ACC Competency Management Committee
Lisa A. Mendes, MD, FACC, Chair
James A. Arrighi, MD, FACC##
John P. Breinholt III, MD, FACC
Jennifer Day, MSN, RN
G. William Dec Jr, MD, FACC∗∗∗
Ali E. Denktas, MD, FACC, FSCAI
David Drajpuch, ACNP-BC, CRNP, FNP-BC, MSN
Nadeen Faza, MD
Sanjeev A. Francis, MD, FACC∗∗∗
Rebecca T. Hahn, MD, FACC
Susan D. Housholder-Hughes, RN, DNP, ACNS-BC, ANP-BC, FACC
Sadiya S. Khan, MD, FACC∗∗∗
Meera Devi Kondapaneni, MBBS, FACC
Kwan S. Lee, MD, FACC
C. Huie Lin, MD, PhD, FACC
Jamal Hussain Mahar, MD
Shannon McConnaughey, MD, FACC∗∗∗
Khusrow Niazi, MBBS, FACC
Dorothy D. Pearson, PA-C, AACC
Lynn R. Punnoose, MD
Risheen S. Reejhsinghani, MD, FACC
Thomas Ryan, MD, FACC∗∗∗
Frank E. Silvestry, MD, FACC
Michael A. Solomon, MD, MBA, FACC
Robert L. Spicer, MD, FACC∗∗∗
Gaby Weissman, MD, FACC
Steven W. Werns, MD, FACC
Preamble
Since publication of its first Core Cardiovascular Training Statement (COCATS) in 1995,1 the American College of Cardiology (ACC) has defined the knowledge, experiences, skills, and behaviors expected of clinical cardiologists. Subsequent revisions have moved toward competency-based training based on the 6-domain competency structure promulgated by the Accreditation Council for Graduate Medical Education (ACGME) and the American Board of Medical Specialties and endorsed by the American Board of Internal Medicine (ABIM).2,3 The ACC has taken a similar approach to describe the aligned general cardiology lifelong learning competencies that practicing cardiologists are expected to maintain. Many hospital systems now use the 6-domain structure as part of medical staff privileging, peer review, and professional competence assessments.
Whereas COCATS and the associated Lifelong Learning Competencies for General Cardiologists4 focus on general clinical cardiology, ACC Advanced Training Statements and associated Lifelong Learning Statements define selected competencies beyond those expected of all cardiologists and that typically require training beyond a standard cardiovascular disease fellowship curriculum. This includes, but is not limited to, those disciplines for which there is an ABIM subspecialty certification. The Advanced Training Statements describe key experiences and outcomes necessary to acquire competency in a defined subspecialty area of cardiology in a structured training program. These are supplemented by Lifelong Learning Statements that address the commitment to sustaining and enriching competency over the span of a career.
The ACC Competency Management Committee oversees the development and periodic revision of the cardiovascular training and competency statements. A key feature of competency-based training and performance is an outcome-based evaluation system. Although specific areas of training may require a minimum number of procedures or duration of training to ensure adequate exposure to the range of clinical disorders, the objective assessment of proficiency and outcomes demonstrates the achievement of competency. Evaluation tools include examinations, direct observation, procedure case logs, simulation, conference presentations, and multisource (360°) evaluations. For practicing physicians, these tools also include professional society registry or hospital quality data, peer-review processes, and patient satisfaction surveys. A second feature of competency-based training is recognition that learners gain competency at different rates. For multiyear training programs, assessment of representative curricular milestones during training can identify learners or areas that require additional focused attention.
The recommendations in ACC Cardiovascular Training and Lifelong Learning Statements are based on available evidence and, where evidence is lacking, reflect consensus expert opinion. The writing committees are broad-based and typically include early-, mid-, and later-career specialists; general cardiology and subspecialty training directors; practicing cardiologists; people working in institutions of various sizes and in diverse practice settings across the United States; and nonphysician members of the cardiovascular care team. All documents undergo a rigorous process of peer review and public comment. Recommendations are intended to guide the assessment of competence of cardiovascular care providers beginning independent practice as well as those undergoing periodic reviews to ensure that competence is maintained.
This Advanced Training Statement addresses the core competencies required of interventional cardiologists, including competencies related to coronary, peripheral vascular, and structural heart interventions. The competencies for coronary interventions in adults serve as the foundation for cardiologists who wish to pursue training in peripheral vascular or structural heart interventions. Furthermore, this statement identifies select competencies for interventional cardiologists who choose to focus their careers on peripheral vascular or structural heart interventions that may be acquired by some advanced trainees either during formal fellowship training or through subsequent training experiences. This document provides examples of appropriate measures for assessing competence in the context of training.
The work of the writing committee was supported exclusively by the ACC without commercial support. Writing committee members volunteered their time to this effort. Conference calls of the writing committee were confidential and attended only by committee members. To avoid actual, potential, or perceived conflicts of interest resulting from relationships with industry and other entities (RWI) held by writing committee members or peer reviewers of the document, individuals were required to disclose all current health care–related relationships, including those existing 12 months before initiation of the writing effort. The ACC Competency Management Committee reviewed these disclosures to identify products (currently marketed or under development) pertinent to the document topic. Based on this information, the writing committee was selected to ensure that the majority of members, including the chair, had no relevant RWI. RWI was reviewed at the start of all meetings and conference calls and was updated as changes occurred. Relevant RWI for authors is disclosed in Appendix 1. To ensure transparency, comprehensive RWI for authors, including RWI not pertinent to this document, is available in a Supplemental Appendix. Employment information and affiliations of the peer reviewers are shown in Appendix 2. There are no RWI restrictions for participation in peer review, in the interest of encouraging comments from a variety of constituencies to ensure that a broad range of viewpoints inform final document content. Reviewers are required, however, to disclose all health care–related RWI and other entities, and their disclosure information is posted online. Disclosure information for the ACC Competency Management Committee is available online at http://www.acc.org/guidelines/about-guidelines-and-clinical-documents/guidelines-and-documents-task-forces, and the ACC disclosure policy for document development is posted at http://www.acc.org/guidelines/about-guidelines-and-clinical-documents/relationships-with-industry-policy.
Lisa A. Mendes, MD, FACC
Chair, ACC Competency Management Committee
1. Introduction
1.1. Document Development Process
1.1.1. Writing Committee Organization
The writing committee consisted of a broad range of members representing the ACC, American Heart Association (AHA), Society for Cardiovascular Angiography and Interventions (SCAI), American Association for Thoracic Surgery, American Society of Echocardiography, Heart Failure Society of America, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, Society of Interventional Radiology, Society of Thoracic Surgeons, Society for Vascular Medicine, and the Society for Vascular Surgery. Each writing committee member performs at least 1 of the following roles: 1) early-, mid-, and later-career interventional cardiologists specializing in coronary, peripheral vascular, structural heart, and adult congenital interventions who work in institutions and catheterization laboratories of various sizes, representing both academic and community-based practice settings; 2) cardiovascular disease and interventional cardiology training program directors, including those who direct structural heart disease (SHD) and peripheral vascular intervention (PVI) programs; 3) specialists representing cardiac anesthesiology, cardiothoracic and vascular surgery, cardiovascular computed tomography (CCT), cardiovascular magnetic resonance (CMR), echocardiography, electrophysiology, general cardiology, geriatric cardiology, heart failure, interventional radiology, pediatric interventions, valvular heart disease, and vascular medicine, as well as those with expertise in quality assurance and systems of care; 4) nurse practitioners, physician associates, and interventional cardiology fellows-in-training; and 5) diversity in geographic region, gender, ethnicity, and race. The writing committee also included physicians experienced in defining and applying training standards according to the 6 general competency domains promulgated by the ACGME and the American Board of Medical Specialties and endorsed by the ABIM. This writing committee met the ACC’s disclosure requirements for relationships with industry, as described in the Preamble.
1.1.2. Document Development and Approval
The writing committee convened by conference call and email to finalize the document outline, develop the initial draft, revise the draft based on committee feedback, and ultimately approve the document for external peer review. In addition, the committee conducted a survey of interventional cardiology training program directors to obtain additional insight into procedural numbers to consider in writing committee deliberations.
The document was reviewed by 24 official representatives from the ACC, AHA, SCAI, American Association for Thoracic Surgery, American Society of Echocardiography, Heart Failure Society of America, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, Society of Interventional Radiology, Society of Thoracic Surgeons, Society for Vascular Medicine, and the Society for Vascular Surgery, as well as by 39 additional content reviewers (see Appendix 2). The document was simultaneously posted for public comment from November 5, 2021, to November 29, 2021. A total of 748 comments were submitted on the document, which were reviewed and addressed by the writing committee. A member of the ACC Competency Management Committee served as lead reviewer to ensure a fair and balanced peer review resolution process. Both the writing committee and the ACC Competency Management Committee approved the final document to be sent for organizational approval. The ACC, AHA, and SCAI approved the document for publication with endorsement from the American Association for Thoracic Surgery, American Society of Echocardiography, Heart Failure Society of America, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, Society of Thoracic Surgeons, and Society for Vascular Medicine. This document is considered current until the ACC Competency Management Committee revises or withdraws it from publication.
1.2. Background and Scope
The original 1995 ACC recommendations for training in adult cardiology evolved from a Core Cardiology Training Symposium.1 After several iterations, COCATS 4 focuses on trainee outcomes that require delineation of specific components of competency within the subspecialty, definition of the tools necessary to assess training, and establishment of milestones documenting the trainee’s progression toward independent competency.5 Ultimately, the goal is for the trainee to develop the professional skill set to be able to evaluate, diagnose, and treat patients with acute and chronic cardiovascular diseases.
Each COCATS 4 document included individual task force reports that address subspecialty areas in cardiology, each of which is an important component in training a fellow in cardiovascular disease. Task Force 10 of that document addressed training in cardiac catheterization and updated previous standards for general cardiovascular training for fellows enrolled in cardiovascular fellowship programs.6 It addressed faculty, facilities, equipment, and additional support. It also addressed training components, including didactic, clinical, and hands-on experience, and the number of procedures and duration of training. Importantly, the COCATS 4 Task Force 10 report did not provide detailed guidelines for advanced training in cardiovascular interventions.
This document focuses on training requirements for advanced training in interventional cardiology for adult patients, including coronary, peripheral vascular, and structural heart interventions. For training standards related to pediatric cardiac catheterization (diagnostic and interventional), readers should refer to the SPCTPD/ACC/AAP/AHA Training Guidelines for Pediatric Cardiology Fellowship Programs “Task Force 3: Pediatric Cardiology Fellowship Training in Cardiac Catheterization”7 and to the “SCAI Expert Consensus Statement for Advanced Training Programs in Pediatric and Congenital Interventional Cardiac Catheterization.”8
1.2.1. Evolution of Interventional Cardiology
Since the first percutaneous coronary balloon angioplasty was performed in 1977, the evolution of endovascular technologies and procedures has allowed interventional cardiologists to treat an expanding population of patients with cardiovascular disease. This population now includes patients presenting with more complex coronary artery disease (CAD), advanced age, heart failure, peripheral vascular disease, and valvular disease, as well as other forms of SHD. Expanding cognitive and procedural competencies are required for practitioners to safely and effectively treat this increasingly diverse and complex patient population. A focus on cardiovascular health equity through understanding differences in care and outcomes related to patients’ sex, gender, race, ethnicity, and age, as well as social determinants of health are also integral to training a competent interventional cardiologist.9,10 Reevaluation of the current interventional cardiology training curriculum is necessary to adequately address the changing clinical challenges that present in practice.
The ABIM requires 3 years of general cardiovascular fellowship in an ACGME-approved program to be eligible to take the certification examination in cardiovascular disease. Successful completion of this fellowship is a requirement for trainees to enter the 1-year interventional cardiology fellowship required for certification in this subspecialty. The competencies developed during general fellowship serve as a strong platform to support the additional knowledge and procedural skills acquired through interventional cardiology training. One year of advanced fellowship training focused predominantly on coronary interventions will not likely provide adequate clinical exposure and procedural experience to achieve competency in all other areas of interventional cardiology. Additional fellowship or postfellowship training will be needed to gain the experience necessary to become a competent, independent expert in most aspects of peripheral vascular or structural heart interventions, depending on the trainee’s career focus. This document provides the framework for training across the expanse of interventional procedures (see Section 4.2.1. Training Pathway and Procedural Number Guidance).
1.2.2. Levels of Training
COCATS 4 updated standards for training fellows in cardiovascular medicine and established consistent training criteria across all aspects of cardiovascular diseases, including cardiac catheterization.6 For the cardiovascular fellowship, the following 3 levels of training have been delineated for training in cardiac catheterization.
Level I training, the basic training required of trainees to become competent consultant cardiologists, is required of all cardiovascular fellows and can be accomplished as part of a standard 3-year training program in cardiology. In the case of cardiac catheterization, Level I represents training for those who will practice noninvasive cardiology and whose invasive activities will be confined to critical care unit procedures.6 This level will also provide training in the indications for the procedure and in the accurate interpretation of data obtained in the catheterization laboratory.
Level II training, also described in COCATS 4, refers to additional training in 1 or more areas that enables some cardiologists to perform or interpret specific procedures or render more specialized care for patients with certain conditions. Level II training in selected areas may be achieved by some trainees during the standard 3-year cardiovascular fellowship, depending on their career goals and use of elective rotations. In the case of cardiac catheterization and peripheral angiography, Level II is defined as training for those who will either practice diagnostic cardiovascular catheterization or pursue further training in interventional cardiology.6 Notably, no certification examination currently exists to assess Level II competency in this field.
Level III training, the primary focus of this document, requires additional training and experience beyond the cardiovascular fellowship for the acquisition of specialized knowledge and experience in performing, interpreting, and training others to perform specific procedures or render advanced, specialized care for specific procedures at a high level of skill. In the case of interventional cardiology, Level III training is for those who will practice diagnostic and interventional cardiac catheterization. In addition to coronary angiography and interventions, certain aspects of peripheral vascular and structural heart interventions can generally be addressed during an ACGME-dedicated interventional cardiovascular training year. Further training may frequently be required should fellows choose to pursue a career focus in peripheral vascular or structural heart interventions. Level II training in vascular medicine (see COCATS 4 Task Force 9 report11) is also recommended before or in conjunction with Level III training in catheter-based PVI.
1.2.3. Methods for Determining Procedural Numbers
The recommended number of procedures performed and interpreted by trainees under faculty supervision has been developed based on published studies and guidelines, competency statements, and the experience and opinions of the members of the writing group. In addition, the writing committee surveyed interventional cardiology training program directors to gain additional insight into procedural volumes. Of 169 directors of ABIM-recognized interventional cardiology training programs, 54 responded. The procedural volumes suggested in this document were determined to be the minimum numbers sufficient to provide trainees with exposure to a variety and spectrum of complexity of clinical case material and to give supervising faculty sufficient opportunity to evaluate the competency developed by each trainee. The numbers of procedures that should be performed to achieve competence (see Section 4.2) are intended as general guidance. Notably, in assessing these volume numbers, the fundamental nature of educational milestones is proficiency and outcomes rather than length of exposure or the exact number of procedures performed. Flexibility is inherent to this concept, and the ACGME mandates that all programs establish milestones for the acquisition of various competencies by trainees during the course of fellowship training.
2. General Standards
2.1. Coronary Interventions
2.1.1. Faculty
Dedicated faculty who are committed to teaching trainees are the most important resource for a high-quality interventional cardiology training program. Faculty serve as role models for professionalism and promote a positive learning environment to foster the education of fellows in clinical, procedural, and scholarly activities. Faculty must include specialists from diverse backgrounds with a broad range of expertise in knowledge base areas of interventional cardiology and related fields; noninvasive and invasive diagnostic testing; and therapeutic options, including medical management and percutaneous and surgical revascularization. The 2020 “ACGME Program Requirements for Graduate Medical Education in Interventional Cardiology” require a single designated program director and at least 1 additional ABIM- or American Osteopathic Board of Internal Medicine–certified core clinical faculty member or a ratio of 1.5 core clinical faculty members to fellows for programs with more than 2 fellows.12 Faculty members include board-certified specialty/subspecialty physicians approved by the program director. Core clinical faculty members should be committed to educating fellows and demonstrate a strong interest in education and scholarly activities. They should also have experience and/or undergo professional development in teaching, mentoring, and assessing procedural competency and have sufficient time to fulfill the teaching, mentoring, and administrative responsibilities required for participation as active faculty in the interventional cardiology training program.
2.1.2. Facilities
An interventional suite includes a dedicated cardiac catheterization laboratory, a sterile area for diagnostic and therapeutic procedures, and a separate space for evaluating and managing patients preprocedure and postprocedure. Institutions should have stress testing and imaging facilities such as CCT and CMR. The “2012 ACC/SCAI Expert Consensus Document on Cardiac Catheterization Laboratory Standards Update” provides detailed information on how to achieve maximal safety and efficiency in a traditional or hybrid cardiac catheterization suite.13 General recommendations for staffing, informed consent, infection control, reporting, and continuous quality assessment and improvement are provided in the 2021 “SCAI Expert Consensus Update on Best Practices in the Cardiac Catheterization Laboratory.”14 In addition, practitioners and staff need to be aware of the risks and hazards of radiation injury and institute practices to minimize patient and operator radiation exposure as well as other occupational health hazards.15 Complementary services (eg, cardiothoracic surgery, anesthesia, pharmacy, advanced practice providers, cardiovascular technologists, critical care medicine) and relevant medical subspecialties (eg, occupational therapy, physical therapy, and supervised exercise rehabilitation programs) should also be available. Finally, hospitals are encouraged to have a system in place for accurate registry reporting and quality assurance and to meet requirements for participation in national coverage determinations.
2.1.3. Equipment
Cardiac catheterization suites require several systems in place to provide safe and thorough evaluation of the patient. Imaging capabilities with fluoroscopy, angiography, and real-time hemodynamic and electrocardiography monitoring are a staple of this environment. Equipment must be optimized to reduce patient and operator radiation exposure. A full complement of diagnostic and guide catheters, wires, balloons, and stents are vital to the function of a well-run catheterization suite, as is the availability of equipment and devices to treat life-threatening complications. Specialized imaging equipment, such as intravascular ultrasound (IVUS) and/or optical coherence tomography (OCT), and hemodynamic testing capabilities, including fractional flow reserve or a nonhyperemic pressure ratio system, should be available to operators to optimize diagnostic accuracy and improve patient outcomes. An inventory system must be in place to continually evaluate and replace equipment that has been used. Resuscitation equipment must be immediately available. Additional necessary and optional equipment can also be identified in the “2012 ACC/SCAI Expert Consensus Document on Cardiac Catheterization Laboratory Standards Update” and the 2021 “SCAI Expert Consensus Update on Best Practices in the Cardiac Catheterization Laboratory.”13,14 In this rapidly evolving, innovative specialty, it is vital that a system is in place to evaluate and implement newly approved technology and equipment used on a regular basis.
2.1.4. Additional Resources
Proficient interventional cardiology training requires multidisciplinary collaboration. Trainees should develop working relationships with cardiac surgeons and cardiologists who have advanced training in electrophysiology, echocardiography, CMR, CCT, heart failure, and advanced practice providers, as well as with relevant multispecialty teams. Trainees should participate in both formal multidisciplinary patient-management conferences and informal consultations with a variety of subspecialists. Physicians from other fields of medical and surgical practice should be available for consultation. Access to other health care professionals, including genetic counselors, pharmacists, dieticians, occupational therapists, physical therapists, exercise physiologists, and social workers, is required.
2.2. Peripheral Vascular Interventions
2.2.1. Faculty
Dedicated faculty who are committed to teaching PVI are the most important resource for a high-quality training program. Faculty serve as role models for professionalism and promote a positive learning environment to foster the education of fellows in clinical, procedural, and scholarly activities. Faculty must include specialists who are knowledgeable about basic vascular biology and clinical aspects of peripheral vascular diseases, including anatomy, physiology, and pathophysiology; both noninvasive and invasive diagnostic strategies and tests; and therapeutic options, including medical management, percutaneous interventional therapies, and surgical revascularization. Each training program should have a designated program director who is board-certified in interventional cardiology. Additional faculty should include an adjunct program director board-certified in interventional cardiology with expertise in PVIs or board-certified in vascular medicine, interventional radiology, or vascular surgery. Additional clinical faculty from these specialties and cardiovascular imaging or other disciplines may also be engaged in the training program. Furthermore, it is recommended that the number of faculty equal or exceed the number of trainees enrolled in the training program. In addition to subject knowledge, faculty should be committed to educating fellows and demonstrate a strong interest in education and scholarly activities. Faculty should also have experience and/or undergo professional training in teaching, mentoring, and assessing procedural competency and have sufficient time to fulfill the teaching, mentoring, and administrative responsibilities required for participation as active faculty members in the PVI training program.
2.2.2. Facilities
Training institutions must provide comprehensive facilities for the care of patients with vascular disease, including areas for outpatient care and hospital-based treatment. An outpatient area that allows for longitudinal management of patients with peripheral vascular disease is essential for training. Institutions should also have an accredited noninvasive vascular laboratory and imaging facilities, including CCT and/or CMR. In addition, the institution should have wound-care management facilities, including equipment to assess limb perfusion with techniques such as ankle-brachial index, toe-brachial index, transcutaneous oximetry, skin perfusion pressure measurement, and other similar techniques. In the hospital environment, a dedicated catheterization laboratory or hybrid operating room that provides a safe and sterile environment for performing endovascular interventions is necessary. The catheterization laboratory must be equipped and staffed to function in accordance with the “2012 ACC/SCAI Expert Consensus Document on Cardiac Catheterization Laboratory Standards Update.”13 Ambulatory facilities (eg, ambulatory surgery centers, office-based laboratories) that provide the appropriate standard of care may increasingly provide a venue where PVI is performed by interventional cardiology trainees under supervision. See Section 2.1.2 for additional catheterization laboratory requirements. Complementary services, including vascular surgery, vascular medicine, anesthesia, interventional radiology, pharmacy, advanced practice providers, cardiovascular technologists, critical care medicine, wound care, and podiatry, and relevant medical subspecialties, including endocrinology and infectious disease, occupational therapy, physical therapy, and supervised exercise rehabilitation programs, should also be available.
2.2.3. Equipment
Catheterization laboratories, operating rooms, and hybrid operating rooms that provide a safe environment for peripheral vascular angiography and intervention require imaging capabilities, including high-resolution fluoroscopy, angiography with iodinated contrast agents or dye-sparing agents such as carbon dioxide, and equipment for recording electrical and hemodynamic signals.6,13,14 Specialized equipment is necessary for performing safe and effective endovascular interventions and includes the presence of extravascular ultrasound equipment for assistance with complex access techniques and IVUS for invasive assessment of vascular structures.16 Digital subtraction angiography and appropriately sized imaging panels, specialized vascular access sheaths, angiographic catheters, ample guidewire selection, balloon dilation catheters, balloon-expandable and self-expandable stents (eg, drug-eluting stents), stent grafts, atherectomy devices, thrombectomy/thrombolysis devices, embolic protection devices, and vascular closure devices should also be readily available. Equipment will continue to evolve, and systems must be present to allow prompt assessment and integration of new technologies. In addition, appropriate resuscitation equipment must be immediately available and radiation exposure control systems must be in place to minimize patient and staff radiation exposure.
2.2.4. Additional Resources
Proficient PVI training requires trainees to develop working relationships with advanced practice providers and relevant multispecialty teams. Direct access to radiologists with advanced training in vascular imaging can be especially helpful in cases with complex anatomy. Vascular surgeons, cardiothoracic surgeons, and anesthesiologists should be available for consultation and, in many cases, for collaboration on hybrid procedures. Close collaboration with specialized wound care teams (eg, experts in hyperbaric therapy, podiatry, and orthopedics) and other specialists, such as occupational/physical therapists and rehabilitation teams, is essential, especially in the management of patients with chronic limb-threatening ischemia (CLTI). In addition, practitioners from other fields, such as endocrinology, infectious disease, medical genetics, geriatrics, nephrology, rheumatology, and neurology/neurosurgery, should also be available for consultation in selected cases. Where available, additional multidisciplinary teams, such as for the management of cerebrovascular disease and stroke, aortic disease, anesthesia, critical care medicine, and pulmonary embolism, provide collaborative programs in which interventional cardiologists or vascular specialists may need to engage for the optimal care of vascular patients. Development of these programs is encouraged.
