Abstract
The circadian clock and gut microbiome play integral roles in preserving metabolic homeostasis. Circadian rhythms represent an endogenous time-keeping system that regulates cell and organ functions and synchronizes physiology with external cues to establish metabolic homeostasis. A variety of functions throughout the gastrointestinal tract and liver are under circadian control, including nutrient transport, processing, and detoxification. The gut microbiota also plays an essential role in host metabolism, regulating processes such as digestion, inflammatory modulation, and bile acid metabolism. Both the circadian clock and the gut microbiota influence each other in a reciprocal fashion, as gut dysbiosis can precipitate circadian asynchrony, and vice-versa. Disruption of either system impacts homeostasis in a bidirectional manner and can contribute to metabolic dysfunction. Evidence suggests such disruptions can lead to the development of metabolic diseases, including obesity, diabetes, nonalcoholic fatty liver disease, cirrhosis, and hepatocellular carcinoma. This review will provide a basic overview of the circadian and gut microbial systems, how they are intertwined, and their impact on the liver and gastrointestinal tract and in the development of metabolic disease. Particular areas of discussion include epigenetic regulation of circadian pathways as well as a mechanistic overview of microbial dysbiosis. In addition, therapeutic targets of these systems, including dietary modifications, behavioral modifications, and microbial-directed therapies, will be explored.
Keywords: Circadian rhythms, Circadian clock, Gut microbiome, Metabolic disorders, Metabolic homeostasis
Introduction to Circadian Rhythms and Metabolism
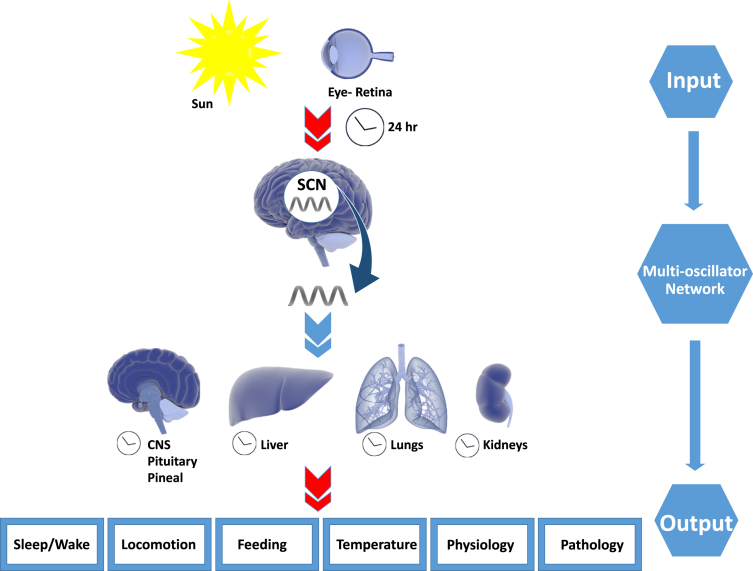
Circadian rhythms, from the Latin circa dies or “around a day”, are biological processes that recur on a 24-hour cycle.1 Organisms have evolved a biological clock built to anticipate and adapt to the cyclical nature of the environment on Earth, notably based on the 24-hour daily rotation period with diurnal variations in sunlight.2 Such a system is crucial for mammalian systems in aligning endogenous rhythms with predictable environmental cues to optimize behaviors into optimal patterns for foraging, feeding, fasting, or sleeping.3 Nearly all physiological and behavioral functions occur on some sort of rhythmic basis, requiring tight coordination to maintain homeostasis.4 Broadly speaking, this system is based on an endogenous time keeping system which produces cyclic physiologic rhythms with an autonomous biologic output that is modulated by environmental cues (Figure 1).5 This endogenous time keeping system is organized as a hierarchical network of biologic and molecular clocks, with a central clock located within the suprachiasmatic nucleus (SCN) of the hypothalamus.1 This neurologic nucleus oscillates autonomously on a 24-hour schedule and is entrained to the day-night cycle based on environmental cues or zeitgebers (German for “time givers”), most notably from light intake via ocular photoreceptors6 and other cues including nutrient intake and temperature. The SCN thereby acts as a sort of “orchestra director”, with output via neuronal and endocrine systems to act on peripheral clocks to coordinate metabolic rhythms through several peripheral systems.3 Circadian clocks input control on multiple disparate systems including sleep-wake cycles, hormone secretion, nutrient metabolism, and memory formation, providing hierarchical control to optimize organismal functioning.7
Figure 1.
Inputs and outputs of the circadian timing system. Light stimuli (zeitgeber input) synchronize with the internal multioscillator network. The suprachiasmatic nucleus coordinates the rhythmic output to central and peripheral organs to maintain normal homeostasis. Mismatch due to abnormal social rhythms increases risk of metabolic dysfunction. Library of Science & Medical Illustrations were utilized in part to create this figure. https://creativecommons.org/licenses/by-nc-sa/4.0/.
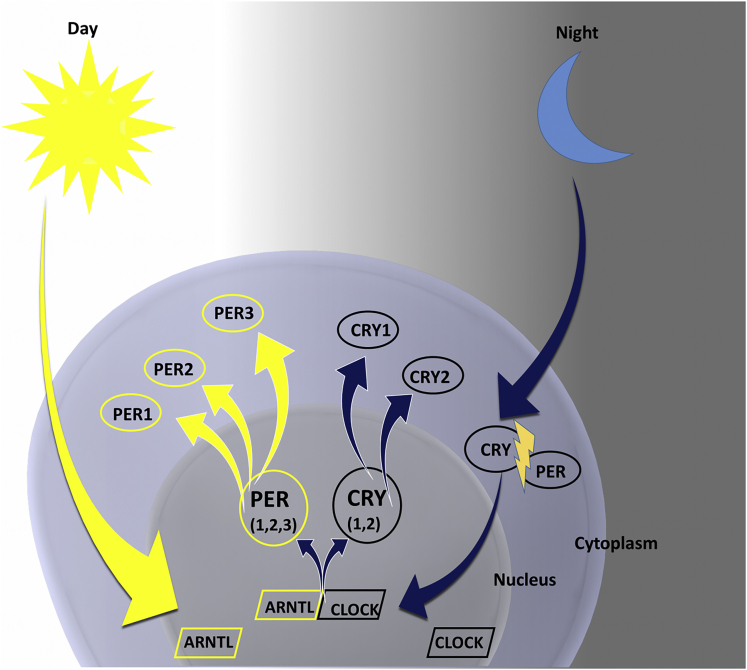
Circadian peripheral clock mechanisms have been established at the genetic and molecular level based on a translational/transcriptional apparatus with input from the conducting SCN via neuronal and humoral pathways.6 The emerging cellular picture from multiple mammalian models is a circadian rhythmicity supported by intracellular feedback loops that perpetuate oscillations in gene expression.8 This rhythmic gene expression is tightly controlled by feedback mechanisms based on the degradation of regulatory enzymes allowing for recurring cycles. The system is also highly plastic, with ability to modify gene output based on changes in environment and protein modification through several post-translational mechanisms including acetylation and phosphorylation.8 Central genes to this mechanism include Clock and Bmal1, which encode transcription activators that induce expression of 2 main oscillators Per and Cry.9 These gene products provide key control of numerous metabolic pathways, most notably the enzyme nicotinamide phosphoribosyltransferase, which acts as the rate-limiting step in the nicotinamide adenine dinucleotide salvage pathway through modulation of sirtuin enzymes.1 Plasticity of this system is derived by expanding or restricting regulation of key proteins within these pathways depending on nutritional, metabolic, or epigenetic status that allows for entrainment and feedback on the autonomous cellular machinery. Careful feedback control is built into these pathways, where buildup of protein products CRY and PER from transcription during daytime has been shown to inhibit further metabolic production at nighttime until these gene products have degraded (Figure 2).
