereas ICC was the most frequent cause of death among the BTC subtypes. BTC mortality increased during the defined time period in the majority of countries analyzed. These findings differ from the study by Torre et al,11 in which mortality caused by gallbladder and other types of BTC decreased in most countries, including the Republic of Korea and several countries in Europe, which had increased mortality in this analysis. These discrepancies may be attributed to the exclusion of ICC data from the study of Torre et al11 as ICC had the highest mortality rate in the majority of countries in the present study. Furthermore, Bertuccio et al10 found that from 2002 to 2012, ICC mortality increased globally while ECC mortality decreased, further highlighting the importance of differentiating between BTC subtypes in global epidemiological studies. The mortality rate of BTC was approximately 5- to 10-fold higher in elderly patients than the overall BTC mortality rate in each country, which may coincide with diagnosis of BTC subtypes averaging in the sixth and seventh decades of life.18,19 In addition, comorbidity in elderly patients may limit curative treatment options, and therefore have an adverse effect on patient outcomes.20
The higher BTC incidence and mortality rates observed in Asian countries compared with other regions is consistent with well-known infection-related risk factors. Hepatitis B and C viral infections are prevalent in many Asian countries and associated with an increased risk of ICC.21, 22, 23 ECC and ICC incidence is high in East Asian countries, where infection with liver flukes, a well-established risk factor, is endemic.2 Although we report the BTC incidence rate in Thailand as one of the highest among the countries included in this analysis, a recent study showed a decrease in cholangiocarcinoma incidence between 2002 and 2013 in a northern Thailand province, which coincided with preventative measures concerning liver flukes.24 ECC and ICC had the top 2 incidence rates for Thailand in our analysis, indicating that it is still an important driver of BTC development.
Comorbidities such as obesity, nonalcoholic fatty liver disease, and diabetes may also contribute to the risk of developing BTC,25, 26, 27, 28 and their increasing prevalence may contribute to high BTC incidence.29,30 A meta-analysis of observational studies found that body mass index status of overweight and obese patients was associated with increased risk of ECC and GBC,26 while a different study suggested that diabetes and obesity increased the risk of ICC.27 Nonalcoholic fatty liver disease is associated with increased risk of ICC.28 In addition, data from 26 pooled prospective studies demonstrated that smoking increased risk of all subtypes of BTC, except GBC, with observed dose-response effects for smoking pack-years, duration, and intensity.6 Meanwhile, alcohol consumption was associated with ICC, with evidence of a dose-response trend.6 This study highlights how each subtype may be vulnerable to specific risk factors and emphasizes the value of separating epidemiologic data by subtype in order to better understand disease etiology.
Variation of incidence and mortality of overall BTC and its subtypes is also partly driven by genetic predisposition. Gallstones are linked to GBC development,31 as evidenced in Chilean native females (approximately 50% gallstone prevalence and 27/100,000 cancer incidence) compared with white American females (approximately 17% and 2/100,000).32 This is especially striking, as GBC is a rare cancer worldwide, yet in Chile, it was the number one cause of cancer death in females (2003–2007).33 On the other hand, the high incidence of GBC in India is disproportionate to the low prevalence of gallstones in the country, and the geographic variation in incidence consistent with the route of the Ganges river indicates that other risk factors may be involved, such as soil and water contamination by industrial wastes, agricultural runoff, and human sewage.34 Asian countries included in our analysis had high overall BTC incidence rates, which can be explained by these environmental factors. However, the higher rates found in Asian populations may not be entirely geographic, as Asian-Americans also suffered higher BTC incidence than the overall US population, as reported here.
A higher incidence and mortality rate for GBC was observed for females vs males in most countries, consistent with prior studies.5,11 In nearly all countries in this analysis, AVC incidence and mortality were higher in males. Less epidemiologic information is available to determine the potential risk factors for AVC, as it is often considered part of ECC or GBC.
While we selected countries providing sufficient-quality data, with population sizes large enough to limit excessive random variation issues, difficulties controlling the quality of the data collected by each nation are evident. For example, data on China in the WHO Mortality Database are based on very low coverage and sampling. Certain countries known to have high BTC incidence, such as Chile and Thailand, are missing from the mortality data collection, limiting our ability to link incidence and mortality. Discrepancies in incidence between neighboring countries with similar socioeconomical environments, for example, Thailand and Vietnam, also highlight reporting issues. Variation in diagnostic techniques could partially explain the differences in BTC subtypes, with better classification of different forms of intrahepatic neoplasms available in some countries.10 Additionally, countries with previously reported high incidence (Bolivia, Peru, Laos, Cambodia, Vietnam)35,36 did not have high rates (Vietnam), or did not meet the inclusion criteria (Bolivia, Peru, Laos, Cambodia) in this analysis, which could be due to poor quality of incidence data. A final limitation of this analysis is the lack of survival data provided by the databases, which can inform the effectiveness of treatments used across countries.