2.3. Structural Heart Interventions
2.3.1. Faculty
Dedicated faculty who are committed to teaching trainees are the most important resource for a high-quality structural interventional training program. Faculty serve as role models for professionalism and promote a positive learning environment to foster the education of fellows in clinical, procedural, and scholarly activities. Faculty includes board-certified specialty/subspecialty physicians approved by the program director. Faculty must include specialists with a broad range of expertise in knowledge base areas of SHD and related fields; noninvasive and invasive diagnostic testing; and therapeutic options, including medical management, transcatheter therapeutics, and surgery. Each training program should have a designated program director who is board-certified in interventional cardiology. Additional faculty should include an adjunct program director who is board-certified in interventional cardiology with expertise in structural heart interventions. Key clinical faculty should be board-certified in their specialties of interest, whether that be interventional cardiology, cardiothoracic surgery, cardiovascular imaging, advanced heart failure, or other disciplines. Key clinical faculty members should be committed to educating fellows and demonstrate a strong interest in education and scholarly activities. They should also have experience and/or undergo professional development in teaching, mentoring, and assessing procedural competency and have sufficient time to fulfill the teaching, mentoring, and administrative responsibilities required for participation as active faculty members in the structural heart interventional training program. Furthermore, it is recommended that the number of faculty equal or exceed the number of trainees enrolled in the training program.
2.3.2. Facilities
Facilities must include dedicated areas for both outpatient care and hospital-based treatment. The outpatient area should allow for longitudinal management of patients and appropriate preprocedural and postprocedural imaging, including transthoracic and transesophageal echocardiography and CCT and CMR imaging, as necessary. In the hospital, a cardiac catheterization laboratory or hybrid room that provides a safe and sterile environment for performing invasive transcatheter procedures is necessary. Access to noninvasive imaging (ie, transthoracic, transesophageal, and/or intracardiac echocardiography) as well as general anesthesia is essential, requiring onsite imaging experts and anesthesiologists. Onsite cardiopulmonary bypass, perfusion, and both cardiothoracic and vascular surgery services should be readily available. See Section 2.1.2 for additional catheterization laboratory requirements. Complementary services, including pharmacy, advanced practice providers, cardiovascular technologists, critical care medicine, and relevant medical subspecialties, including occupational therapy, physical therapy, and supervised exercise rehabilitation programs, should also be available.
2.3.3. Equipment
Cardiac catheterization laboratories or hybrid suites that provide a safe and ergonomically appropriate environment for invasive structural heart interventions require fluoroscopic, angiographic, and echocardiographic imaging capabilities and equipment for monitoring and recording hemodynamic and other procedural data. Specialized equipment, including additional imaging modalities, (eg, intracardiac echocardiography, IVUS) vascular plugs, device implants, covered stents, snares, and emergent access to cardiopulmonary bypass should be readily available. Appropriate resuscitation equipment must be immediately available, and specific emergency preparedness protocols for various complications should be developed and readily available.17 Appropriate radiation shielding for all personnel involved in the procedure and practices to both minimize and quantify cumulative provider exposure must be present.15
2.3.4. Additional Resources
For an optimal training experience, the interventional cardiology trainee focused on structural heart interventions needs to work closely with other disciplines. The multidisciplinary team, including cardiothoracic surgeons, cardiac anesthesiologists, general cardiologists, electrophysiologists, heart failure specialists, advanced cardiac imagers, advanced practice providers, and program coordinators, provides a framework for optimal patient care and trainee education. The multidisciplinary team seeks input from various disciplines and the patient to determine the best therapeutic option. In selected patients, it may be appropriate to consult with other specialties such as geriatrics, palliative care, neurology, infectious diseases, nephrology, critical care medicine, vascular surgery, rehabilitation services, or pulmonary medicine. Preprocedural planning also requires broad input to ensure an appropriate location for performing the procedure (catheterization laboratory versus hybrid room), the level of anesthesia support required, and the optimal place of recovery for the patient to ensure cost-effective clinical care. The trainee needs to be involved in the quality assurance/performance improvement process of each institution. For structural heart interventional training encompassing adult congenital interventions, integrating with an adult congenital heart disease (ACHD) team is imperative. Multidisciplinary, case-based conferences are one venue with which to achieve this interactive discussion.
3. Training Components
3.1. Coronary Interventions
3.1.1. Didactic Program
Level III training in interventional cardiology includes didactic instruction, which is a component of the curriculum that may take place in numerous formats, including lectures, online modules, journal clubs, grand rounds, multidisciplinary clinical and technical case conferences, research conferences, simulator-based training, and patient safety or quality improvement conferences. Additionally, regular participation in catheterization laboratory peer review processes, such as patient safety or quality improvement conferences, random case reviews, and quality registries, is strongly recommended to educate trainees in peer review practices that emphasize multidisciplinary collaboration, benchmarking, anonymization, and concordance with guidelines.18 Topics for didactic discussion should include normal and variant anatomy, pathophysiology of atherosclerosis, thrombosis, coronary ischemia and response to intervention, pharmacology, periprocedural assessment and diagnostic testing, identifying and treating coronary clinical syndromes, interpretation of angiograms and intracoronary imaging, invasive hemodynamic assessment, procedural techniques, procedural complication management, interventional devices, radiation safety, health equity, and patient-centered care.19 Didactic sessions and case reviews are important mechanisms for training in the interpretation of complex coronary anatomy and interventional techniques and in the evaluation and management of hospitalized patients and outpatients with CAD. Journal clubs bring advances in the field to trainees and provide opportunities to critically review published literature, including assessments of methodology, validity, and generalizability. Weekly didactic sessions are a reasonable goal for training in interventional cardiology and can be supplemented by independent or group learning activities. Moreover, training programs should emphasize the importance of lifelong learning that does not end on completion of the fellowship, especially as new technologies and procedures are developed.
3.1.2. Clinical Experience
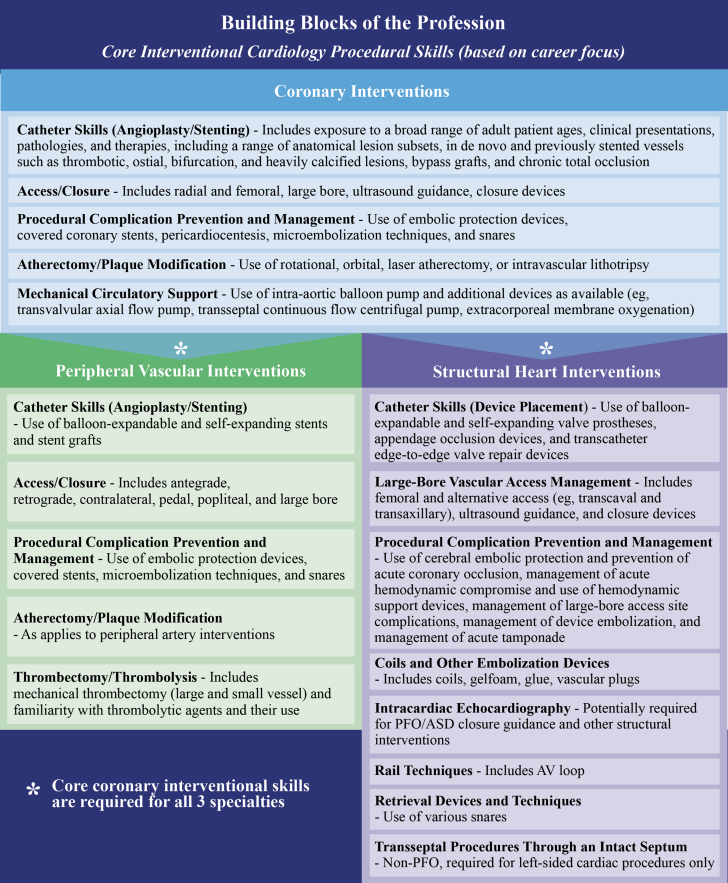
Level III training in interventional cardiology requires prerequisite completion of a 3-year cardiovascular disease fellowship and competency in diagnostic cardiac catheterization. Level III training is accomplished in a 12-month program with robust clinical and procedural experiences in the cardiac catheterization laboratory as well as in the inpatient and outpatient settings. In the cardiac catheterization laboratory, trainees should participate in supervised procedures with graduated responsibility and autonomy in procedural performance, ultimately to achieve clinical independence and the ability to function as an operator in increasingly complex patients and anatomy, with appropriate oversight from an attending interventionalist. Level III trainees are expected to develop the skills necessary to competently perform the diagnostic and therapeutic procedures considered essential to the management of patients with chronic and acute coronary syndromes. In addition to procedural skills, the Level III trainee in interventional cardiology must acquire clinical experience in the care of patients who are being evaluated for interventional therapy. This should include case selection; planning interventional procedures in terms of approaches, limitations, outcomes, complications, and longitudinal follow-up in the outpatient setting; preprocedure and postprocedure patient care in the inpatient setting, the setting of acute coronary syndrome, and as an outpatient procedure. The Level III trainee must have experience with subspecialty consultation in interventional cardiology in the setting of the hospitalized patient, the patient in the emergency room, and the patient in the critical care unit. Finally, the Level III trainee must have experience functioning as a part of a multidisciplinary team approach to the management of the patient with complex cardiac disease. See Section 4.2 for a discussion of minimum procedural volume and core interventional cardiology procedural skills regarding commonly performed coronary interventions.
3.1.3. Hands-On Procedural Experience
Hands-on experience is the foundation for training in interventional cardiology. Level III training in interventional cardiology requires that the majority of time be allocated to obtaining experience in the cardiac catheterization laboratory performing diagnostic heart catheterization, percutaneous coronary intervention (PCI), and adjunctive procedures.
The Level III trainee should have experience with a range of anatomical and ultrasound-guided vascular access, including, but not limited to, radial, distal radial, ulnar, brachial, axillary, and femoral arteries as well as hemostasis techniques with the use of each access site. The trainee must have experience in selection and use of diagnostic and guiding catheters, guidewires, and diagnostic and therapeutic coronary equipment. The trainee is required to have experience performing diagnostic coronary and graft angiography and left ventriculography in addition to procedural experience with invasive hemodynamic monitoring by performing and interpreting left and right heart catheterization. Core devices for hands-on experience in Level III training include balloon and stent delivery systems (including stent grafts), microcatheters, guide extension catheters, atherectomy platforms, embolic protection devices, thrombectomy catheters, intracoronary imaging catheters, pressure wires, and mechanical circulatory support devices.
The Level III trainee must have hands-on experience with the management of complex CAD through exposure to a wide range of coronary anatomical lesion subsets, including de novo and restenotic, bypass grafts, ostial, left main, bifurcation, chronic total occlusions (CTOs), calcified, tortuous, thrombotic, and multivessel disease lesions. Trainees should have hands-on experience performing procedures that are elective, urgent/emergent, and salvage, including in settings of acute myocardial infarction and cardiac arrest. Mechanical circulatory support device use for high-risk interventions and cardiogenic shock should include intra-aortic balloon counterpulsation and additional percutaneous ventricular assist devices (eg, transvalvular or transeptal axial or continuous flow devices), and extracorporeal membrane oxygenation, as available. The trainee should be experienced in the choice and use of plaque modification devices and have hands-on experience with rotational, orbital, and laser atherectomy, and intravascular lithotripsy, as available. The availability of some technologies for hands-on training, and experimental/non-FDA–approved devices, is not universal in training programs; therefore, education may require skills laboratories, simulation training, or proctoring after fellowship. The complexity of coronary procedures has evolved over time with the advent of specialized equipment and techniques for complex coronary interventions. During the 12-month interventional cardiology training, fellows are expected to gain competence in knowledge of the indications and procedural risks for complex coronary interventions. However, training in skills to perform these procedures will vary among programs. Further training in coronary interventions may therefore be needed in centers specializing in these procedures in advanced fellowships or may be obtained post-training through courses, proctoring, or local mentorship.
3.1.4. Diagnosis and Management of Emergencies and Complications
Major complications during PCI are reported to range from 0.2% to 3.2%; however, in acute myocardial infarction or high-risk PCI, the rate may be substantially higher.20 Laboratory complications and emergencies resulting from PCI or as a result of a patient’s clinical presentation include coronary perforation, coronary artery dissection, retained devices (eg, balloons, stents, atherectomy burrs, and wires), coronary no-reflow, hemodynamic compromise, and anaphylaxis. Most of the more serious complications of PCI are uncommon. Vascular access site complications include ischemic limbs, retroperitoneal bleeding, arteriovenous fistulas, pseudoaneurysms, and expanding hematomas, and complication rates are lower with radial access compared with femoral access.20 Other periprocedural complications include contrast-induced nephropathy, acute embolic events (ie, stroke), compartment syndrome, and nonaccess site bleeding.
Managing emergencies and complications of PCI and other percutaneous procedures is critical to providing comprehensive care to patients referred to the catheterization laboratory. Learning these skills is an integral part of Level III training in interventional cardiology. Trainees should be able to recognize those at risk for periprocedural emergencies and complications and understand the processes that can be used to minimize this risk. Trainees should be familiar with the equipment used to manage such events, including covered stents, coils, snares, devices used for thrombus aspiration, and microcatheters. Additionally, trainees must develop skills in insertion of hemodynamic support devices, performing pericardiocentesis, and transvenous pacing. Included in the competency of managing complications is a detailed knowledge of the mechanism of action and dosing of the pharmacologic agents used to treat hemodynamic compromise, cardiac arrhythmias, no-reflow, and in situ thrombosis. Trainees need to know when to consult specialists for procedural complications outside of their interventional cardiology expertise (eg, compartment syndrome). The Level III trainee should be expected to follow institutional requirements for reporting complications, to present and discuss them at patient safety or quality improvement conferences, and to learn from such experiences.
Due to the rarity of many of these conditions, trainees may never gain direct experience in managing some complications during their interventional training. To ensure an adequate education in the management of all emergencies and complications, training programs should encourage the involvement of fellows in any in-laboratory event. This can include direct hands-on experience (when the trainee is assisting in the case); indirect but real-time observation of the events (for trainees not directly involved in the case but present in the catheterization laboratory); or participation in immediate postprocedure huddles, patient safety or quality improvement conferences, and other such drill downs. Other methods to ensure a comprehensive educational experience might include didactic lectures and/or workshops that outline the management of such complications. When necessary, some of this education can be achieved by attendance at society-sponsored training courses for fellows or online learning sessions taught by recognized experts. Additionally, when available, simulation techniques can provide further technical training in managing procedural complications and approximate real-time experiences. Irrespective of the modality used, the training should focus on the importance of properly identifying those patients at risk of procedural emergencies and complications, the process that can be implemented to avoid such events, and prompt recognition of early clinical and angiographic findings. Education should always include a detailed algorithm for management of each of these circumstances. Education should also emphasize the need to understand those situations that might mandate a more conservative approach to treatment and those that require escalation of care (eg, treating a coronary perforation with prolonged balloon inflation versus covered stenting versus emergency surgery), and the circumstances permitting continuation of the PCI procedure or termination of the procedure after successful treatment of a complication.
3.1.5. Diagnosis and Management of Less Common Clinical Conditions and Syndromes
Although most coronary procedures performed during Level III training in interventional cardiology involve patients with well-recognized clinical syndromes resulting from coronary atherosclerosis, it is important that the Level III trainee is able to recognize and, when necessary, treat those patients presenting to the cardiac catheterization laboratory with less common diagnoses, including spontaneous coronary artery dissection, coronary artery vasospasm (or other vasomotor disorders), microvascular dysfunction, myocardial infarction with nonobstructive coronary arteries, takotsubo cardiomyopathy, inflammatory vasculitis, myocardial bridging, coronary artery fistula, and coronary artery aneurysm. Although all of these conditions have the potential to result in varying degrees of ischemia, many of these syndromes do not require revascularization and are best treated with more conservative care, including pharmacologic therapies. Furthermore, 5% to 6% of patients with acute myocardial infarction undergoing angiography are found to have nonobstructive CAD and require additional testing to uncover the underlying etiology for their presentation.21 Level III training in interventional cardiology should include education surrounding the underlying pathophysiology and angiographic findings characteristic of each condition. The Level III trainee should be educated on the role of additional diagnostic testing to uncover said diagnosis (eg, coronary flow reserve, index of microvascular resistance, coronary vasoreactivity testing, intracoronary imaging, endomyocardial biopsy, serologic testing, shunt calculation, CMR, and coronary computed tomography angiography) as well as the potential risks for and treatment of complications associated with some of these procedures (ie, expansion of dissection with use of intracoronary imaging of a spontaneous coronary artery dissection or refractory spasm with vasoreactivity testing in patients with coronary vasospasm). Additionally, trainees should be educated on the strengths and limitations of various invasive and noninvasive methods for treating these disorders. This should include, but is not limited to, the role of PCI in patients with spontaneous coronary artery dissection who have symptoms refractory to conservative care, the role of coil embolization for patients with fistulas, as well as the surgical management of large coronary artery fistulas or aneurysms. Unfortunately, given the rarity of many of these syndromes, trainees may never gain direct hands-on experience in the diagnosis and treatment of patients with these uncommon and sometimes rare disorders. Other methods to ensure a comprehensive educational experience for Level III training might include didactic lectures focusing on angiographic and intracoronary imaging case reviews or simulation-based training. The Level III trainee is not expected to be an expert in the management of patients with these conditions but should know when to use noninvasive imaging and consultation with cardiac surgeons and other experts to diagnose and manage affected patients.
3.2. Peripheral Vascular Interventions
3.2.1. Didactic Program
Level III training in interventional cardiology includes aspects of peripheral vascular disease as they pertain to advanced knowledge in diagnostic and therapeutic modalities for evaluating, managing, and treating patients with vascular disease. Training pathways dedicated to the development of expertise broadly or within a focused area of peripheral vascular disease may be pursued for expansive or narrowed competency and independence in PVIs. Dependent on career focus, this may require additional training beyond the 12-month interventional cardiology fellowship program or postfellowship training through courses, proctoring, or direct mentorship.
Didactic activities for trainees may take place in a variety of formats, including lectures, online modules, clinical conferences, clinical case presentations, journal clubs, research conferences, grand rounds, simulator-based training, and patient safety and quality improvement conferences. It is expected that a significant portion of the didactic curriculum is addressed during the core cardiovascular disease and interventional cardiology fellowship training programs. The educational content should cover the anatomy, pathophysiology, genetics, risk factors, evaluation, noninvasive diagnosis and management, hemodynamic assessment, procedural techniques, invasive management, and management of procedural complications of vascular patients. Special emphasis should be placed on review of noninvasive vascular imaging and integration with the vascular laboratory as well as cross-sectional imaging. Clinical areas of competency may include vascular access site complication management; lower extremity peripheral artery disease (PAD); chronic and acute limb-threatening ischemia; renovascular disease; mesenteric vascular disease; upper extremity PAD; vertebral and carotid artery disease; venous disease, including venous thromboembolism (acute and chronic), deep venous occlusive and compressive disease, and superficial venous disease; aortic and peripheral artery aneurysm; microvascular disease; and nonatherosclerotic vascular disease. Expertise in one area does not necessarily imply mastery in all areas. Educational content should also include training in radiation safety and patient-centered care. Additionally, regular participation in catheterization laboratory peer-review processes, such as patient safety or quality improvement conferences, random case reviews, and quality registries, is strongly recommended to educate trainees in peer-review practices that emphasize multidisciplinary collaboration, benchmarking, anonymization, and concordance with guidelines.18 It is expected that the trainee will embark on a lifelong journey of education and learning that continues after completion of the fellowship, especially as new technologies and procedures are developed.
3.2.2. Clinical Experience
Level III training in PVI requires prerequisite completion of fellowship training and competency in diagnostic cardiac catheterization. Level III interventional cardiology training is required to achieve competency in advanced PVI. Advanced training in PVI could potentially be started before and completed in conjunction with or after Level III training in interventional cardiology, depending on trainee experience and programmatic availability. Advanced PVI training requires robust clinical experiences in both the ambulatory and inpatient settings as well as in the catheterization laboratory. In each of these domains, trainees should assist in patient care in a supervised setting that provides patient-centered education in all aspects of invasive and noninvasive management of patients with vascular disease. Level III training should provide the knowledge and skills to function as an independent endovascular specialist, including the ability to recognize patients’ clinical presentation, plan and interpret diagnostic testing (invasive and noninvasive), and formulate appropriate management plans for patients across the entire spectrum of vascular disease. This may include, but is not limited to, lower extremity PAD; chronic and acute limb-threatening ischemia; renovascular disease; mesenteric vascular disease; upper extremity PAD; vertebral and carotid artery disease; venous disease, including venous thromboembolism (acute and chronic), superficial venous disease, deep venous occlusive and thrombotic disease; aortic and peripheral artery aneurysm disease; and nonatherosclerotic vascular disease. Level III training for PVI requires extensive knowledge of procedural indications and contraindications, management of complications, and the risks versus benefits of conservative therapy (eg, guideline-directed medical therapy) versus intervention/revascularization and, if revascularization is being considered, the risks versus benefits of surgical, hybrid, or percutaneous revascularization. Trainees should understand the pathophysiology of restenosis lesions and strategies for management, including laser atherectomy/plaque modification techniques, covered stent grafts and drug-eluting devices, the role of intravascular image guidance, and the appropriate use of intravascular brachytherapy. When treating patients with CLTI or acute limb ischemia (ALI), Level III trainees are required to have the ability to assess the risks versus benefits of amputation versus revascularization. Level III PVI trainees, when treating patients with ALI, must be able to assess the viability of the affected limb and tailor the percutaneous and/or surgical management accordingly. The ability to recognize the early signs of compartment syndrome is required. When treating patients with venous disease (acute and chronic), Level III trainees are required to understand the appropriate use of devices used in the venous circulation, including inferior vena cava filter insertion and timely removal, stents, and thrombectomy catheters.
Trainees should acquire the skills necessary to identify patients who will benefit from endovascular interventions while weighing the risks and benefits of alternative management strategies. During Level III training, the trainee should acquire the skills to competently and independently perform the diagnostic and therapeutic procedures considered essential to the practice of PVI. The trainee must also be aware of potential complications of these procedures and acquire the skills to manage them. See Section 4.2 for a discussion of minimum procedural volume and core interventional cardiology procedural skills regarding commonly performed PVIs. Appreciating the many diverse procedural skills required in the broad field of PVI, it is reasonable that trainees might choose to pursue a narrower set of skills involving focused areas of competency aligned with their planned clinical practice and level of expertise.
3.2.3. Hands-On Procedural Experience
Hands-on experience is essential for training in PVI and requires robust experience in the catheterization laboratory performing peripheral vascular diagnostic angiography, including noniodinated contrast media alternatives (eg, carbon dioxide) and therapeutic peripheral vascular procedures in both arteries and veins (eg, balloon angioplasty, stent placement [bare-metal, drug-eluting, and covered], use of plaque-modifying debulking devices, cutting balloons, inferior vena cava filter placement/removal, catheter-based thrombolysis, or catheter-based thrombectomy/embolectomy). Training should also include experience and mastery of image-guided procedures (extravascular and IVUS and/or other modalities). The number of supervised procedures that need to be completed during PVI training is summarized in Section 4.2.
Level III trainees require hands-on experience in using a variety of guidewires for crossing lesions to deliver devices, including treating CTOs and performing complex infrapopliteal limb-salvage procedures. Performance of PVI procedures for claudication, CLTI, ALI, renal and mesenteric artery disease, subclavian, vertebral, and carotid disease, venous disease (acute and chronic), and aortic and peripheral artery aneurysm disease requires hands-on experience with a range of percutaneous vascular access skills (with and without external ultrasound imaging), including radial artery, antegrade and retrograde common femoral artery/vein, retrograde popliteal artery/vein, and tibio-pedal artery/vein access. Demonstrated skill in large-bore femoral artery access is required for the management of aortic aneurysm exclusion.
Managing complications requires hands-on experience with placement of covered stents, catheter-based thrombectomy/embolectomy, stent/device retrieval, pseudoaneurysm management (compression and thrombin injection), and therapeutic embolization (eg, coils). Hands-on skills with embolic protection devices are necessary for atherectomy or debulking procedures and for carotid stent procedures.
