Abstract
Background and Aims
The 2012 and 2020 US Multi-Society Task Force postpolypectomy guidelines have recommended progressively longer surveillance intervals for patients with low-risk adenomas (LRAs). These guidelines require data from past colonoscopies. We examined the impact of the 2012 guidelines for second surveillance on clinical practice, including the availability of prior colonoscopy data, with the aim of informing the implementation of the 2020 guidelines.
Methods
We identified surveillance colonoscopies at Stanford Health Care and the Palo Alto Veterans Affairs Health Care System in 3 periods: preguideline (March–August 2012), postguideline (January–June 2013), and delayed postguideline (July–September 2017). We collected data on the most recent previous colonoscopy, findings at the study entry surveillance colonoscopy, and recommendations for subsequent surveillance.
Results
Among 977 patients, the most recent prior colonoscopy data were available in 78% of preguideline, 78% of postguideline, and 61% of delayed postguideline cases (P < .001). The fraction of surveillance colonoscopy reports that deferred recommendations awaiting pathology increased from 6% to 11% in preguideline and postguideline to 59% in delayed postguideline cases (P < .001). Overall adherence to guidelines for subsequent surveillance was similar in all 3 periods (54%–67%; P = .089). In the postguideline and delayed postguideline periods combined, a 10-year subsequent surveillance interval was recommended in 0 of 29 cases with LRA followed by normal surveillance colonoscopy.
Conclusion
In patients undergoing surveillance, prior colonoscopy data were not always available and recommendations were often deferred awaiting pathology. Adherence to subsequent surveillance guidelines was suboptimal, especially for LRA followed by normal colonoscopy. Strategies addressing these gaps are needed to optimize implementation of the updated 2020 postpolypectomy guidelines.
Keywords: Colorectal Adenoma, Colorectal Polyps, Guidelines, Implementation, Surveillance
See editorial on page 156
Introduction
Colorectal cancer (CRC) remains a leading cause of cancer-related mortality in the United States.1 There is robust evidence that screening decreases CRC incidence and mortality.2, 3, 4, 5, 6, 7 In contrast, although postpolypectomy colonoscopy surveillance is well established in practice, evidence of its impact on CRC outcomes is still developing.8 As the spectrum of postpolypectomy CRC risk has been clarified, guidelines have endorsed progressively longer surveillance intervals, especially for patients with low-risk adenomas (LRAs).9, 10, 11 With surveillance accounting for approximately 20% of US colonoscopy volume,12 efforts are needed to optimize surveillance programs.
Adherence to medical guidelines is often suboptimal.13, 14, 15 Despite growing evidence that individuals with only LRA have relatively low risk for interval CRC and CRC mortality,5,16 adherence to surveillance guidelines has been variable in the past17, 18, 19 with overutilization in low-risk individuals and underutilization in high-risk individuals.20, 21, 22 The US Multi-Society Task Force (MSTF) 2012 guidelines10 for the first time provided recommendations for second surveillance, based on findings of high-risk adenomas (HRAs) or LRA at baseline and first surveillance colonoscopies. This requirement of detailed knowledge of preceding colonoscopy findings introduced new complexity. The 2020 MSTF guidelines further increased the need for detailed information on polyp number, size, and histology to inform surveillance recommendations.9
Our aim was to determine adherence to recommendations for subsequent (as opposed to initial) surveillance following publication of the 2012 MSTF guidelines. Our motivation was to inform strategies to promote adherence with the latest 2020 MSTF guidelines.9 Thus, we performed a study comparing clinical practice patterns of subsequent surveillance following vs preceding publication of the 2012 guidelines, and we contrasted immediate vs delayed postguideline periods to account for the time it takes to implement new guidelines in practice. We hypothesized that detailed prior colonoscopy information to inform subsequent surveillance recommendations may often be lacking; that adherence to new guidelines might be poor, particularly to longer intervals after LRA removal followed by normal colonoscopy; and that adherence with new guidelines might improve over time.
Methods
Study Design
We focused on recommendations for subsequent surveillance, and not for first surveillance after baseline screening colonoscopy. We conducted a retrospective review of electronic health records (EHR) for postpolypectomy surveillance colonoscopies performed at Stanford Health Care (SHC) and the Palo Alto Veterans Affairs (PAVA) Health Care System for discrete periods from March 2012 through September 2017. These surveillance colonoscopies were considered the study entry colonoscopies. The 2012 guidelines were published in September 2012.10 We defined 3 periods of interest: 1) preguideline (March–August 2012), 2) postguideline (January–June 2013), and 3) delayed postguideline (July–September 2017). September–December 2012 were excluded to allow for initial guideline dissemination. 5 years was chosen as a reasonable time period to observe a change from an initial to a delayed postguideline period. No specific institutional-level education or instructions were provided to endoscopists for surveillance interval recommendations. Thus, this study was an observation of natural guideline dissemination in practice.
Data were extracted from 2 distinct EHR systems, Epic at SHC and Computerized Patient Record System (CPRS) at PAVA, using the following colonoscopy indications for the study entry colonoscopy in both EHR systems: 1) high risk colon cancer surveillance: personal history of colonic polyps, 2) surveillance: personal history of adenomatous polyps on the last colonoscopy, 3) surveillance: personal history polyps unknown histology last colonoscopy, 4) follow-up for history of adenomatous polyps in the colon, and 5) established adenomatous polyps in the colon. Data were managed in a REDCap database designed and maintained by the Stanford Quantitative Sciences Unit. The study protocol was performed in accordance with the Declaration of Helsinki and approved by the Stanford University Institutional Review Board.
Study Population
Patients identified for inclusion were adults of age at least 50 years presenting for postpolypectomy surveillance colonoscopy during the time periods of interest. Exclusion criteria included inflammatory bowel disease history, piecemeal resection of large polyp at the study entry colonoscopy, personal or family history of colorectal cancer, inadequate bowel preparation, incomplete colonoscopy, and those who would be > 85 years old at the time of the next surveillance.