Figure 2.
The molecular organization of the cellular circadian clock. Proteins encoded by clock genes PER, CLOCK, ARNTL, and CRY interact to create a negative transcription-translation feedback loop. Intracellular levels of CLOCK are maintained during the 24-hour period. High levels of ARNTL at dawn promote the formation of ARNTL-CLOCK heterodimers, which activate the transcription of PER and CRY. Ubiquitylation degrades the accumulated PER in the cytoplasm. CRY accumulates in the cytoplasm during dusk and translocates to the nucleus to inhibit ARNTL-CLOCK–mediated transcription. During the night, the PER-CRY complex is degraded, and the cycle restarts. Library of Science & Medical Illustrations were utilized in part to create this figure. https://creativecommons.org/licenses/by-nc-sa/4.0/.
Metabolic pathways play a crucial role in entraining circadian clocks and are in turn tightly regulated by output from these systems.7 Alignment of internal clocks with external zeitgebers appears energetically favorable to an organism based on multiple animal models, and derangements of these systems in mouse models have been found to lead to metabolic disorders.10 Intake and metabolism of glucose, lipids, and amino acids impact activity of peripheral clocks and lead to alternating periods of nutrient supply throughout the day. Robust, synchronized circadian peripheral rhythms are required for proper metabolic utilization, and disruptions of these rhythms by gene mutations or food restriction are detrimental to metabolic function and can lead to a variety of illnesses.11
Epigenetic Regulation of Circadian Pathways
Clock-controlled genes play an important role in regulating circadian rhythms. These genes help to maintain homeostasis via regulatory feedback loops that involve epigenetic modulators. In fact, epigenetic mechanisms are essential for the integration of environmental signals, both endogenous and exogenous from the external environment.12 Furthermore, RNA and protein expression of genes can be altered by epigenetic changes that are induced by the environment. These primary environmental factors are cyclic changes that are responsible for the creation of circadian rhythms. Light is the strongest stimulus for circadian rhythms, as sunlight triggers activation of retinal ganglion cells which subsequently leads to clock gene activation in the hypothalamus.13
Among the most important regulators of circadian rhythms are the Clock and Bmal1 clock genes, whose transcription can induce activation of additional clock genes, such as Per and Cry. Epigenetic modification of these genes can precipitate changes to circadian as well as metabolic systems. For example, Per is believed to be phosphorylated by casein kinase I. Mutation in this enzyme leads to accumulation of undegraded Per protein, resulting in a circadian rhythm acceleration and a subsequent shortening of the daily period.14 In addition, another study using a gene-based approach determined a significant association of CpG methylation patterns in the Per2 gene with both blood glucose metabolism and insulin resistance.15 In general, variable methylation patterns of DNA genes have been demonstrated for different severities of metabolic and cardiovascular diseases, even among monozygotic twins.16 Furthermore, an individual’s chronotype, or the time-of-day preference for daily activity, is set up by a combination of genetic and epigenetic factors and has influence on both physical and mental health.17 Ultimately, the close link between epigenetics, circadian rhythms, and metabolism is an important concept.
Circadian Regulation of Satiety
Circadian rhythms are closely linked with the regulation of satiety and nutritional metabolism. Various hormones involved in such processes are under circadian control. One example is with glucagon-like peptide 1 (GLP-1), which lowers glycemia via stimulation of glucose-dependent insulin secretion on nutrient ingestion. One study demonstrated the existence of a diurnal rhythm in GLP-1 secretory response to oral glucose loads. The pattern of GLP-1 release correlated with that of insulin secretion, and both rhythms were completely inverted in animals subjected to a 12-hour feeding cycle disruption.18 These findings indicated that an independent peripheral clock in the intestine drives a circadian rhythm in GLP-1 secretory responses and suggested that GLP-1 acts to entrain the pancreatic β-cell response to nutrient intake.
Peptide YY (PYY) is another gut hormone involved with feeding and acts to inhibit appetite after meal ingestion. Circadian disruption in the form of variable fasting affects PYY function, where PYY is less effective in reducing food consumption during fasting periods in daytime compared with nighttime.19 Another study found that obese and lean mice with similar patterns of food intake displayed a difference in levels and rhythms of gut hormones, with GLP-1 and PYY levels highest during the light cycle in lean mice.20 This suggests a close relation between body habitus, feeding cycles, and the day-light cycle in regulation of satiety hormones.
Lipid pathways are also under circadian control in metabolic organs. In peripheral tissues, genes involved in lipid biosynthesis and fatty acid oxidation are rhythmically activated and repressed by clock proteins.21 In the liver, peroxisome-proliferator–activated receptors (PPARs) are diurnally regulated, including PPARα, which promotes mitochondrial fatty acid β-oxidation.22 The circadian clock also plays an important role in regulating both lipid absorption and lipolysis via the Bmal1-Clock transcriptional complex.23,24 In general, the circadian system is essential to the coordination of satiety and nutritional metabolism, including the processes involving gut hormones and lipid metabolism. The following sections explore the impact of circadian rhythms on gastrointestinal and liver health, followed by an in-depth exploration of the gut microbiome and its role in impacting circadian rhythms and metabolic health.
Impact of Circadian Rhythms on Liver and Gastrointestinal Physiology
Multiple processes throughout the gastrointestinal tract and liver are under circadian control, including metabolic functions for nutrient uptake, processing, and detoxification.9 Circadian control allows synchrony of these organ functions based on nutrient supply and demand for optimal function. The overt rest and or activity and feeding and or fasting cycles necessitate alterations in nutrient intake, which are absorbed, processed, and distributed through the gastrointestinal tract and subsequently metabolized by the liver. Proactive circadian alignment anticipates recurring organismal needs. In the rodent gastrointestinal (GI) tract, gradual increases in the expression levels of clock genes, including Clock, Bmal1, and Per 1/2/3, have been observed from the duodenum to the colon,25 supporting the role of these peripheral clock genes in orchestrating GI circadian rhythms.