Accurate diagnosis of BTC is challenging due to geographic differences in diagnostic strategies, access to resources, and anatomic location; also, misdiagnosis between intrahepatic BTC and hepatocellular carcinoma may result in miscalculation of global incidences.37,38 In addition, inaccurate or delayed diagnosis of BTC contributes to poor patient outcomes, as therapeutic options are limited and surgery is less frequently an option at late stages of disease.39 Improved diagnosis of BTC is also relevant to advances in targeted treatment as BTC subtypes may respond differently to treatments as suggested by studies showing that some subtypes have targetable molecular alterations.40 This is especially relevant, since targeted therapies have recently shown remarkable results in biomarker-selected patient populations,41,42 a potential which could be exploited in combination with the more recent proposed schemas of immunotherapy.40
In conclusion, the reported incidence and mortality rates, including the overall increase in mortality rates, highlight an existing unmet need for improved prevention of, and treatment for, BTC. These findings may provide important public health guidance for intervention strategies and the development of therapeutics related to geographic groups with differing BTC rates.
Acknowledgments:
The authors would like to thank the patients, their families and caregivers, and all investigators involved in this study. The authors would also like to thank the WHO scientific and administrative contributors, as well as the directors and staff of all the registries who submitted data. Without their collective hard work and dedication in obtaining information on the incidence of cancer, this type of study would not be possible.
Authors' Contributions:
Katherine Baria: Conception and design of the study; generation, collection, assembly, analysis, and/or interpretation of data; drafting or revision of the manuscript; and approval of the final draft of the manuscript. Enrico N. De Toni: Conception and design of the study, drafting or revision of the manuscript, and approval of the final draft of the manuscript. Binbing Yu: Generation, collection, assembly, analysis, and/or interpretation of data; drafting or revision of the manuscript; and approval of the final draft of the manuscript. Zhouxin Jiang: Generation, collection, assembly, analysis, and/or interpretation of data; drafting or revision of the manuscript; and approval of the final draft of the manuscript. Shaum M. Kabadi: Generation, collection, assembly, analysis, and/or interpretation of data; drafting or revision of the manuscript; and approval of the final draft of the manuscript. Matteo Malvezzi: Conception and design of the study, drafting or revision of the manuscript, and approval of the final draft of the manuscript.
Footnotes
Conflicts of Interest: These authors disclose the following: Katherine Baria, Binbing Yu, and Zhouxin Jiang are employees and stockholders of AstraZeneca. Enrico N. De Toni has served as a paid consultant for AstraZeneca, Bayer, Bristol-Myers Squibb, EISAI, Eli Lilly, IPSEN, Pfizer, and Roche; has received reimbursement of meeting attendance fees and travel expenses from ArQule, AstraZeneca, Bayer, Bristol-Myers Squibb, Celsion, and Roche; has received lecture honoraria from Bristol-Myers Squibb and Falk; and has received third-party funding for scientific research from ArQule, AstraZeneca, Bayer, Bristol-Myers Squibb, Eli Lilly, and Roche. Shaum M. Kabadi was an employee of AstraZeneca during the manuscript development and is currently an employee of Sanofi and a shareholder of AstraZeneca and Sanofi. The remaining author discloses no conflicts.
Funding: This study was funded by AstraZeneca.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data-sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Writing Assistance: Medical writing support, which was in accordance with Good Publication Practice (GPP3) guidelines, was provided by Anne-Marie Manwaring (Parexel, Littlehampton, UK) and Nicole Seneca, PhD, (Parexel, Hackensack, NJ) and was funded by AstraZeneca. Editorial assistance was provided by Laura Park, MSc, (CMC Connect, Glasgow, UK) in accordance with GPP3 guidelines and funded by AstraZeneca.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2022.04.007.
