Abstract
Background
Transcatheter mitral valve-in-valve (MViV) replacement has emerged as an alternative to redo surgical mitral valve replacement (redo-SMVR) in patients with failed mitral bioprostheses deemed to be at a high surgical risk. The aim of this analysis was to compare the outcomes of MViV replacement with those of redo-SMVR in patients with a failed bioprosthetic mitral valve.
Methods
We performed a study-level meta-analysis that compared MViV replacement with redo-SMVR in patients with failed mitral bioprostheses. Seven observational studies, with a total of 5083 patients, were included (1138 patients [22.4%] in the MViV replacement arm). The primary focus was all-cause mortality. Additional outcomes included major bleeding, stroke, vascular complications, and mean mitral valve gradient at follow-up.
Results
The in-hospital mortality was lower in patients who underwent MViV replacement than in those who underwent redo-SMVR (odds ratio [OR], 0.64; 95% CI, 0.53-0.78; P = .0023). The short-term mortality (<1 year) was numerically lower in the MViV replacement group (OR, 0.45; 95% CI, 0.18-1.13; P = .069). At 1 year, the risk of mortality was similar in the 2 groups (OR, 0.99; 95% CI, 0.69-1.40; P = .906), and at midterm follow-up (≥1 year), there was a numerically higher risk of mortality in the MViV replacement group (OR, 1.51; 95% CI, 1.00-2.29; P = .051). The risk of major bleeding was significantly lower in the MViV replacement group (OR, 0.23; 95% CI, 0.10-0.56; P = .01). Additionally, stroke and vascular complications were similar between the 2 groups.
Conclusions
The in-hospital mortality was lower in the MViV replacement group than in the redo-SMVR group. There were no differences in mortality at short-term (<1 year), 1-year, or midterm (≥1 year) follow-ups.
Keywords: failed bioprosthetic mitral valve, redo surgical aortic valve replacement, transcatheter mitral valve replacement
Central Illustration
Highlights
-
•
In patients with failed mitral bioprostheses, transcatheter mitral valve replacement is associated with lower short-term mortality.
-
•
Transcatheter mitral valve replacement using a transseptal approach has midterm mortality comparable to redo surgical replacement.
-
•
The risk of major bleeding was significantly lower in the transcatheter mitral valve replacement arm.
-
•
The risk of stroke and vascular complications were similar in both the arms.
Introduction
Surgery is the standard treatment option for degenerative mitral valve disease. After 7 to 8 years of implantation, bioprosthetic valves start to degenerate and eventually fail.1 Some patients with failed mitral bioprostheses or annuloplasty rings are at a particularly high risk of death or major complications after redo surgery.2 This risk is particularly high in patients with advanced age, severe comorbidities, and unfavorable anatomic conditions.2 Over the last few years, transcatheter mitral valve-in-valve (MViV) replacement has become an alternative to redo surgical mitral valve replacement (redo-SMVR) in patients with failed mitral bioprostheses at a high surgical risk.3
Currently, there are limited data comparing mortality between surgical and transcatheter approaches in patients with failed mitral bioprostheses. The results at 1 year in patients who underwent MViV replacement showed a low burden of symptoms and excellent prosthesis function.4 A meta-analysis of 270 patients showed a lower risk of major adverse cardiac events in patients who underwent MViV replacement than in those who underwent redo-SMVR, with a similar rate of in-hospital mortality between the 2 groups.5 A recent multicenter analysis showed increased early survival in patients who underwent MViV replacement.6 The present meta-analysis was performed to evaluate mortality in patients who underwent MViV replacement or redo-SMVR for degenerated mitral valve bioprostheses.
Methods
The meta-analysis was performed in accordance with the Cochrane Collaboration guidelines, reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines,7 and registered at PROSPERO (CRD42022320317).
The Population, Intervention, Comparison, Outcome, and Study design strategy was used, and our inclusion criteria included the following:
-
1.
The study included adult patients with a failed bioprosthetic mitral valve.
-
2.
One group of patients underwent MViV replacement.
-
3.
Another group of patients underwent redo-SMVR.
-
4.
The study reported mortality, major bleeding, major vascular complications, stroke, and/or mean valve gradient at follow-up.
-
5.
The study design was retrospective, prospective, randomized, nonrandomized, and single or multicenter with matched or unmatched populations.
We conducted a comprehensive literature review using the PubMed, Embase, and Cochrane databases through January 2022. The following search terms were included: failed bioprosthetic mitral valve, transcatheter mitral replacement, transcatheter mitral valve-in-valve implantation, TMVR, redo mitral valve surgery, redo-SMVR, redo surgical mitral valve implantation, mortality, stroke, major bleeding, and vascular complications.