Study Variables
We collected findings at the study entry surveillance colonoscopy and documented recommendations for subsequent surveillance. We then searched back in time for any available information on the most recent prior colonoscopy, including colonoscopy reports, pathology results, clinical notes, and communications to patients, and referring physicians. Information collected included patient demographics (age, sex), endoscopist, polyp characteristics (number, size, histology), colonoscopy quality indicators (extent of exam, preparation quality), surveillance recommendations and where they were found (physician note, colonoscopy report, pathology report, patient letter), and whether endoscopist recommendations changed after pathology was available.
Outcomes analyzed included: 1) availability of information for the most recent prior colonoscopy and 2) level of adherence with applicable surveillance guidelines. In the preguideline group, we assessed adherence to the previous 2008 MSTF guidelines.11 In the postguideline and delayed postguideline groups, we assessed adherence to the 2012 MSTF guidelines.10 In the 2012 but not the 2008 guidelines, recommendations for future surveillance depend on the initial surveillance and the preceding baseline screening colonoscopy. We therefore performed analyses stratifying by whether information on the most recent prior colonoscopy was available and whether the presence or absence of HRA or LRA was documented.
Recommendations for HRA vs LRA
As in the 2012 guidelines, LRA was defined as 1–2 nonadvanced, <10 mm tubular adenomas, and HRA was defined as 3–10 tubular adenomas, tubular adenoma ≥10 mm, tubulovillous or villous adenoma, or adenoma with high-grade dysplasia. The 2008 guideline did not address sessile serrated lesions (SSLs). In the 2012 guideline, advanced serrated lesions (SSL ≥10 mm or with dysplasia, or traditional serrated adenoma) were treated like advanced adenomas, with a 3-year surveillance interval recommended; for non-advanced SSLs <10 mm, a 5-year surveillance interval was recommended, which is encompassed by the 5–10-year interval that was recommended for 1–2 small (<10 mm) adenomas. Therefore, in assessing adherence to the guidelines, nonadvanced SSLs <10 mm were grouped with LRA, and advanced SSLs were grouped with HRA.
The 2012 guidelines did not provide guidance on surveillance beyond the second surveillance. We acknowledge that in our study, the most recent prior colonoscopy may not have been a baseline screening colonoscopy, and that if the most recent prior colonoscopy did not document HRA, it is possible that the patient had HRA on an even earlier colonoscopy. Evidence suggests that patients whose colonoscopies have only ever revealed LRA may require little or no surveillance, especially after normal examinations.23, 24, 25, 26, 27, 28 However, patients with any prior history of HRA may remain at increased risk for subsequent future HRA.29,30 We therefore performed exploratory analyses in which we considered Scenario 1 (most stringent) in which we assumed that these patients did not have a previous HRA at any point, and Scenario 2 (most liberal) in which we assumed these patients had a previous HRA at some point before the most recent past colonoscopy.
Data Analysis
We compared demographic characteristics and information about preceding colonoscopy and surveillance findings and recommendations by the 3 periods of interest. Age at study entry surveillance colonoscopy was compared using analysis of variance; categorical variables were compared using chi-square tests (or Fisher's exact test for variables with small counts). We used descriptive statistics for assessing recommendations at surveillance and presenting adherence to guidelines. Statistical analyses were conducted using SAS software version 9.4 (SAS, Cary, NC).
Results
Study Population
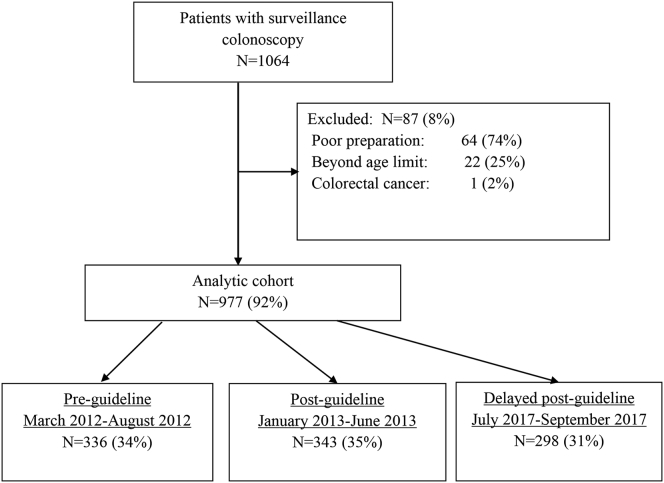
A total of 1064 patients were identified for possible inclusion. After the exclusion of 87 patients because of poor preparation, age, or CRC diagnosis, 977 patients with colonoscopies performed by 47 endoscopists remained in the analytic cohort (Figure 1). There were comparable numbers of patients in the preguideline, postguideline, and delayed postguideline groups (Figure 1), and these groups had similar distributions for age and sex (Table 1).
Figure 1.
Study flow diagram.
Table 1.