Supplementary Materials
References
- 1.Tariq N.U., McNamara M.G., Valle J.W. Biliary tract cancers: current knowledge, clinical candidates and future challenges. Cancer Manag Res. 2019;11:2623–2642. doi: 10.2147/CMAR.S157092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banales J.M., Cardinale V., Carpino G., et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 3.Jackson S.S., Van Dyke A.L., Zhu B., et al. Anthropometric risk factors for cancers of the biliary tract in the biliary tract cancers pooling project. Cancer Res. 2019;79:3973–3982. doi: 10.1158/0008-5472.CAN-19-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirstein M.M., Vogel A. Epidemiology and risk factors of cholangiocarcinoma. Visc Med. 2016;32:395–400. doi: 10.1159/000453013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salazar M., Ituarte C., Abriata M.G., et al. Gallbladder cancer in South America: epidemiology and prevention. Chin Clin Oncol. 2019;8:32. doi: 10.21037/cco.2019.07.12. [DOI] [PubMed] [Google Scholar]
- 6.McGee E.E., Jackson S.S., Petrick J.L., et al. Smoking, alcohol, and biliary tract cancer risk: a pooling project of 26 prospective studies. J Natl Cancer Inst. 2019;111:1263–1278. doi: 10.1093/jnci/djz103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marin J.J.G., Prete M.G., Lamarca A., et al. Current and novel therapeutic opportunities for systemic therapy in biliary cancer. Br J Cancer. 2020;123:1047–1059. doi: 10.1038/s41416-020-0987-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepage C., Capocaccia R., Hackl M., et al. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999-2007: results of EUROCARE-5. Eur J Cancer. 2015;51:2169–2178. doi: 10.1016/j.ejca.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 9.Wang H., Yu B., Baria K. Biliary tract cancer (BTC) epidemiology in the United States. Pharmacoepidemiol Drug Saf. 2020;29(Suppl 3):2469. [Google Scholar]
- 10.Bertuccio P., Malvezzi M., Carioli G., et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71:104–114. doi: 10.1016/j.jhep.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Torre L.A., Siegel R.L., Islami F., et al. Worldwide burden of and trends in mortality from gallbladder and other biliary tract cancers. Clin Gastroenterol Hepatol. 2018;16:427–437. doi: 10.1016/j.cgh.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Bray F., Colombet M., Mery L., et al. CI5 XI: cancer incidence in five continents, volume XI (electronic version) 2017. https://ci5.iarc.fr Available from:
- 13.Ouyang G., Liu Q., Wu Y., et al. The global, regional, and national burden of gallbladder and biliary tract cancer and its attributable risk factors in 195 countries and territories, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study 2017. Cancer. 2021;127:2238–2250. doi: 10.1002/cncr.33476. [DOI] [PubMed] [Google Scholar]
- 14.Khan S.A., Emadossadaty S., Ladep N.G., et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56:848–854. doi: 10.1016/j.jhep.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention National Program of Cancer Registries (NPCR) - about the program. 2018. https://www.cdc.gov/cancer/npcr/about.htm Available from:
- 16.World Health Organization WHO Mortality Database. 2019. https://www.who.int/data/data-collection-tools/who-mortality-database Available from:
- 17.Newsome J.R., Venkatramani R., Heczey A., et al. Cholangiocarcinoma among children and adolescents: a review of the literature and surveillance, epidemiology, and end results program database analysis. J Pediatr Gastroenterol Nutr. 2018;66:e12–e18. doi: 10.1097/MPG.0000000000001749. [DOI] [PubMed] [Google Scholar]
- 18.Kim B.W., Oh C.M., Choi H.Y., et al. Incidence and overall survival of biliary tract cancers in South Korea from 2006 to 2015: using the National Health Information Database. Gut Liver. 2019;13:104–113. doi: 10.5009/gnl18105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rawla P., Sunkara T., Thandra K.C., et al. Epidemiology of gallbladder cancer. Clin Exp Hepatol. 2019;5:93–102. doi: 10.5114/ceh.2019.85166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahara N., Nakai Y., Saito K., et al. The impact of age and comorbidity in advanced or recurrent biliary tract cancer receiving palliative chemotherapy. J Gastroenterol Hepatol. 2020;35:1828–1835. doi: 10.1111/jgh.15066. [DOI] [PubMed] [Google Scholar]
- 21.Shaib Y.H., El-Serag H.B., Nooka A.K., et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol. 2007;102:1016–1021. doi: 10.1111/j.1572-0241.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 22.Hong C.Y., Sinn D.H., Kang D., et al. Incidence of extrahepatic cancers among individuals with chronic hepatitis B or C virus infection: a nationwide cohort study. J Viral Hepat. 2020;27:896–903. doi: 10.1111/jvh.13304. [DOI] [PubMed] [Google Scholar]