Two authors (A.A.-A. and Y.S.) independently reviewed the search results, extracted potential articles, and assessed their eligibility. Any disagreements between the 2 authors were resolved by mutual discussion. This study was exempted from institutional review board oversight because the meta-analysis was performed at the study level.
Data abstraction, risk-of-bias assessment, and outcomes
Data were abstracted using standard data collection forms. We collected the characteristics of each study: first author’s name; year of publication; single versus multicenter; number of participants in each group; follow-up duration; and demographic, clinical, and procedural characteristics between the competing interventions. We used the Newcastle-Ottawa Scale for a risk-of-bias assessment (Table 1). The primary focus was on all-cause mortality, which was stratified into in-hospital mortality, short-term mortality (≤1 year), 1-year mortality, and midterm (≥1 year) all-cause mortality. Additional outcomes included stroke, major bleeding, vascular complications, and mean mitral valve gradient at the maximum available follow-up.
Table 1.
The Newcastle-Ottawa Scale for quality assessment of studies comparing transcatheter mitral valve-in-valve versus redo surgical mitral valve replacement in patients with degenerated mitral prostheses.
| Selection |
Comparability |
Outcome |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of nonexposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at the start of the study | Adjust for the most important risk factors | Adjust for other risk factors | Assessment of outcome | Follow-up length | Loss to follow-up rate | Total quality score | ||
| Simard et al,12 2022 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 8 | ||
| Zubarevich et al,11 2021 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 8 | ||
| Khan et al,9 2021 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||||
| Simonetto et al,6 2021 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 8 | ||
| Osman et al,13 2020 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||||
| Kamioka et al,10 2018 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 8 | ||
| Murzi et al,8 2017 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 8 | ||
Two authors (A.A.-A. and Y.S.) were involved in data abstraction and the risk-of-bias assessment. Discrepancies among investigators were resolved by a consensus.
Statistical analysis
We used the R package, Metafor, version 4.1.3 (R foundation) for all analyses. The Mantel-Haenszel random-effects models were used to estimate the odds ratio (OR) and corresponding 95% CIs for binary outcomes. We used the inverse variance method to estimate the standardized mean difference for continuous outcomes. I2 statistics were used to assess statistical heterogeneity; I2 >50% indicated a high degree of heterogeneity. A subgroup analysis was performed in patients who underwent MViV replacement using a transseptal (TS) approach. For all analyses, P < .05 was considered statistically significant.
Results
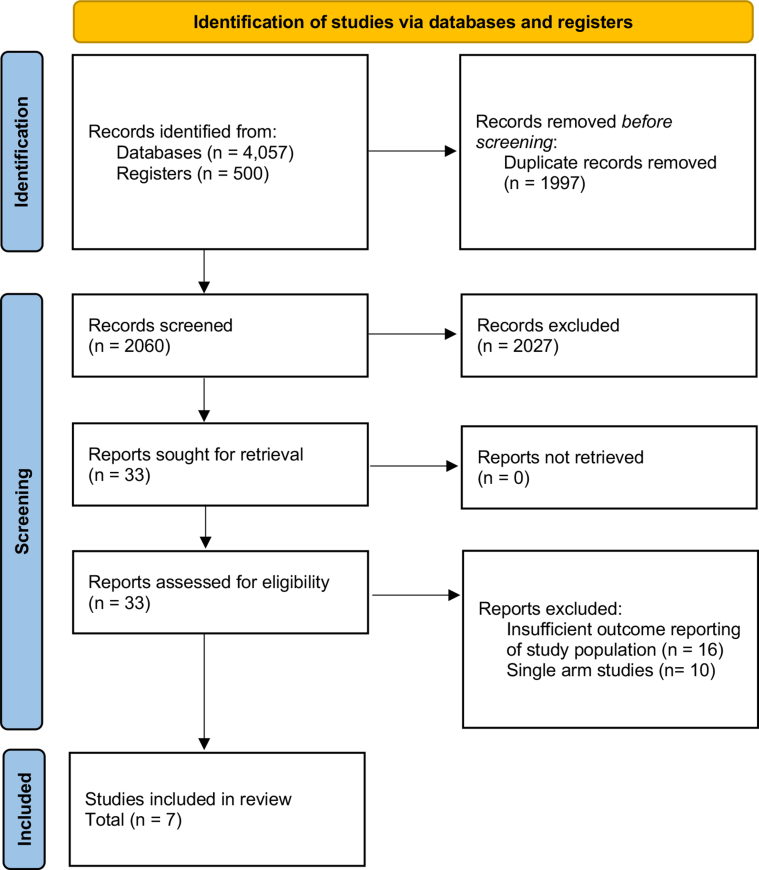