Surveillance Colonoscopy Demographics, Findings, and Characteristics of Recommendations at Surveillance
| Patient demographics, colonoscopy information and surveillance recommendations | Preguidelines n = 336 |
Postguidelines n = 343 |
Delayed postguidelines n = 298 |
P-value |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Age, mean (SD) | 66 (8) | 65 (8) | 66 (7) | .142 |
| Gender, male | 280 (83) | 280 (82) | 230 (77) | .144 |
| Most recent prior colonoscopy information readily availablea | 261 (78) | 266 (78) | 182 (61) | <.001 |
| Most recent preceding colonoscopy availability | n = 261 | n = 266 | n = 182 | |
| Information sources | ||||
| Colonoscopy report | 250 (96) | 257 (97) | 182 (100) | .024 |
| Pathology report | 68 (26) | 91 (34) | 112 (62) | <.001 |
| Note or record | 15 (6) | 11 (4) | 0 | .193 |
| Letter to patient or physician | 46 (18) | 71 (27) | 108 (59) | <.001 |
| Other | 20 (8) | 14 (5) | 0 | <.001 |
| Surveillance colonoscopyb findings and recommendations | n = 336 | n = 343 | n = 298 | |
| Surveillance colonoscopy findings | ||||
| High-risk adenoma | 75 (22) | 78 (23) | 73 (25) | .876 |
| Low-risk adenoma | 121 (36) | 117 (34) | 103 (35) | .777 |
| No adenoma (or hyperplastic polyps only) | 140 (42) | 148 (43) | 122 (41) | .888 |
| Surveillance recommendation in colonoscopy report | ||||
| “Await pathology report” | 21 (6) | 37 (11) | 175 (59) | <.001 |
| Discrete interval (eg 5 y) | 291 (87) | 271 (79) | 95 (32) | <.001 |
| Range (eg 3–5 y) | 18 (5) | 27 (8) | 26 (9) | .229 |
| No recommendation | 6 (2) | 8 (2) | 2 (1) | .244 |
| Second surveillance interval recommendations among those with documented HRA or LRA at most recent preceding colonoscopyc | n = 109 | n = 128 | n = 58 | |
| Recommendation adherent to guidelines | 73 (67) | 69 (54) | 38 (66) | .089 |
| Recommendation interval too short | 28 (26) | 50 (39) | 15 (26) | .056 |
| Recommendation interval too long | 1 (1) | 1 (1) | 1 (2) | .791 |
| Recommendation interval unknown | 7 (6) | 8 (6) | 4 (7) | 1.000 |
| Second surveillance interval recommendations among those with no HRA or LRA at most recent preceding colonoscopyc -- assuming no prior advanced adenoma or advanced sessile serrated lesion ever (Scenario 1) | n = 152 | n = 138 | n = 124 | |
| Recommendation adherent to guidelines | 121 (80) | 70 (51) | 66 (53) | <.001 |