Nutrient transport and GI uptake are also closely regulated by circadian systems. The circadian clock genes are involved in rhythmic cycles of ion, sugar, and peptide transporter expression in small intestine cells. The sugar transporters SGLT1, Glut2, and Glut5 exhibit diurnal rhythmicity in mouse enterocytes, with high levels of mRNA expression observed in the afternoon and evening.26 Both lipid absorption and peptide absorption demonstrate diurnal variations, with the highest levels of peptide cotransporter expression during the dark phase in nocturnal animals.27 The gut barrier is another important GI function under circadian control. Tight junctions that form the gut barrier, such as occludin and claudin-1, have been shown to exhibit circadian variations in the colonic epithelia.28 Mice with a mutant Clock gene had increased intestinal permeability compared with wild-type mice,29 suggesting that proper circadian functioning is essential for maintaining gut barrier integrity. Many important GI hormones also demonstrate circadian patterns. Ghrelin, leptin, gastrin, and insulin have all been shown to oscillate in daily cycles, with anticipation of feeding being a key trigger for these oscillations.30
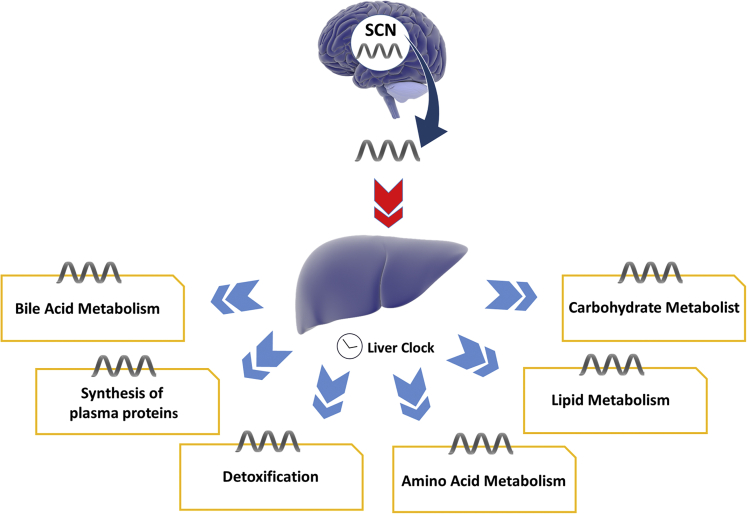
The liver controls energy homeostasis, and many rate-limiting enzymes in nutrient metabolism have been found to be expressed in a circadian manner to match energy needs. Expression levels of glucose transporters, glucagon receptors, and enzymes regulating sugar metabolism have shown peak levels in the early evening, which coincides with expected daily ingestion.1 Enzymes involved with lipid metabolism, including key enzymes in the glycerol 3-phosphate pathway, are expressed in a circadian manner.25 This pathway regulates glycerol and lipid metabolism as well as triglyceride accumulation. The liver is crucial in maintenance of glucose homeostasis, along with the pancreas and skeletal muscle. Although direct glucose bloodstream signaling is the main process allowing rapid changes in glucose availability, circadian signaling provides rhythmic baseline regulation in accordance with predictable feeding and starvation events. The liver’s role in this circadian cycle is to provide a buffering system to maintain blood glucose in the setting of these fluctuations in intake.31 This is hypothesized to occur through clock-dependent positive and negative regulators that act on signaling pathways converting on multiple rate-limiting enzymes of glucose anabolism and catabolism such as pyruvate kinase.26 Liver detoxification pathways are also under rhythmic circadian control, designed to peak key transcription of detoxification enzymes at expected timing of food ingestion27 (Figure 3).
Figure 3.
Circadian regulation of liver physiology. The liver is synchronized by the hypothalamic master clock, which then drives cyclic expression of regulators and rate-limiting enzymes in key hepatic pathways. Library of Science & Medical Illustrations were utilized in part to create this figure. https://creativecommons.org/licenses/by-nc-sa/4.0/.
Circadian Rhythms and Their Influence on Gastrointestinal and Hepatic Illnesses
Alterations in circadian rhythms have been implicated with several GI and liver illnesses (Table 1). Asynchrony in lifestyle rhythms, seen in a variety of settings in modern society including shift work and poor sleep hygiene, may disrupt energy metabolism and the circadian clock in such a way as to predispose to metabolic diseases including diabetes, nonalcoholic fatty liver disease (NAFLD), and the metabolic syndrome.11 The metabolic syndrome is a cluster of metabolic abnormalities with numerous health implications which is increasing substantially in the Western world, with incidence now estimated between 25% and 40% of adults.2 Disruptions of the finely tuned metabolic circadian clock have been shown to disrupt normal oscillatory characteristics of this system and as such have provided a clear link between circadian disruptions and metabolic disease. Studies on shift workers and in those with social jetlag have shown positive correlation with markers of metabolic disease, including increase in body mass index, elevated triglycerides, hyperglycemia, and even elevated inflammatory markers in comparison with controls.32 Circadian rhythms and sleep disruption have also been implicated in the pathogenesis of inflammatory bowel disease (IBD). Shift workers and patients with sleep interruption exhibited higher risks for the development of IBD.33 Biopsies of colonic mucosa in patients with IBD have demonstrated changes in key circadian genes including Bmal1 and downregulation of Per 1/3 compared with healthy controls.34
Table 1.
Circadian Implications for Various Gastrointestinal and Hepatic Diseases
| Studies | GI/Hepatic disease | Circadian implication |
|---|---|---|
| Canakis et al.33 | IBD | -Shift workers exhibit higher risk for disease development |
| Palmieri et al.34 | -Colonic mucosa biopsies demonstrate Bmal1 upregulation and Per1/3 downregulation | |
| Gnocchi et al.35 | NAFLD | -Deregulation of circadian transcription factors (ie, RORα, nocturnin) is implicated in NAFLD progression to NASH |
| Montagnese et al.36 | Cirrhosis | -Cirrhotics exhibit delayed sleep-wake cycles and changes in melatonin circulation |
| Ma et al.37 | -Per1 and Per2 double-knockout mice demonstrate cholestatic livers | |
| Filipski et al.38 | -Jet-lagged mice precipitated accelerated diethylnitrosamine-induced liver cancer |
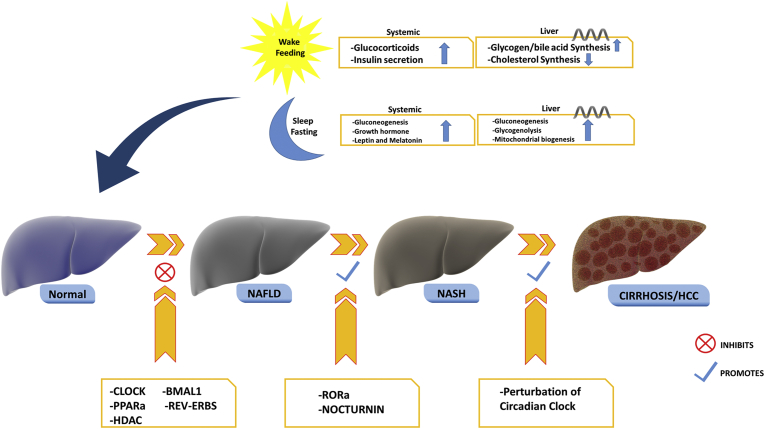
Circadian disturbance has been associated with diseases of the liver, including NAFLD, cirrhosis, and hepatocellular carcinoma. Via disruptions in liver metabolic pathways described previously, disruption in hepatic triglyceride accumulation and inflammation via circadian disruption conceivably contributes to the pathogenesis of nonalcoholic steatohepatitis (NASH).31 Patients with cirrhosis have evidence of abnormal circadian clock systems, leading to daytime sleepiness, delayed sleep-wake cycles, and changes in melatonin circulation.36 Normal levels of circadian gene products CLOCK, BMAL1, PPARa, and HDAC have been implicated with slowing onset of NAFLD, whereas deregulation of certain circadian transcription factors, including RORa and nocturnin, has been implicated in the progression from NAFLD to NASH35 (Figure 4). Mice with Per1 and Per2 double knockout demonstrated elevated serum and hepatic bile acid levels and cholestatic livers.37 Hepatocellular carcinoma has been linked in animal models with altered circadian patterns, possibly from influence of the circadian clock on cell proliferation genes, oncogenes, and tumor suppressor genes. This has been demonstrated in Per2 mutant mice, which exhibit increased tumorigenesis compared with wild-type mice. Another mouse model simulated chronic jet lag by advancing light-dark cycles by 8 hours every 2 days, which resulted in accelerated diethylnitrosamine-induced liver cancer.38 Abnormal expression of core clock genes has also been found in hepatocellular carcinoma biopsy tissue samples and correlated with increased tumor size.39 As with other metabolic systems, circadian rhythms are essential to the natural regulation of gastrointestinal processes, and disruption of these rhythms is implicated in various GI and hepatic illnesses.