PVI is a continuously evolving field, and the ongoing introduction of new technology and new procedures can be expected. These evolving technologies/procedures include antirestenotic drug-eluting devices, bioabsorbable stents, aneurysm exclusion devices, covered stents, CTO crossing devices, plaque-modifying/debulking devices, catheter-based thrombectomy/embolectomy devices, percutaneous bypass procedures, renal denervation, transcarotid stent delivery, and deep vein arterialization. Performance of procedures in the special populations of patients who require these new approaches may be limited to certain centers that expose trainees to a larger number of these patients.
3.2.4. Diagnosis and Management of Emergencies and Complications
Major complications during PVI vary in severity and frequency depending on the vascular territory being treated. Nevertheless, vascular access site complications occurring in the laboratory or postprocedure, including ischemic limbs, retroperitoneal bleeding, arteriovenous fistulas, pseudoaneurysms, and expanding hematomas, remain the most frequently encountered. Other periprocedural complications include contrast-induced nephropathy or acute embolic events (ie, stroke) and nonaccess site bleeding. The most critical emergencies resulting from PVI include vascular perforation, vascular dissection, retained or embolized devices (eg, balloons, stents, atherectomy burrs, and wires), arterial no-reflow, and hemodynamic collapse due to obstructive or hemorrhagic shock and anaphylaxis.
Managing emergencies and complications of PVI is critical to providing comprehensive care to patients referred to the catheterization laboratory, including mobilization of the multidisciplinary team. Learning these skills is an integral part of Level III training in interventional cardiology and advanced training in PVI. Trainees should be able to recognize those at risk for periprocedural emergencies and complications and understand the processes that can be used to minimize this risk. In collaboration with a multidisciplinary team that is well-versed in PVI, serious and long-term complications can be appropriately managed. Trainees should be familiar with the equipment used to manage such events, including covered stents, occlusion balloons, coils, snares, devices used for thrombus aspiration, and thrombolytic infusion catheters. The Level III trainee should be expected to follow institutional requirements for reporting complications, to present and discuss them at patient safety or quality improvement conferences, and to learn from such experiences. See Section 3.1.4 for addressing training options for less commonly encountered emergencies and complications that can be similarly applied to the PVI field.
3.2.5. Diagnosis and Management of Less Common Clinical Conditions and Syndromes
There are a variety of rare conditions and syndromes that peripheral vascular interventionalists may encounter, and it is therefore essential that peripheral vascular interventionalists are well-versed in vascular medicine and the noninvasive diagnostic evaluation preceding interventional therapy. Cognitive competencies include a comprehensive understanding of the physiology of arterial and venous circulation and their relationship to end-organ derangements. In addition, peripheral vascular interventionalists must possess a detailed understanding of thrombophilia and hypercoagulability as well as nonatherosclerotic causes of vascular disease, such as extrinsic vascular compression (eg, popliteal artery entrapment syndromes, endofibrosis), abnormalities of the arterial wall (eg, fibromuscular dysplasia, idiopathic mid-aortic syndrome, pseudoxanthoma elasticum, segmental arterial mediolysis, and cystic adventitial disease), medium and large-vessel vasculitides (Takayasu’s arteritis, giant-cell arteritis, and Behçet’s disease), vasospastic disease, connective tissue disorders such as Ehlers-Danlos Syndrome, and tobacco-related disorders (thromboangiitis obliterans [Buerger’s disease]). It is prudent for Level III interventional cardiology trainees to have basic knowledge of such; however, when dealing with less common conditions, 1 year of interventional training is not sufficient to make trainees experts, and further focused training may be necessary to gain expertise and competence in the treatment of such conditions. Although Level III trainees are not expected to be expert in the management of patients with these conditions and syndromes, they should be able to use information technology or other available methodologies, including consultation with other experts in these conditions, to diagnose and manage affected patients.
3.3. Structural Heart Interventions
3.3.1. Didactic Program
Level III training in interventional cardiology includes aspects of SHD pertaining to advanced knowledge in diagnostic and therapeutic modalities for its evaluation, management, and treatment. Level III training for SHD interventions requires extensive knowledge of procedural indications and contraindications, management of complications, and the risks versus benefits of conservative therapy versus surgical or transcatheter intervention. Training pathways dedicated to the development of expertise broadly or within a focused area of the SHD field (eg, aortic stenosis) may be pursued for expansive or narrow competency and independence in structural heart interventions. Dependent on the trainee’s career focus, additional training beyond the 12-month interventional cardiology fellowship program may be required.
Didactic instruction is a component of the curriculum that may take place in numerous formats, including lectures, online modules, journal clubs, grand rounds, clinical and technical case conferences, research conferences, simulator-based training, and patient safety or quality improvement conferences. Additionally, regular participation in catheterization laboratory peer review processes, such as patient safety or quality improvement conferences, random case review, and quality registries is strongly recommended to educate trainees in peer review practices that emphasize multidisciplinary collaboration, benchmarking, anonymization, and concordance with guidelines.18 It is expected that a significant portion of the didactic curriculum will be addressed during the core cardiovascular disease and interventional cardiology fellowship training programs. Given the rapidly expanding nature of the SHD field, topics for discussion include use of noninvasive cardiovascular imaging (ie, echocardiography, CCT, or CMR) and invasive hemodynamic assessment to inform diagnosis and management decisions for SHD. Furthermore, topics to address intraprocedural guidance include, but are not limited to, radiation safety; utility of a multidisciplinary team approach to decision-making; indications and treatment options for aortic, mitral, tricuspid, and pulmonic valve disease; pathophysiology, evaluation, and treatment options for more common congenital heart anomalies and consequent repairs; and indications and patient selection for a broad range of structural heart interventions, including interatrial septal interventions, paravalvular leak closure, alcohol septal ablation, left atrial appendage occlusion, and pulmonary vein stenting. Case-based simulations in the cardiac catheterization laboratory should be used to prepare for infrequent emergencies that can occur with coronary or structural interventions, including pericardial effusion/tamponade, aortic dissection, stroke, device embolization, coronary obstruction, myocardial infarction, conduction block, tachyarrhythmias, hemodynamic compromise, and anaphylaxis. Management of vascular access site complications occurring in the laboratory or remote from the procedure, including ischemic limbs, retroperitoneal bleeding, arteriovenous fistulas, pseudoaneurysms, and expanding hematomas, should be distinctly addressed, especially with the frequent large-bore access required for SHD interventions. Finally, trainees should be able to competently manage anticoagulation and antiplatelet therapy following left-sided SHD interventions to mitigate the risks of both bleeding and device-associated thrombosis. It is expected that the trainees will embark on a lifelong journey of education and learning that does not end with the completion of the training, especially as new technologies, procedures, and technical refinements are developed.
3.3.2. Clinical Experience
Level III training in SHD interventions requires prerequisite completion of training and competency in diagnostic cardiac catheterization and hemodynamic assessment. Level III interventional cardiology training is required to achieve competency in advanced structural heart interventions. Advanced training in SHD interventions could potentially be started before and completed in conjunction with or after Level III training in interventional cardiology, depending on trainee experience and programmatic availability. Advanced training in structural heart interventions requires robust clinical experiences in the outpatient and inpatient consultation settings, imaging laboratories, hybrid suites, and in the cardiac catheterization laboratory.
Dependent on the scope of clinical training, the advanced trainee is expected to act as a first-line consultant in SHD management with appropriate on-site attending backup. In this capacity, the advanced trainee is expected to gather accurate, essential information from all sources, including medical interviews, physical examinations, records, and diagnostic/therapeutic procedures; make informed recommendations about preventive, diagnostic, and therapeutic options, including interventions and surgical options, on the basis of clinical judgment, scientific evidence, and patient preferences; develop, negotiate, and implement patient management plans; and perform competently the diagnostic and therapeutic procedures considered essential to the practice of an interventional cardiologist with expertise in the specific area of SHD. Emphasis is also placed on patient-centered education and shared decision-making in the context of a multidisciplinary team.
Level III training should provide the trainee with the knowledge and skills to function as an independent SHD specialist, including the ability to recognize patients’ clinical presentations, plan and interpret diagnostic testing (invasive and noninvasive), and formulate appropriate management plans for patients across the range of SHD expertise that a specific interventionalist desires. As the range of SHD is vast, expertise in specific procedures can be obtained by appropriate training. This includes, but is not limited to, procedures involving valvular heart disease (ie, the aortic, mitral, tricuspid, and/or pulmonic valves), patent foramen ovale, atrial or ventricular septal defects, hypertrophic cardiomyopathy, pulmonary venous disease, left atrial appendage closure, transcatheter heart failure devices, and ACHD interventions. Incorporating invasive and noninvasive cardiac imaging into the practice of transcatheter interventions is an expected competency. Trainees should acquire the skills necessary to identify patients who will benefit from SHD interventions while weighing the risks and benefits of alternative management strategies. During Level III training, the trainee should acquire the skills to competently perform the diagnostic and therapeutic procedures considered essential to the practice of SHD interventions, as described in more detail in Section 3.3.3. The trainee must also be aware of potential complications of these procedures and acquire the skills to manage them. See Section 4.2 for a discussion of minimum procedural volume and core interventional cardiology procedural skills regarding commonly performed structural heart interventions. Appreciating the many diverse procedural skills required in the broad field of structural heart intervention, it is reasonable that trainees may choose to pursue a more narrow set of skills involving a focused area of competency aligned with their planned clinical practice (eg, limiting SHD training and content to transcatheter aortic valve replacement [TAVR] or left atrial appendage closure, which might be acquired during the formal ACGME-accredited year of interventional cardiology fellowship training or acquired with a short period of additional training).
3.3.3. Hands-On Procedural Experience
Hands-on experience for structural heart interventions is an essential component of advanced training. For Level III training, in addition to specific procedural requirements, there are several areas that require skill sets shared among many procedures. This includes extensive experience in the catheterization laboratory for hemodynamic assessment of various SHD lesions, including crossing stenotic valves and developing techniques for accurately measuring shunts and valve competency. In addition, large-bore vascular access techniques, from proper selection of the entry site to using ultrasound-guided access, preprocedure and postprocedure closure techniques, and bailout techniques for failed closure (eg, contralateral access and balloon tamponade), are required. Many procedures require transseptal access, and this skill set requires hands-on experience with different equipment as well as integrating imaging techniques to achieve safe crossing of the interatrial septum and in specific portions of the septum, dependent on procedural needs. Experience in using snares and the techniques for creating the arteriovenous rails necessary for many procedures is also required. Interpreting noninvasive echocardiographic and CCT imaging is imperative in procedure planning and should be done in conjunction with cardiac and radiology imaging experts. Training in the performance and interpretation of intracardiac echocardiography should be acquired in relevant cases. Most of these skills are acquired by working with patients in the catheterization laboratory or hybrid room but the use of simulators for less commonly encountered situations is warranted. Expertise in valvular heart disease interventional procedures varies based on the valve being treated. For aortic valve procedures, trainees must have experience with balloon aortic valvuloplasty, which is sometimes part of the TAVR procedure, including the use of rapid pacing techniques to stabilize devices across the valve. Performance of TAVR requires experience with advancement of catheters and correct placement and positioning across the native and prosthetic aortic valve as well as experience with the proper deployment of valves, which differ depending on the platform. Exposure to multiple transcatheter platforms specific to a particular procedure (eg, balloon-expanding as well as self-expanding valves for TAVR) is ideal.
To gain experience in left-sided cardiac procedures, trainees require supervised experience with transseptal access, as outlined earlier. Trainees performing mitral valvuloplasty require hands-on experience with preparation, advancement, deployment, and retrieval of the commonly used devices for balloon mitral valvuloplasty. Transcatheter edge-to-edge repair (TEER) requires familiarity with the commercially available systems. Advancement of both the delivery sheath and the device requires supervised experience to allow safe advancement of the device into the left atrium and subsequent positioning across the mitral valve. Accurate deployment of mitral valve devices also requires an understanding of the 3-dimensional structure of the valve and how that structure can be imaged both on fluoroscopy and echocardiography. The latter also requires clear communication between the proceduralist and the imager. Understanding this anatomy also enables trainees to obtain expertise in native and mitral valve-in-valve and mitral valve-in-ring replacement. These skills can also be translated to treatment of the tricuspid valve with emerging interventional devices. Left atrial appendage closure requires an integrated skill set in transseptal puncture, noninvasive cardiac imaging, and an understanding of the risks and benefits of various closure devices.
When working with a collaborative ACHD team, experience can be obtained for competency in simple or complex ACHD interventions, including atrial or ventricular septal defect closure, patent foramen ovale closure, coil embolization, and pulmonic valve replacement. Trainees need to recognize the unique challenges presented by adult patients with congenital heart disease (CHD), including limited vascular access, potential developmental delays, and anxiety associated with multiple prior interventions.
SHD interventions comprise a broad range of procedures, and the field is constantly evolving. New technologies are constantly being introduced, including many new techniques for treatment of valvular heart disease. Delineation of specific requirements for hands-on experience with every procedure is not possible. Implicit in this discussion should be the understanding that each center may not provide experience in every currently performed structural heart procedure. Examples might include bioprosthetic aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction (BASILICA), ventricular septal defect closure techniques, paravalvular leak closure, or alcohol septal ablation. Nevertheless, hands-on experience should give trainees the opportunity to develop the basic skill sets to effectively perform the more common of these procedures and set the stage for further professional development in clinical practice with appropriate training and proctorship.
3.3.4. Diagnosis and Management of Emergencies and Complications
Although management of most vascular and ischemic coronary complications is among the core skillsets of interventional cardiologists, there are some unique complications associated with structural heart interventions that need to be recognized and managed. Complications vary based on the specific types of SHD interventions, which are delineated in Section 4.2.3.
Laboratory complications and emergencies resulting from the intervention or from a patient’s clinical presentation include pericardial effusion/tamponade, aortic dissection, aortic annular disruption, acute valvular dysfunction, stroke, device embolization, coronary obstruction, myocardial infarction, hemodynamic compromise, and anaphylaxis. Vascular access site complications occurring in the laboratory or remote from the procedure, including ischemic limbs, retroperitoneal bleeding, arteriovenous fistulas, pseudoaneurysms, and expanding hematomas, are seen infrequently. As large-bore access is almost universally used in most SHD interventions, ultrasound-guided vascular access should be routinely used, along with techniques for both suture- and collagen-based vascular closure. Prompt recognition of vascular injury and retroperitoneal bleeding is critical for patient management with either arterial or venous access. Other periprocedural complications include neurologic compromise, heart block, contrast-induced nephropathy, and nonaccess site bleeding. Based on the clinical presentation, paravalvular regurgitation after TAVR can be managed by additional balloon dilation, closure plugs, or rarely, a second valve implantation. Appropriate recognition of patients at risk and immediate management with temporary transvenous pacemaker insertion is a mandatory skill.
A new pericardial effusion during any SHD intervention is especially a cause for concern, as it can rapidly progress to cardiac tamponade. Prompt recognition and management of the potential complications that can lead to cardiac perforation are critical. These complications can usually be handled by pericardiocentesis, reversal of anticoagulation, and escalation to expeditious cardiac surgery with exploration and repair as indicated. A pericardial effusion that occurs when SHD interventions are being performed in the left atrium is usually managed with a pericardial drain and reversal of anticoagulation without cardiac surgery, although consideration for surgery should not be excluded.
Device embolization can occur with any implanted valve or closure device. Techniques for removal of small and larger devices with snares from both the left- and right-sided cardiac circulation can be learned in actual cases or, due to the rarity of such a complication, via simulation training. See Section 3.1.4 for addressing training options for less commonly encountered emergencies and complications that can be similarly applied to the SHD interventions. Prompt recognition of a patient with low ejection fraction, hemodynamic compromise, or in cardiogenic shock during or after a procedure helps in early mobilization of the multidisciplinary team. Appropriate treatment, including temporary hemodynamic support and urgent/emergency cardiothoracic surgical therapies, helps to address most of the rare but catastrophic complications. Importantly, in collaboration with a multidisciplinary team that is well-versed in the relevant disease process, serious complications can be appropriately managed.
3.3.5. Diagnosis and Management of Less Common Clinical Conditions and Syndromes
There are a variety of rare conditions and syndromes that the structural interventionalist may encounter, such as repaired and unrepaired CHD, genetic syndromes that may lead to valvular or vascular pathology (eg, Turner or Marfan syndrome), and others that may be associated with myopathies or sudden death (eg, anomalous coronary arterial anatomy). It is prudent for Level III interventional cardiology trainees to have a basic knowledge of such conditions. However, when dealing with less common conditions, it is imperative to recognize that 1 year of interventional training is insufficient for mastery of rare conditions, and further focused training will be necessary to gain expertise and competence in the treatment of such conditions. Although Level III trainees are not expected to be expert in the management of patients with these conditions and syndromes, they should be able to consult with specialists (eg, CHD specialists, vascular medicine specialists, or clinical geneticists), and use evidence-based guidelines and expert consensus documents to diagnose and manage affected patients.
3.4. Research and Scholarly Activity
A strong foundation of scholarly activity for all areas of interventional cardiology should be instilled during training to prepare the trainee for a lifetime of learning and evidence-based practice. There are 3 levels to which this can be achieved during training. The basic expectation for all trainees is that they will integrate habits of self-learning into their clinical training and practice. This includes a scholarly approach to answering clinical questions through literature reviews relevant to patient care, reading journals, attending scholarly conferences, and participating in quality improvement activities. Some trainees may take this a step further by generating their own novel scholarly research, which should be encouraged. To achieve this, seeking mentorship with appropriate faculty to guide their research and scholarly growth is essential, along with oversight from program directors. Lastly, trainees should be both supported and encouraged to present their findings at local, regional, and/or national meetings, and submit them for publication in peer-reviewed journals.
4. Training Requirements
4.1. Competency Development and Evaluation
Training requirements in interventional cardiology address the 6 general competencies promulgated by the ACGME and endorsed by the ABIM. These competency domains are Medical Knowledge, Patient Care and Procedural Skills, Practice-Based Learning and Improvement, Systems-Based Practice, Interpersonal and Communication Skills, and Professionalism. The ACC has used this structure to define and depict the components of the clinical competencies for cardiology. The curricular milestones for each competency and domain also provide a developmental roadmap for fellows as they progress through various levels of training and serve as an underpinning for the ACGME reporting milestones. The ACC has adopted this format for its competency and training statements, career milestones, lifelong learning, and educational programs.
Table 1 depicts the Medical Knowledge competencies and Patient Care and Procedural Skills competencies specifically related to interventional cardiology as well as examples of evaluation tools suitable for assessing competence in each domain that are discussed in more detail in Section 6. The focus of this document is on delineation of the core competencies expected of all trainees in interventional cardiology based on successful completion of a standard 1-year ACGME-accredited interventional cardiology fellowship. These competencies are marked under the “All” column. Certain areas of advanced knowledge or procedural skills may not be encountered in sufficient volume during a standard 12-month training period to develop and demonstrate competence. Therefore, these competencies would require additional dedicated exposure and are designated in the “Add” column of the table, representing a skill level beyond that necessary for coronary interventional cardiology certification. These additional competencies could potentially be started before and completed in conjunction with or after the standard interventional cardiology fellowship, depending on the trainee’s career focus and the opportunities available at the training program. Although the competency components included in the “All” column should be achieved by all trainees and are appropriate areas for assessment, not every component need be individually assessed in every trainee. Rather, as with all educational activities, assessment is a sampling process that should be tailored to the needs of the individual trainee and program.
Table 1.