Of 4057 records retrieved, 33 were reviewed for eligibility after excluding duplicates and screening at the title and abstract levels. Ultimately, 7 observational studies were included for the analysis. The bias assessment showed the included studies to be of good quality, as determined using the Newcastle-Ottowa Scale. A total of 5083 patients were included, 1138 (22.4%) of whom were in the MViV replacement arm (Figure 1).6,8, 9, 10, 11, 12, 13 The patients in the MViV replacement arm were older (mean age, 73.6 vs 67.7 years in the MViV replacement and redo-SMVR groups, respectively; P < .01). There were 2713 women (53.4%) included in the entire population, with similar distribution between the 2 arms (54.4% in the MViV replacement arm vs 53% in the redo-SMVR arm, P = .26). The numbers of patients with a high burden of symptoms (New York Heart Association functional class III and IV) were reported in 5 studies, and they had a similar distribution between the 2 arms (79% in the MViV replacement arm vs 61% in the redo-SMVR arm, P = .77). In the transcatheter mitral valve replacement (TMVR) group, the majority of patients underwent the valve-in-valve procedure (99.8% underwent the valve-in-valve procedure and 0.2% underwent the valve-in-ring procedure). Additionally, only 3 studies reported the surgical risk of their population, and patients in the MViV replacement group were at a higher surgical risk in all studies. Three studies reported the type of valve and the approach used in the MViV replacement group. The most utilized valves were SAPIEN XT and SAPIEN 3, and the TS approach was used more frequently (63%). Additional characteristics of the included studies and population are shown in Tables 2 and 3.
Figure 1.
The Preferred Reporting Items for Systematic Reviews and Meta-analysesflow diagram.
Table 2.
Characteristics of included studies comparing mitral valve-in-valve versus redo surgical mitral valve replacement in patients with degenerated mitral prostheses.
| Simard et al,12 2022 | Zubarevich et al,11 2021 | Khan et al,9 2021 | Simonetto et al,6 2021 | Osman et al,13 2020 | Kamioka et al,10 2018 | Murzi et al,8 2017 | |
|---|---|---|---|---|---|---|---|
| Type of the study | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective |
| Follow-up duration | 5 y | 3 y | In-hospital | 1 y | 30 d | 1 y | 2 y |
| Location | United States | Germany | United States | Italy | United States | United States | Italy |
| Number of centers | Single center | Single center | National registry | 4 centers | National registry | 3 centers | Single center |
| Sample size | 215 | 74 | 2745 | 78 | 1789 | 121 | 61 |
| TMVR access site | Transseptal (97.7%), transapical (2.3%) | Transapical (100%) | N/A | Transseptal (55%), transapical (45%) | N/A | Transseptal (77%), transapical (23%) | Transapical (100%) |
| TMVR valve type | SAPIEN, SAPIEN XT, SAPIEN 3, SAPIEN 3 Ultra | SAPIEN XT, SAPIEN 3 | N/A | N/A | N/A | SAPIEN, SAPIEN XT, SAPIEN 3 | SAPIEN XT, SAPIEN 3 |
TMVR, transcatheter mitral valve replacement; N/A; not available.
Table 3.
Baseline characteristics of patients included in the studies comparing mitral valve-in-valve versus redo surgical mitral valve replacement in patients with degenerated mitral prostheses.