| Recommendation interval too short | 28 (18) | 63 (46) | 49 (40) | <.001 |
| Recommendation interval too long | 1 (1) | 1 (1) | 3 (2) | .450 |
| Recommendation interval unknown | 2 (1) | 4 (3) | 6 (5) | .227 |
| Second surveillance interval recommendations among those with no HRA or LRA at most recent preceding colonoscopyc -- assuming ≥1 prior advanced adenoma or advanced sessile serrated lesion ever (Scenario 2) | n = 152 | n = 138 | n = 124 | |
| Recommendation adherent to guidelines | 120 (80) | 95 (69) | 92 (74) | .146 |
| Recommendation interval too short | 28 (18) | 26 (19) | 12 (10) | .070 |
| Recommendation interval too long | 2 (1) | 13 (9) | 15 (12) | .0003 |
| Recommendation interval unknown | 2 (1) | 4 (3) | 5 (4) | .346 |
Statistically significant P values are indicated in bold.
1 High-risk adenoma defined as 3 or more adenomas (including sessile serrated adenomas/polyps [SSA/P]), tubular adenomas or SSA/SSP >10 mm, adenoma or SSA/SSP with villous histology or HGD, as well as traditional serrated adenomas.
2 Low-risk adenoma defined as 1–2 tubular adenomas or SSA/SSP <10 mm.
Readily available defined as the presence of the baseline colonoscopy report in the medical record or detailed history on the past colonoscopy reported in the pathology report or as a note in the medical record.
Surveillance colonoscopy defined as first colonoscopy following prior polypectomy.
Adenomas at most recent preceding and first surveillance colonoscopies were classified using information from the pathology report/letter if available. If a pathology report was not available, then information from the colonoscopy report was used.
Availability of Data on the Most Recent Prior Colonoscopy
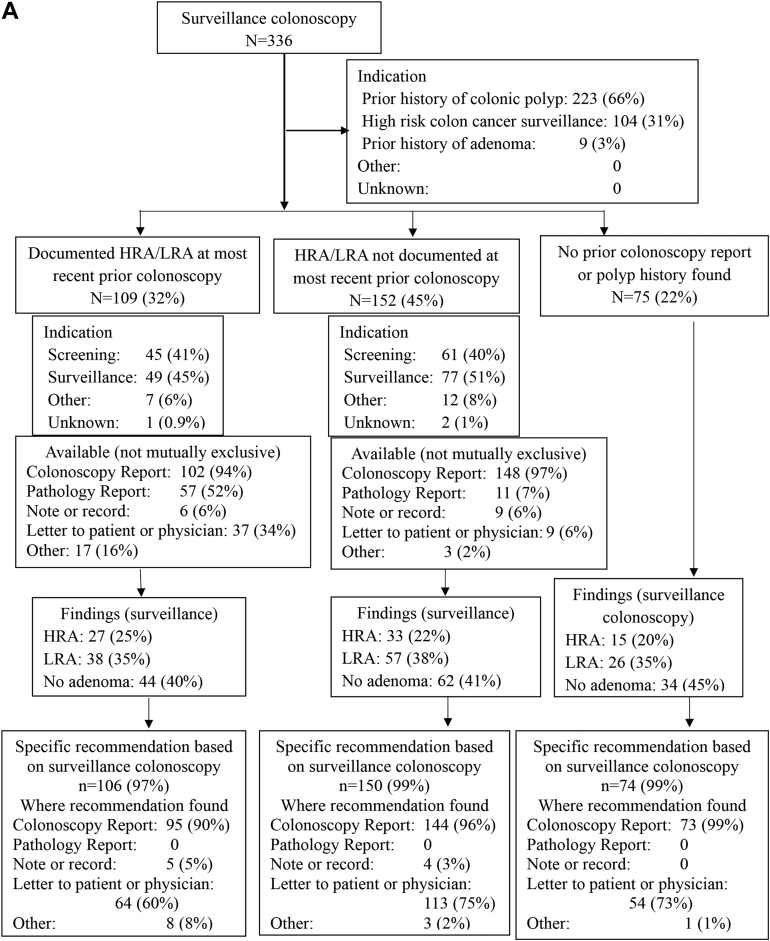
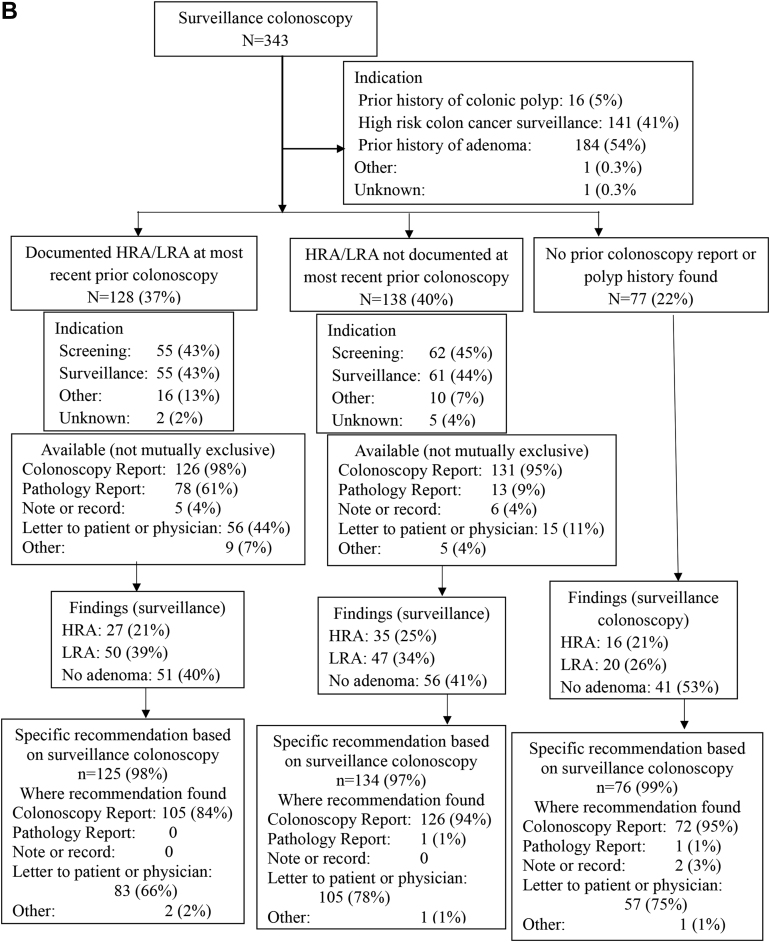
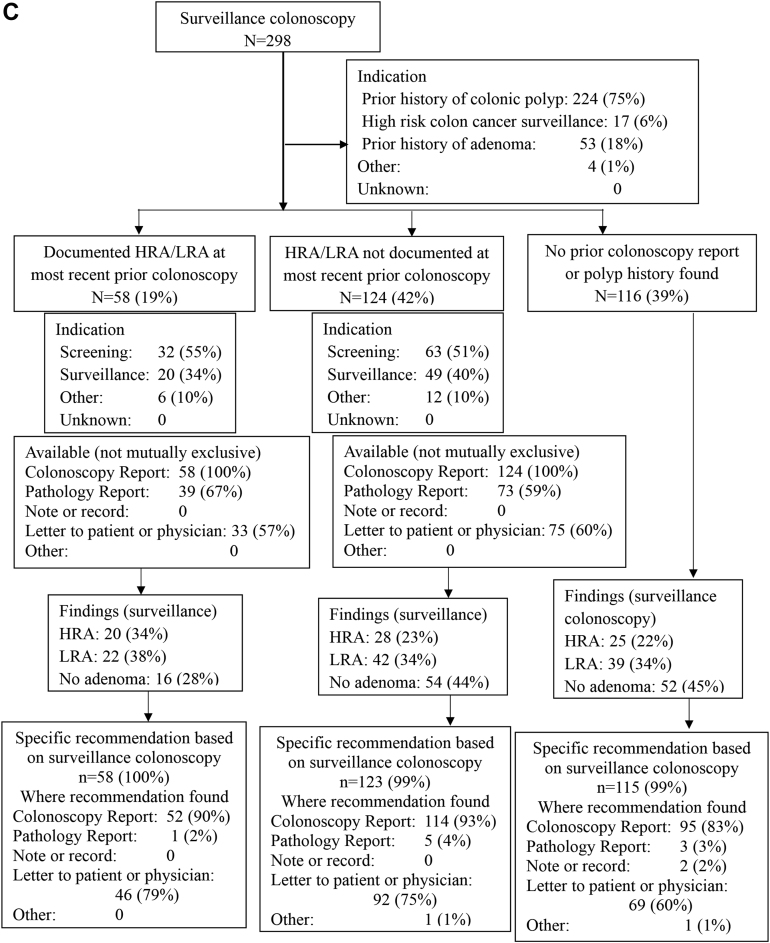
Data from the most recent prior colonoscopy were available more often in the preguideline (261/336, 78%) and postguideline (266/343, 78%) groups than in the delayed postguideline group (182/298, 61%) (P < .001) (Table 1). These overall trends were observed at SHC (Table A1) but not at the PAVA (Table A2). When data were available, the most common sources were the prior colonoscopy report itself (96%–100%), the pathology report (26%–62%), and a letter to the referring provider or patient (18%–59%), with increasing availability of each of these sources over time (Table 1, Figure 2).
Figure 2.