Components and Curricular Milestones for Level III Training in Interventional Cardiology (Coronary, Peripheral Vascular, and Structural Heart Interventions)
| MEDICAL KNOWLEDGE | Milestones (Months) |
||
|---|---|---|---|
| All (12) | Add∗ | ||
| CORONARY INTERVENTIONS | |||
| Anatomy and Pathophysiology of Coronary Ischemia and Intervention | |||
| 1. | Know the normal coronary and extracardiac arterial anatomy, variations, and congenital abnormalities. | X | |
| 2. | Know the anatomy and physiology of congenital and surgical shunts. | X | |
| 3. | Know the classification and/or scoring system for coronary lesion subsets, including bifurcation, chronic total occlusion, multivessel disease, and restenotic lesions. | X | |
| 4. | Know the physiology and angiographic assessment of coronary and myocardial blood flow. | X | |
| 5. | Know the causes, pathophysiology, and differential diagnosis of myocardial ischemia and infarction. | X | |
| 6. | Know the causes, pathophysiology, and management of pericardial diseases, constrictive pericarditis, and restrictive cardiomyopathy. | X | |
| 7. | Know the pathology of atherosclerotic diseases, vascular remodeling, and response to coronary intervention. | X | |
| 8. | Know the pathology of acute and chronic nonatherosclerotic and/or nonobstructive coronary diseases, including microvascular dysfunction, endothelial dysfunction, coronary spasm, myocardial bridging, spontaneous coronary artery dissection, takotsubo cardiomyopathy, and myocardial infarction with nonobstructive coronary artery disease. | X | |
| 9. | Know the coagulation cascade and pharmacology of antiplatelet, anticoagulant, and fibrinolytic drugs. | X | |
| 10. | Know the pathophysiology, risk factors, diagnostic criteria, and preventive and treatment strategies for adverse reactions to contrast agents. | X | |
| Periprocedural Assessment and Diagnostic Testing | |||
| 11. | Know the indications and appropriate use of noninvasive evaluations (eg, stress testing, imaging, and viability assessment) in patients undergoing percutaneous coronary intervention. | X | |
| 12. | Know the indications for surgical correction of coronary anomalies. | X | |
| 13. | Know the indications and interpretation of right heart catheterization. | X | |
| 14. | Know the indications for and methods to assess vascular function abnormalities, including microvascular dysfunction and coronary vasospasm. | X | |
| 15. | Know the appropriate use criteria classification for diagnostic cardiac catheterization and revascularization in patients with ischemic heart disease (ie, appropriate care, may be appropriate care, and rarely appropriate care). | X | |
| 16. | Know the effects of normative aging on cardiovascular structure and function and the implications for patient selection and expected outcomes for interventional procedures. | X | |
| 17. | Know the limitations and contraindications of percutaneous coronary intervention, particularly as relevant to comorbid systemic diseases and special anatomic subsets. | X | |
| 18. | Know the indications and methods to assess the functional significance of coronary lesions in the catheterization laboratory in patients with and without preprocedural noninvasive ischemic testing. | X | |
| 19. | Know the indications and methods to perform and interpret intracoronary imaging. | X | |
| 20. | Know the options for arterial and venous access and corresponding advantages and limitations. | X | |
| Clinical Syndromes | |||
| 21. | Know the strategies to improve cardiovascular health equity by understanding sex; gender; race; social determinants of health; and ethnicity-related differences in clinical epidemiology, causes, and outcomes of cardiovascular disease. | X | |
| 22. | Know the evaluation and treatment of arrhythmias and hemodynamic instability during coronary interventions, including drugs and devices. | X | |
| 23. | Know the pharmacokinetics and indications for antiplatelet and anticoagulant agents in the context of coronary interventions for chronic and acute coronary syndromes. | X | |
| 24. | Know the indications and timing of revascularization of nonculprit lesions and complete revascularization in multivessel disease across the spectrum of clinical coronary syndromes. | X | |
| 25. | Know the indications for percutaneous coronary intervention and the adjunctive and alternative uses of medical therapy and surgery for patients with stable ischemic heart disease. | X | |
| 26. | Know the roles of time of presentation, facility capability, anticipated door-to-device time, presence or absence of ongoing symptoms, and electrocardiographic abnormalities on the selection of reperfusion therapy for patients with ST-elevation myocardial infarction. | X | |
| 27. | Know the diagnostic criteria for nonatherosclerotic causes of acute coronary syndromes. | X | |
| 28. | Know the causes, diagnostic criteria, scoring systems, and treatments for cardiogenic shock. | X | |
| Devices and Equipment | |||
| 29. | Know the x-ray imaging systems and safety measures to minimize radiation exposure of patients, operators, and staff, including during pregnancy. | X | |
| 30. | Know the structure, polymer, and pharmacologic characteristics of coronary stents. | X | |
| 31. | Know the equipment, techniques, and devices used to treat coronary stenosis, thrombosis, tortuosity, and calcification. | X | |
| 32. | Know the selection and technical considerations of percutaneous mechanical support devices. | X | |
| Procedural Techniques | |||
| 33. | Know the levels of sedation, airway management, and medications used for conscious sedation and their reversal agents. | X | |
| 34. | Know the indications for placement and management of mechanical circulatory support. | X | |
| 35. | Know the technical approaches to complex coronary anatomy, including ostial, bifurcation, calcified, chronic total occlusion, left main, and restenotic lesions. | X | |
| 36. | Know the indications and devices available for vascular access management. | X | |
| PERIPHERAL VASCULAR INTERVENTIONS | |||
| 37. | Know the indications and appropriate use of noninvasive and invasive evaluations in patients undergoing peripheral vascular intervention. | X | |
| 38. | Know the guideline-directed therapies for primary and secondary prevention in patients with peripheral artery and venous disease, including lifestyle modification, pharmacologic therapies, and supervised exercise. | X | |
| Lower Extremity Peripheral Artery Disease | |||
| 39. | Know the anatomy, pathophysiology, and clinical presentation of lower extremity peripheral artery disease. | X | |
| 40. | Know the indications and surgical and endovascular treatment options for lower extremity peripheral artery disease. | X | |
| 41. | Know the complications associated with treatment of patients with lower extremity peripheral artery disease. | X | |
| Chronic and Acute Limb-Threatening Ischemia | |||
| 42. | Know the anatomy, pathophysiology, and clinical presentation of chronic and acute limb-threatening ischemia. | X | |
| 43. | Know the indications and surgical and endovascular treatment options for chronic and acute limb-threatening ischemia. | X | |
| 44. | Know the complications associated with treatment of patients with chronic and acute limb-threatening ischemia. | X | |
| Renal and Mesenteric Artery Disease | |||
| 45. | Know the anatomy, pathophysiology, and clinical presentation of renal and mesenteric artery disease. | X | |
| 46. | Know the indications and surgical and endovascular treatment options for renal and mesenteric artery disease. | X | |
| 47. | Know the complications associated with treatment of patients with renal and mesenteric artery disease. | X | |
| Subclavian, Vertebral, and Carotid Artery Disease | |||
| 48. | Know the anatomy, pathophysiology, and clinical presentation of subclavian, vertebral, and carotid artery disease. | X | |
| 49. | Know the indications and surgical and endovascular treatment options for subclavian, vertebral, and carotid artery disease. | X | |
| 50. | Know the complications associated with treatment of patients with subclavian, vertebral, and carotid artery disease. | X | |
| Venous Disease | |||
| 51. | Know the anatomy, pathophysiology, and clinical presentation of deep venous occlusive and thrombotic disease, superficial venous disease, and pulmonary embolism. | X | |
| 52. | Know the indications for surgical and endovascular treatment of deep venous occlusive and thrombotic disease, superficial venous disease, and pulmonary embolism. | X | |
| 53. | Know the complications associated with treatment of patients with deep venous occlusive and thrombotic disease, superficial venous disease, and pulmonary embolism. | X | |
| Aortic and Peripheral Artery Aneurysm Disease | |||
| 54. | Know the anatomy, pathophysiology, and clinical presentation of aortic and peripheral artery aneurysm disease. | X | |
| 55. | Know the indications and surgical and endovascular treatment options for aortic and peripheral artery aneurysm disease. | X | |
| 56. | Know the complications associated with treatment of patients with aortic and peripheral artery aneurysm disease. | X | |
| Nonatherosclerotic Vascular Disease | |||
| 57. | Know the anatomy, pathophysiology, and clinical presentation of nonatherosclerotic and inflammatory vascular disease. | X | |
| 58. | Know the indications and surgical and endovascular treatment options for nonatherosclerotic vascular disease. | X | |
| 59. | Know the complications associated with treatment of patients with nonatherosclerotic vascular disease. | X | |
| STRUCTURAL HEART INTERVENTIONS | |||
| 60. | Know the guideline-directed therapies for patients with structural heart disease, including lifestyle modification and pharmacologic therapies. | X | |
| 61. | Know the 3-dimensional cardiac anatomy relevant to structural heart interventions. | X | |
| 62. | Know the indications and appropriate use of noninvasive cardiac and noncardiac studies, including echocardiography, cardiovascular computed tomography, and cardiovascular magnetic resonance for structural heart interventions. | X | |
| 63. | Know the role of noninvasive and invasive hemodynamic studies in structural heart disease. | X | |
| 64. | Know the preprocedural evaluation and longitudinal care of patients under consideration for structural heart interventions. | X | |
| 65. | Know the utility of the multidisciplinary team and team-based decision-making, both among clinicians and between clinicians and their patients. | X | |
| 66. | Know the hemodynamic interactions and effects of multivalve disease and outcomes related to treatment. | X | |
| 67. | Know the indications and patient selection for paravalvular leak closure. | X | |
| Aortic Valve Interventions | |||
| 68. | Know the pathophysiology and clinical presentation of native and prosthetic aortic valve disease, including bicuspid aortic valve disease. | X | |
| 69. | Know the indications and transcatheter and surgical treatment options for aortic valve disease. | X | |
| 70. | Know the intraprocedural and postprocedural complications of aortic valve interventions, both transcatheter and surgical, such as paravalvular leak, stroke, coronary obstruction, aortic regurgitation, conduction abnormalities, indication for permanent pacemaker, pericardial effusion, major bleeding, and vascular and device-specific complications. | X | |
| 71. | Know the longitudinal history, therapeutic options, and outcomes of bioprosthetic valve dysfunction following transcatheter or surgical aortic valve replacement. | X | |
| Mitral Valve Interventions | |||
| 72. | Know the pathophysiology and clinical presentation of native and prosthetic mitral valve disease, anatomy and classification of mitral regurgitation, anatomy of the interatrial septum, and proper location for transeptal puncture. | X | |
| 73. | Know the indications and treatment options for native valve mitral regurgitation, including medical therapy, surgery, and transcatheter treatment. | X | |
| 74. | Know the indications and treatment options for prosthetic mitral valve disease, including transcatheter and surgical options. | X | |
| 75. | Know the indications and treatment options for native valve mitral stenosis, including medical therapy, surgery, and transcatheter treatment. | X | |
| 76. | Know the intraprocedural and postprocedural complications of mitral valve interventions, both transcatheter and surgical, such as left ventricular outflow tract obstruction, mitral regurgitation and stenosis, stroke, major bleeding, vascular complications, left ventricular dysfunction, pericardial effusion, and device-specific complications. | X | |
| Tricuspid Valve Interventions | |||
| 77. | Know the pathophysiology and clinical presentation of native and prosthetic tricuspid valve disease. | X | |
| 78. | Know the medical treatment options for tricuspid valve disease. | X | |
| 79. | Know the indications and treatment options for tricuspid valve disease, including surgery and transcatheter treatment. | X | |
| 80. | Know the intraprocedural and postprocedural complications of tricuspid valve interventions both transcatheter and surgical, including right ventricular failure, tricuspid regurgitation, major bleeding, vascular complications, pericardial effusion, and device-specific complications. | X | |
| Nonvalvular Structural Heart Interventions | |||
| 81. | Know the indications and patient selection for alcohol septal ablation. | X | |
| 82. | Know the indications and patient selection for left atrial appendage closure. | X | |
| 83. | Know the indications and patient selection for pulmonary vein angioplasty/stenting. | X | |
| 84. | Know the indications and patient selection for implantable hemodynamic monitor deployment. | X | |
| 85. | Know the indications and patient selection for patent foramen ovale and atrial septal defect closure. | X | |
| Adult Congenital Heart and Pulmonary Valve Interventions | |||
| 86. | Know the pathophysiology and clinical presentation of native and prosthetic pulmonic valve disease. | X | |
| 87. | Know the indications and treatment options for transcatheter and surgical replacement of pulmonic valve disease. | X | |
| 88. | Know the indications and treatment options for pulmonic valvuloplasty. | X | |
| 89. | Know the intraprocedural and postprocedural complications of pulmonic valve interventions, including right ventricle-pulmonary artery conduit rupture, valve embolization, pulmonary arterial perforation, coronary artery compression, pericardial effusion, and device-specific complications. | X | |
| 90. | Know the pathophysiology, clinical presentation, noninvasive advanced cardiac imaging, and invasive hemodynamic evaluation of simple adult congenital heart disease, including patent foramen ovale, anomalous origins of the coronary arteries, anomalous pulmonary venous connection, atrial septal defect, patent ductus arteriosus, ventricular septal defect, coarctation of the aorta, and pulmonary valve stenosis. | X | |
| 91. | Know the indications and treatment options for transcatheter and surgical intervention for simple adult congenital heart disease, including patent foramen ovale, anomalous origins of the coronary arteries, anomalous pulmonary venous connection, atrial septal defect, patent ductus arteriosus, ventricular septal defect, coarctation of the aorta, and pulmonary valve stenosis. | X | |
| 92. | Know the intraprocedural and postprocedural complications of transcatheter interventions in simple congenital heart disease, including patent foramen ovale, anomalous origins of the coronary arteries, anomalous pulmonary venous connection, atrial septal defect, patent ductus arteriosus, ventricular septal defect, coarctation of the aorta, and pulmonary valve stenosis. | X | |
| 93. | Know the pathophysiology, clinical presentation, noninvasive advanced cardiac imaging, and invasive hemodynamic evaluation of complex adult congenital heart disease such as tetralogy of Fallot, transposition of the great arteries, Eisenmenger syndrome, and single-ventricle/Fontan physiology. | X | |
| 94. | Know the indications and treatment options for percutaneous and surgical intervention for complex adult congenital heart disease such as tetralogy of Fallot, transposition of the great arteries, Eisenmenger syndrome, and single-ventricle/Fontan physiology. | X | |
| 95. | Know the intraprocedural and postprocedural complications of transcatheter interventions in complex congenital heart disease such as tetralogy of Fallot, transposition of the great arteries, Eisenmenger syndrome, and single-ventricle/Fontan physiology. | X | |
| 96. | Know the intraprocedural and postprocedural complications of right ventricle–pulmonary artery conduit intervention, Fontan fenestration, and collateral embolization. | X | |
| Evaluation Tools: chart review, conference presentation, direct observation, multisource evaluation, preliminary report review | |||
| PATIENT CARE AND PROCEDURAL SKILLS | Milestones (Months) |
||
|---|---|---|---|
| All (12) | Add∗ | ||
| CORONARY INTERVENTIONS | |||
| Consultation and Management | |||
| 1. | Skill to perform comprehensive consultations for interventional management of patients in outpatient and inpatient settings. | X | |
| 2. | Skills to determine and coordinate timing of coronary procedures based on patients’ comorbidities and clinical presentations. | X | |
| 3. | Skill to assess frailty, cognitive impairment, and delirium in older adults in the context of interventional procedures and integrate these conditions into patient management. | X | |
| 4. | Skills to evaluate and manage patients in the emergency, intensive care, and postoperative coronary procedural and surgical care units. | X | |
| 5. | Skill to integrate clinical and testing data in selecting candidates for percutaneous coronary intervention, incorporating evidence-based guidelines and clinical trial information. | X | |
| 6. | Skill to determine risks/benefits of percutaneous coronary intervention (and type of percutaneous coronary intervention) versus alternative revascularization or medical treatments. | X | |
| 7. | Skill to achieve volume and quality outcome benchmarks for percutaneous coronary intervention in training and in practice. | X | |
| 8. | Skill to carry out postprocedural evaluations. | X | |
| 9. | Skills to recognize and manage a patient with cardiogenic shock in and out of the catheterization laboratory. | X | |
| 10. | Skill to provide guideline-directed therapies for primary and secondary prevention in patients with ischemic heart disease, including lifestyle modification, pharmacologic therapies, and cardiac rehabilitation. | X | |
| Invasive Diagnostic Testing | |||
| 11. | Skills to perform and analyze coronary angiograms of normal and anomalous coronary artery origin as well as coronary artery bypass grafting. | X | |
| 12. | Skills to perform and interpret intracoronary imaging and functional assessment. | X | |
| Devices and Procedural Techniques | |||
| 13. | Skills to operate and manipulate intravascular guidewires, balloons and stents, guide extensions, microcatheters, atherectomy devices, thrombectomy devices, embolic protection devices, and closure devices. | X | |
| 14. | Skills to operate and manipulate percutaneous mechanical circulatory devices. | X | |
| 15. | Skills to identify and manage complications of diagnostic and interventional procedures including use of pericardiocentesis, anticoagulants/procoagulants, snares, coils, and covered stents. | X | |
| 16. | Skills to identify and manage peripheral complications of cardiac catheterization or percutaneous coronary intervention, including arteriovenous fistula, retroperitoneal bleeding, arterial ischemia, refractory spasm, pseudoaneurysm, and artery dissection. | X | |
| 17. | Skill to perform percutaneous arterial and venous access (including large bore), including use of ultrasound guidance and placement of closure and hemostatic devices. | X | |
| 18. | Skills to perform percutaneous coronary intervention in a range of lesion subsets. | X | |
| 19. | Skills to promptly recognize, identify, and treat hemodynamic instability, including the use of hemodynamic data, pharmacological agents, and/or percutaneous mechanical circulatory assist devices. | X | |
| 20. | Skills to place and manage a transvenous pacemaker. | X | |
| 21. | Skill to perform elective and emergency pericardiocentesis. | X | |
| PERIPHERAL VASCULAR INTERVENTIONS | |||
| 22. | Skill to provide guideline-directed therapies for primary and secondary prevention in patients with atherosclerotic vascular disease, including lifestyle modification, pharmacologic therapies, and supervised exercise. | X | |
| 23. | Skill to integrate clinical and testing data in selecting candidates for peripheral vascular intervention, incorporating evidence-based guidelines and clinical trial information. | X | |
| 24. | Skill to perform diagnostic angiography of the aorta with runoff of the lower extremities. | X | |
| 25. | Skill to perform renal and mesenteric angiography. | X | |
| 26. | Skill to perform aortic arch and upper extremity angiography. | X | |
| 27. | Skill to perform 4-vessel cervicocerebral angiography. | X | |
| 28. | Skill to obtain alternate antegrade femoral and retrograde tibiopedal access. | X | |
| 29. | Skills to perform endovascular revascularization of the aortoiliac arteries and identify and manage complications of the procedure. | X | |
| 30. | Skills to perform endovascular revascularization of the femoropopliteal arteries and identify and manage complications of the procedure. | X | |
| 31. | Skills to perform endovascular revascularization of infrapopliteal arteries and identify and manage complications of the procedure. | X | |
| 32. | Skills to perform endovascular revascularization of the renal and mesenteric arteries and identify and manage complications of the procedure. | X | |
| 33. | Skills to perform endovascular revascularization of the subclavian, vertebral, and carotid arteries and identify and manage complications of the procedure. | X | |
| 34. | Skills to perform deep vein thrombectomy and thrombolysis and identify and manage complications of the procedure. | X | |
| 35. | Skills to perform inferior vena cava filter placement and retrieval and identify and manage complications of the procedures. | X | |
| 36. | Skills to perform venous stenting with intravascular imaging and identify and manage complications of the procedure. | X | |
| 37. | Skills to perform superficial vein ablations and identify and manage complications of the procedure. | X | |
| 38. | Skills to perform pulmonary embolism thrombectomy and thrombolysis and identify and manage complications of the procedures. | X | |
| 39. | Skills to perform endovascular aortic aneurysm repair and identify and manage complications of the procedures. | X | |
| 40. | Skill to perform endovascular procedures such as embolization/ exclusion procedures for visceral artery aneurysms. | X | |
| 41. | Skill to perform interventional management (embolization) of postendograft endoleaks. | X | |
| 42. | Skills to perform and interpret external and intravascular noncoronary ultrasound. | X | |
| 43. | Skill to perform balloon occlusion or tamponade for management of large-bore access hemostasis or of a femoral access site complication. | X | |
| 44. | Skills to perform stroke thrombectomy and identify and manage complications of the procedure. | X | |
| STRUCTURAL HEART INTERVENTIONS | |||
| 45. | Skill to provide guideline-directed therapies for patients with structural heart disease, including lifestyle modification and pharmacologic therapies. | X | |
| 46. | Skill to integrate clinical and testing data in selecting candidates for structural heart intervention, incorporating evidence-based guidelines and clinical trial information. | X | |
| 47. | Skill to integrate clinical presentation, preprocedural work-up, and noninvasive cardiac imaging in patient selection for less common structural heart interventions, including tricuspid repair/replacement, pulmonary valve replacement, use of devices for heart failure, and other adult congenital heart disease interventions. | X | |
| 48. | Skills to perform transcatheter aortic valve replacement and identify and manage complications of the procedure. | X | |
| 49. | Skills to perform transcatheter mitral valve repair/replacement and identify and manage complications of the procedure. | X | |
| 50. | Skills to perform transcatheter tricuspid repair/replacement and identify and manage complications of the procedure. | X | |
| 51. | Skills to perform transcatheter pulmonic replacement and identify and manage complications of the procedure. | X | |
| 52. | Skills to perform and interpret intracardiac echocardiography. | X | |
| 53. | Skills to perform transcatheter patent foramen ovale/atrial septal defect closure and identify and manage complications of the procedure. | X | |
| 54. | Skills to close a paravalvular leak with vascular coils or plugs and identify and manage complications. | X | |
| 55. | Skill to utilize noninvasive cardiac and noncardiac studies, including echocardiography, cardiovascular computed tomography, and cardiovascular magnetic resonance for structural heart interventions. | X | |
| 56. | Skills to perform balloon aortic valvuloplasty and identify and manage complications of the procedure. | X | |
| 57. | Skills to perform balloon mitral valvuloplasty and identify and manage complications of the procedure. | X | |
| 58. | Skills to perform balloon pulmonic valvuloplasty and identify and manage complications of the procedure. | X | |
| 59. | Skills to perform alcohol septal ablation and identify and manage complications of the procedure. | X | |
| 60. | Skills to perform left atrial appendage closure and identify and manage complications of the procedure. | X | |
| 61. | Skills to perform pulmonary vein angioplasty/stenting and identify and manage complications of the procedure. | X | |
| 62. | Skills to perform invasive catheterization, angiography, hemodynamic evaluation, and interpretation to identify and manage complications in simple and complex adult congenital heart disease. | X | |
| 63. | Skill to implant a hemodynamic monitoring device. | X | |
| Evaluation Tools: chart review, clinical and patient safety and quality improvement conference presentation, direct observation, fellow-acquired image review, case logs, multisource evaluation, simulation | |||
Add = additional competencies that extend beyond the core expectations and that may be achieved by some interventional cardiologists based on career focus, either during or following formal interventional cardiology fellowship training (see text for details).
Table 1 encompasses both the Medical Knowledge competencies and Patient Care and Procedural Skills competencies specifically related to interventional cardiology. Table 2 specifies a common set of professional behavior competencies that fall under the ACGME competency domains titled Systems-Based Practice, Practice-Based Learning and Improvement, Interpersonal and Communication Skills, and Professionalism. Although these competencies are relevant to all clinical cardiovascular disease specialists, they should be interpreted within the context of interventional cardiology practice.
Table 2.
Common Professional Behavior Competencies Relevant to All Clinical Cardiovascular Disease Specialists
| SYSTEMS-BASED PRACTICE | All Specialists | |
|---|---|---|
| 1. | Incorporate risk-benefit analysis, cost, resource, and value considerations into care of patients with cardiovascular disease. | X |
| 2. | Identify and address financial, cultural, and social barriers to adherence with patient care recommendations, including social and economic determinants of health. | X |
| 3. | Participate in practice-based continuous quality improvement and safety initiatives. | X |
| 4. | Maintain continuity of care with efficient and effective handoffs through transitions of care. | X |
| 5. | Identify barriers to learning and prioritize education for patients with cardiovascular disease. | X |
| 6. | Develop, implement, and evaluate individualized, patient-centered educational strategies. | X |
| 7. | Incorporate new therapies and clinical trial data into individualized patient care plans, including strategies to ensure that minorities and women receive the same evidence-based care as majority groups. | X |
| 8. | Participate in hospital-based and regional systems of care for patients with urgent and emergent cardiovascular conditions. | X |
| 9. | Identify and address barriers that affect the ability of health care professionals to provide optimal cardiovascular care. | X |
| 10. | Collaborate with all cardiovascular care team members to reduce avoidable hospitalizations for cardiovascular disease. | X |
| 11. | Collaborate with physicians and health care professionals in other disciplines to optimize the care of patients with complex and multisystem disease. | X |
| 12. | Recognize and reduce the role of implicit and explicit biases in access to care within health care systems. | X |
| Evaluation Tools: chart review, direct observation, multisource evaluation | ||
| PRACTICE-BASED LEARNING AND IMPROVEMENT | All Specialists | |
|---|---|---|
| 1. | Identify personal knowledge gaps and seek educational and training opportunities to improve knowledge, skills, and performance. | X |
| 2. | Utilize clinical practice guidelines, appropriate use criteria, and other information tools at the point of care to improve clinical decision-making. | X |
| 3. | Maintain current standards of care by performing literature searches, interpreting data, and applying results to clinical care. | X |
| 4. | Solicit and incorporate feedback from patients, colleagues, and other health care team members to improve clinical performance. | X |
| 5. | Use health record and registry data to assess appropriateness, quality, equity, and safety of cardiovascular care. | X |
| 6. | Develop the practice of lifelong learning, including regular review of journals and practice guidelines/appropriate use criteria/consensus statements and attendance at scientific and continuing medical education meetings. | X |
| 7. | Recognize and reduce the role of implicit and explicit biases in clinical decision-making. | X |
| Evaluation Tools: case logs, chart review, conference presentation, direct observation, multisource evaluation, quality improvement project, reflection and self-assessment | ||
| PROFESSIONALISM | All Specialists | |
|---|---|---|
| 1. | Demonstrate respect, consideration, and empathy for patients, families, and all members of the health care team. | X |
| 2. | Purposefully engage with individuals of different demographic and socioeconomic backgrounds to enhance cultural awareness. | X |
| 3. | Recognize and reduce the role of implicit and explicit biases in interpersonal relationships. | X |
| 4. | Practice within the scope of personal expertise, training, and technical skills. | X |
| 5. | Recognize the need for and obtain consultations in a timely manner. | X |
| 6. | Know current evidence-based clinical practice guidelines, consensus statements, appropriate use criteria, and performance measures relevant to scope of practice. | X |
| 7. | Identify, disclose, and manage relationships with industry and other entities to minimize bias and undue influence on clinical decision-making. | X |
| 8. | Demonstrate high ethical standards in personal and professional conduct. | X |
| 9. | Take responsibility and follow through on professional commitments and obligations in a timely manner. | X |
| 10. | Identify potential for impaired professional performance in oneself and colleagues and take action to mitigate. | X |
| 11. | Attend to one’s own health, well-being, and abilities to maximize personal and professional performance. | X |
| Evaluation Tools: direct observation, multisource evaluation, reflection and self-assessment | ||
| INTERPERSONAL AND COMMUNICATION SKILLS | All Specialists | |
|---|---|---|
| 1. | Assess and manage human responses experienced by individuals with cardiovascular disease (eg, depression, spiritual distress, nonadherence, decisional conflict). | X |
| 2. | Communicate with patients, families, and other health care professionals in an effective, timely, and culturally competent manner. | X |
| 3. | Engage patients in shared decision-making based on balanced presentation of potential risks, benefits, and alternatives, factoring in patients’ values and preferences. | X |
| 4. | Review medical records, complete documentation, and communicate results of diagnostic findings and management strategies to patients and collaborating health care professionals in a timely manner. | X |
| 5. | Lead and collaborate in interdisciplinary and cardiovascular care teams to promote a culture of well-being, diversity, and inclusion. | X |
| 6. | Compassionately discuss sensitive/difficult topics, including end-of-life care and care of critically ill patients. | X |
| 7. | Provide emotional support to patients and families. | X |
| Evaluation Tools: direct observation, multisource evaluation | ||
4.2. Procedural and Technical Experience
4.2.1. Training Pathway and Procedural Number Guidance
The required cognitive knowledge base and skills for interventional cardiology are acquired throughout the continuum of training in cardiovascular diseases and interventional cardiology fellowship programs. The cognitive knowledge base for a career in interventional cardiology is initially obtained in the internal medicine residency, followed by a fellowship in general cardiovascular diseases. The foundational technical skills for interventional cardiology are obtained during the general cardiovascular disease training with a focus on cardiac catheterization procedures, including vascular access, performing optimal angiography, and measuring and interpreting cardiovascular hemodynamic data. These skills are further developed and expanded during interventional cardiology fellowship training. Core procedural skills acquired for diagnostic coronary angiography and hemodynamic assessment provide a foundation for coronary, peripheral vascular, and structural heart interventions. Core procedural skills in interventional cardiology serve as prerequisites for developing ACHD procedural skills. Once Level II training in diagnostic cardiac catheterization has been obtained during the Cardiovascular Disease Fellowship, trainees can participate in cardiovascular interventions, which may count toward the cumulative training minimum volume requirements for structural heart or PVI. However, for these procedural volumes to count toward training in either select peripheral vascular or structural heart interventions, the Cardiovascular Disease Fellowship must be followed by completion of an interventional cardiology fellowship program. This continuum represents the training pathway for interventional cardiology (Figure 1).