Figure 4.
Role of the circadian clock in the pathogenesis and progression of NAFLD and NASH. Normal levels of circadian clock gene products inhibit the onset of NAFLD, whereas dysregulation in transcription products of circadian cycles is implicated in progression to NASH as well as progression to HCC. Library of Science & Medical Illustrations were utilized in part to create this figure. https://creativecommons.org/licenses/by-nc-sa/4.0/.
Introduction to the Gut Microbiome
Trillions of microorganisms, known as the gut microbiota, exist within the human gastrointestinal tract. As a whole, the genetic information contained within these organisms form what is referred to as the gut microbiome. With a collective genome that outnumbers the human genome by 150-fold, the gut microbiome plays an integral role in both human physiology and pathology.40,41 These functions involve proper regulation of host metabolism through influence on digestion, maintenance of the immune system, and production of vitamins and enzymes.42 Balance in this host-microbiotal ecosystem contributes to homeostasis and normal physiologic functioning. A disruption in this balance, otherwise known as dysbiosis, can be associated with various metabolic and gastrointestinal illnesses. The following sections explore this relationship between the gut microbiome and metabolic disorders, its impact on circadian rhythms, and therapeutic microbiotal targets.
Mechanisms of Microbiotal Symbiosis and Dysbiosis
The gut microbiota plays several roles in maintaining local ecological homeostasis, also called symbiosis. Significant alterations to the microbiota lead to dysbiosis and subsequent changes in host metabolism. One of these key roles is acting to preserve gut barrier health. The normal gut barrier serves to prevent unwanted pathogens and damaging substances from crossing the mucosal surface. In metabolic diseases such as diabetes and obesity, this gut barrier function has been shown to be damaged,43 implicating local inflammatory disruption of the normal microbiotal-gut interface. In studying intestinal permeability in patients with NAFLD, Miele et al44 showed that patients with NAFLD also had significantly increased gut permeability compared with healthy patients.
Gut microorganisms also play a role in energy metabolism, where bacterial metabolism aids in the breakdown of ingested nutrients that can then be extracted by the human host. The microbiota breaks down dietary carbohydrates to produce short-chain fatty acids (SCFAs), which play a role in energy homeostasis and have been implicated in metabolic disease in conditions of dysbiosis.45 There are 3 SCFAs involved in these pathways: butyrate, acetate, and propionate. Butyrate provides energy for the colonic epithelium, demonstrates anti-inflammatory effects, and is known to increase insulin sensitivity.46,47 It is the predominant product of carbohydrate fermentation by the Firmicutes phylum, which includes Clostridium, Roseburia, and Fecalibacteria.48 Low Firmicutes prevalence and subsequently low butyrate levels have been identified in several metabolic disorders, particularly diabetes mellitus.49, 50, 51, 52 This highlights the importance of butyrate for blood glucose metabolism, suggesting that low levels of butyrate could lead to impaired glucose tolerance. Acetate and propionate are mainly produced by the Bacteroidetes phylum.47 These 2 SCFAs act as substrates for lipogenesis and gluconeogenesis, and alterations in these pathways have been linked to the development of metabolic disorders.45
The gut microbiota plays an important role in modulating inflammation through the regulation of endotoxins and lipopolysaccharides. In normal symbiosis, excess release and absorption of these inflammatory mediators are limited by healthy gut bacteria, whereas in states of dysbiosis, these inflammatory markers are produced and absorbed at an increased rate. One study in mouse models demonstrated that changes in the gut microbiota led to increased endotoxemia, which was then associated with increased systemic inflammation, weight gain, and hyperglycemia.53 This increase in metabolic endotoxemia also depended on an increase in intestinal permeability to allow endotoxin translocation.53 Other studies have shown that translocation of endotoxins and lipopolysaccharides into the portal circulation activates proinflammatory cytokines, which in turn contributes to hepatic inflammation and the development of NASH.54,55 Splanchnic vasodilation mediated by tumor necrosis factor-alpha and nitric oxide release has been shown to cause portal hypertension, which in turn exacerbates intestinal permeability with further bacterial translocation.56 One prospective cohort directly correlated endotoxin markers and the development of NAFLD.57
The liver is intricately linked to the gut microbiome via the hepatic portal system as well as enterohepatic circulation of bile acids. Extensive crosstalk is facilitated via these pathways between the gut microbiota and the host’s immune, metabolic, and neuroendocrine systems.58 Dysbiosis has been linked to metabolic disease through several mechanisms, including alterations in bile acid metabolism. Normal gut microbiota metabolizes bile acids into primary and secondary bile acids. Primary bile acids serve as ligands for the nuclear transcription factor farnesoid X receptor (FXR). FXR activation stimulates secretion of gut-derived hormones, such as fibroblast growth factor-19,59 which regulates bile acid synthesis as well as lipid and glucose metabolism. Specifically, FXR activation has been shown to suppress hepatic gluconeogenesis and lipogenesis, enhance fatty acid oxidation and lipoprotein clearance, and improve insulin sensitivity.60 Several studies have shown that type 2 diabetes, obesity, and NAFLD are associated with changes in the bile acid pool size and composition.61 In studies of gut microbial depletion in germ-free or antibiotic-treated mice, there were increases in the proportion of taurine-conjugated primary bile acids, including tauro-beta-muricholic acid, which is a known FXR antagonist.62
Impact of the Gut Microbiome on Metabolic Disorders and Liver Diseases
The gut microbiota has been linked to the development of metabolic illnesses including obesity, diabetes, and NAFLD by disruption of many of the mechanisms described previously. Studies of obese mice microbiomes have found an increased capacity to harvest energy from their diet, likely in part due to changes in the relative abundance of certain dominant bacterial species.63 The effect of microbial abundance has also been evaluated in human subjects, in which stool studies from a small series of obese subjects showed that the low bacterial gene count was associated with an unhealthy metabolic phenotype, including higher insulin resistance and dyslipidemia, compared with those with high bacterial gene counts.64 Another interesting study involved direct manipulation of the microbiome, by infusing intestinal microbiota from lean donors to recipients with metabolic syndrome and examining how this altered microbial composition and glucose metabolism. They found that obese patients who received allogenic microbiota infusion from lean donors demonstrated significant improvement in insulin sensitivity and increased gut microbial diversity.49
Diabetes pathogenesis has been linked to gut dysbiosis, with variations in intestinal bacterial abundance seen between diabetics and nondiabetics. Metagenomic studies have demonstrated that diabetic patients show an increase in Bacteroidetes species and decreases in Firmicutes species such as Clostridium50,51 and that this altered composition correlates with hyperglycemia. One study comparing children with type 1 diabetes mellitus with healthy controls found the Bacteroidetes to Firmicutes ratio in diabetic children was significantly increased and that greater increases in the proportion of Bacteroidetes species correlated with higher hemoglobin A1C levels.52 In a study of adults with type 2 diabetes, the proportions of Firmicutes phylum and Clostridia class were significantly reduced in the diabetic group compared with the control group. Similar to the correlation seen in children, the Bacteroidetes to Firmicutes ratio correlated positively and significantly with plasma glucose concentration.65
Variations in the gut microbiota have also been associated with metabolic disorders of the liver and the development of NAFLD and NASH as well as hepatocellular carcinoma.56 Previous animal reports have indicated the role of gut microbiota in the pathogenesis in NAFLD.66 In human studies, patients with NAFLD have been shown to harbor lower gut microbial diversity than healthy controls.67 Stool samples from patients with biopsy-proven NASH have shown significantly increased concentrations of Bacteroides and decreased concentrations of Prevotella.54 When adjusting for multiple metabolic factors, Bacteroides were found to be independently associated with NASH.68 Other analyses of microbiotal content in patients with moderate to severe NAFLD have identified correlation with Firmicutes content.69
In addition to its impact on metabolic diseases of the liver, the microbiome has been implicated in the development of cirrhosis and hepatocellular carcinoma. Disruptions in the gut microbiota have been associated with a cycle of hepatocyte injury and regeneration, in a pattern often seen with other chronic liver diseases on a pathway toward cirrhosis.58 Several mechanisms have been implicated, including the “leaky gut” pathway and the inflammatory impact of microbiotal-associated molecular patterns and metabolites via the portal system, as well as parenchymal changes inflicted by altered metabolism of bile acids and SCFAs.58 The gut microbiome is also implicated in hepatocarcinogenesis, where changes in the liver microenvironment resulting from dysbiosis and subsequent chronic inflammation and reduced immune surveillance have been hypothesized to contribute to pathways leading to hepatocellular carcinoma.70 Although these relationships are being established in animal studies, further investigation into human microbiotal composition and linkage to hepatocellular carcinoma is needed.