| Simard et al,12 2022 |
Zubarevich et al,11 2021 |
Khan et al,9 2021 |
Simonetto et al,6 2021 |
Osman et al,13 2020 |
Kamioka et al,10 2018 |
Murzi et al,8 2017 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TMVR | Redo-SMVR | TMVR | Redo-SMVR | TMVR | Redo-SMVR | TMVR | Redo-SMVR | TMVR | Redo-SMVR | TMVR | Redo-SMVR | TMVR | Redo-SMVR | |
| Sample size | 86 | 129 | 41 | 33 | 495 | 2250 | 49 | 29 | 384 | 1404 | 62 | 59 | 21 | 40 |
| Age, y | 74.9 | 64.5 | 73.6 | 63.7 | 77 | 68 | 77.6 | 67.7 | 76 | 68 | 74.9 | 63.7 | 77 | 67 |
| Female, n (%) | 54 (62.8) | 81 (62.8) | 19 (46.3) | 22 (66.7) | 260 (52.5) | 1150 (51.1) | 20 (40.8) | 13 (44.8) | 215 (56) | 769 (54.8) | 38 (61.3) | 36 (61) | 13 (61.9) | 23 (56.1) |
| Diabetes mellitus | 15 (17.4) | 24 (18.6) | 14 (34.1) | 4 (12.1) | 40 (8.1) | 185 (8.2) | 9 (18.3) | 4 (14.8) | 133 (34.6) | 543 (38.7) | 15 (24.2) | 7 (11.9) | 5 (23.8) | 4 (9.8) |
| Chronic lung disease | 30 (34.9) | 26 (20.2) | 17 (41.5) | 5 (15.2) | 140 (28.3) | 575 (25.6) | 4 (8.2)a | 3 (10.7)a | n/a | n/a | 21 (33.9)b | 8 (13.6)b | 3 (14.2) | 5 (12.2) |
| NYHA class III or IV | 85 (98.8) | 89 (69) | 41 (100) | 24 (72.7) | n/a | n/a | 42 (85.7) | 16 (57.2) | n/a | n/a | Class IV: 19 (30.7) | Class IV: 19 (32.2) | 18 (85.7) | 29 (70.7) |
| STS score | n/a | n/a | 11.9 | 10.2 | n/a | n/a | 8.7 | 3.6 | n/a | n/a | 12.7 | 8.7 | n/a | n/a |
| Logistic EuroSCORE | n/a | n/a | 42.3 | 32.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 39 | 23 |
| EuroSCORE II | n/a | n/a | 21.2 | 18.2 | n/a | n/a | 12.1 | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
| Ejection fraction | 58.7 | 59.3 | 45.4 | 52.2 | n/a | n/a | 59.7 | 59.7 | n/a | n/a | 54.6 | 55.7 | 50 | 53 |
EuroSCORE, European System for Cardiac Operative Risk Evaluation; n/a, not available; NYHA, New York Heart Association; SMVR, surgical mitral valve replacement; STS, Society of Thoracic Surgeons; TMVR, transcatheter mitral valve replacement.