Findings at the study entry surveillance colonoscopy and recommendations for subsequent surveillance, stratified by findings preceding the study entry colonoscopy, for preguideline (A), postguideline (B), and delayed post guideline (C) periods.
Data Pertaining to the Most Recent Prior Colonoscopy
When data from the most recent prior colonoscopy were available, the most common indications for that prior colonoscopy were screening (40%–41% preguideline, 43%–45% postguideline, 51%–55% delayed postguideline) or surveillance (45%–51% preguideline, 43%–44% postguideline, 34%–40% delayed postguideline) (Figure 2). Documentation at the most recent prior colonoscopy showed HRA or LRA in 19%–37% of cases, and no HRA or LRA in 40%–45% of cases, depending on the study period, with no pertinent available documentation in the remaining 22%–39% of cases (Figure 2).
Findings at the Study Entry Surveillance Colonoscopy
HRA were detected in 22%–25% and LRA in 34%–36% of study entry surveillance colonoscopies, depending on the study period, with no statistically significant differences between periods (Table 1). Figure 2 shows the detection of HRA or LRA by study period, stratified by the availability and content of data pertaining to the most recent prior colonoscopy.
Recommendations for Subsequent Surveillance Colonoscopy at the Time of Study Entry Surveillance Colonoscopy
A recommendation regarding subsequent surveillance colonoscopy was found following the entry surveillance colonoscopy in nearly all cases (97%–99% preguideline and postguideline, and 99%–100% delayed postguideline) (Figure 2). There was almost always some recommendation in the colonoscopy report (90%–99% preguideline, 97%–99% postguideline, and 83%–93% delayed postguideline) and often also in a letter to the patient or referring provider (60%–75% preguideline, 66%–78% postguideline, and 60%–79% delayed postguideline) (Figure 2).
For recommendations provided in the study entry surveillance colonoscopy report itself, there was a substantial increase over study periods in the number of cases deferring a final recommendation until pathology was available: use of “await pathology report” increased from 6% of preguideline and 11% of postguideline cases to 59% of delayed postguideline cases (P < .001, Table 1). Complementing this trend, there was a substantial decrease over time in the number of cases with a recommendation in the colonoscopy report listing a discrete surveillance interval (87% preguideline, 79% post guideline, and 32% delayed post guideline; P < .001) (Table 1, Tables A1 and A2). A recommendation for an interval range (eg 3–5 years) was rarely made in the colonoscopy report (Table 1).
Recommendations for Subsequent Surveillance Colonoscopy Once Study Entry Surveillance Colonoscopy Pathology was Available: HRA or LRA at the Most Recent Prior Colonoscopy
Among those with HRA or LRA documented at the most recent prior colonoscopy, the final recommendations for subsequent surveillance colonoscopy adhered with guidelines in 67% of preguideline cases, 54% of postguideline cases, and 66% of delayed postguideline cases (P = .089) (Table 1). Recommendations in which the interval was too short vs guideline recommendations were made in 26% of preguideline cases, 39% of postguideline cases, and 26% or delayed postguideline cases (P = .056) (Table 1, Tables A1 and A2).
Recommendations that were not adherent with guidelines were most common in the postguideline and delayed postguideline periods for cases with LRA on the most recent prior colonoscopy followed by no LRA or HRA at the study entry surveillance colonoscopy (26/29 with interval too short; 3/29 with no future surveillance recommendation provided) (Table 2; Tables A3 and A4). A recommendation for a 10-year surveillance interval was made in 0/29 of these cases (Table 2).
Table 2.
Recommendations at Surveillance Depending on Most Recent Preceding Colonoscopy and Surveillance Findings
| Most recent preceding colonoscopy findings | First surveillance findings | Guideline-recommended interval for second surveillance (y) | N | Adherent with guidelines N (%) |
Recommendations earlier than guidelines N (%) |
Recommendations later than guidelines N (%) |
Recommendations not available N (%) |
|---|---|---|---|---|---|---|---|
| Pre-2012 guidelinesa | |||||||
| Low-risk adenoma | High-risk adenoma | 3 | 5 | 5 (100) | NA | NA | NA |
| Low-risk adenoma | 5–10 implied | 15 | 10 (67) | 4 (27) | 0 | 1 (7) | |
| No adenoma | 5–10 implied | 23 | 19 (83) | 2 (9) | 0 | 2 (9) | |
| High-risk adenoma | High-risk adenoma | 3 | 22 | 16 (73) | 6 (27) | 0 | 0 |
| Low-risk adenoma | 5 | 23 | 11 (48) | 10 (43) | 1 (4) | 1 (4) | |
| No adenoma | 5 | 21 | 12 (57) | 6 (29) | 0 | 3 (14) | |
| No adenoma | High-risk adenoma | 3 | 33 | 23 (70) | 8 (24) | 1 (3) | 1 (3) |
| Low-risk adenoma | 5–10 implied | 57 | 42 (74) | 14 (25) | 0 | 1 (2) | |
| Stringent Scenario: No adenoma, assuming no prior advanced adenoma or advanced sessile serrated lesion ever |
5–10 implied | 62 | 56 (90) | 6 (10) | 0 | 0 | |
| Liberal Scenario: No adenoma, assuming ≥1 prior advanced adenoma or advanced sessile serrated lesion ever |
5 implied | 62 | 55 (89) | 6 (10) | 1 (2) | 0 | |
| Post-2012 guidelines | |||||||
| Low-risk adenoma | High-risk adenoma | 3 | 5 | 2 (40) | 1 (20) | 0 | 2 (40) |
| Low-risk adenoma | 5 | 19 | 15 (79) | 3 (16) | 0 | 1 (5) | |
| No adenoma | 10 | 26 | 0 | 23 (88) | 0 | 3 (12) | |
| High-risk adenoma | High-risk adenoma | 3 | 22 | 16 (73) | 6 (27) | 0 | 0 |
| Low-risk adenoma | 5 | 31 | 20 (65) | 10 (32) | 0 | 1 (3) | |
| No adenoma | 5 | 25 | 16 (64) | 7 (28) | 1 (4) | 1 (4) | |