Bidirectional Influences Between the Gut Microbiome and Circadian Rhythms
With an understanding of the association between how both the gut microbiome and circadian rhythms impact metabolic disorders, attention can turn to how the gut microbiome and circadian rhythms impact one another in a reciprocal fashion (Table 2). Two-way interactions between these systems allow for a centrally entrained circadian clock to synchronize peripheral organs and metabolic pathways, which are additionally influenced by signaling from the gut microbiota. Metagenomic studies have identified inherent circadian rhythms exhibited by intestinal bacteria. Studies on human stool samples of Enterobacter aerogenes have demonstrated responsivity to the circadian hormone melatonin as well as a daily rhythm.71 Up to 20% of intestinal bacteria have diurnal fluctuations in abundance and activity, suggesting circadian fluctuations in concert with host physiology.72 It has been proposed that the gut microbiome influences the rhythmic expression of the host’s internal clock by signaling molecules such as butyrate and by oscillations in microbiotal bacterial content in response to feeding patterns.73 The gut epithelium experiences differential bacterial species and metabolites depending on the time of day and expresses toll-like receptors which sense microbiotal metabolites in a rhythmic pattern.74 These signals act to reset hepatic circadian and intestinal peripheral clock gene expression and tailor metabolic enzymes to meet peripheral needs. Bile acid signaling plays an important part in this pathway, where microbes in the intestinal lumen help deconjugate bile salts into unconjugated bile acids which act as a signaling molecule in host metabolic pathways as previously described. Within enterocytes of the ileum, these unconjugated bile acids have been shown to influence the amplitude and periodicity of host circadian gene expression in the ileum, colon, and liver.75 Another study by Thaiss et al identified the biogeography and metabolome of the intestinal microbiota as 2 features that undergo diurnal oscillations. Abrogation of these rhythms, via antibiotic treatment or in germ-free mice, leads to alterations in intestinal epigenetic and transcriptional programs.76 Furthermore, the microbiota metabolome was demonstrated to drive transcriptional oscillations in the liver as well. One example is with hepatic metabolism of acetyl-para-aminophenol, in which antibiotic-treated or germ-free mice disrupted diurnal variations in the severity of acetyl-para-aminophenol–induced hepatotoxicity. This example highlights the importance of the microbiota in maintenance of the circadian transcriptome for hepatic drug metabolism.
Table 2.
Microbial and Circadian Influences on One Other as Well as on Metabolism
| Study authors | Influencing factor | Resulting effect |
|---|---|---|
| Govindarajan et al.75 | Microbial deconjugation of bile salts | -Unconjugated bile acids regulate the amplitude and periodicity of circadian gene expression in the ileum, colon, and liver |
| Leone et al.77 | High-fat diet | -Impaired central and hepatic circadian clock gene expression |
| -Dampened circadian oscillations of gut microbiota | ||
| -Altered microbial diversity | ||
| Zeb et al78, 79 | Time-restricted feeding | -Increased gut microbial diversity |
| -Reduced total cholesterol and triglyceride levels | ||
| Voigt et al.29 | Altered light-dark cycle and mutation of the Clock gene | -Microbiota dysbiosis: increase in proinflammatory bacteria and decrease in anti-inflammatory bacteria |
| Thaiss et al.76 | Germ-free mice receiving stool from jet-lagged humans | -Enhanced weight gain -Higher blood glucose levels after oral glucose challenge |
Timing and nutrient content are important factors in microbiotal content and diversity, which thereby signal changes in hepatic clock rhythms and metabolism. Diets with a high fat content have been studied extensively in relation to circadian rhythms and impact on gut organisms. Leone et al77 demonstrated that mice that fed high fat diets exhibit markedly impaired central and hepatic circadian clock gene expression, dampened circadian oscillations of gut microbiota, and altered microbial diversity. The timing of meals is also important, as variations in timing can alter circadian patterns and create shifts in bacterial populations,71 which have implications for the development of metabolic disease. Time-restricted feeding, a form of intermittent fasting, refers to time-restricted food consumption with a daily fasting duration of at least 12 hours, regardless of the quality and quantity of food. In a multicenter study of adult males, patients who underwent time-restricted feeding had increased gut microbial diversity (P < .005) as well as upregulation of Bmal1 and Clock mRNA (P = .002 and .0302, respectively) compared with controls without time constriction. Patients in the time-restricted feeding group demonstrated significant reduction in total cholesterol and triglyceride levels and increase in high-density lipoprotein cholesterol levels.78 Variation in meal timing is thus a key regulator of circadian rhythms, gut microbiome, and metabolic homeostasis.
Studies in germ-free mice highlight the 2-way relationship between gut microbes and circadian patterns, as these mice lack a microbiota in comparison with healthy controls. One such study demonstrated changes in circadian gene expression in response to dietary manipulations, including less responsiveness in circadian gene levels in the hypothalamus and liver to diets high in fat compared with mice with healthy micriobiota.77 Other studies have shown how manipulating circadian rhythms can lead to changes in the microbiome and subsequent metabolic modifications. Voigt et al induced both environmental and genetic disruption of circadian rhythms in healthy mice via weekly changes in the light-dark cycle and mutation of the core molecular Clock gene. These alterations lead to microbiota dysbiosis, characterized by an increase in proinflammatory bacteria, a decrease in anti-inflammatory, butyrate-producing bacteria, and a shift in the Bacteroidetes to Firmicutes ratio.29 One interesting study attempted to analyze the impact of jet lag, a common form of circadian disruption, by transfer of stool from jet-lagged humans to germ-free mice. Mice colonized with microbiota from jet-lagged individuals developed enhanced weight gain (P < .01) and featured higher blood glucose levels after oral glucose challenge (P < .05) compared with samples taken before the time shift.71,78 Interestingly, this metabolic alteration was reversed after a 2-week recovery from jet lag and repeat stool transplant. These studies highlight the significance of the bidirectional circadian-microbiome relationship in shaping metabolism.