Severe.
≥Moderate.
All-cause mortality
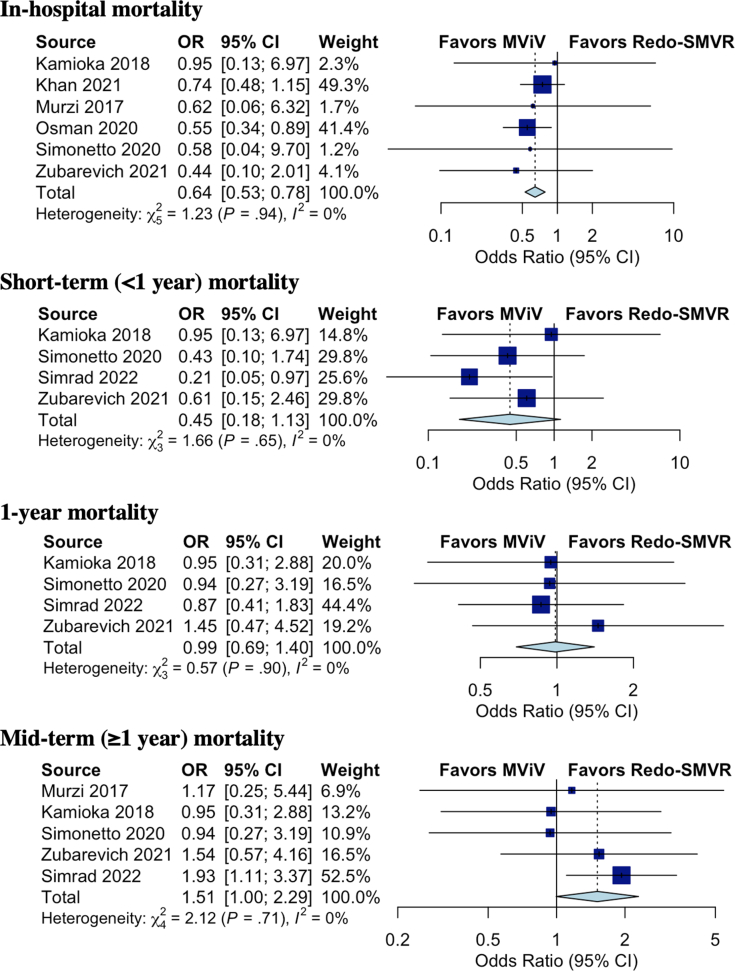
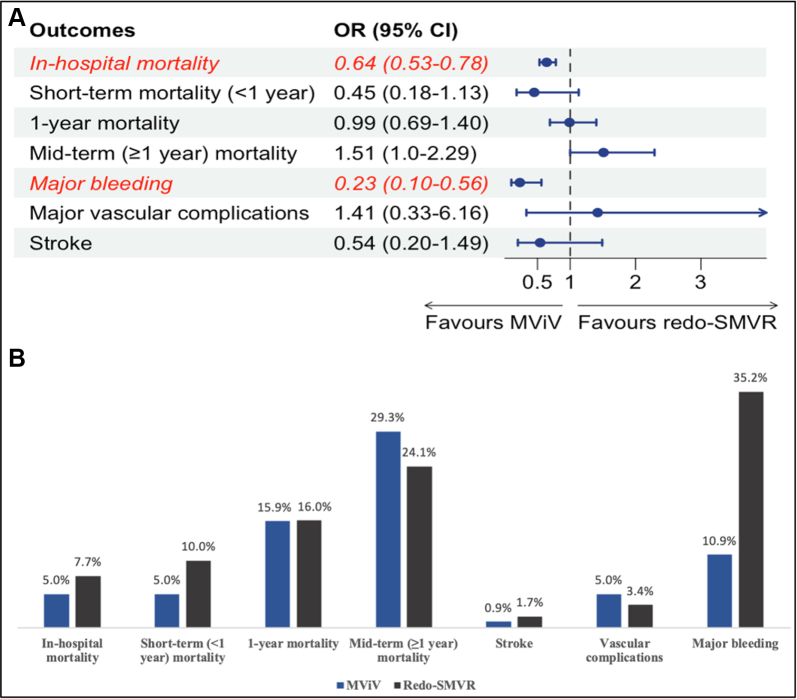
The in-hospital mortality was lower in the MViV replacement group than in the redo-SMVR group (5% in the MViV replacement group vs 7.7% in the redo-SMVR group; OR, 0.65; 95% CI, 0.53-0.78; P = .0023; I2 = 0%). The short-term mortality (<1 year) was numerically lower in the MViV replacement group and did not reach statistical significance (5% in the MViV replacement group vs 10% in the redo-SMVR group; OR, 0.45; 95% CI, 0.18-1.13; P = .069; I2 = 0%).
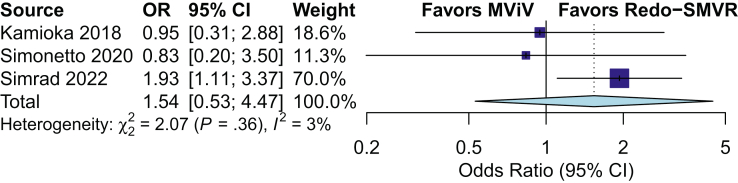
The rate of 1-year mortality was 16% in the entire population and similar between the 2 groups (15.9% in the MViV replacement group vs 16% in the redo-SMVR group; OR, 0.99; 95% CI, 0.69-1.40; P = .906; I2 = 0%). There was a trend toward higher midterm mortality (≥1 year) at a median-weighted follow-up of 2.94 years in the MViV replacement group, and it did not reach significance (29.3% in the MViV replacement group vs 24.1% in the redo-SMVR group, P = .051) (Figure 2). The subgroup analysis in patients who underwent MViV replacement using the TS approach showed similar midterm mortality (30.9% in the MViV replacement group vs 25.8% in the redo-SMVR group; OR, 1.54; 95% CI, 0.53-4.47; P = .22; I2 = 3%) (Figure 3).
Figure 2.
Forest plot of in-hospital, short-term (<1 year), 1-year, and midterm mortality in patients with failed bioprostheses who underwent mitral valve-in-valve (MViV) replacement versus those in patients who underwent redo surgical mitral valve replacement (SMVR). OR, odds ratio.
Figure 3.
Forest plot of midterm mortality in the subgroup of patients who underwent mitral valve-in-valve (MViV) using the transseptal approach versus that in patients who underwent redo surgical mitral valve replacement (SMVR). OR, odds ratio.