| No adenoma | High-risk adenoma | 3 | 35 | 22 (63) | 12 (34) | 1 (3) | 0 |
| Low-risk adenoma | 5–10 implied | 47 | 36 (77) | 9 (19) | 0 | 2 (4) | |
| Stringent Scenario: No adenoma, assuming no prior advanced adenoma or advanced sessile serrated lesion ever |
10 | 56 | 12 (21) | 42 (75) | 0 | 2 (4) | |
| Liberal Scenario: No adenoma, assuming ≥1 prior advanced adenoma or advanced sessile serrated lesion ever | 5 implied | 56 | 37 (66) | 5 (9) | 12 (21) | 2 (4) | |
| Delayed post-2012 guidelines | |||||||
| Low-risk adenoma | High-risk adenoma | 3 | 3 | 3 (100) | NA | NA | NA |
| Low-risk adenoma | 5 | 3 | 1 (33) | 1 (33) | 1 (33) | 0 | |
| No adenoma | 10 | 3 | 0 | 3 (100) | NA | NA | |
| High-risk adenoma | High-risk adenoma | 3 | 17 | 12 (71) | 4 (24) | 0 | 1 (6) |
| Low-risk adenoma | 5 | 19 | 12 (63) | 5 (26) | 0 | 2 (11) | |
| No adenoma | 5 | 13 | 10 (77) | 2 (15) | 0 | 1 (8) | |
| No adenoma | High-risk adenoma | 3 | 28 | 22 (79) | 3 (11) | 3 (11) | 0 |
| Low-risk adenoma | 5–10 implied | 42 | 33 (79) | 6 (14) | 0 | 3 (7) | |
| Stringent Scenario: No adenoma, assuming no prior advanced adenoma or advanced sessile serrated lesion ever |
10 | 54s | 11 (20) | 40 (74) | 0 | 3 (6) | |
| Liberal Scenario: No adenoma, assuming ≥1 prior advanced adenoma or advanced sessile serrated lesion ever |
5 implied | 54 | 37 (69) | 3 (6) | 12 (22) | 2 (4) |
For colonoscopies performed prior to September 2012, provider surveillance recommendations were compared to the 2006 USMSTF guidelines.
Recommendations for Subsequent Surveillance Colonoscopy Once Study Entry Surveillance Colonoscopy Pathology was Available: No HRA or LRA at the Most Recent Prior Colonoscopy
Among those in whom the absence of HRA or LRA was documented at the most recent prior colonoscopy, when we assumed that these patients had never had prior HRA (stringent Scenario 1), the final recommendations for future, repeat surveillance colonoscopy adhered with guidelines in 80% of cases preguideline, 51% of cases postguideline, and 53% or cases delayed postguideline (P < .001) (Table 1). There was a statistically significant difference in the distribution of recommendations in which the interval was too short in the 3 time periods (Table 1), Tables A1 and A2.
When it was assumed that all of these patients had prior HRA at some point further in the past (liberal Scenario 2), however, no differences in overall adherence with guidelines were observed for the 3 time periods (Table 1).
Discussion
This study addressing the implementation of the 2012 MSTF postpolypectomy surveillance guidelines10 identifies themes that are relevant for the successful implementation of the more recent 2020 updated guidelines.9 As clinical practice has moved away from sustained, every 5-year surveillance given a history of only 1 nonadvanced adenoma on multiple colonoscopies, the need for comprehensive information on a patient's polyp history has intensified in order to allow guideline-adherent surveillance recommendations.
We found that, contrary to one of our initial hypotheses, information on the most recent prior colonoscopy was often available to providers to inform subsequent surveillance recommendations. Nonetheless, this information was lacking in a substantial minority of cases. Prior colonoscopy information was available in 78% of preguideline and 78% of postguideline cases, but only in 61% of delayed postguideline cases. This finding may relate to the increased embrace of open access colonoscopy over time, especially at SHC. This finding highlights the challenge of providing surveillance recommendations that depend on present and past colonoscopy findings when the available information on past findings is incomplete, which is most likely to occur in open access colonoscopy services. This situation may be ameliorated by increased data sharing (eg “Care Everywhere” function in Epic and “Joint Legacy Viewer” in the VA EHR system).
The 2012 guidelines also increased the need to know details of polyp histology at the current colonoscopy. We observed that the majority of recommendations in delayed postguideline colonoscopy reports (59% vs 6%–11% in the other 2 periods) were to await the results of pathology. Along with this finding, there was a lower proportion in the fraction of delayed postguideline colonoscopy reports with a recommendation listing a discrete surveillance interval (eg 5 years) compared to the preguideline and postguideline periods (32% vs 79%–87%). This finding highlights the need for robust systems to review pathology postcolonoscopy and communicate surveillance intervals to patients and referring physicians, which is even more relevant under the 2020 guidelines.
Even in the 61%–78% of cases with complete current and recent polyp information, adherence to guidelines for subsequent surveillance was not optimal. When the most recent prior colonoscopy revealed HRA or LRA, surveillance recommendations adhered with guidelines in 54%–67% of cases, without a statistically significant difference between study periods. The most striking lack of adherence with the 2012 guidelines was in cases with LRA followed by normal colonoscopy: a 10-year surveillance interval was not recommended in even a single case in the combined postguideline or delayed postguideline periods. We would expect that the number of patients who might have had HRA in the more distant past (information that was not captured in our data extraction) would be a small minority. This finding reflects the challenges of instituting longer surveillance intervals in practice when clinicians and patients may be used to shorter intervals, and when some may believe that more surveillance must always be desirable.