Figure 1.
Interventional Cardiology Training Pathway
ACC = American College of Cardiology; ACHD = adult congenital heart disease; CVD = cardiovascular disease; IC = interventional cardiology; PAI = peripheral artery intervention; PCI = percutaneous coronary intervention; PFO = patent foramen ovale; PVI = peripheral vascular intervention; SHI = structural heart intervention.
This document also provides guidance on professional building blocks and minimum volume requirements for coronary, peripheral vascular, and structural heart interventions (Figures 1 and 2, Tables 3 and 4). The building blocks of the profession—core interventional procedures across coronary interventions (required by all) as well as peripheral vascular and structural heart interventions (required based on practice focus)—are delineated (Figure 2). Notably, procedural numbers are not assigned in Figure 2, appreciating that these competencies include techniques that may be acquired at any time during cardiovascular training and are performed at varying frequencies dependent on local device availability and practice. Importantly, mastering core procedural skills in one procedural area of focus may directly contribute to skills required to perform other interventional procedures and serve as the basis for continued skill acquisition after training. For low-frequency procedures, development of procedural skills may be supplemented with simulation training that is either available on site or through interventional courses or industry-sponsored programs. Guidance for minimum procedural volume requirements and case mix for coronary, peripheral vascular, and structural heart interventions (Table 3) and for ACHD interventions (Table 4) is provided. These numbers are meant to provide guidance for interventional cardiology trainees, recognizing that competency to perform each procedure must be based on evaluation by the training director/supervising physician.
Figure 2.
Building Blocks of the Profession
ASD = atrial septal defect; AV = arteriovenous; PFO = patent foramen ovale.
Table 3.
Minimum Procedural Volume Typically Necessary for the Development and Demonstration of Interventional Cardiology Competencies
| Procedure/Technical Skill | Minimum Number of Procedures∗ | Notes |
|---|---|---|
| INTERVENTIONAL PROCEDURES | 250 | Includes a minimum of 200 percutaneous coronary interventions. The remaining 50 procedures could include any combination of coronary, peripheral artery, or structural heart interventions. |
| CORONARY INTERVENTIONS (required) | ||
| Percutaneous coronary interventions | 200 | Includes exposure to a broad range of adult patient ages, clinical presentations, pathologies, and therapies, including a range of anatomical lesion subsets, in de novo and previously stented vessels, such as thrombotic, ostial, bifurcation, and heavily calcified lesions, bypass grafts, and chronic total occlusion. |
| Adjunctive procedures | ||
|
25 | Includes fractional flow reserve and nonhyperemic pressure ratio. |
|
25 | Use of intravascular ultrasound and additional devices as available (optical coherence tomography). |
| PERIPHERAL VASCULAR INTERVENTIONS (optional based on practice focus) | ||
| Peripheral artery interventions | 100 diagnostic procedures 50 interventional procedures (with half as primary operator†) |
Includes exposure to lower extremity, subclavian, innominate, renal, mesenteric, and chronic limb ischemia. |
| Carotid stenting | 25 (with 13 as primary operator†) | Carotid numbers can count toward numbers for peripheral artery interventions. |
| Endovascular aortic aneurysm repair | 20 (with 10 as primary operator†) | Specialized advanced training for endovascular aneurysm repair is required in addition to the required peripheral artery interventions. |
| Peripheral venous interventions | 20 | Includes broad exposure to acute and chronic pulmonary embolism interventions, deep vein thrombosis intervention, dialysis access angioplasty and stenting, and inferior vena cava filter placement and retrieval. A minimum of 5 cases of any individual procedure is required to demonstrate competence in that specific procedure in addition to the required peripheral artery interventions. |
| STRUCTURAL HEART INTERVENTIONS (optional based on practice focus) | ||
| Transcatheter aortic valve replacement | 50 (with 25 as primary operator†) | For practitioners solely focused on TAVR, this minimum volume is required. |
| Left atrial appendage closure | 10 | In addition to the minimum number shown, a foundation of 50 lifetime transcatheter structural heart procedures, including a minimum of 20 transseptal procedures (10 as primary operator†) through an intact septum (ie, non-PFO), is required to demonstrate competency for these procedures. |
| Transcatheter mitral valve edge-to-edge repair | 20 | |
| Mitral balloon valvuloplasty | 5 | |
| Patent foramen ovale closure | 12 | |
| Atrial septal defect closure | 15 | |
PFO = patent foramen ovale; TAVR = transcatheter aortic valve replacement.
Numbers are based on consensus, are intended as general guidance, and represent the cumulative experience that may occur at any time during training. Competency to perform each procedure must be based on evaluation and documentation by the Interventional Cardiology Training Program Director through direct observation and feedback from the Accreditation Council for Graduate Medical Education Clinical Competency Committee or the program director or training supervisor for peripheral vascular and structural heart interventions. Industry standards should be met when available.
The primary operator is defined as the interventionalist performing the critical portions of the procedure, either independently or under direct supervision.
Table 4.
Minimum Procedural Volume Typically Necessary for the Development and Demonstration of Interventional Cardiology Competencies for Additional, Optional Adult Congenital Heart Interventions∗
| Procedure/Technical Skill | Minimum Number of Procedures† |
|---|---|
| Device Closures | |
|
15 |
|
12 |
|
2 |
|
8 |
| Angioplasty/Stenting Procedures | |
|
8 |
|
12 |
|
5 |
|
5 |
|
5 |
|
5 |
|
2 |
|
2 |
Adapted from Aboulhosn et al.44
These numerical requirements pertain to interventional cardiologists who perform congenital heart interventions in adult patients.
Numbers are based on consensus and intended as general guidance for structural heart intervention trainees who wish to specialize in adult congenital heart disease interventions and represent cumulative experience that may occur at any time during training. Competency to perform each procedure must be based on evaluation and documentation by the adult congenital heart disease interventional program director or training supervisor through direct observation and feedback from the Accreditation Council for Graduate Medical Education Clinical Competency Committee. Industry standards should be met when available.
During the 12-month interventional cardiology fellowship, trainees should participate in a minimum of 250 interventional procedures by completion of training, including at least 200 percutaneous coronary interventions. The requirement for 50 additional interventional procedures is more flexible and can include any combination of coronary, peripheral vascular, and/or structural procedures. Trainees should consider aligning their training with their area of clinical career goals when choosing the focus for these 50 procedures. In addition to the requirement for 250 interventional procedures, 25 diagnostic coronary physiology and 25 intracoronary imaging procedures are also recommended. Dependent on career focus, trainees may require additional training beyond the 12-month interventional cardiology fellowship and 36-month cardiovascular diseases fellowship. Procedural participation as a primary trainee operator for structural heart or PVIs during the cardiovascular diseases fellowship may count toward the minimum procedural volume requirements for select structural heart or peripheral vascular interventional procedures, provided the minimum volume requirements are met during the 12-month interventional cardiology training period. The primary operator is the interventionalist performing the critical portions of the procedure, either independently or under direct supervision. Examples of procedures that count toward the minimum case volumes are:
-
▪
Intravascular imaging or assessment of coronary physiology for a procedure followed by an intervention counts as 1 imaging and/or physiology procedure and 1 intervention
-
▪
Multivessel interventions during the same procedure count as 1 procedure
-
▪
Coronary and peripheral vascular interventional procedures during the same episode of care count as a single procedure
-
▪
Coronary and structural heart interventional procedures during the same episode of care count as a single procedure
-
▪
Any structural heart or peripheral vascular interventional procedure as a primary operator during the cardiovascular diseases fellowship can only be counted toward procedural competency if an interventional cardiology fellowship is subsequently completed with 250 cardiovascular interventions and 25 coronary physiology and 25 intracoronary imaging procedures.
As indicated in Section 1.2.3, procedural volumes in this document are based on judgment about the minimum experience required to provide most trainees with a sufficient variety of clinical situations and allow faculty ample opportunity to evaluate the trainee’s emerging competency. Societal guidance for coronary interventional procedures,6,22, 23, 24, 25 PVI,26, 27, 28, 29, 30, 31, 32, 33, 34 structural heart interventions,23,35, 36, 37, 38, 39, 40, 41, 42, 43 and ACHD interventions8,44,45 further informed these recommendations. Proficiency and procedural outcomes, rather than length of exposure or the exact number of procedures performed, are the dominant criteria for the evaluation of competency in the context of educational milestones. In addition, absolute mastery of all aspects of interventional cardiology is not likely to be achieved based on the fellowship experience alone. For common and straightforward procedures, mastery can occur, but for very complex or infrequently performed procedures, lower levels of proficiency are anticipated for new graduates. Complete proficiency in advanced techniques may develop after additional years of independent practice and clinical experience.
Lastly, it is expected that training is directed by an appropriately trained and board-certified program director in an ACGME-accredited program, as defined in Section 2.1. Training directors must assess and attest to trainee competence through direct observation, documentation of procedural numbers and complexity, and input from the Clinical Competency Committee.
4.2.2. Coronary Interventions
4.2.2.1. Anatomy and Pathophysiology of Coronary Ischemia and Interventions
All interventional cardiologists should know the normal cardiac and extracardiac arterial anatomy and its variations. This includes a thorough knowledge of the anatomy and physiology of the surgical and congenital shunts and baffles as well as the classification and scoring systems applicable to lesion subsets, such as bifurcations, total occlusions, multivessel disease, and restenosis lesions. In some of these subsets, scoring will help a practicing interventional cardiologist determine the relative success and complication rates of the procedures they undertake. Trainees must be well-versed in the causes, pathophysiology, and differential diagnosis of myocardial ischemia and infarction and the atherosclerotic and nonatherosclerotic causes of myocardial ischemia and infarction. These conditions include, but are not limited to, ruptured plaques, dissections, and myocardial bridges. They should also know the vascular response to percutaneous intervention, such as response to injury, mechanisms of restenosis, and stent thrombosis to ensure the optimization of the intervention to limit future adverse events. They should be able to recognize the appearance of major contributors to stent thrombosis and restenosis on intravascular imaging.
In most interventional cardiology procedures, anticoagulants and antiplatelet agents are used, and these procedures may result in bleeding complications. Trainees should therefore know the coagulation cascade, antiplatelet agents, anticoagulants, fibrinolytic drugs, and the interactions between these agents and other medications and conditions. Occasionally during interventions, patients might experience coronary vasospasm. Interventional trainees should be able to reverse this condition by using appropriate medications for epicardial and microvascular spasm. They should also know the hemodynamics and etiologies of shock as well as the indications for and use of vasopressor/inotropic agents. Trainees should know the indications and the limitations of intra-aortic balloon pumps. Although they might not have experience with all of the mechanical support devices due to local availability, they should know the indications, limitations, expected hemodynamic response, and complications of other current mechanical support devices such as transvalvular pumps, left atrial to femoral artery bypass pumps, intra-aortic balloon counterpulsation pumps, extracorporeal membrane oxygenation systems, and future devices as they become available over time.
Interventional cardiology trainees should have the necessary knowledge to limit patient and cardiac catheterization laboratory staff exposure to x-ray radiation. To prevent acute kidney injury postprocedure, they should also know strategies for limiting the volume of contrast media used in patients. They need to understand contrast-induced acute kidney injury, including the risk factors, diagnostic criteria, prevention, and treatment strategies. Trainees should know how to prevent and treat allergic reactions to contrast media, including anaphylaxis.
4.2.2.2. Periprocedural Assessment and Diagnostic Testing
Interventional cardiology trainees should know the currently accepted methods of assessing patients in the periprocedural period. Trainees should know the indications and limitations of noninvasive evaluations, including, but not limited to, treadmill exercise testing, nuclear perfusion scans, positron emission tomography, and magnetic resonance imaging, as well as invasive evaluations such as intracoronary imaging and functional lesion assessment. Trainees should be able to synthesize these data with an understanding of the patient’s functional status and comorbidities to develop an appropriate management plan. One way to ensure proper patient selection is to use Appropriate Use Criteria.46 The interventional cardiology curriculum should include references to Appropriate Use Criteria and its uses in identifying appropriate patients for each procedure. This will ensure that procedures are limited to those who need them, thereby limiting complications due to unnecessary interventions. A clear understanding of the latest ACC/AHA guidelines for percutaneous and surgical revascularization is also important for practicing evidence-based care.20,47 Interventional cardiology fellows should demonstrate knowledge and competence in patient management before, during, and after interventional cardiology procedures. They should know how to triage and manage patients in different clinical settings, such as the cardiac care unit, emergency department, outpatient clinics, and the cardiac catheterization laboratory.
Interventional cardiology fellows should be well-versed in understanding the indications, contraindications, complications, and effects of different patient and lesion characteristics on the procedural outcome. The patient and lesion assessment does not end at the preprocedural period; assessment should be revised as new images or data become available during the procedure.
4.2.2.3. Consultation and Management
Consultative cardiology is an integral responsibility of the interventional cardiologist that should be valued and prioritized. Interventional trainees should be involved and proficient in all aspects of patient care, including history and physical examination, medical therapy, shared decision-making, informed consent, and postprocedure care. Synthesis of clinical data, including patient history, physical examination, laboratory evaluation, electrocardiogram, echocardiography, and noninvasive stress testing, should inform a patient-focused discussion of the risks, benefits, and alternatives of invasive and noninvasive management strategies. Numerous risk scores are available to stratify patients at risk for contrast nephropathy, periprocedural complications, and outcomes and should be considered when therapeutic options are discussed. A system should be in place to hold case discussions to critically evaluate patient care and outcomes based on integration of clinical data. Ultimately, invasive testing results, comorbidities, and integration of clinical trial data should guide shared decision-making in choosing a medical management, catheter-based intervention, or cardiovascular surgical approach to care.
4.2.2.4. Invasive Diagnostic Testing
Invasive diagnostic testing, including intracoronary imaging and physiologic testing, is fundamental to the practice of interventional cardiology. Trainees must know the different types of imaging (eg, IVUS and OCT) and physiological testing (eg, adenosine fractional flow reserve and nonhyperemic pressure ratios) and acquire the skills to perform each. Trainees must learn the indications for invasive imaging versus physiological testing and demonstrate the ability to choose the best modality for a specific situation dependent on whether anatomical or functional information is primarily needed. Trainees also need to learn the roles of each of these modalities preprocedurally, intraprocedurally, and postprocedurally, as well as the outcomes data that support imaging and physiology in each of these settings. Trainees must acquire the skills to set up, deploy, and troubleshoot imaging and physiological testing devices. For imaging, these include appropriately connecting devices, flushing lines, and image optimization. For physiological testing, these include zeroing the system, equalizing the wire, and recognizing and correcting techniques that may result in erroneous information, such as drift or a deeply seated guide.
In addition, the ability to accurately interpret the data provided by these invasive tests is paramount to providing optimal patient care. For intracoronary imaging, this includes recognition of normal vessel anatomy, different types of plaque, calcium, thrombus, dissection, bridging, stent underexpansion, stent malapposition, and mechanisms of restenosis. It also includes the ability to measure vessel/lesion length, diameter, and area, and choose appropriately sized equipment, as well as knowing the preferred cutpoints to indicate optimized stenting. Finally, it includes the ability to choose the next appropriate step based on the information gathered, such as using atherectomy for excessive calcification or further postdilation for an underexpanded stent. For physiological testing, this includes knowledge of cutpoints used for indicating functional significance based on the physiological modality being used (such as hyperemic versus resting); understanding the effects of anatomical subsets on epicardial physiological data, including acute lesions, serial lesions, collateral vessels, and microvascular dysfunction; and the ability to appropriately interpret and respond to post-PCI physiological data to optimize outcomes.
4.2.2.5. Clinical Syndromes
Advanced training in interventional cardiology includes the development of comprehensive knowledge regarding the identification and management of the various clinical coronary syndromes, ranging from stable ischemic heart disease to acute coronary syndromes (ie, unstable angina, ST-elevation myocardial infarction, and non–ST-elevation acute coronary syndromes) and cardiogenic shock. Stable ischemic heart disease includes a range of patients, from those with nonobstructive CAD to those with complex 3-vessel disease. Trainees should understand the presentation, available diagnostic tests, and management strategies for patients with ischemia and nonobstructive coronary arteries. For obstructive CAD, trainees should know the indications for, the risks and benefits of, as well as the alternatives to PCI, coronary artery bypass grafting, and medical management so that they can appropriately guide patients through evidence-based shared decision-making, in conjunction with a multidisciplinary team, as appropriate.
Acute coronary syndromes, including unstable angina, non–ST-elevation myocardial infarction, and ST-elevation myocardial infarction can present with nonobstructive or obstructive CAD. Trainees should be able to identify the different types of myocardial infarction as well as understand their underlying pathology and best management. Along with the universal definition of myocardial infarction, this includes myocardial infarction with nonobstructive coronary arteries and spontaneous coronary artery dissection. Trainees should also be highly proficient in the identification and management of ST-elevation myocardial infarction, demonstrating a working knowledge of associated guideline-based quality metrics. Finally, trainees must be able to accurately identify the clinical signs and hemodynamic findings of cardiac decompensation and demonstrate the ability to quickly choose the appropriate lifesaving measures and when to consider engaging other specialists, such as anesthesia, cardiac surgery, or vascular surgery, among others. For cardiogenic shock, this would include anticipating the possibility of shock based on the clinical scenario and knowing the diagnostic criteria (such as hypotension, cool extremities, acidosis, and an elevated pulmonary capillary wedge pressure) and scoring systems and identifying the appropriate therapy (medical therapy and/or mechanical circulatory support) as well as the risks and benefits of these therapies.
4.2.2.6. Devices
All trainees should have a strong understanding of how to evaluate and treat complex coronary lesions using the spectrum of devices available as well as have the skills to address potential complications. Trainees should know how to select the most appropriate invasive diagnostic studies, such as intracoronary imaging (eg, IVUS and OCT) and physiological testing (eg, fractional flow reserve, nonhyperemic pressure ratios) to best evaluate lesions for appropriateness of the intervention and to facilitate optimal procedural outcomes. For example, trainees should know how to use intravascular imaging to elucidate the cause of stent thrombosis or restenosis. They should understand the appropriate cutoffs for hemodynamic significance and the advantages and disadvantages of each of these methods of evaluation under a variety of anatomical circumstances, such as the limitations of OCT in ostial left main disease and how to interpret fractional flow reserve with tandem lesions.
When intervention is indicated, trainees should understand features of stent structure, sizing, and deployment, and the drugs incorporated into them to allow for stent selection tailored to lesion and patient characteristics. All trainees should have experience using a variety of guide catheters, including knowledge of catheter shapes and sizing as they relate to patient anatomy and the radial versus femoral approach. They should have experience in the safe use of guide extenders and an understanding of the techniques for accessing complex coronary anatomy, including coronary intervention in bypass grafts, anomalous coronary arteries, and in post-TAVR patients. To maximize procedural success, all trainees should have a strong working experience with various guidewires across the spectrum of pushability, steerability, and torque. If trainees are at centers with CTO training opportunities, they should be encouraged to gain experience using specialized CTO wires and devices. All trainees should gain experience using a variety of balloon types, and they should understand when the use of atherectomy is necessary to achieve appropriate lesion preparation. They should be knowledgeable about atherectomy devices (ie, rotational, orbital, laser), intravascular lithotripsy, and cutting balloons for calcified and/or nondilatable lesions. They should also gain experience in the use of distal protection devices in vein graft PCI and thrombus extraction when needed.
All trainees should be skilled in recognizing and managing potential cardiac complications of diagnostic and interventional procedures, including use of snares, coils, covered stents, and pericardiocentesis needles. Experience with a variety of hemostasis devices is important for all trainees, along with knowing how to prevent and manage access site complications at the wrist and groin (such as radial artery occlusion and retroperitoneal hemorrhage). Skills in the appropriate selection and use of a variety of mechanical support options tailored to specific clinical conditions are expected of all trainees.
4.2.2.7. Procedural Techniques
By the completion of training, all trainees should be able to independently perform all aspects of a coronary intervention, demonstrating the skills to perform safe vascular access, determine the significance of lesions, apply appropriate technical approaches to complex anatomy, evaluate the adequacy of interventions, and manage potential complications.
Trainees should have proficiency in vascular access techniques from both the radial and femoral approach (including the use of ultrasound for both and the micropuncture technique for the femoral approach) and for both arterial and venous access. Knowledge of the procedural advantages and disadvantages of the radial versus femoral approach should inform the access site decision, and the trainee should be able to demonstrate skills in maneuvering through challenging peripheral anatomy. Appropriate use of intravenous and oral antithrombotic therapy during the preprocedural, periprocedural, and postprocedural periods to support PCI should be understood, including dosing, timing, and special circumstances. Skill to visualize the coronary anatomy through use of different fluoroscopy views is essential. Techniques for minimizing radiation to both patients and operators is required, as well as experience with methods to reduce the use of contrast media, particularly in patients at increased risk for contrast nephropathy.
All trainees should be skilled in a variety of approaches for performing PCIs for complex coronary anatomy, including, but not limited to, ostial, bifurcation, calcified, left main, restenotic, and bypass graft lesions. All trainees should have a basic understanding of approaches to CTOs, with trainees encouraged to gain experience using specialized CTO techniques, if possible.
The skills to promptly detect and manage PCI complications, both in the laboratory and postprocedure, are vital for all trainees. They should understand the risks and recognize the signs of major PCI procedural complications, including coronary vascular (eg, dissection, thrombosis, perforation, embolization) and other vascular (eg, dissection, pseudoaneurysm, retroperitoneal hemorrhage, arteriovenous fistula, and stroke) complications. They should also be skilled in the use of procedural techniques to treat these complications, such as pericardiocentesis, thrombectomy, and angiography of peripheral vessels, and know when to engage the multidisciplinary team. Trainees should be able to identify causes of hemodynamic instability and treat them accordingly with emergent use of pharmacologic agents and/or percutaneous mechanical circulatory assist devices, as needed.
4.2.3. Peripheral Vascular Interventions
4.2.3.1. Lower Extremity PAD
Interventional cardiology trainees must have an in-depth understanding of the epidemiology, risk factors, pathophysiology, and associated morbidity and mortality of lower extremity PAD. Given the variable presentation of PAD, fellows must be facile in identifying the wide spectrum of disease. This may include, but is not limited to, patients with PAD who have a nonhealing wound in the setting of CLTI or the rapid onset of lower-extremity pain and lack of pulses seen in ALI. To accomplish this, trainees must be familiar with both the history and physical examination findings suggestive of PAD.48 In conjunction with the aforementioned, trainees must know the indications, limitations, and interpretation of appropriate subsequent confirmatory tests (ankle-brachial index, pulse volume recording, toe-brachial index, transcutaneous oximetry, duplex ultrasound, computed tomography angiography, magnetic resonance angiography, peripheral angiography, and invasive hemodynamic assessment).48,49 These data will be used to plan all vascular procedures.