Additional end points
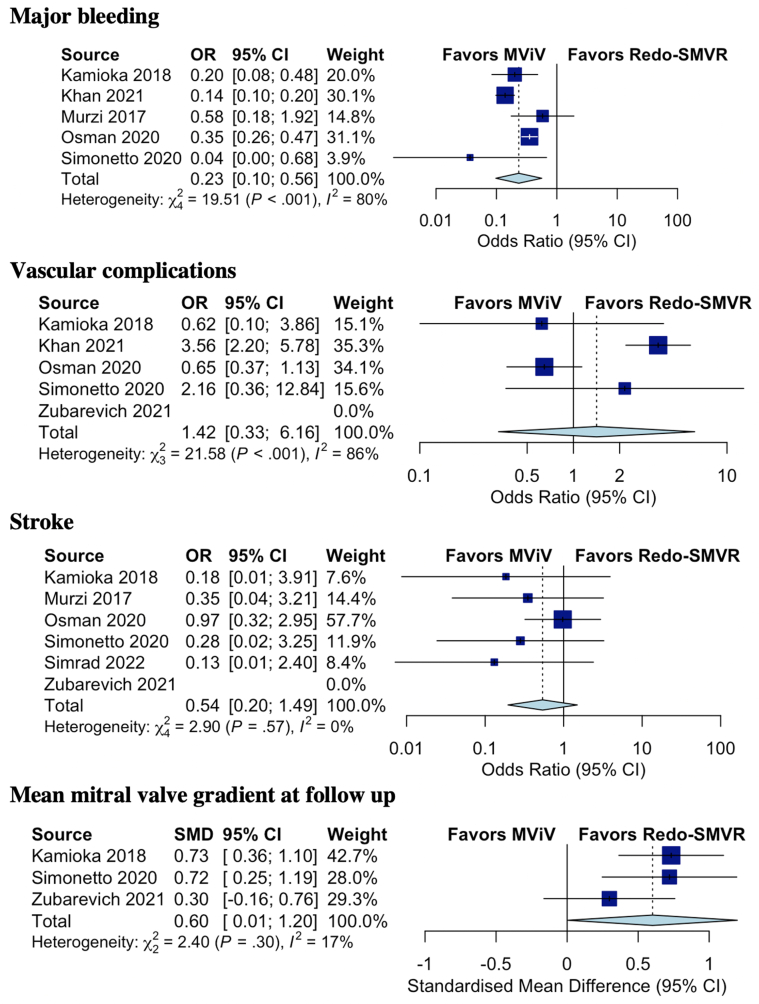
The mean mitral valve gradient at follow-up was higher in the MViV replacement group than in the redo-SMVR group (mean gradient, 6.3 mm Hg in the MViV replacement group vs 5.1 mm Hg in the redo-SMVR group) (mean difference of 0.60; 95% CI, 0.01-1.20; P = .049; I2 = 17%). The rates of stroke (0.9% in the MViV replacement group vs 1.7% in the redo-SMVR group; OR, 0.54; 95% CI, 0.20-1.49; P = .168; I2 = 0%) and vascular complications (5% in the MViV replacement group vs 3.4% in the redo-SMVR group; OR, 1.42; 95% CI, 0.36-6.16; P = .506; I2 = 86%) were similar between the 2 groups; however, the rate of major bleeding was significantly lower in the MViV replacement group (10.9% in the MViV replacement group vs 35.2% in the redo-SMVR group; OR, 0.23; 95% CI, 0.10-0.56; P = .01; I2 = 80%) (Figure 4).
Figure 4.
Forest plot of the rate of major bleeding, vascular complications, stroke, and mean mitral valve gradient at follow-up in patients with failed bioprostheses who underwent mitral valve-in-valve (MViV) versus those in patients who underwent redo surgical mitral valve replacement (SMVR). OR, odds ratio.
Discussion
In this meta-analysis of 7 observational studies, with a total of 5083 patients with failed mitral bioprostheses, we compared the outcomes of MViV replacement with those of redo-SMVR. The main findings of this study are as follows: (1) the in-hospital mortality and the rates of major bleeding were lower in the MViV replacement group than in the redo-SMVR group; (2) the 1-year mortality, rates of stroke, and vascular complications were similar in the 2 groups; and (3) there was a trend toward higher mortality in the MViV replacement group than in the redo-SMVR group at a median-weighted follow-up of 2.94 years (Central Illustration).
Central Illustration.
(A) Meta-analysis of clinical outcomes in patients with a failed bioprosthesis who underwent transcatheter mitral valve replacement (MViV) versus those in patients who underwent redo surgical mitral valve replacement (SMVR). (B) The incidence of the outcomes with transcatheter mitral valve replacement versus redo surgical mitral valve replacement in patients with a failed mitral bioprosthesis. OR, odds ratio.
Recently, few studies have investigated the safety and efficacy of MViV replacement. A multicenter registry study that included 1529 high-risk patients (Society of Thoracic Surgeons predicted risk of mortality [STS PROM], 11.1%) with failed mitral bioprostheses who underwent MViV replacement with a SAPIEN 3 valve reported a procedural mortality of 4%, 30-day mortality of 5.4%, and 1-year mortality of 16.7%. Additionally, the TS approach was associated with lower mortality compared with the transapical approach.14 The Mitral Implantation of Transcatheter Valves trial included 30 patients with failed surgical mitral bioprostheses who underwent MViV replacement via the TS approach with a SAPIEN 3 valve and reported a technical success rate of 100%.15 At 1 year, the all-cause mortality was 3.3%, and 89.3% of the patients were in New York Heart Association functional class I or II. Moreover, no significant mitral regurgitation or stenosis was achieved in 96.6% of the patients at 30 days and 82.8% of the patients at 1 year. Because of favorable outcomes, TS access became the preferred approach for MViV replacement. A systematic review of single-arm studies reporting the outcomes of MViV replacement included 11 studies and reported a 30-day mortality of up to 8% and 1-year mortality rate of up to 16%.16 This is similar to our results of 8% mortality in the short term and 16% at 1 year.