Circadian Rhythms and the Gut Microbiome as Therapeutic Targets
Studies of circadian rhythms and the gut microbiome have offered several promising targets for prevention and treatment of metabolic diseases. Considering the extensive interplay described between circadian systems and the gut microbiome, therapies targeting either system will promote organismal homeostasis in a bidirectional fashion. Multiple interventions involving the circadian clock have been proposed and preliminarily investigated, with some promising targets involving restoration of normal rhythms by light exposure, timed food regimens, reduction in shift work, and schedule exercise. Targets for altering the microbiome and altering disease course have also been studied, which include the use of dietary modification, prebiotics, probiotics, and fecal microbiota transplant (FMT). Many of the most promising data, particularly regarding circadian clock therapy, come from animal studies, highlighting the need for human series and trials to establish therapies as well as the difficulties studying and implementing many of the involved interventions (especially behavioral interventions) for both microbiotal illnesses and disorders of circadian rhythms.
Dietary Modifications
In terms of clock-directed therapies, the impact of time-restricted feeding on mice has been described, with improvements in cholesterol and triglyceride levels.78 This intervention involves limiting caloric intake to certain time intervals to rectify clock oscillation. Mice on time-restricted feeding diets show improvement in obesity, insulin levels, and hepatic steatosis compared with mice allowed to eat without schedule, despite similar allowed levels of caloric intake. It has been shown that time-restricted feeding also changes gut microbial composition and relative abundance, highlighting the interplay of these 2 systems.79 Altering diet content has been investigated as well. One study of obese adults found that a 6-week intervention of a low-calorie, high-protein diet increased gut microbial diversity and improved insulin resistance and systemic inflammation.80
Behavioral Adjustments
Scheduled exercise is another suggested intervention, with proven improvements in insulin sensitivity through shifting the phase of clock oscillation in peripheral skeletal muscle.81 Considering the suggested relationship between shift work (including night work) and cardiovascular and metabolic disease, ongoing studies will attempt to determine the absolute risk that circadian dyssynchrony plays and guidelines for patient counseling.82 Light exposure therapy, particularly light avoidance in evening hours, has shown promising improvement in total sleep time and sleep hygiene, including in patients with neuropsychiatric disorders with assumed high prevalence of circadian dysregulation.83
Microbial-directed Therapies
Microbiotal therapeutic targets also have implications for metabolic disease. Prebiotic and probiotic formulations offer therapeutic benefit from treatment of the microbiome and are gaining rapid popularity over the last decade for the treatment of several gastrointestinal and metabolic diseases.84 Prebiotics are nondigestible polysaccharides that can mitigate gut dysbiosis by promoting the growth of healthy bacterial species, whereas probiotics are live microorganisms that theoretically act to balance out healthy bacterial concentrations. Given their newfound popularity, several analyses have attempted to determine their safety and efficacy in treating several gastrointestinal and metabolic illnesses. Meta-analysis involving prebiotic supplementation showed an overall reduced postprandial hyperglycemia and insulin secretion, as well as boosting satiety.85 Meta-analysis of probiotics, which included both animal studies as well as 19 randomized trials in humans, reported a beneficial effect of Lactobacillus-enriched probiotics on weight, and a strong species-dependent antiobesity effect was observed with Lactobacillus gasseri and Lactobacillus plantarum.86 In a meta-analysis regarding impact on gastrointestinal illnesses, probiotics were found to be generally beneficial in the treatment of several diseases, including pouchitis, infectious diarrhea, irritable bowel syndrome, Clostridium difficile infection, and antibiotic-associated diarrhea.87 FMT is another potential method of intervening on microbiotal health. It involves the administration of fecal material from a healthy donor into the gastrointestinal tract of a diseased patient to restore a healthy gut microbiota. FMT is a highly effective therapeutic option for recurrent Clostridioides difficile infection and is guideline-directed therapy for refractory Clostridioides difficile infection.88 It has also been studied on a limited basis for other disorders including metabolic disorders, with improvements in insulin sensitivity based on a suggested improvement in microbial diversity.49
Future Directions
Considering the importance of circadian clock pathways in homeostasis, the list of clock-associated diseases is bound to grow further, as is the search for new interventions and therapies to target disease and physiological decline. We anticipate further study and implementation of behavioral therapies into existing clinical models for many metabolic diseases, including light exposure therapy, caloric restriction, and time-restricted feeding as this field continues to grow. In addition, there are many promising pharmacologic agents acting on clock gene pathways being studied that hold potential in future human treatment. Many of these potential agents act to support the robust regulation of circadian amplitude, described as the difference between peak and trough levels of gene expression.89 Molecules that enhance this amplitude provide a possible avenue for therapeutic intervention and are being explored in animal models. Expanding therapeutic trials to humans on these clock-amplitude enhancing small molecules, which include compounds such as nobiletin (a natural flavonoid isolated from citrus peels) and a close analog tangeritin, provides an exciting future target for circadian functionality in metabolic disease.90 Nobiletin has also been shown to ameliorate lipid metabolism in hepatocytes via influence on Bmal1, which holds future potential as therapy for fatty liver disease.91 Selenium-rich diets have demonstrated cancer-preventive effects by acting as a modulator of circadian BMAL1 upregulation and warrant additional exploration as a disease modifier.92 Significant gaps in the understanding of the mechanisms of these pharmacologist agents remain and will need to be established in future studies.
Conclusion
Circadian rhythms are recurring metabolic cycles entrained to external signals with crucial implications for organismal homeostasis and metabolic output. Both the gut microbiome and circadian rhythms play an integral role in maintaining normal metabolism and influence each other in a reciprocal fashion. Disruption of either system impacts homeostasis in a bidirectional manner and has been shown to lead to metabolic changes that can impact health and potentially contribute to chronic metabolic diseases including obesity, diabetes, NAFLD, cirrhosis, and hepatocellular carcinoma. The gut microbiota influences metabolism through several mechanisms including gut barrier integrity, energy regulation via SCFAs, inflammatory modulation, and bile acid metabolism. Considering the impact of gut dysbiosis or circadian dyssynchrony on health, interest in therapeutic targets for these systems is growing. Examples of these interventions include time-restricted feeding, dietary modification, light exposure therapy, prebiotics, probiotics, and FMT.
Footnotes
Conflicts of Interest: The authors disclose no conflicts.
Funding: The authors report no funding.
Ethical Statement: The study did not require the approval of an institutional review board.
Authors' Contributions: All authors were involved in data collection, writing the manuscript, and editing the manuscript.