Trainees need to be proficient in the medical and noninvasive management of patients with PAD, both symptomatic and asymptomatic48 and must be able to understand the indications for and timing of revascularization in the setting of claudication, CLTI, and ALI.48,50 The decision to select surgical and/or endovascular revascularization should be made on a unique patient level and based on the risk-benefit ratio and treatment durability.50 This requires knowledge of lower extremity anatomy and physiology, in addition to familiarity with societal documents that provide guidance for optimal surgical versus endovascular revascularization strategies based on lesion anatomy: aortoiliac, femoropopliteal, and infrapopliteal.48,50, 51, 52, 53, 54 Furthermore, the field of PVI is constantly evolving, with new patency-enhancing balloons and stents and adjunctive devices that include, but are not limited to, atherectomy, scoring balloons, lasers, and lithotripsy. Interventional fellows must be adept in assessing the benefit and safety of such advancements in conjunction with thoughtful analysis of the available supporting peer-reviewed literature. Last, effective communication strategies will be required to optimize revascularization outcomes by aligning high-quality care across multiple specialties (eg, vascular medicine, surgery, imaging, podiatry, wound management).
The goal of PVI should be to perform the correct procedure for each patient at the correct time. Trainees should be aware of periprocedural complications, which vary based on the clinical presentation. PVI-related complications include major adverse cardiovascular events: death, myocardial infarction, and stroke/transient ischemic attack; and major adverse limb events: emergency vascular surgery, amputation, vascular complications requiring treatment, or compartment syndrome. Other complications associated with PVI include vascular access site complications. Interventional fellows should be well-versed in best practices with regard to vascular access, management, and closure.55 This includes, but is not limited to, proficiency in adjunctive vessel access techniques (eg, ultrasound, fluoroscopy, and micropuncture needles) that mitigate common complications such as bleeding, pseudoaneurysm, arteriovenous fistula, and hematoma. Trainees must be able to both diagnose and manage these events in a timely manner.56
4.2.3.2. Chronic and Acute Limb-Threatening Ischemia
Although CLTI and ALI are unique manifestations of peripheral vascular disease, both require timely identification and treatment to mitigate amputation risk and reduce cardiovascular morbidity and mortality. CLTI is predominantly a manifestation of systemic atherosclerosis where patients present with ischemic rest pain or tissue loss. ALI, in contrast, occurs due to a variety of medical conditions as well as some iatrogenic sources. Trainees should understand the pathophysiology and varied presentations of these conditions to facilitate appropriate diagnostic testing, identify the underlying etiology, and initiate appropriate therapy.
Endovascular and surgical treatment are established and effective therapies for CLTI and ALI. Decisions regarding revascularization strategy are complex and often depend on the severity of ischemia present, anatomical characteristics, patient comorbidities, and resources available. Trainees should understand the indications for endovascular and surgical therapies to treat CLTI and ALI and select revascularization strategies tailored to individual patients. Many patients may require multiple procedures and modalities, often necessitating frequent longitudinal reassessment and collaboration with specialists from other disciplines.
Trainees should understand the complications associated with CLTI and ALI. Such events may be related to the natural history of the conditions themselves or result from the therapy provided. From a procedural standpoint, endovascular procedures may result in bleeding, vessel injury, embolization, kidney injury, or other cardiovascular events. Unique to ALI, revascularization may lead to compartment syndrome, rhabdomyolysis, and systemic inflammatory response syndrome. Prompt recognition and treatment of these complications has a favorable impact on patient outcomes.
4.2.3.3. Renal and Mesenteric Artery Disease
Trainees should be knowledgeable in the epidemiology, pathophysiology, and clinical manifestations of various common renal and mesenteric artery diseases. These include renovascular hypertension related to both atherosclerotic and nonatherosclerotic renal artery stenosis (including fibromuscular dysplasia) and chronic and acute mesenteric ischemia. Trainees should also be familiar with the noninvasive tests required to diagnose these conditions and indications for interventional or surgical treatment. Familiarity with the evidence base on the interventional management of renal artery stenosis, including the use of invasive hemodynamic assessment, is also recommended.50,57
Depending on the expertise and opportunities available during the 1-year interventional cardiology training period, it may not be possible for all trainees to acquire skills in selective angiography or percutaneous treatment of renal or mesenteric disease; however, the skills to obtain a nonselective abdominal aortic angiogram are recommended. Trainees who wish to pursue renal and mesenteric artery interventions as part of their career focus should obtain advanced procedural skills through additional formal or informal training. The trainee should be knowledgeable in the use of digital subtraction imaging equipment for this purpose.
4.2.3.4. Subclavian, Vertebral, and Carotid Artery Disease; Acute Stroke
Subclavian, vertebral, and carotid artery disease are common in individuals with atherosclerosis. Nonatherosclerotic conditions like fibromuscular dysplasia and some forms of vasculitis also frequently target these vascular distributions. Whereas symptoms of arm claudication, transient ischemic attack, and stroke are commonly recognized, disease in these locations may manifest as unique clinical entities such as steal syndromes. Trainees should understand the pathophysiology of diseases affecting these territories and recognize their unique clinical presentations.58
The role of acute intervention in ischemic stroke has emerged as preferred strategy for these patients. Although most patients in the United States have access to prompt primary PCI for acute myocardial infarction, less than half have similar access to primary neurovascular intervention for acute stroke. In some centers, interventional cardiologists, after appropriate training and with appropriate mentorship and oversight, may be able to contribute to teams performing acute neurovascular interventions for ischemic stroke. Additional training is required.
Medical therapy to mitigate cardiovascular risk is first-line treatment for individuals with subclavian, vertebral, and carotid artery disease. Revascularization is also indicated for many patients, and the decision to treat with surgical or endovascular techniques is often influenced by symptom status, anatomical characteristics, patient comorbidities, and other variables. Trainees must know the indications for surgical and endovascular treatment in these territories and understand the factors that influence the selection of the revascularization modality for individual patients. Moreover, trainees should understand the complications associated with medical and invasive treatment of these conditions.
4.2.3.5. Venous Disease
Interventional cardiology fellows should be well-acquainted with the epidemiology, risk factors, pathophysiology, associated morbidity and mortality, and preventive measure of venous thromboembolism. Trainees must be adept in recognizing the clinical presentation across the entire spectrum of venous thromboembolism. Timely identification of acute venous thrombotic disease is often crucial in maximizing the benefit of therapy and minimizing the long-term sequelae; therefore, trainees must have the required skill set to use biomarkers, imaging, history, and physical examination for diagnosis and subsequent treatment. Fellows should be well-versed in the nuances of inherited and acquired thrombophilias, including common settings in which they are likely to occur, such as in pregnancy or malignancy. Furthermore, trainees must be familiar with all classes of anticoagulation therapies, including dosing, contraindications, and reversal agents used as first-line treatment of thrombosis in the venous system.59
It is crucial for interventional cardiology fellows to recognize the indications and timing for advanced therapies in regard to invasive management for acute venous disease. Catheter-directed thrombolysis, mechanical or surgical thrombectomy, vena cava filters, and extracorporeal membrane oxygenation should be selected on an individual patient basis among those presenting with venous thromboembolism. To best accomplish this, trainees must understand the indications, benefits, and complications associated with advanced therapies, in particular bleeding and access site complications. In particular, trainees must become proficient in directing/participating in pulmonary embolism response teams that aid in promptly mobilizing multidisciplinary teams to guide care in the setting of acute pulmonary embolism. To accomplish this effectively, interventional fellows will need to be facile in identifying patients with acute pulmonary embolism who are at heightened risk of decompensation and short-term mortality and stand to benefit from advanced therapies.60,61
With respect to deep and superficial venous disease, trainees must be familiar with the physiology and pathophysiology of the venous circulation. In particular, the etiology of venous hypertension and sequelae should be studied in the ambulatory setting, vascular laboratory, and in the catheterization laboratory. Management of venous occlusive disease, compression, and reflux should be part of the curriculum.
4.2.3.6. Aortic and Peripheral Artery Aneurysm Disease
Trainees should be knowledgeable in the epidemiology, pathophysiology, and clinical manifestations of common thoracic and abdominal aortic aneurysmal disease. They should also understand the indications for repair, including the advantages and disadvantages of endovascular versus open surgical repair. Trainees should also be familiar with the epidemiology and clinical manifestations of nonaortic peripheral artery aneurysmal disease (eg, iliac, popliteal, and visceral artery aneurysms) and indications/options for treatment. Familiarity with noninvasive diagnostic testing for these diseases is also recommended. The ACC and other societal guidelines are good sources of information.62, 63, 64, 65
Trainees in a traditional 1-year training program will likely not be able to acquire the case numbers and procedural skills to perform endovascular repair of aneurysmal disease, and additional training will likely be required through non–ACGME-accredited advanced fellowships or postfellowship training through courses, proctoring, or direct mentorship. The presence of aortoiliac aneurysmal disease may pose significant challenges for the interventional management of CAD, and all interventional cardiology trainees should be skilled in addressing these challenges, including the choice of vascular access and guide-catheter support issues, as well as the potential risk of embolization from catheter manipulation.
4.2.3.7. Nonatherosclerotic Vascular Disease
Nonatherosclerotic PAD is much less common than atherosclerotic PAD. The lower extremity ischemia is often manifest by exertional lower extremity discomfort consistent with typical or atypical intermittent claudication, and there may be physical signs suggestive of arterial ischemia. Nonatherosclerotic PAD should be suspected in patients with signs or symptoms of lower extremity ischemia who: 1) are without risk factors for atherosclerosis (eg, younger age, tobacco use, diabetes mellitus, hypercholesterolemia, hypertension); 2) do not have other clinical manifestations of atherosclerosis (prior heart attack, prior stroke); or 3) lack the typical characteristics on physical examination, lesion distribution, or vascular imaging for atherosclerosis (calcification).
The etiology of nonatherosclerotic PAD includes extrinsic vascular compression, as referenced in Section 3.2.5. The trainee should also be aware of congenital anatomical variants of the external iliac artery. The treatment of these conditions is optimized by a multidisciplinary vascular team. When revascularization is deemed necessary, surgical therapies are frequently preferred with the exception of fibromuscular dysplasia, which is often amenable to balloon angioplasty.
4.2.4. Structural Heart Interventions
4.2.4.1. Aortic Valve Interventions
The trainee will acquire the knowledge and skills to manage aortic valve disease as part of a multidisciplinary team approach, including geriatric and palliative care consultations when appropriate. These include a thorough understanding of the indications for intervention and the relative benefits and risks of transcatheter techniques, surgical interventions, and medical management. The trainee will develop a working understanding of echocardiographic measurements as well as proficiency in invasive measurements of hemodynamics and understand the physiology and workup of other presentations of aortic disease, such as low-flow and low-gradient aortic stenosis, and the appropriate steps to confirm severe aortic stenosis. Central to invasive management of aortic stenosis is the ability to interpret CCT scans with specific emphasis on the appropriate choice of valve size, characteristics that may represent special challenges to implantation, and assessment of vascular features conducive to transfemoral versus alternative access sites. The trainee will obtain proficiency in the performance of balloon aortic valvuloplasty and transcatheter valve implantation in both native and prior surgical prostheses, including both balloon-expandable and self-expanding valves. The trainee must also have proficiency in the evaluation of satisfactory valve positioning, with emphasis on diagnosing paravalvular leak and high residual gradient as well as steps to address these issues. The trainee will also be proficient in diagnosing and treating immediate complications of valve implantation, including heart block, stroke, coronary obstruction, valve embolization, pericardial effusion, and disruption of annular or subannular structures. The trainee will acquire knowledge of patient management after their index hospitalization, including teamwork and communication, appropriate anticoagulation strategies, assessment for prosthetic valve dysfunction (valve thrombosis, degeneration, or infection), infectious endocarditis prophylaxis, and knowledge of techniques for coronary catheterization in the setting of a prior transcatheter valve.
4.2.4.2. Mitral Valve Interventions
The etiology of mitral valve disease, its clinical presentation, diagnosis, and progression, should be appreciated, in addition to knowledge of appropriate management strategies, including medical therapy and percutaneous or surgical intervention. Currently approved interventions for mitral valve disease include balloon valvuloplasty for mitral stenosis, TEER for mitral regurgitation, and transcatheter valve-in-valve and transcatheter valve-in-ring replacement for prosthetic structural valve dysfunction. Each of these diseases has a different pathophysiology and clinical presentation that must be understood, interpreted, and applied to the patient’s clinical presentation, diagnostic workup, and management.66
Patients with mitral stenosis may or may not have a history of rheumatic fever, but the classic pathology and echocardiographic morphology must be appreciated. After initial medical management, the criteria for timing of intervention may include pulmonary hypertension in addition to symptoms of dyspnea. More importantly, physicians should know the impact of valve morphology, various scoring systems, and their corresponding hemodynamic and echocardiographic features that influence the short- and long-term outcomes of the valvuloplasty or valve replacement and help in informed decision-making.
For mitral regurgitation, physicians must know the insidious nature of this disease in many patients who may be asymptomatic to provide medical management and decide on the appropriate timing for percutaneous or surgical treatment. It is also essential to understand the differences in the pathophysiology of primary and secondary mitral regurgitation and the role of noninvasive imaging in monitoring asymptomatic patients. The indications for TEER in primary mitral regurgitation (in severely symptomatic patients with high or prohibitive surgical risk) and in secondary mitral regurgitation (based on the inclusion and exclusion criteria of the COAPT [Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation] trial) are key.66 Understanding the rapid evolution of the field, newer options for goal-directed medical therapy, as well as newer devices for percutaneous interventions is important in making an informed decision. It also emphasizes the role of a multidisciplinary team in the management of these patients. Finally, the advent of recent-generation TEER devices has expanded the initial morphologic considerations for successful TEER, but the importance of experienced operators in patients who are not ideal for TEER should be considered.35
The most recent transcatheter mitral valve intervention involves transseptal placement of a balloon-expandable TAVR prosthesis in a deteriorating surgical mitral bioprosthesis or surgical mitral ring in patients who are at high risk for repeat surgery. Understanding the nuances of each surgical prosthesis and the infrequent performance of this procedure should limit it to highly experienced structural heart centers that are equipped to provide and interpret advanced imaging assessments preprocedure (CT angiography and transesophageal echocardiography) and capable of dealing with unique complications, including acute left ventricular outflow tract obstruction, valve embolization, paravalvular regurgitation, and hemolysis.
4.2.4.3. Pulmonic Valve Interventions
Dysfunction of the right ventricular outflow tract is almost always related to a congenital etiology. There are rare circumstances where patients without CHD develop pulmonary valve obstruction (eg, due to infective endocarditis) or pulmonary valve regurgitation (eg, due to severe pulmonary hypertension), but most pulmonary valve dysfunction cases are due to native or palliated/repaired CHD. Congenital obstruction of the right ventricular outflow tract can occur at multiple levels: below, at, or above the level of the pulmonary valve. Subpulmonic stenosis is typically treated surgically, whereas isolated valvular stenosis may be amenable to balloon dilation. Postoperative situations where patients may have conduit or prosthetic pulmonary valve stenosis or regurgitation are typically amenable to transcatheter interventions, including transcatheter pulmonary valve replacement. Main and branch pulmonary artery stenoses can also benefit from transcatheter interventions.
The training received during a 1-year interventional cardiology fellowship would not be expected to appropriately cover the variety of transcatheter pulmonary valve interventions in a heterogeneous group of adult patients with CHD. Therefore, it is not expected that the trainee will have sufficient cognitive knowledge regarding the complex variety of potential right ventricular outflow tract lesions, nor sufficient procedural training to perform these complex ACHD interventions without further dedicated ACHD training.
4.2.4.4. Tricuspid Valve Interventions
Causes of tricuspid valve disease include rheumatic disease, infective endocarditis, CHD, tricuspid regurgitation due to left-sided pathology, atrial fibrillation, and iatrogenic tricuspid valve injury (biopsy and pacemaker lead). The tricuspid valve has long been considered the “forgotten valve” due to the insidious clinical nature and nonspecific symptoms of tricuspid regurgitation and infrequent surgical treatment. The latter is due, in part, to the risk of surgery related to postoperative right ventricular and hepatic failure. Nonetheless, severe tricuspid regurgitation is associated with reduced survival. Interventional cardiologists should be familiar with the clinical presentation, diagnosis (echocardiographic and hemodynamic), grading, and dynamic nature of this disease.
There are no targeted treatments for tricuspid regurgitation, but medical therapies like diuretic agents and afterload reduction with pulmonary vasodilators can be useful in providing symptomatic relief.66 Recently, a number of less-invasive investigational therapies have been developed and are undergoing clinical trial evaluation, including TEER, annuloplasty, and valve replacement. Although some of these approaches may provide patients with an alternative option to surgery, clinicians at the current time must be more attuned to the medical and surgical management of patients with tricuspid valve disease. In this regard, trainees should understand the appropriate timing and indications for surgical intervention in patients with both asymptomatic and symptomatic disease and appreciate the influence of right ventricular failure and tricuspid annular dilatation on this decision. Finally, the indications for concomitant tricuspid intervention with left-sided valve surgery should be considered in patients with annular dilation or right ventricular dysfunction.
4.2.4.5. Nonvalvular Structural Heart Interventions
The field of SHD continues to expand and encompasses a broad range of disease conditions with transcatheter therapeutic options. Comprehensive knowledge of the indications and key aspects of patient selection for SHD interventions is essential for Level III interventional cardiology training. Further training in SHD is required to provide a more nuanced opinion on harms, benefits, and potential adverse outcomes to guide case selection and shared decision-making with patients, families, and multidisciplinary team members, as well as to perform the procedures and guide preprocedure and postprocedure management.
Interventional cardiology trainees must understand the indications and clinical trial data to support patent foramen ovale closure for stroke prevention, as well as the extent and limitations of data on closure for clinical presentations such as migraine, hypoxia, or those engaged in deep-sea diving. Trainees must also be able to differentiate the workup of a patient with atrial septal defect from that of a patent foramen ovale to include additional imaging for CHD lesions. Knowledge of the limitations of current devices in closure of atrial septal defects and potential harms of closure, in addition to surgical closure options, is necessary. Exposure to the basic views and uses of intracardiac echocardiography in the closure of intra-atrial septal defects is encouraged.
Further knowledge of interventional options for stroke prevention must include the indications for left atrial appendage closure in patients with atrial fibrillation at elevated risk of stroke and those intolerant of anticoagulation. This includes up-to-date knowledge of the available devices and current FDA indications for device closure, understanding of the limitations of data on surgical closure and best practices in this area, as well as facility with postprocedure management, including antiplatelet and/or anticoagulant therapy.
Advanced training in interventional cardiology includes a focus on complex hemodynamic assessment in the catheterization laboratory. Given that the interventional cardiologist will help lead the evaluation of unexplained dyspnea with complex hemodynamic studies in the catheterization laboratory, the ability to generate a broad differential diagnosis of conditions that can be difficult to assess with echocardiography is essential. This includes knowledge of prosthetic valve dysfunction, left ventricular outflow obstruction, and pulmonary vein occlusion and their potential remedies. Trainees must understand the indications and patient selection for paravalvular leak closure in the setting of prosthetic valvular heart disease as well as for alcohol septal ablation in the context of hypertrophic obstructive cardiomyopathy. Finally, trainees will gain an understanding of clinical scenarios leading to a need for rarer interventions, including pulmonary vein interventions.
4.2.4.6. Select Adult Congenital Heart Interventions
CHD is the most common congenital anomaly and occurs in ∼0.8% of all live births.67 Medical and surgical advancements over the past century have resulted in markedly improved survival, and most patients with CHD are now surviving to adulthood.67 Along with the rise in the number of adult patients with CHD, there has been an increase in the volume and variety of transcatheter diagnostic and interventional procedures applicable to this population. The rapid rise in the number and complexity of transcatheter procedures has prompted the publication of recommendations from stakeholder organizations regarding the delivery of transcatheter procedures, including recommendations within the ACC/AHA ACHD guidelines explicitly stating that interventional procedures should be performed at regional ACHD centers by qualified specialists with training and experience in ACHD interventional care and in laboratories with appropriate staffing and experience in ACHD treatment.68
In recognition of the need for specific recommendations regarding interventional training, expert consensus statements from SCAI were published in 2010 and updated in 2020.44,69,70 The authors of the 2010 document recognized that fellowship training in structural and congenital interventions is but “a foundation for a lifetime of learning and maturation, and very few trainees will master more than either the basics of a very select number of complex procedures during a 1- or even 2-year program.”69 The SCAI position statement from 2020 gives recommendations for procedural numbers and strongly encourages ongoing mentorship and continued learning thereafter. This consensus document also highlighted the importance of integrated multidisciplinary care in partnership with established centers of excellence in ACHD.
It is important to recognize that ACHD represents a heterogeneous group of disorders, both operated and unoperated, that can range immensely in degree of complexity and clinical presentation. ACHD training received during the adult interventional cardiovascular fellowship is not comprehensive or focused enough to suffice as an alternative to more focused training specific to CHD. Therefore, it is highly unlikely that trainees completing a 1-year interventional cardiovascular fellowship will have the cognitive knowledge or sufficient procedural training to perform ACHD interventions. However, trainees should receive appropriate training and be capable of performing diagnostic procedures during the general cardiovascular disease and interventional cardiology fellowships. This should include knowledge regarding the common unoperated types of ACHD, the clinical manifestations thereof, the indications for operation and intervention, and calculation of shunt fractions and arterial resistances.
5. Leadership and Administrative Skills
In addition to clinical competency, interventional cardiology trainees are expected to function effectively as leaders in allied efforts to ensure high-quality care and promote individual and population health. Some of these activities and attributes fall outside the realm of clinical knowledge and skill and instead involve administrative roles in clinical practice, hospitals, health systems, professional societies, or other organizations. Examples of how advanced interventional cardiology fellows can gain experience in laboratory administration include developing familiarity with accreditation standards for cardiac catheterization laboratories and participation in an accreditation process or internal and external quality improvement programs such as the National Cardiovascular Data Registry, Vascular Quality Initiative, or Transcatheter Valve Therapy Registry. The intention of training is to provide a foundation of leadership and administrative skills that are enhanced and refined throughout one’s career after fellowship. Specific competencies expected of all general cardiologists and cardiovascular specialists, including those whose careers require greater involvement in administrative, managerial, or advocacy positions, are delineated in Table 12 of the “2016 ACC Lifelong Learning Competencies for General Cardiologists.”4
6. Evaluation of Proficiency
Evaluation of interventional cardiology trainee proficiency involves multiple assessments of the trainee’s ability to clinically diagnose and manage patients across the broad spectrum of diseases, including patients with acute and chronic coronary disease, PAD, and valvular and structural heart disease.
Trainees must be evaluated regularly with respect to clinical judgment, case management, and procedural skills, including the integration of clinical findings and the results of noninvasive testing and invasive coronary or peripheral angiography to make an accurate diagnosis and formulate a comprehensive management plan. Trainees should be directly assessed on their procedural technique, including their competency in determining the most appropriate approach to revascularization, selecting the proper equipment, gaining arterial (or venous) access, manipulating catheters, and delivering wires and devices. Trainees should also be assessed on their ability to recognize and manage complications. Trainees should be evaluated on their interactive behavior with the patient and family members as well as their commitment to working in a multidisciplinary environment with other members of the health care team.
Assessment of trainees should include multisource evaluations, direct observation by instructors, case logs, chart reviews (including adherence to guideline recommendations, Appropriate Use Criteria and patient outcomes), simulation training, the trainee’s portfolio (including scholarly productivity and quality improvement projects), and assessment of leadership skills. Online assessment methods are available through the ACGME with guidance on a practical approach to the assessment of trainees in all 6 core competencies.71, 72, 73 The trainee’s organization of, and participation in, didactic conferences and case presentations also provides opportunities to evaluate the trainee’s proficiency. Self-assessment programs are available. Program directors and trainees are encouraged to incorporate these resources into the course of training.
Trainees must maintain records of participation and advancement using an electronic database or procedure case logs that are Health Insurance Portability and Accountability Act (HIPAA)–compliant and contain pertinent clinical information, including number of cases, clinical diagnoses, disease severity, procedures performed, outcomes, and disposition.
The interventional cardiology training program director is responsible for providing faculty development on use of assessment methods, effective feedback, coaching, and confirming trainee experience and competence. Online resources for assessing clinical competence and providing feedback are available and can be adapted to the needs of a particular program.73,74 The program director will collaborate with a formal Clinical Competency Committee, working to verify and document trainee performance. Reviewing the overall progress of trainees is important to ensure achievement of training milestones3 and identification of areas in which additional focused training may be required. On a periodic basis, the program director should review each trainee’s case logs to ensure adequate exposure to a broad spectrum of pathology and trainee experience in acquiring and interpreting diagnostic and interventional cardiac and peripheral vascular procedures. Program directors should provide feedback to fellows on transitioning to independent practice. In addition, the program director should provide feedback to faculty on their teaching skills, assessment methods, and evaluations.