Meanwhile, several studies have directly compared MViV replacement with redo-SMVR. Kamioka et al10 reported 1-year outcomes in 121 patients, 51% of whom underwent MViV replacement. Patients in the MViV replacement group were older, had more comorbidities, and had a higher STS PROM (8.7% in the redo-SMVR group vs 12.7% in the MViV replacement group; P < .001). The TS approach was more frequently utilized (77.4%). The in-hospital, 30-day, and 1-year mortality was similar between the 2 groups despite a higher surgical risk and comorbidities in the MViV replacement group (1-year mortality, 11.9% in the redo-SMVR group vs 11.3% in the MViV replacement group; P = .92).10 Zubarevich et al11 reported clinical outcomes in 74 patients with failed bioprosthetic mitral valves (55.4% of the population underwent MViV replacement). All patients in the MViV replacement group underwent the procedure via the transapical approach. The patients in the MViV replacement arm were older, had more comorbidities, and had a higher STS PROM (10.2% in the redo-SMVR group vs 11.9% in the MViV group; P = .003). The mortality was similar between the 2 arms at 30-day, 1-year, and 3-year follow-ups.11 Most recently, Simard et al12 reported the clinical outcomes of TMVR versus those of redo-SMVR in 215 patients. Patients in the TMVR arm were older, had more comorbidities, and were more symptomatic at baseline. The TS approach was used in 97.7% of the patients in the TMVR arm. The mortality was lower in the TMVR arm at 30 days (2.4% in the TMVR arm vs 10.2% in the redo-SMVR arm), similar between the 2 arms at 1 year (14.7% in the TMVR arm vs 17.5% in the redo-SMVR arm), and higher in the TMVR arm at 5 years (49.9% in the TMVR arm vs 34.0% in the redo-SMVR arm). In the TMVR group, the effective orifice area and right ventricular systolic pressure remained stable at 3 years of follow-up.12
Our pooled results showed significantly lower in-hospital mortality and a trend toward lower short-term (<1 year) mortality in the MViV replacement group. The mortality was similar in both the groups at 1 year, with a trend toward higher mortality in the MViV replacement group at 2.9 years of follow-up. However, patients in the MViV replacement group across all studies were at a high surgical risk, significantly older, and had more comorbid conditions, which likely contributed to higher midterm mortality. The role of MViV in nonhigh-surgical risk patients is not known. The PARTNER 3 Mitral Valve in Valve Registry (NCT03193801) is a prospective, single-arm study evaluating the safety and effectiveness of mitral valve-in-valve in patients with failed mitral bioprostheses who have intermediate surgical risk.17 The results of this ongoing trial will provide further insights into the role of MViV in lower-risk patients.
Limitations
This study has multiple limitations. First, this analysis involved retrospective observational studies. There are no randomized data comparing MViV replacement with redo-SMVR for failed mitral bioprostheses, and all evidence thus far has been derived from retrospective data. Second, although most of the studies utilized a SAPIEN valve, different generations of valves were used across the studies, making comparisons challenging. Third, hemodynamic and echocardiographic outcomes were only reported in few studies. Fourth, only 3 studies, with a total of 414 patients, reported the outcomes of the TS approach using subgroup analyses; thus, they should be interpreted with caution. Lastly, the pooled analysis was derived from the aggregate data from all studies and not from individual-level patient data.
Conclusion
Our pooled analysis showed lower in-hospital mortality in the MViV replacement group than in the redo-SMVR group for failed mitral bioprostheses, despite a greater comorbidity burden in the MViV replacement group. There was no difference in mortality at short-term (<1 year), 1-year, or midterm (≥1 year) follow-up between the 2 groups.
Acknowledgments
Declaration of competing interest
Sachin S. Goel is a consultant for Medtronic, Speaker’s Bureau, and Abbott Structural Heart. Mayra Guerrero has received institutional research grant support from Edwards Lifesciences. The remaining authors reported no financial interests.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement and patient consent
This research adheres to relevent ethical guidelines.