References
- 1.Asher G., Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell. 2015;161:84–92. doi: 10.1016/j.cell.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Bae S.A., Fang M.Z., Rustgi V., et al. At the interface of lifestyle, behavior, and circadian rhythms: metabolic implications. Front Nutr. 2019;6:132. doi: 10.3389/fnut.2019.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saini C., Suter D.M., Liani A., et al. The mammalian circadian timing system: synchronization of peripheral clocks. Cold Spring Harb Symp Quant Biol. 2011;76:39–47. doi: 10.1101/sqb.2011.76.010918. [DOI] [PubMed] [Google Scholar]
- 4.Vitaterna M.H., Takahashi J.S., Turek F.W. Overview of circadian rhythms. Alcohol Res Health. 2001;25:85–93. [PMC free article] [PubMed] [Google Scholar]
- 5.Pilorz V., Helfrich-Forster C., Oster H. The role of the circadian clock system in physiology. Pflugers Arch. 2018;470:227–239. doi: 10.1007/s00424-017-2103-y. [DOI] [PubMed] [Google Scholar]
- 6.Freedman M.S., Lucas R.J., Soni B., et al. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 7.Eckel-Mahan K., Sassone-Corsi P. Metabolism control by the circadian clock and vice versa. Nat Struct Mol Biol. 2009;16:462–467. doi: 10.1038/nsmb.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakahata Y., Kaluzova M., Grimaldi B., et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reppert S.M., Weaver D.R. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 10.Sahar S., Sassone-Corsi P. Regulation of metabolism: the circadian clock dictates the time. Trends Endocrinol Metab. 2012;23:1–8. doi: 10.1016/j.tem.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Minh N., Damiola F., Tronche F., et al. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 2001;20:7128–7136. doi: 10.1093/emboj/20.24.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaenisch R., Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33 Suppl:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 13.Hudec M., Dankova P., Solc R., et al. Epigenetic regulation of circadian rhythm and its possible role in diabetes mellitus. Int J Mol Sci. 2020;21:E3005. doi: 10.3390/ijms21083005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowrey P.L., Shimomura K., Antoch M.P., et al. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng H., Zhu Y., Goldberg J., et al. DNA methylation of five core circadian genes jointly contributes to glucose metabolism: a gene-set analysis in monozygotic twins. Front Genet. 2019;10:329. doi: 10.3389/fgene.2019.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaminsky Z.A., Tang T., Wang S.-C., et al. DNA methylation profiles in monozygotic and dizygotic twins. Nat Genet. 2009;41:240–245. doi: 10.1038/ng.286. [DOI] [PubMed] [Google Scholar]
- 17.Etchegaray J.-P., Mostoslavsky R. Interplay between metabolism and epigenetics: a nuclear adaptation to environmental changes. Mol Cell. 2016;62:695–711. doi: 10.1016/j.molcel.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gil-Lozano M., Mingomataj E.L., Wu W.K., et al. Circadian secretion of the intestinal hormone GLP-1 by the rodent L cell. Diabetes. 2014;63:3674–3685. doi: 10.2337/db13-1501. [DOI] [PubMed] [Google Scholar]
- 19.Maroni M.J., Capri K.M., Cushman A.V., et al. The timing of fasting leads to different levels of food consumption and PYY3-36 in nocturnal mice. Hormones (Athens) 2020;19:549–558. doi: 10.1007/s42000-020-00221-x. [DOI] [PubMed] [Google Scholar]
- 20.Moghadam A.A., Moran T.H., Dailey M.J. Alterations in circadian and meal-induced gut peptide levels in lean and obese rats. Exp Biol Med (Maywood) 2017;242:1786–1794. doi: 10.1177/1535370217732041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gooley J.J. Circadian regulation of lipid metabolism. Proc Nutr Soc. 2016;75:440–450. doi: 10.1017/S0029665116000288. [DOI] [PubMed] [Google Scholar]
- 22.Yang X., Downes M., Yu R.T., et al. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 23.Shostak A., Meyer-Kovac J., Oster H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes. 2013;62:2195–2203. doi: 10.2337/db12-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan X., Zhang Y., Wang L., et al. Diurnal regulation of MTP and plasma triglyceride by CLOCK is mediated by SHP. Cell Metab. 2010;12:174–186. doi: 10.1016/j.cmet.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoogerwerf W.A., Hellmich H.L., Cornelissen G., et al. Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology. 2007;133:1250–1260. doi: 10.1053/j.gastro.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Rhoads M.K., Balagee V., Thomas S.J. Circadian regulation of blood pressure: of mice and men. Curr Hypertens Rep. 2020;22:40. doi: 10.1007/s11906-020-01043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan A., Hu F.B. Effects of carbohydrates on satiety: differences between liquid and solid food. Curr Opin Clin Nutr Metab Care. 2011;14:385–390. doi: 10.1097/MCO.0b013e328346df36. [DOI] [PubMed] [Google Scholar]
- 28.Butler T.D., Gibbs J.E. Circadian host-microbiome interactions in immunity. Front Immunol. 2020;11:1783. doi: 10.3389/fimmu.2020.01783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voigt R.M., Summa K.C., Forsyth C.B., et al. The circadian clock mutation promotes intestinal dysbiosis. Alcohol Clin Exp Res. 2016;40:335–347. doi: 10.1111/acer.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patton D.F., Katsuyama A.M., Pavlovski I., et al. Circadian mechanisms of food anticipatory rhythms in rats fed once or twice daily: clock gene and endocrine correlates. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reinke H., Asher G. Circadian clock control of liver metabolic functions. Gastroenterology. 2016;150:574–580. doi: 10.1053/j.gastro.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 32.Suwazono Y., Dochi M., Oishi M., et al. Shiftwork and impaired glucose metabolism: a 14-year cohort study on 7104 male workers. Chronobiol Int. 2009;26:926–941. doi: 10.1080/07420520903044422. [DOI] [PubMed] [Google Scholar]
- 33.Canakis A., Qazi T. Sleep and fatigue in IBD: an unrecognized but important extra-intestinal manifestation. Curr Gastroenterol Rep. 2020;22:8. doi: 10.1007/s11894-020-0746-x. [DOI] [PubMed] [Google Scholar]
- 34.Palmieri O., Mazzoccoli G., Bossa F., et al. Systematic analysis of circadian genes using genome-wide cDNA microarrays in the inflammatory bowel disease transcriptome. Chronobiol Int. 2015;32:903–916. doi: 10.3109/07420528.2015.1050726. [DOI] [PubMed] [Google Scholar]
- 35.Gnocchi D., Custodero C., Sabba C., et al. Circadian rhythms: a possible new player in non-alcoholic fatty liver disease pathophysiology. J Mol Med (Berl) 2019;97:741–759. doi: 10.1007/s00109-019-01780-2. [DOI] [PubMed] [Google Scholar]
- 36.Montagnese S., Russo F.P., Amodio P., et al. Hepatic encephalopathy 2018: a clinical practice guideline by the Italian Association for the Study of the Liver (AISF) Dig Liver Dis. 2019;51:190–205. doi: 10.1016/j.dld.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 37.Ma K., Xiao R., Tseng H.T., et al. Circadian dysregulation disrupts bile acid homeostasis. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filipski E., Subramanian P., Carriere J., et al. Circadian disruption accelerates liver carcinogenesis in mice. Mutat Res. 2009;680:95–105. doi: 10.1016/j.mrgentox.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Lin Y.M., Chang J.H., Yeh K.T., et al. Disturbance of circadian gene expression in hepatocellular carcinoma. Mol Carcinog. 2008;47:925–933. doi: 10.1002/mc.20446. [DOI] [PubMed] [Google Scholar]