Following the completion of advanced training in interventional cardiology, trainees are eligible to take the certification examination in interventional cardiology offered by the ABIM or another nationally recognized certification body. Information concerning eligibility and prerequisites for this certification can be obtained online.75,76 An important component of the certification process requires the interventional cardiology program director to certify trainee competency in procedural skills as a prerequisite for sitting for the board certification exam. After initial certification, it is the responsibility of the practicing cardiologist to ensure continued maintenance of certification and competence throughout their careers.
Presidents and Staff
American College of Cardiology
Edward T.A. Fry, MD, FACC, President
Cathleen C. Gates, Chief Executive Officer
Janice Sibley, MS, MA, Executive Vice President, Education and Publishing
Teresa V. Callahan, Document Production Specialist
Grace D. Ronan, Team Leader, Clinical Policy Publications
American Heart Association
Michelle A. Albert, MD, MPH, FAHA, President
Nancy Brown, Chief Executive Officer
Mariell Jessup, MD, FAHA, Chief Science and Medical Officer
Radhika Rajgopal Singh, PhD, Senior Vice President, Office of Science and Medicine
Paul St. Laurent, DNP, RN, National Senior Director, Office of Science and Medicine
Jody Hundley, Senior Production and Operations Manager, Scientific Publications, Office of Science Operations
Society for Cardiovascular Angiography and Interventions
Sunil V. Rao, MD, FSCAI, President
Robert C. Bartel, CAE, FACEHP, Vice President, Education, Publications and Quality
Emily Senerth, Assistant Director, Standards, Guidelines and Quality
Peer review statement
Given his role as associate editor, Sahil A. Parikh had no involvement in the peer review of this article and has no access to information regarding its peer review.
Developed in collaboration with and endorsed by the American Association for Thoracic Surgery, American Society of Echocardiography, Heart Failure Society of America, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, Society of Interventional Radiology, Society of Thoracic Surgeons, Society for Vascular Medicine, and the Society for Vascular Surgery
The document was approved by the American College of Cardiology Lifelong Learning Oversight Committee, the American Heart Association Science Advisory and Coordinating Committee, and the Society for Cardiovascular Angiography and Interventions Executive Committee in November 2022 and the American Heart Association Executive Committee in December 2022.
The Society for Cardiovascular Angiography and Interventions requests that this document be cited as follows: Bass TA, Abbott JD, Mahmud E, Parikh SA, Aboulhosn J, Ashwath ML, Baranowski B, Bergersen L, Chaudry HI, Coylewright M, Denktas AE, Gupta K, Gutierrez JA, Haft J, Hawkins BM, Herrmann HC, Kapur NK, Kilic S, Lesser J, Lin CH, Mendirichaga R, Nkomo VT, Park LG, Phoubandith DR, Quader N, Rich MW, Rosenfield K, Sabri SS, Shames ML, Shernan SK, Skelding KA, Tamis-Holland J, Thourani VH, Tremmel JA, Uretsky S, Wageman J, Welt F, Whisenant BK, White CJ, Yong CM. 2023 ACC/AHA/SCAI advanced training statement on interventional cardiology (coronary, peripheral vascular, and structural heart interventions): a report of the ACC Competency Management Committee. J Soc Cardiovasc Angiogr Interv. 2023;2:100575.
This article has been copublished in the Journal of the American College of Cardiology and Circulation: Cardiovascular Interventions.
Copies: This document is available on the websites of the American College of Cardiology (www.acc.org), American Heart Association (www.professional.heart.org), and Society for Cardiovascular Angiography and Interventions (www.scai.org). For copies of this document, please contact Elsevier Inc. Reprint Department via fax (212-633-3820) or e-mail (reprints@elsevier.com).
Permissions: Multiple copies, modification, alteration, enhancement, and/or distribution of this document are not permitted without the express permission of the American College of Cardiology. Requests may be completed online via the Elsevier site (http://www.elsevier.com/about/policies/author-agreement/obtaining-permission).
Former Competency Management Committee chair; chair during this writing effort.
Former Competency Management Committee member; member during this writing effort.
Appendix
Appendix 1.
Author Relationships With Industry and Other Entities (Relevant)—2023 ACC/AHA/SCAI Advanced Training Statement on Interventional Cardiology (Coronary, Peripheral Vascular, and Structural Heart Interventions)
| Committee Member | Employment | Consultant | Speakers Bureau | Ownership/Partnership/Principal | Personal Research |
Institutional/Organizational or Other Financial Benefit | Expert Witness |
|---|---|---|---|---|---|---|---|
| Theodore A. Bass (Chair) | University of Florida, Division of Cardiology, UF Health Jacksonville—Professor of Medicine | None | None | None | None | None | None |
| J. Dawn Abbott (IC Vice Chair) | Lifespan Cardiovascular Institute—Director, Interventional Cardiology and Structural Fellowship Program; The Warren Alpert Medical School of Brown University—Associate Professor of Medicine, Division of Cardiology |
|
None | None | None | None | |
| Ehtisham Mahmud (SHD Vice Chair) | University of California, San Diego—Professor and Division Chief of Cardiovascular Medicine; Edith and William Perlman Chair in Cardiology UCSD Cardiovascular Institute—Executive Director | None | None | None | None | ||
| Sahil A. Parikh (PVI Vice Chair) | Columbia University Irving Medical Center—Director of Endovascular Services; Associate Professor of Medicine |
|
None | None | None | None | |
| Jamil Aboulhosn | David Geffen School of Medicine at the University of California—Professor of Medicine and Pediatrics |
|
None | None |
|
None | None |
| Mahi L. Ashwath | University of Iowa Health Care—Director, Cardiac MRI; University of Iowa Hospitals and Clinics—Clinical Professor of Medicine and Radiology | None | None | None | None | None | None |
| Bryan Baranowski | Cleveland Clinic—Cardiac Electrophysiologist | None | None | None | None | None | None |
| Lisa Bergersen | Harvard Medical School, Heart Center, Boston Children’s Hospital—Associate Professor of Pediatrics | None | None | None | None | None | None |
| Hannah I. Chaudry | Dartmouth Hitchcock Medical Center—Interventional Cardiologist; Geisel School of Medicine at Dartmouth—Assistant Professor of Medicine‡ |
None | None | None | None | None | None |
| Megan Coylewright | The Erlanger Heart and Lung Institute—Director, Structural Heart Program, Vice Chief, Cardiology; University of Tennessee—Associate Professor |
|
None | None | None | None | |
| Ali E. Denktas | Michael E. Debakey VA Medical Center—Director, Cardiac Catheterization Laboratory; Baylor College of Medicine—Professor of Medicine | None | None | None | None | None | None |
| Kamal Gupta | University of Kansas Medical Center—Program Director, Interventional Cardiology Fellowship; Director, Vascular Medicine Department of Cardiovascular Medicine; University of Kansas School of Medicine—Professor of Medicine and Vice Chair (Academics) |
None | None | None | None | None | |
| J. Antonio Gutierrez | Duke Cardiology South Durham—Cardiologist; Duke University School of Medicine—Assistant Professor of Medicine |
|
None | None | None | None | None |
| Jonathan Haft | University of Michigan—Associate Section Head of Clinical Quality; ECMO Medical Director, Department of Cardiac Surgery Robert H. Bartlett, MD, Collegiate Professor | None | None | None | None | None | None |
| Beau M. Hawkins | University of Oklahoma Health Sciences Center—Program Director, Cardiovascular Disease Fellowship; Associate Professor of Medicine | None | None | None | None | None | None |
| Howard C. Herrmann | Hospital of the University of Pennsylvania—Health System Director for Interventional Cardiology, Director, Cardiac Catheterization Labs; Perelman School of Medicine at the University of Pennsylvania—John W. Bryfogle Professor of Cardiovascular Medicine and Surgery | None |
|
|
None | ||
| Navin K. Kapur | Tufts Medical Center—Executive Director, The Cardiovascular Center for Research and Innovation; Director, Acute Circulatory Support Program; Director, Interventional Research Laboratories; Investigator, Molecular Cardiology Research Institute; Associate Professor, Department of Medicine/Division of Cardiology |
|
|
|
|
|
None |
| Sena Kilic | Oregon Health and Science University Medical Center—Interventional Cardiologist | None | None | None | None | None | None |
| John Lesser | Minneapolis Heart Institute—Director of Advanced Imaging | None | None | None | None | None | None |
| C. Huie Lin | Methodist Debakey Heart Center—Associate Program Director, Fellowship in Cardiovascular Disease; Director, Adult Congenital Heart Disease Program Assistant Professor |
|
None | None | None |
|
None |
| Rodrigo Mendirichaga | Wentworth-Douglass Hospital—Interventional Cardiologist§ | None | None | None | None | None | None |
| Vuyisile T. Nkomo | Mayo Clinic—Director, Valvular Heart Disease Clinic; Vice Chair, Clinical Practice, Division of Structural Heart Disease, Department of Cardiovascular Medicine; Professor of Medicine |
None | None | None | None | None | None |
| Linda G. Park | UCSF School of Nursing Department of Community Health Systems—Associate Professor; San Francisco VA Medical Center—Research Health Science Specialist; John Muir Medical Center—Nurse Practitioner | None | None | None | None | None | None |
| Dawn R. Phoubandith|| | American College of Cardiology—Director, Competency Management | None | None | None | None | ACC salaried employee | None |
| Nishath Quader | Washington University, St. Louis—Director Cardiovascular Imaging Fellowship; Director, Interventional Echocardiography; Associate Professor of Medicine | None | None | None | None | None | None |
| Michael W. Rich | Washington University School of Medicine, St. Louis—Professor of Medicine; Associate Program Director for Research |
None | None | None | None | None | None |
| Kenneth Rosenfield | Massachusetts General Hospital—Section Head, Vascular Medicine and Interventions, Division of Cardiology | None | None | None | None | ||
| Saher S. Sabri | MedStar Georgetown University Hospital—Chief of Interventional Radiology; Professor of Radiology |
|
None | None |
|
None | None |
| Murray L. Shames | University of South Florida Health Morsani School of Medicine—Professor and Chief, Division of Vascular Surgery; Vice-Chair of Clinical Operations, Department of Surgery; Tampa General Hospital Heart and Vascular Institute—Co-Director; Tampa General Hospital Aortic Center—Director |
|
|
None |
|
None | None |
| Stanton K. Shernan | Brigham and Women’s Hospital—Anesthesiologist, Department of Anesthesiology, Perioperative, and Pain Medicine; Harvard Medical School—Professor of Anesthesia | None | None | None | None |
|
None |
| Kimberly A. Skelding | Confluence Health—Interventional Cardiologist; University of Washington—Interventional Cardiologist |
|
None | None | None | None | None |
| Jacqueline Tamis-Holland | Mount Sinai Morningside-BronxCare—Program Director, Cardiovascular Disease Training Program; Mount Sinai Morningside—Associate Director, Cardiac Catheterization Laboratory; Icahn School of Medicine at Mount Sinai—Professor of Medicine | None | None |
|
None | None | None |
| Vinod H. Thourani | Piedmont Heart Institute—Marcus Chief of Cardiovascular Surgery; Structural Heart Center of Excellence and Marcus Heart Valve Center—Director |
|
None | None | None |
|
None |
| Jennifer A. Tremmel | Stanford University Medical Center—Susan P. and Riley P. Bechtel Endowed Medical Director, Women’s Heart Health Program; Associate Professor of Cardiovascular Medicine |
|
None | None | None | None | None |
| Seth Uretsky | Atlantic Health System—Medical Director, Cardiovascular Imaging; Associate Director, Cardiovascular Fellowship Program; Sidney Kimmel Medical College Thomas Jefferson University—Associate Professor of Medicine |
None | None | None | None | None | None |
| Jessica Wageman | Park Nicollet Heart and Vascular Center—Coordinator of Structural Heart Programs | None | None | None | None | None | None |
| Frederick Welt | University of Utah Health Sciences Center—Margaret Amundsen Professor of Cardiology; Vice Chair, Clinical Affairs, Department of Medicine; Associate Chief, Division of Cardiovascular Medicine; Director, Cardiac Catheterization Laboratory |
|
None | None | None | None | None |
| Brian K. Whisenant | Intermountain Medical Center Heart Institute—Medical Director, Heart Valve and Structural Heart Disease | None |
|
None | None | None | |
| Christopher J. White | Ochsner Health—System Chair for Cardiology; Medical Director of Value Based Care; The Ochsner Clinical School at the University Queensland, Australia—Professor and Chair of Medicine | None | None | None | None | None | None |
| Celina M. Yong | Palo Alto VA Healthcare System—Director of Interventional Cardiology; Stanford University School of Medicine—Assistant Professor of Medicine | None | None | None | None | None | None |
This table represents relationships of committee members with industry and other entities that were determined to be relevant to this document. These relationships were reviewed and updated in conjunction with all meetings and/or conference calls of the writing committee during the document development process. The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of ≥5% of the voting stock or share of the business entity, ownership of ≥$5,000 of the fair market value of the business entity, or if funds received by the person from the business entity exceed 5% of the person’s gross income for the previous year. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted. Please refer to http://www.acc.org/guidelines/about-guidelines-and-clinical-documents/relationships-with-industry-policy for definitions of disclosure categories or additional information about the ACC Disclosure Policy for Writing Committees.
According to the ACC, a person has a relevant relationship if: a) the relationship or interest relates to the same or similar subject matter, intellectual property or asset, topic, or issue addressed in the document; b) the company/entity (with whom the relationship exists) makes a drug, drug class, or device addressed in the document, or makes a competing drug or device addressed in the document; or c) the person or a member of the person’s household has a reasonable potential for financial, professional, or other personal gain or loss as a result of the issues/content addressed in the document.
ACC = American College of Cardiology; AHA = American Heart Association; DSMB = data and safety monitoring board; ECMO = extracorporeal membrane oxygenation; IC = interventional cardiology; MRI = magnetic resonance imaging; PVI = peripheral vascular interventions; RWI = Relationships with Industry and Other Entities; SCA = Society of Cardiovascular Anesthesiologists; SCAI = Society for Cardiovascular Angiography and Interventions; SHD = structural heart disease; UCSD = University of California, San Diego; UCSF = University of California, San Francisco; UF = University of Florida; VA = Veterans Affairs.
No financial benefit.
Significant relationship.
Dr. Chaudry was employed by Brown University and Lifespan Rhode Island Hospitals and Health Services as an Interventional Cardiology Fellow in Training during most of this writing effort.
Dr. Mendirichaga was employed by the University of California, San Diego as an Interventional Cardiology Fellow in Training, Division of Cardiovascular Medicine, during most of this writing effort.
Ms. Phoubandith is an ACC staff member and served as the document advisor for the 2023 ACC/AHA/SCAI Advanced Training Statement on Interventional Cardiology (Coronary, Peripheral Vascular, and Structural Heart Interventions). No relevant relationships to report. Nonvoting author and not included/counted in the RWI balance for this committee.
Dr. Skelding reported this new disclosure to the writing committee at the time of writing committee sign-off on the document for publication and recused herself from voting on the final document.
Appendix 2.
Peer Reviewer Information—2023 ACC/AHA/Scai Advanced Training Statement on Interventional Cardiology (Coronary, Peripheral Vascular, and Structural Heart Interventions)
| Name | Employment | Representation in Peer Review Process |
|---|---|---|
| Tayo Addo | UT Southwestern—Associate Professor, Medical Director, Internal Medicine at Parkland Program Director, Interventional Cardiology Fellowship Program |
Official Reviewer, AHA |
| Michael Foster | MUSC Columbia Downtown Hospital Heart and Vascular—Medical Director, Catheterization Laboratory and Cardiac Rehabilitation | Official Reviewer, ACC Board of Governors |
| Kwan Seung Lee | University of Arizona—Professor of Medicine, Interim Chief, Division of Cardiology; Banner University Medical Group—Cardiovascular Service Line Director | Official Reviewer, ACC Competency Management Committee Lead Reviewer |
| Sunil V. Rao | Duke University Health System—Professor of Medicine | Official Reviewer, SCAI |
| Toniya Singh | St. Louis Heart and Vascular—Managing Partner; Christian NorthEast Hospital—Chief of Medicine | Official Reviewer, AHA |
| Ada C. Stefanescu Schmidt | Massachusetts General Hospital—Structural and Adult Congenital Heart Disease Interventional Cardiologist; Harvard Medical School—Instructor in Medicine | Official Reviewer, ACC Lifelong Learning Oversight Committee |
| Molly Szerlip | Baylor Scott and White The Heart Hospital Plano—Associate Director, Education and Research, Program Director, Cardiology and Structural Heart Fellowship Program | Official Reviewer, SCAI |
| Anthony Aizer | New York University Langone Health—Cardiac Electrophysiologist; New York University Grossman School of Medicine—Associate Professor | Organizational Reviewer, Heart Rhythm Society |
| Lauren Baldassarre | Yale University School of Medicine—Associate Professor of Cardiovascular Medicine and Radiology and Biomedical Imaging | Organizational Reviewer, Society for Cardiovascular Magnetic Resonance |
| Ruma Bose | Beth Israel Deaconess Medical Center—Program Director Adult Cardiothoracic Anesthesiology | Organizational Reviewer, Society of Cardiovascular Anesthesiologists |
| Keith D. Calligaro | Pennsylvania Hospital—Chief, Vascular Surgery and Endovascular Therapy | Organizational Reviewer, Society for Vascular Surgery |
| Richard Cheng | University of California San Francisco—Health Sciences Assistant Clinical Professor | Organizational Reviewer, Heart Failure Society of America |
| Milind Desai | Cleveland Clinic—Haslam Family Endowed Chair in Cardiovascular Medicine, Professor of Medicine; Cleveland Clinic Lerner College of Medicine—Director, Clinical Operations; Director, HCM Center, Medical Director, Aorta Center Department of Cardiovascular Medicine, Heart and Vascular Institute | Organizational Reviewer, Society of Cardiovascular Computed Tomography |
| Christopher K. Dyke | National Jewish Health—Associate Professor of Medicine, Director of Cardiac Imaging | Organizational Reviewer, Society for Cardiovascular Magnetic Resonance |
| Tsuyoshi Kaneko | Brigham and Women's Hospital—Hybrid Cardiac Surgeon; Harvard Medical School—Assistant Professor of Surgery | Organizational Reviewer, Society of Thoracic Surgeons |
| Minaj S. Khaja | University of Virginia Health—Associate Professor of Radiology; Program Director, Independent Interventional Radiology Residency | Organizational Reviewer, Society of Interventional Radiology |
| Omar Khalique | Columbia University Medical Center/Saint Francis Hospital and Heart Center—Director, Multimodality Cardiac Imaging, Structural Heart and Valve Center | Organizational Reviewer, Society of Cardiovascular Computed Tomography |
| Stephen H. Little | Houston Methodist Hospital—Director, Cardiovascular Fellowship Program, Director of Structural Heart; Weill Cornell Medical College, Cornell University—John S. Dunn Chair in Clinical Cardiovascular Research and Education | Organizational Reviewer, American Society of Echocardiography |
| S. Chris Malaisrie | Northwestern—Cardiac Surgeon, Professor of Surgery | Organizational Reviewer, American Association for Thoracic Surgery |
| Charles B. Nyman | Brigham and Women's Hospital—Fellowship Program Director Adult Cardiothoracic Anesthesiology, Associate Director Interventional Cardiac Anesthesiology | Organizational Reviewer, Society of Cardiovascular Anesthesiologists |
| Vera H. Rigolin | Northwestern University Feinberg School of Medicine—Professor of Medicine | Organizational Reviewer, American Society of Echocardiography |
| Jean Marie Ruddy | Medical University of South Carolina—Associate Professor of Surgery | Organizational Reviewer, Society for Vascular Surgery |
| T. Gregory Walker | Massachusetts General Hospital—Assistant Professor of Radiology | Organizational Reviewer, Society of Interventional Radiology |
| Mitchell D. Weinberg | Staten Island University Hospital, Northwell Health—Cardiology Chair, Cardiology System Director Peripheral Intervention; Zucker School of Medicine at Hofstra—Associate Professor of Cardiology | Organizational Reviewer, Society for Vascular Medicine |
| Vishal Arora | Augusta University—Professor of Medicine: Cardiology; Augusta University Medical Center—Interventional Cardiologist | Content Reviewer, Interventional Cardiology Program Director |
| Jordan D. Awerbach | Phoenix Children's Hospital—Director, Adult Congenital Heart Disease Program; University of Arizona College of Medicine-Phoenix—Assistant Professor, Departments of Child Health and Internal Medicine | Content Reviewer, ACC Adult Congenital and Pediatric Cardiology Section Leadership Council |
| Duane Berkompas | Michigan State University College of Human Medicine at Spectrum Health Hospital—Program Director Interventional Cardiology Fellowship | Content Reviewer, Interventional Cardiology Program Director |
| Anna E. Bortnick | Montefiore Medical Center—Program Director, Interventional Cardiology Fellowship; Albert Einstein College of Medicine—Associate Professor | Content Reviewer, Interventional Cardiology Program Director and ACC Interventional Cardiology Section Leadership Council |
| John P. Breinholt | Phoenix Children's Hospital—Division Chief, Pediatric Cardiology | Content Reviewer, ACC Competency Management Committee |
| Ashok Chaudhary | Rutgers Robert Wood Johnson Medical School—Structural Heart Fellowship Program Director | Content Reviewer, Structural Heart Program Director |
| G. William Dec | Massachusetts General Hospital—Chief Emeritus, Cardiology Division and Roman W. DeSanctis Professor of Medicine | Content Reviewer, Former ACC Competency Management Committee Member |
| Douglas E. Drachman | Massachusetts General Hospital, Division of Cardiology—Heart Center Director of Education | Content Reviewer, Interventional Cardiology Program Director |
| Bailey Ann Estes | Hendrick Medical Center—Cardiac Catheterization Laboratory Nurse and Research Coordinator | Content Reviewer, ACC Cardiovascular Team Section Leadership Council |
| Kendra J. Grubb | Emory University—Associate Professor of Surgery and Medicine; Surgical Director Structural Heart and Valve Center | Content Reviewer, ACC Cardiac Surgery Team Section Leadership Council |
| Rebecca T. Hahn | Columbia Structural Heart and Valve Center—Chief Scientific Officer of the Echocardiography Core Lab at the Cardiovascular Research Foundation and Director of Interventional Echocardiography; Columbia University Irving Medical Center—Professor of Medicine | Content Reviewer, ACC Competency Management Committee |
| Uzoma Ibebuogu | Methodist University Hospital—Director of Structural Heart Disease Intervention; University of Tennessee Health Sciences Center, Division of Cardiovascular Diseases—Associate Professor of Medicine | Content Reviewer, ACC Interventional Cardiology Section Leadership Council |
| Ashequl Islam | University of Massachusetts Chan Medical School-Baystate—Program Director, Interventional Cardiology Fellowship | Content Reviewer, Interventional Cardiology Program Director |
| Henry S. Jennings III | Vanderbilt University Medical Center, Vanderbilt Heart & Vascular Institute/Interventional Cardiology Section—Assistant Professor of Medicine | Content Reviewer, ACC Cardiovascular Imaging Section Leadership Council |
| Tara L. Jones | University of Utah—Assistant Professor of Medicine, Interventional Cardiology | Content Reviewer, Individual Contributor |
| Sabeeda Kadavath | Vanderbilt University—Structural Heart Disease Fellow | Content Reviewer, ACC Interventional Cardiology Section Leadership Council |
| Clifford J. Kavinsky | Rush University Medical Center—Professor of Medicine and Pediatrics, Chief, Section of Structural and Interventional Cardiology, Director, Rush Center for Congenital and Structural Heart Disease, Director, Interventional Fellowship |
Content Reviewer, ACC Interventional Cardiology Section Leadership Council |
| Sadiya S. Khan | Northwestern University Feinberg School of Medicine—Assistant Professor of Medicine (Cardiology) and Preventive Medicine (Epidemiology) | Content Reviewer, Former ACC Competency Management Committee Member |
| Rami Khouzam | University of Tennessee Health Science Center—Professor of Medicine, Vice Chair, Internal Medicine Department, Program Director, Interventional Cardiology, Associate Program Director, Cardiology Fellowship; Methodist University Hospital and Methodist Le Bonheur Healthcare System—Director, Cardiac Catheterization Laboratory | Content Reviewer, Interventional Cardiology Program Director |
| Viet Le | Intermountain Healthcare—Cardiology Research PA | Content Reviewer, ACC Cardiovascular Team Section Leadership Council |
| Alexander Lee | Northwell Health - Long Island Jewish Medical Center—Program Director, Interventional Cardiology Fellowship | Content Reviewer, Interventional Cardiology Program Director |
| Stamatios Lerakis | Mount Sinai Hospital—Director of Noninvasive Cardiology; Icahn School of Medicine at Mount Sinai—Director of Imaging for Structural and Valve Interventions | Content Reviewer, ACC Cardiovascular Imaging Section Leadership Council |
| Ryan Mallory | Indiana University—Cardiology Fellow in Training | Content Reviewer, ACC Fellows in Training Section Leadership Council |
| John Mulrow | Methodist Cardiology Clinic of San Antonio—Cardiologist | Content Reviewer, ACC Geriatric Cardiology Section Leadership Council |
| Abhiram Prasad | Mayo Clinic, Department of Cardiovascular Diseases—Consultant, Program Director, Interventional Cardiology Fellowship; Mayo Clinic College of Medicine—Professor of Medicine | Content Reviewer—Interventional Cardiology Program Director |
| Tanveer Rab | Emory University—Professor of Medicine, Interventional Cardiology | Content Reviewer, ACC Interventional Cardiology Section Leadership Council |
| Michael Ragosta | University of Virginia Health System—Professor of Medicine, Director, Cardiac Catheterization Laboratories | Content Reviewer, Interventional Cardiology Program Director |
| Bharath Rajagopalan | Prairie Heart Institute—Cardiac Electrophysiologist | Content Reviewer, ACC Electrophysiology Section Leadership Council |
| Robert F. Riley | Overlake Medical Center—Director, Complex Coronary Therapeutics Program | Content Reviewer, ACC Interventional Cardiology Section Leadership Council |
| Shubha Deep Roy | University of Iowa Hospitals and Clinics—Interventional Cardiology Fellow, Division of Cardiovascular Medicine, Department of Internal Medicine, University of Iowa Hospitals and Clinics | Content Reviewer, ACC Fellows in Training Section Leadership Council |
| Matthew Sherwood | Inova Heart and Vascular Institute—Co-Director of Structural Heart Program, Co-Director of Cardiac Catheterization Laboratory | Content Reviewer, ACC Interventional Section Leadership Council |
| Adhir Shroff | University of Illinois College of Medicine at Chicago—Interventional Cardiology Program Director; Professor of Medicine | Content Reviewer, Interventional Cardiology Program Director |
| Scott Shurmur | Texas Tech University Health Sciences Center—Chairman, Internal Medicine; Division Chief, Cardiology; Interventional Cardiology Program Director; Professor | Content Reviewer, Interventional Cardiology Program Director |
| Michael A. Solomon | National Institutes of Health Clinical Center—Senior Research Physician | Content Reviewer, ACC Competency Management Committee Member |
| Anwar Tandar | University of Utah School of Medicine—Director of Structural Cardiac Intervention; Associate Professor of Medicine | Content Reviewer, Interventional Cardiology Program Director |
| Alex Truesdell | Inova Heart and Vascular Institute—Interventional Cardiologist | Content Reviewer, ACC Interventional Cardiology Section Leadership Council |
| Poonam Velagapudi | University of Nebraska Medical Center—Structural Interventional Cardiologist; Associate Program Director for Cardiovascular Medicine Fellowship; Assistant Professor of Medicine | Content Reviewer, ACC Early Career Professionals Section Leadership Council |
| Eric Yang | University of California at Los Angeles—Associate Clinical Professor of Medicine | Content Reviewer, ACC Interventional Cardiology Section Leadership Council |
| Alan C. Yeung | Stanford University—Li Ka Shing Professor of Medicine | Content Reviewer, ACC Academic Cardiology Section Leadership Council |
This table represents the individuals, organizations, and groups that peer reviewed this document. A comprehensive list of health care–related disclosures for each reviewer can be found in the Supplemental Appendix.