When the most recent prior colonoscopy revealed no HRA or LRA, implying that at least one adenoma was removed at some point in the more distant past because all patients entered the study with a surveillance colonoscopy, the recommendations for subsequent surveillance adhered with guidelines in 51%–80% of cases, depending on whether these patients might have had HRA in the past. Our results highlight the clinical implications of not knowing the results of all prior colonoscopies, namely shorter interval recommendations and increased resourced utilization. In clinical practice, even if information on the most recent colonoscopy is available, it often still remains uncertain whether a patient ever had HRA on even earlier colonoscopies.
The need to know detailed information on all of a patient's past colonoscopies continues to grow as stronger evidence for risk stratification based on polyp characteristics develops.8 While those with any prior history of HRA may remain at increased risk for subsequent future HRA, those with LRA or no adenoma on serial examinations may require little or no surveillance.15, 16, 17, 18, 19, 20, 21, 22 However, practice can be slow to change. Our findings regarding adherence to the 2012 guidelines suggest that widespread acceptance in the US of a 10-year interval for patients with LRA, particularly with subsequent normal colonoscopies9 may take time. Evidence from randomized controlled trials31,32 is eagerly awaited, as this may impact future guidelines and their implementation. It remains a challenge to devise physician outreach and education programs, as well as clinical workflows, to accelerate the incorporation of updated guidelines into clinical practice.33, 34, 35, 36
The strengths of this study include that we were able to analyze a diverse group of patients from 2 different health care systems, with comprehensive extraction of data pertinent to the study entry surveillance colonoscopy, and the most recent prior colonoscopy. We acknowledge some study limitations. First, our sample size was modest given the need for detailed chart review leading to feasibility constraints regarding a larger sample size, and there may be limited generalizability to other very different practice settings. Second, some clinically relevant prior data were unavailable, but measuring the magnitude of this problem was a primary objective of our study. Third, unmeasured confounders may be present, including institutional policies, administrative pressures, and endoscopist characteristics. Given the large number of endoscopists relative to the number of colonoscopies, a wide range of colonoscopy number by endoscopist, and the fact that many endoscopists contributed colonoscopies in only one or 2 study periods, analyses accounting for clustering by endoscopist were not feasible. Fourth, we did not attempt to ascertain a complete past screening and surveillance history for each patient, and instead focused on the most recent prior colonoscopy. We addressed potential uncertainty regarding the spectrum of past neoplasia by analyzing liberal vs stringent scenarios to account for the possibility of prior HRA. Finally, guidelines do not capture all the clinical considerations for each individual patient, and we were not able to ascertain justifications for practice that varied from guideline recommendations. Future studies can address this important dimension of clinical practice in the context of guidelines.
In conclusion, this study highlights key themes in postcolonoscopy surveillance. These include the need for comprehensive information on all prior colonoscopies in order to make guideline-adherent surveillance recommendations, the often-observed imperfect adherence to guidelines, and the typical delays that occur in guideline uptake in routine clinical practice. A salient finding is the lack of uptake of a 10-year surveillance interval in patients with LRA and then normal colonoscopy. Future efforts should concentrate on strategies to implement evidence-based postpolypectomy surveillance guidelines into practice.
Footnotes
Authors' Contributions: Ulysses S. Rosas: Acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content. Jennifer Y. Pan: Acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. Vandana Sundaram: Study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis. Andrew Su: Study concept and design, acquisition of data, Muhammad Fazal: Acquisition of data. Philip Dinh: Acquisition of data. Uri Ladabaum: Study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, study supervision.
Conflicts of Interest: This author discloses the following: UL, advisory board (UniversalDx, Lean Medical), consultant (Covidien, Motus GI, Quorum, Clinical Genomics). The remaining authors disclose no conflicts.
Funding: The authors report no funding.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: The analytic methods, but not the primary data and study materials, are available upon request to the corresponding author.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2022.07.014.
Supplementary Materials
References
- 1.Carethers J.M., Doubeni C.A. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354–367. doi: 10.1053/j.gastro.2019.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zauber A.G., Winawer S.J., O'Brien M.J., et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer Deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardcastle J.D., Chamberlain J.O., Robinson M.H., et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 4.Mandel J.S., Church T.R., Bond J.H., et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343:1603–1607. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 5.Nishihara R., Wu K., Lochhead P., et al. Long-Term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–1105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holme Ø., Løberg M., Kalager M., et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA. 2014;312:606–615. doi: 10.1001/jama.2014.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoen R.E., Pinsky P.F., Weissfeld J.L., et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–2357. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lieberman D., Gupta S. Does colon polyp surveillance improve patient outcomes? Gastroenterology. 2020;158:436–440. doi: 10.1053/j.gastro.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta S., Lieberman D., Anderson J.C., et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US multi-society task force on colorectal cancer. Gastroenterology. 2020;158:1131–1153.e5. doi: 10.1053/j.gastro.2019.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lieberman D.A., Rex D.K., Winawer S.J., et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US multi-society task force on colorectal cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Levin B., Lieberman D.A., McFarland B., et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US multi-society task force on colorectal cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Lieberman D.A., Williams J.L., Holub J.L., et al. Colonoscopy utilization and outcomes 2000 to 2011. Gastrointest Endosc. 2014;80:133–143.e3. doi: 10.1016/j.gie.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Cyhaniuk A., Coombes M.E. Longitudinal adherence to colorectal cancer screening guidelines. Am J Manag Care. 2016;22:105–111. [PubMed] [Google Scholar]