- 40.Nicholson J.K., Holmes E., Kinross J., et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 41.Qin J., Li R., Raes J., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guarner F., Malagelada J.R. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 43.Sharma R., Young C., Neu J. Molecular modulation of intestinal epithelial barrier: contribution of microbiota. J Biomed Biotechnol. 2010;2010:305879. doi: 10.1155/2010/305879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miele L., Valenza V., La Torre G., et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 45.Rosenbaum M., Knight R., Leibel R.L. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol Metab. 2015;26:493–501. doi: 10.1016/j.tem.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leung C., Rivera L., Furness J.B., et al. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412–425. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 47.Chakraborti C.K. New-found link between microbiota and obesity. World J Gastrointest Pathophysiol. 2015;6:110–119. doi: 10.4291/wjgp.v6.i4.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tai N., Wong F.S., Wen L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev Endocr Metab Disord. 2015;16:55–65. doi: 10.1007/s11154-015-9309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vrieze A., Van Nood E., Holleman F., et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916.e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 50.Karlsson F.H., Tremaroli V., Nookaew I., et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 51.Qin J., Li Y., Cai Z., et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 52.Murri M., Leiva I., Gomez-Zumaquero J.M., et al. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med. 2013;11:46. doi: 10.1186/1741-7015-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cani P.D., Bibiloni R., Knauf C., et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 54.Henao-Mejia J., Elinav E., Jin C., et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miura K., Ohnishi H. Role of gut microbiota and toll-like receptors in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:7381–7391. doi: 10.3748/wjg.v20.i23.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shen T.D., Pyrsopoulos N., Rustgi V.K. Microbiota and the liver. Liver Transpl. 2018;24:539–550. doi: 10.1002/lt.25008. [DOI] [PubMed] [Google Scholar]
- 57.Wong V.W., Wong G.L., Chan H.Y., et al. Bacterial endotoxin and non-alcoholic fatty liver disease in the general population: a prospective cohort study. Aliment Pharmacol Ther. 2015;42:731–740. doi: 10.1111/apt.13327. [DOI] [PubMed] [Google Scholar]
- 58.Rattan P., Minacapelli C.D., Rustgi V. The microbiome and hepatocellular carcinoma. Liver Transpl. 2020;26:1316–1327. doi: 10.1002/lt.25828. [DOI] [PubMed] [Google Scholar]
- 59.Kir S., Beddow S.A., Samuel V.T., et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Flint H.J., Bayer E.A., Rincon M.T., et al. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 61.Lake A.D., Novak P., Shipkova P., et al. Decreased hepatotoxic bile acid composition and altered synthesis in progressive human nonalcoholic fatty liver disease. Toxicol Appl Pharmacol. 2013;268:132–140. doi: 10.1016/j.taap.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sayin S.I., Wahlstrom A., Felin J., et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Turnbaugh P.J., Ley R.E., Mahowald M.A., et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 64.Aron-Wisnewsky J., Prifti E., Belda E., et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut. 2019;68:70–82. doi: 10.1136/gutjnl-2018-316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Larsen N., Vogensen F.K., van den Berg F.W., et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le Roy T., Llopis M., Lepage P., et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 67.Shen F., Zheng R.D., Sun X.Q., et al. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2017;16:375–381. doi: 10.1016/S1499-3872(17)60019-5. [DOI] [PubMed] [Google Scholar]
- 68.Boursier J., Mueller O., Barret M., et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsai H.J., Tsai Y.C., Hung W.W., et al. Gut microbiota and non-alcoholic fatty liver disease severity in type 2 diabetes patients. J Pers Med. 2021;11:238. doi: 10.3390/jpm11030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwabe R.F., Greten T.F. Gut microbiome in HCC - mechanisms, diagnosis and therapy. J Hepatol. 2020;72:230–238. doi: 10.1016/j.jhep.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 71.Paulose J.K., Wright J.M., Patel A.G., et al. Human gut bacteria are sensitive to melatonin and express endogenous circadian rhythmicity. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thaiss C.A., Zeevi D., Levy M., et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159:514–529. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 73.Paschos G.K., FitzGerald G.A. Circadian clocks and metabolism: implications for microbiome and aging. Trends Genet. 2017;33:760–769. doi: 10.1016/j.tig.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mukherji A., Kobiita A., Ye T., et al. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153:812–827. doi: 10.1016/j.cell.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 75.Govindarajan K., MacSharry J., Casey P.G., et al. Unconjugated bile acids influence expression of circadian genes: a potential mechanism for microbe-host crosstalk. PLoS One. 2016;11 doi: 10.1371/journal.pone.0167319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thaiss C.A., Levy M., Korem T., et al. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell. 2016;167:1495–1510.e12. doi: 10.1016/j.cell.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 77.Leone V., Gibbons S.M., Martinez K., et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe. 2015;17:681–689. doi: 10.1016/j.chom.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeb F., Wu X., Chen L., et al. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br J Nutr. 2020;123:1216–1226. doi: 10.1017/S0007114519003428. [DOI] [PubMed] [Google Scholar]
- 79.Zeb F., Wu X., Chen L., et al. Time-restricted feeding is associated with changes in human gut microbiota related to nutrient intake. Nutrition. 2020;78:110797. doi: 10.1016/j.nut.2020.110797. [DOI] [PubMed] [Google Scholar]
- 80.Cotillard A., Kennedy S.P., Kong L.C., et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 81.Wolff G., Esser K.A. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med Sci Sports Exerc. 2012;44:1663–1670. doi: 10.1249/MSS.0b013e318255cf4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X.S., Armstrong M.E., Cairns B.J., et al. Shift work and chronic disease: the epidemiological evidence. Occup Med (Lond) 2011;61:78–89. doi: 10.1093/occmed/kqr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Faulkner S.M., Bee P.E., Meyer N., et al. Light therapies to improve sleep in intrinsic circadian rhythm sleep disorders and neuro-psychiatric illness: a systematic review and meta-analysis. Sleep Med Rev. 2019;46:108–123. doi: 10.1016/j.smrv.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 84.Lee G.R., Maarouf M., Hendricks A.J., et al. Topical probiotics: the unknowns behind their rising popularity. Dermatol Online J. 2019;25 13030/qt2v83r5wk. [PubMed] [Google Scholar]
- 85.Kellow N.J., Coughlan M.T., Reid C.M. Metabolic benefits of dietary prebiotics in human subjects: a systematic review of randomised controlled trials. Br J Nutr. 2014;111:1147–1161. doi: 10.1017/S0007114513003607. [DOI] [PubMed] [Google Scholar]
- 86.Million M., Angelakis E., Paul M., et al. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathog. 2012;53:100–108. doi: 10.1016/j.micpath.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 87.Ritchie M.L., Romanuk T.N. A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McDonald L.C., Gerding D.N., Johnson S., et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis. 2018;66:987–994. doi: 10.1093/cid/ciy149. [DOI] [PubMed] [Google Scholar]
- 89.Gloston G.F., Yoo S.H., Chen Z.J. Clock-enhancing small molecules and potential applications in chronic diseases and aging. Front Neurol. 2017;8:100. doi: 10.3389/fneur.2017.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Z., Yoo S.H., Park Y.S., et al. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc Natl Acad Sci U S A. 2012;109:101–106. doi: 10.1073/pnas.1118034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.He B., Nohara K., Park N., et al. The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab. 2016;23:610–621. doi: 10.1016/j.cmet.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu Y., Spengler M.L., Kuropatwinski K.K., et al. Selenium is a modulator of circadian clock that protects mice from the toxicity of a chemotherapeutic drug via upregulation of the core clock protein, BMAL1. Oncotarget. 2011;2:1279–1290. doi: 10.18632/oncotarget.411. [DOI] [PMC free article] [PubMed] [Google Scholar]