- 23.Huy T.T.T., Abe K. Molecular epidemiology of hepatitis B and C virus infections in Asia. Pediatr Int. 2004;46:223–230. doi: 10.1046/j.1442-200x.2004.01867.x. [DOI] [PubMed] [Google Scholar]
- 24.Kamsa-Ard S., Luvira V., Suwanrungruang K., et al. Cholangiocarcinoma trends, incidence, and relative survival in Khon Kaen, Thailand from 1989 through 2013: a population-based cancer registry study. J Epidemiol. 2019;29:197–204. doi: 10.2188/jea.JE20180007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steele J.A., Richter C.H., Echaubard P., et al. Thinking beyond Opisthorchis viverrini for risk of cholangiocarcinoma in the lower Mekong region: a systematic review and meta-analysis. Infect Dis Poverty. 2018;7:44. doi: 10.1186/s40249-018-0434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L., Gan Y., Li W., et al. Overweight, obesity and the risk of gallbladder and extrahepatic bile duct cancers: a meta-analysis of observational studies. Obesity (Silver Spring) 2016;24:1786–1802. doi: 10.1002/oby.21505. [DOI] [PubMed] [Google Scholar]
- 27.Petrick J.L., Thistle J.E., Zeleniuch-Jacquotte A., et al. Body mass index, diabetes and intrahepatic cholangiocarcinoma risk: the Liver Cancer Pooling Project and meta-analysis. Am J Gastroenterol. 2018;113:1494–1505. doi: 10.1038/s41395-018-0207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wongjarupong N., Assavapongpaiboon B., Susantitaphong P., et al. Non-alcoholic fatty liver disease as a risk factor for cholangiocarcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 2017;17:149. doi: 10.1186/s12876-017-0696-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forte V., Pandey A., Abdelmessih R., et al. Obesity, diabetes, the cardiorenal syndrome, and risk for cancer. Cardiorenal Med. 2012;2:143–162. doi: 10.1159/000337314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurice J., Manousou P. Non-alcoholic fatty liver disease. Clin Med (Lond) 2018;18:245–250. doi: 10.7861/clinmedicine.18-3-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcano-Bonilla L., Mohamed E.A., Mounajjed T., et al. Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin Clin Oncol. 2016;5:61. doi: 10.21037/cco.2016.10.09. [DOI] [PubMed] [Google Scholar]
- 32.Hundal R., Shaffer E.A. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. doi: 10.2147/CLEP.S37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izarzugaza M.I., Fernández L., Forman D., et al. Burden of gallbladder cancer in Central and South America. Cancer Epidemiol. 2016;44 Suppl 1:S82–S89. doi: 10.1016/j.canep.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 34.Dutta U., Bush N., Kalsi D., et al. Epidemiology of gallbladder cancer in India. Chin Clin Oncol. 2019;8:33. doi: 10.21037/cco.2019.08.03. [DOI] [PubMed] [Google Scholar]
- 35.Miranda-Filho A., Piñeros M., Ferreccio C., et al. Gallbladder and extrahepatic bile duct cancers in the Americas: incidence and mortality patterns and trends. Int J Cancer. 2020;147:978–989. doi: 10.1002/ijc.32863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng J., Feng Y., Rinaldi G., et al. The miRNAome of Opisthorchis viverrini induced intrahepatic cholangiocarcinoma. Genom Data. 2014;2:274–279. doi: 10.1016/j.gdata.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li F., Li Q., Liu Y., et al. Distinguishing intrahepatic cholangiocarcinoma from hepatocellular carcinoma in patients with and without risks: the evaluation of the LR-M criteria of contrast-enhanced ultrasound liver imaging reporting and data system version 2017. Eur Radiol. 2020;30:461–470. doi: 10.1007/s00330-019-06317-2. [DOI] [PubMed] [Google Scholar]
- 38.Mueller C., Waldburger N., Stampfl U., et al. Non-invasive diagnosis of hepatocellular carcinoma revisited. Gut. 2018;67:991–993. doi: 10.1136/gutjnl-2017-314981. [DOI] [PubMed] [Google Scholar]
- 39.Benson A.B., D'Angelica M.I., Abbott D.E., et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:541–565. doi: 10.6004/jnccn.2021.0022. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura H., Arai Y., Totoki Y., et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003–1010. doi: 10.1038/ng.3375. [DOI] [PubMed] [Google Scholar]
- 41.Abou-Alfa G.K., Macarulla T., Javle M.M., et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:796–807. doi: 10.1016/S1470-2045(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abou-Alfa G.K., Sahai V., Hollebecque A., et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.