- 14.Bandi P., Minihan A.K., Siegel R.L., et al. Updated review of major cancer risk factors and screening test use in the United States in 2018 and 2019, with a focus on smoking cessation. Cancer Epidemiol Prev Biomarkers. 2021;30:1287–1299. doi: 10.1158/1055-9965.EPI-20-1754. [DOI] [PubMed] [Google Scholar]
- 15.Inadomi J.M., Vijan S., Janz N.K., et al. Adherence to colorectal cancer screening: a randomized clinical trial of Competing strategies. Arch Intern Med. 2012;172:575–582. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Løberg M., Kalager M., Holme Ø., et al. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med. 2014;371:799–807. doi: 10.1056/NEJMoa1315870. [DOI] [PubMed] [Google Scholar]
- 17.Saini S.D., Nayak R.S., Kuhn L., et al. Why don't gastroenterologists follow colon polyp surveillance guidelines? J Clin Gastroenterol. 2009;43:554–558. doi: 10.1097/MCG.0b013e31818242ad. [DOI] [PubMed] [Google Scholar]
- 18.Schoen R.E., Pinsky P.F., Weissfeld J.L., et al. Utilization of surveillance colonoscopy in community practice. Gastroenterology. 2010;138:73–81. doi: 10.1053/j.gastro.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ransohoff D.F., Yankaskas B., Gizlice Z., et al. Recommendations for post-polypectomy surveillance in community practice. Dig Dis Sci. 2011;56:2623–2630. doi: 10.1007/s10620-011-1791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodwin J.S., Singh A., Reddy N., et al. Overuse of screening colonoscopy in the medicare population. Arch Intern Med. 2011;171:1335–1343. doi: 10.1001/archinternmed.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radaelli F., Paggi S., Bortoli A., et al. Overutilization of post-polypectomy surveillance colonoscopy in clinical practice: a prospective, multicentre study. Dig Liver Dis. 2012;44:748–753. doi: 10.1016/j.dld.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Cooper G.S., Kou T.D., Sloan J.S.B., et al. Use of colonoscopy for polyp surveillance in medicare beneficiaries. Cancer. 2013;119:1800–1807. doi: 10.1002/cncr.27990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pilonis N.D., Bugajski M., Wieszczy P., et al. Long-term colorectal cancer incidence and mortality after a single negative screening colonoscopy. Ann Intern Med. 2020;173:81–91. doi: 10.7326/M19-2477. [DOI] [PubMed] [Google Scholar]
- 24.Miller J., Mehta N., Feldman M., et al. Findings on serial surveillance colonoscopy in patients with low-risk polyps on initial colonoscopy. J Clin Gastroenterol. 2010;44:e46–e50. doi: 10.1097/MCG.0b013e3181a7ed2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J.K., Jensen C.D., Levin T.R., et al. Long-term risk of colorectal cancer and related deaths after a colonoscopy with normal findings. JAMA Intern Med. 2019;179:153–160. doi: 10.1001/jamainternmed.2018.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenner H., Altenhofen L., Stock C., et al. Incidence of colorectal adenomas: birth cohort analysis among 4.3 million participants of screening colonoscopy. Cancer Epidemiol Biomarkers Prev. 2014;23:1920–1927. doi: 10.1158/1055-9965.EPI-14-0367. [DOI] [PubMed] [Google Scholar]
- 27.Laiyemo A.O., Pinsky P.F., Marcus P.M., et al. Utilization and yield of surveillance colonoscopy in the continued follow-up study of the polyp prevention trial. Clin Gastroenterol Hepatol. 2009;7:562–567. doi: 10.1016/j.cgh.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson D.J., Burke C.A., Welch H.G., et al. Using the results of a baseline and a surveillance colonoscopy to predict recurrent adenomas with high-risk characteristics. Ann Intern Med. 2009;151:103. doi: 10.7326/0003-4819-151-2-200907210-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan B.A., Redding T.S., Hauser E.R., et al. High-risk adenomas at screening colonoscopy remain predictive of future high-risk adenomas despite an intervening negative colonoscopy. Am J Gastroenterol. 2020;115:1275–1282. doi: 10.14309/ajg.0000000000000677. [DOI] [PubMed] [Google Scholar]
- 30.Duvvuri A., Chandrasekar V.T., Srinivasan S., et al. Risk of colorectal cancer and cancer related mortality after detection of low-risk or high-risk adenomas, compared with no adenoma, at index colonoscopy: a systematic review and meta-analysis. Gastroenterology. 2021;160:1986–1996.e3. doi: 10.1053/j.gastro.2021.01.214. [DOI] [PubMed] [Google Scholar]
- 31.Jover R., Bretthauer M., Dekker E., et al. Rationale and design of the European Polyp Surveillance (EPoS) trials. Endoscopy. 2016;48:571–578. doi: 10.1055/s-0042-104116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US). Five or ten year colonoscopy for 1-2 non-advanced adenomatous polyps (FORTE) https://clinicaltrials.gov/ct2/show/NCT05080673 Available at:
- 33.Melson J.E., Imperiale T.F., Itzkowitz S.H., et al. AGA white paper: roadmap for the future of colorectal cancer screening in the United States. Clin Gastroenterol Hepatol. 2020;18:2667–2678.e2. doi: 10.1016/j.cgh.2020.06.053. [DOI] [PubMed] [Google Scholar]
- 34.Anderson J.C., Baron J.A., Ahnen D.J., et al. Factors associated with shorter colonoscopy surveillance intervals for patients with low-risk colorectal adenomas and effects on outcome. Gastroenterology. 2017;152:1933–1943.e5. doi: 10.1053/j.gastro.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meester R.G.S., Lansdorp-Vogelaar I., Winawer S.J., et al. High-intensity versus low-intensity surveillance for patients with colorectal adenomas: a cost-effectiveness analysis. Ann Intern Med. 2019;171:612. doi: 10.7326/M18-3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cross A.J., Robbins E.C., Pack K., et al. Long-term colorectal cancer incidence after adenoma removal and the effects of surveillance on incidence: a multicentre, retrospective, cohort study. Gut. 2020;69:1645–1658. doi: 10.1136/gutjnl-2019-320036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.