ACC = American College of Cardiology; AHA = American Heart Association; HCM = hypertrophic cardiomyopathy; MUSC = Medical University of South Carolina; SCAI = Society for Cardiovascular Angiography and Interventions; UT = University of Texas.
Appendix 3.
Abbreviations
| ABIM = American Board of Internal Medicine |
| ACGME = Accreditation Council for Graduate Medical Education |
| ACHD = adult congenital heart disease |
| ALI = acute limb ischemia |
| CAD = coronary artery disease |
| CCT = cardiovascular computed tomography |
| CHD = congenital heart disease |
| CLTI = chronic limb-threatening ischemia |
| CMR = cardiovascular magnetic resonance |
| COCATS = Core Cardiovascular Training Statement |
| CTO = chronic total occlusion |
| IVUS = intravascular ultrasound |
| OCT = optical coherence tomography |
| PAD = peripheral artery disease |
| PVI = peripheral vascular intervention |
| RWI = relationships with industry and other entities |
| SHD = structural heart disease |
| TAVR = transcatheter aortic valve replacement |
| TEER = transcatheter edge-to-edge repair |
References
- 1.Alpert J.S. Guidelines for training in adult cardiovascular medicine core cardiology training symposium (COCATS) June 27–28, 1994. J Am Coll Cardiol. 1995;25:1–2. doi: 10.1016/0735-1097(95)96215-k. [DOI] [PubMed] [Google Scholar]
- 2.Swing S.R. The ACGME outcome project: retrospective and prospective. Med Teach. 2007;29:648–654. doi: 10.1080/01421590701392903. [DOI] [PubMed] [Google Scholar]
- 3.The Accreditation Council for Graduate Medical Education Cardiovascular disease milestones. https://www.acgme.org/globalassets/pdfs/milestones/cardiovasculardiseasemilestones.pdf
- 4.Williams E.S., Halperin J.L., Arrighi J.A., et al. 2016 ACC lifelong learning competencies for general cardiologists: a report of the ACC Competency Management Committee. J Am Coll Cardiol. 2016;67:2656–2695. doi: 10.1016/j.jacc.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Halperin J.L., Williams E.S., Fuster V. COCATS 4 introduction. J Am Coll Cardiol. 2015;65:1724–1733. doi: 10.1016/j.jacc.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 6.King S.B., Babb J.D., Bates E.R., et al. COCATS 4 task force 10: training in cardiac catheterization. J Am Coll Cardiol. 2015;65:1844–1853. doi: 10.1016/j.jacc.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Armsby L.B., Vincent R.N., Foerster S.R., et al. Task force 3: pediatric cardiology fellowship training in cardiac catheterization. J Am Coll Cardiol. 2015;66:699–705. doi: 10.1016/j.jacc.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Armsby L., Beekman R.H., 3rd, Benson L., et al. SCAI expert consensus statement for advanced training programs in pediatric and congenital interventional cardiac catheterization. Catheter Cardiovasc Interv. 2014;84:779–784. doi: 10.1002/ccd.25550. [DOI] [PubMed] [Google Scholar]
- 9.Hammond G., Joynt Maddox K.E. A theoretical framework for clinical implementation of social determinants of health. JAMA Cardiol. 2019;4:1189–1190. doi: 10.1001/jamacardio.2019.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itchhaporia D. Paving the way for health equity in cardiology. J Am Coll Cardiol. 2021;77:2613–2616. doi: 10.1016/j.jacc.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creager M.A., Gornik H.L., Gray B.H., et al. COCATS 4 task force 9: training in vascular medicine. J Am Coll Cardiol. 2015;65:1832–1843. doi: 10.1016/j.jacc.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 12.Accreditation Council for Graduate Medical Education ACGME program requirements for graduate medical education in interventional cardiology. https://acgme.org/Portals/0/PFAssets/ProgramRequirements/152_InterventionalCardiology_2020.pdf?ver=2020-06-26-132343-780
- 13.Bashore T.M., Balter S., Barac A., et al. 2012 American College of Cardiology Foundation/Society for Cardiovascular Angiography and Interventions expert consensus document on cardiac catheterization laboratory standards update: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2012;59:2221–2305. doi: 10.1016/j.jacc.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Naidu S.S., Abbott J.D., Bagai J., et al. SCAI expert consensus update on best practices in the cardiac catheterization laboratory: this statement was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), and the Heart Rhythm Society (HRS) in April 2021. Catheter Cardiovasc Interv. 2021;98:255–276. doi: 10.1002/ccd.29744. [DOI] [PubMed] [Google Scholar]
- 15.Klein L.W., Goldstein J.A., Haines D., et al. SCAI multi-society position statement on occupational health hazards of the catheterization laboratory: shifting the paradigm for healthcare workers' protection. J Am Coll Cardiol. 2020;75:1718–1724. doi: 10.1016/j.jacc.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Creager M.A., Goldstone J., Hirshfeld J.W., Jr., et al. ACC/ACP/SCAI/SVMB/SVS clinical competence statement on vascular medicine and catheter-based peripheral vascular interventions: a report of the American College of Cardiology/American Heart Association/American College of Physician Task Force on Clinical Competence (ACC/ACP/SCAI/SVMB/SVS Writing Committee to Develop a Clinical Competence Statement on Peripheral Vascular Disease) J Am Coll Cardiol. 2004;44:941–957. doi: 10.1016/j.jacc.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Naidu S.S., Aronow H.D., Box L.C., et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory (endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology–Association Canadienne de Cardiologie D'Intervention) Catheter Cardiovasc Interv. 2016;88:407–423. doi: 10.1002/ccd.26551. [DOI] [PubMed] [Google Scholar]
- 18.Jennings H.S., III, Rao S.V., Feldman D.N., et al. SCAI core curriculum for adult and pediatric interventional fellowship training in continuous quality assessment and improvement. Catheter Cardiovasc Interv. 2015;86:422–431. doi: 10.1002/ccd.26029. [DOI] [PubMed] [Google Scholar]
- 19.Institute of Medicine Committee on Quality of Health Care in America . National Academies Press; 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. [PubMed] [Google Scholar]
- 20.Lawton J.S., Tamis-Holland J.E., Bangalore S., et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79:e21–e129. doi: 10.1016/j.jacc.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Tamis-Holland J.E., Jneid H., Reynolds H.R., et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139:e891–e908. doi: 10.1161/CIR.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 22.Aengevaeren W.R., Laarman G.J., Suttorp M.J., et al. Dutch guidelines for interventional cardiology: institutional and operator competence and requirements for training. Neth Heart J. 2005;13:416–422. [PMC free article] [PubMed] [Google Scholar]
- 23.Harold J.G., Bass T.A., Bashore T.M., et al. ACCF/AHA/SCAI 2013 update of the clinical competence statement on coronary artery interventional procedures; a report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training (Writing Committee to Revise the 2007 Clinical Competence Statement on Cardiac Interventional Procedures) J Am Coll Cardiol. 2013;62:357–396. doi: 10.1016/j.jacc.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Martial H., Christian P., Carlo Di M., et al. Consensus document on the radial approach in percutaneous cardiovascular interventions: position paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Cardiac Care and Thrombosis of the European Society of Cardiology. EuroIntervention. 2013;8:1242–1251. doi: 10.4244/EIJV8I11A192. [DOI] [PubMed] [Google Scholar]
- 25.Parker D.J., Gray H.H., Balcon R., et al. Planning for coronary angioplasty: guidelines for training and continuing competence. British Cardiac Society (BCS) and British Cardiovascular Intervention Society (BCIS) Working Group on Interventional Cardiology. Heart. 1996;75:419–425. doi: 10.1136/hrt.75.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spittell J.A., Creager M.A., Dorros G., et al. Recommendations for peripheral transluminal angioplasty: training and facilities: American College of Cardiology Peripheral Vascular Disease Committee. J Am Coll Cardiol. 1993;21:546–548. doi: 10.1016/0735-1097(93)90701-2. [DOI] [PubMed] [Google Scholar]
- 27.Aronow H.D., Collins T.J., Gray W.A., et al. SCAI/SVM expert consensus statement on carotid stenting: training and credentialing for carotid stenting. Catheter Cardiovasc Interv. 2016;87:188–199. doi: 10.1002/ccd.26304. [DOI] [PubMed] [Google Scholar]
- 28.Bates E.R., Babb J.D., Casey D.E., Jr., et al. ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents (ACCF/SCAI/SVMB/SIR/ASITN Clinical Expert Consensus Document Committee on Carotid Stenting) J Am Coll Cardiol. 2007;49:126–170. doi: 10.1016/j.jacc.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 29.Calligaro K.D., Amankwah K.S., D'Ayala M., et al. Guidelines for hospital privileges in vascular surgery and endovascular interventions: recommendations of the Society for Vascular Surgery. J Vasc Surg. 2018;67:1337–1344. doi: 10.1016/j.jvs.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Connors J.J., Sacks D., Furlan A.J., et al. Training, competency, and credentialing standards for diagnostic cervicocerebral angiography, carotid stenting, and cerebrovascular intervention: a joint statement from the American Academy of Neurology, the American Association of Neurological Surgeons, the American Society of Interventional and Therapeutic Neuroradiology, the American Society of Neuroradiology, the Congress of Neurological Surgeons, the AANS/CNS Cerebrovascular Section, and the Society of Interventional Radiology. Neurology. 2005;64:190–198. doi: 10.1212/01.WNL.0000148958.34025.09. [DOI] [PubMed] [Google Scholar]
- 31.Cremonesi A., Setacci C., Bignamini A., et al. Carotid artery stenting: first consensus document of the ICCS-SPREAD Joint Committee. Stroke. 2006;37:2400–2409. doi: 10.1161/01.STR.0000236101.09480.b7. [DOI] [PubMed] [Google Scholar]
- 32.Lal B.K., Jordan W., Kashyap V.S., et al. Clinical competence statement of the Society for Vascular Surgery on training and credentialing for transcarotid artery revascularization. J Vasc Surg. 2020;72:779–789. doi: 10.1016/j.jvs.2020.05.053. [DOI] [PubMed] [Google Scholar]
- 33.Pathak A.G.X., Azizi M., et al. Expert consensus: renal denervation for the treatment of arterial hypertension: French Society of Hypertension; French Society of Cardiology; Working Group on Atheroma, Interventional Cardiology; French Society of Radiology. Arch Cardiovasc Dis. 2012;105:386–393. [PubMed] [Google Scholar]
- 34.Rosenfield K., Babb J.D., Cates C.U., et al. Clinical competence statement on carotid stenting: training and credentialing for carotid stenting—multispecialty consensus recommendations: a report of the SCAI/SVMB/SVS Writing Committee to Develop a Clinical Competence Statement on Carotid Interventions. J Am Coll Cardiol. 2005;45:165–174. doi: 10.1016/j.jacc.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Bonow R.O., O’Gara P.T., Adams David H., et al. 2019 AATS/ACC/SCAI/STS expert consensus systems of care document: operator and institutional recommendations and requirements for transcatheter mitral valve intervention. J Am Coll Cardiol. 2020;76:96–117. doi: 10.1016/j.jacc.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura R.A., O’Gara P.T., Bavaria J.E., et al. 2019 AATS/ACC/ASE/SCAI/STS expert consensus systems of care document: a proposal to optimize care for patients with valvular heart disease: a joint report of the American Association for Thoracic Surgery, American College of Cardiology, American Society of Echocardiography, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2019;73:2609–2635. doi: 10.1016/j.jacc.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Horlick E., Kavinsky C.J., Amin Z., et al. SCAI expert consensus statement on operator and institutional requirements for PFO closure for secondary prevention of paradoxical embolic stroke. Catheter Cardiovasc Interv. 2019;93:859–874. doi: 10.1002/ccd.28111. [DOI] [PubMed] [Google Scholar]
- 38.Bavaria J.E., Tommaso C.L., Brindis R.G., et al. 2018 AATS/ACC/SCAI/STS expert consensus systems of care document: operator and institutional recommendations and requirements for transcatheter aortic valve replacement. J Am Coll Cardiol. 2019;73:340–374. doi: 10.1016/j.jacc.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Kavinsky C.J., Kusumoto F.M., Bavry A.A., et al. SCAI/ACC/HRS institutional and operator requirements for left atrial appendage occlusion. J Am Coll Cardiol. 2016;67:2295–2305. doi: 10.1016/j.jacc.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Hijazi Z.M., Ruiz C.E., Zahn E., et al. SCAI/AATS/ACC/STS operator and institutional requirements for transcatheter valve repair and replacement, part III: pulmonic valve. J Am Coll Cardiol. 2015;65:2556–2563. doi: 10.1016/j.jacc.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 41.O'Gara P.T., Calhoon J.H., Moon M.R., et al. Transcatheter therapies for mitral regurgitation: a professional society overview from the American College of Cardiology, the American Association for Thoracic Surgery, Society for Cardiovascular Angiography and Interventions Foundation, and the Society of Thoracic Surgeons. Catheter Cardiovasc Interv. 2014;83:849–863. doi: 10.1002/ccd.25306. [DOI] [PubMed] [Google Scholar]
- 42.Tommaso C.L., Bolman R.M., 3rd, Feldman T., et al. Multisociety (AATS, ACCF, SCAI, and STS) expert consensus statement: operator and institutional requirements for transcatheter valve repair and replacement, part 1: transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59:2028–2042. doi: 10.1016/j.jacc.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 43.Herrmann H.C., Baxter S., Ruiz C.E., et al. Results of the Society of Cardiac Angiography and Interventions survey of physicians and training directors on procedures for structural and valvular heart disease. Catheter Cardiovasc Interv. 2010;76:E106–E110. doi: 10.1002/ccd.22703. [DOI] [PubMed] [Google Scholar]
- 44.Aboulhosn J.A., Hijazi Z.M., Kavinsky C.J., et al. SCAI position statement on adult congenital cardiac interventional training, competencies and organizational recommendations. Catheter Cardiovasc Interv. 2020;96:643–650. doi: 10.1002/ccd.28885. [DOI] [PubMed] [Google Scholar]
- 45.Butera G., Morgan G.J., Ovaert C., et al. Recommendations from the Association of European Paediatric Cardiology for training in diagnostic and interventional cardiac catheterisation. Cardiol Young. 2015;25:438–446. doi: 10.1017/S1047951114001309. [DOI] [PubMed] [Google Scholar]
- 46.Patel M.R., Calhoon J.H., Dehmer G.J., et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69:2212–2241. doi: 10.1016/j.jacc.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Gulati M., Levy P.D., Mukherjee D., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;78:e187–e285. doi: 10.1016/j.jacc.2021.07.053. [DOI] [PubMed] [Google Scholar]
- 48.Gerhard-Herman M.D., Gornik H.L., Barrett C., et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69:e71–e126. doi: 10.1016/j.jacc.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Aboyans V., Criqui M.H., Abraham P., et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 50.Bailey S.R., Beckman J.A., Dao T.D., et al. ACC/AHA/SCAI/SIR/SVM 2018 appropriate use criteria for peripheral artery intervention: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Heart Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, and Society for Vascular Medicine. J Am Coll Cardiol. 2019;73:214–237. doi: 10.1016/j.jacc.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norgren L., Hiatt W.R., Dormandy J.A., et al. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45:S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 52.Jaff M.R., White C.J., Hiatt W.R., et al. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: a supplement to the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Vasc Med. 2015;20:465–478. doi: 10.1177/1358863X15597877. [DOI] [PubMed] [Google Scholar]
- 53.Conte M.S., Bradbury A.W., Kolh P., et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69:3S–125S.e140. doi: 10.1016/j.jvs.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hawkins B.M., Li J., Wilkins L.R., et al. SCAI/ACR/APMA/SCVS/SIR/SVM/SVS/VESS position statement on competencies for endovascular specialists providing CLTI care. J Soc Cardiovasc Angiogr Interv. 2022;1 [Google Scholar]
- 55.Shroff A., Pinto D. Society for Cardiovascular Angiography and Interventions; 2019. Vascular Access, Management, and Closure: Best Practices. Accessed August 21, 2021. https://scai.org/publications/ebooks/vascular-access-management-and-closure-best-practices. [Google Scholar]
- 56.Creager M.A., Hamburg N.M., Calligaro K.D., et al. 2021 ACC/AHA/SVM/ACP advanced training statement on vascular medicine (revision of the 2004 ACC/ACP/SCAI/SVMB/SVS clinical competence statement on vascular medicine and catheter-based peripheral vascular interventions) J Am Coll Cardiol. 2021;77:998–1020. doi: 10.1016/j.jacc.2020.09.579. [DOI] [PubMed] [Google Scholar]
- 57.Anderson J.L., Halperin J.L., Albert N.M., et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:1425–1443. doi: 10.1161/CIR.0b013e31828b82aa. [DOI] [PubMed] [Google Scholar]
- 58.Brott T.G., Halperin J.L., Abbara S., et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. J Am Coll Cardiol. 2011;57:e16–e94. doi: 10.1016/j.jacc.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Ortel T.L., Neumann I., Ageno W., et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Advances. 2020;4:4693–4738. doi: 10.1182/bloodadvances.2020001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jaff M.R., McMurtry M.S., Archer S.L., et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 61.Kearon C., Akl E.A., Ornelas J., et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 62.Hiratzka L.F., Bakris G.L., Beckman J.A., et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55:e27–e129. doi: 10.1016/j.jacc.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 63.Isselbacher E.M., Preventza O., Black J.H., 3rd, et al. 2022 ACC/AHA guidelines for the diagnosis and management of aortic disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;80:e223–e393. doi: 10.1016/j.jacc.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erbel R., Aboyans V., Boileau C., et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult: the Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 65.Chaikof E.L., Dalman R.L., Eskandari M.K., et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77.e72. doi: 10.1016/j.jvs.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 66.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:e25–e197. doi: 10.1016/j.jacc.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 67.Williams R.G., Pearson G.D., Barst R.J., et al. Report of the National Heart, Lung, and Blood Institute Working Group on Research in Adult Congenital Heart Disease. J Am Coll Cardiol. 2006;47:701–707. doi: 10.1016/j.jacc.2005.08.074. [DOI] [PubMed] [Google Scholar]
- 68.Stout K.K., Daniels C.J., Aboulhosn J.A., et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e81–e192. doi: 10.1016/j.jacc.2018.08.1029. [DOI] [PubMed] [Google Scholar]
- 69.Ruiz C.E., Feldman T.E., Hijazi Z.M., et al. Interventional fellowship in structural and congenital heart disease for adults. J Am Coll Cardiol Intv. 2010;3:e1–e15. doi: 10.1016/j.jcin.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 70.Feldman T., Ruiz C.E., Hijazi Z.M. The SCAI Structural Heart Disease Council: toward addressing training, credentialing, and guidelines for structural heart disease intervention. Catheter Cardiovasc Interv. 2010;76:e87–e89. doi: 10.1002/ccd.22701. [DOI] [PubMed] [Google Scholar]
- 71.Holmboe E.S., Iobst W.F. ACGME Assessment Guidebook. https://www.acgme.org/Portals/0/PDFs/Milestones/Guidebooks/AssessmentGuidebook.pdf
- 72.Accreditation Council for Graduate Medical Education Resources for assessment. https://dl.acgme.org/pages/assessment
- 73.ACGME clinical competency committees: a guidebook for programs, 3rd edition. https://www.acgme.org/Portals/0/ACGMEClinicalCompetencyCommitteeGuidebook.pdf
- 74.American College of Cardiology Fellowship Program Tools and Resources. https://www.acc.org/Education-and-Meetings/Products-and-Resources/Fellowship-Program-Director-Tools-and-Resources
- 75.American Board of Internal Medicine Interventional cardiology policies. https://www.abim.org/certification/policies/internal-medicine-subspecialty-policies/interventional-cardiology/
- 76.American Osteopathic Board of Internal Medicine AOBIM subspecialty certification in interventional cardiology: interventional cardiology certification process. https://certification.osteopathic.org/internal-medicine/certification-process-overview/interventional-cardiology
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.