References
- 1.Kostyunin A.E., Yuzhalin A.E., Rezvova M.A., Ovcharenko E.A., Glushkova T.V., Kutikhin A.G. Degeneration of bioprosthetic heart valves: update 2020. J Am Heart Assoc. 2020;9(19):e018506–e018507. doi: 10.1161/JAHA.120.018506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urena M., Himbert D., Brochet E., et al. Transseptal transcatheter mitral valve replacement using balloon-expandable transcatheter heart valves: a step-by-step approach. JACC Cardiovasc Interv. 2017;10(19):1905–1919. doi: 10.1016/j.jcin.2017.06.069. [DOI] [PubMed] [Google Scholar]
- 3.Testa L., Popolo Rubbio A., Casenghi M., Pero G., Latib A., Bedogni F. Transcatheter mitral valve replacement in the transcatheter aortic valve replacement era. J Am Heart Assoc. 2019;8(22):e013352–e013353. doi: 10.1161/JAHA.119.013352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eleid M.F., Whisenant B.K., Cabalka A.K., et al. Early outcomes of percutaneous transvenous transseptal transcatheter valve implantation in failed bioprosthetic mitral valves, ring annuloplasty, and severe mitral annular calcification. JACC Cardiovasc Interv. 2017;10(19):1932–1942. doi: 10.1016/j.jcin.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Zahid S., Ullah W., Sarvepalli D., Inayat A., Salman F., Khan M.Z. Meta-analysis comparing valve in valve transcatheter mitral valve replacement versus redo surgical mitral valve replacement for degenerating bioprosthetic valves. Am J Cardiol. 2021;149:155–156. doi: 10.1016/j.amjcard.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Simonetto F., Purita P.A., Malerba M., et al. Surgical redo versus transseptal or transapical transcatheter mitral valve-in-valve implantation for failed mitral valve bioprosthesis. Catheter Cardiovasc Interv. 2021;97(4):714–722. doi: 10.1002/ccd.29324. [DOI] [PubMed] [Google Scholar]
- 7.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):1–11. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murzi M., Berti S., Gasbarri T., et al. Transapical transcatheter mitral valve-in-valve implantation versus minimally invasive surgery for failed mitral bioprostheses. Interact Cardiovasc Thorac Surg. 2017;25(1):57–61. doi: 10.1093/icvts/ivx067. [DOI] [PubMed] [Google Scholar]
- 9.Zia Khan M., Zahid S., Khan M.U., et al. Redo surgical mitral valve replacement versus transcatheter mitral valve in valve from the national inpatient sample. J Am Heart Assoc. 2021;10(17):e020948–e020949. doi: 10.1161/JAHA.121.020948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamioka N., Babaliaros V., Morse M.A., et al. Comparison of clinical and echocardiographic outcomes after surgical redo mitral valve replacement and transcatheter mitral valve-in-valve therapy. JACC Cardiovasc Interv. 2018;11(12):1131–1138. doi: 10.1016/j.jcin.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Zubarevich A., Szczechowicz M., Arjomandi Rad A., et al. Mitral surgical redo versus transapical transcatheter mitral valve implantation. PLoS One. 2021;16(8):e0256569–e0256570. doi: 10.1371/journal.pone.0256569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simard T., Lloyd J., Crestanello J., et al. Five-year outcomes of transcatheter mitral valve implantation and redo surgery for mitral prosthesis degeneration. Catheter Cardiovasc Interv. 2022;99(5):1659–1665. doi: 10.1002/ccd.30059. [DOI] [PubMed] [Google Scholar]
- 13.Osman M., Al-Hijji M.A., Kawsara A., Patel B., Alkhouli M. Comparative outcomes of mitral valve in valve implantation versus redo mitral valve replacement for degenerated bioprotheses. Am J Cardiol. 2020;132:175–176. doi: 10.1016/j.amjcard.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whisenant B., Kapadia S.R., Eleid M.F., et al. One-year outcomes of mitral valve-in-valve using the SAPIEN 3 transcatheter heart valve. JAMA Cardiol. 2020;5(11):1245–1252. doi: 10.1001/jamacardio.2020.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerrero M., Pursnani A., Narang A., et al. Prospective evaluation of transseptal TMVR for failed surgical bioprostheses: mitral trial valve-in-valve arm 1-year outcomes. JACC Cardiovasc Interv. 2021;14(8):859–872. doi: 10.1016/j.jcin.2021.02.027. [DOI] [PubMed] [Google Scholar]
- 16.Sengupta A., Yazdchi F., Alexis S.L., et al. Reoperative mitral surgery versus transcatheter mitral valve replacement: a systematic review. J Am Heart Assoc. 2021;10(6) doi: 10.1161/JAHA.120.019854. e019854-e019854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.PARTNER 3 trial - mitral valve in valve. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03193801