Abstract
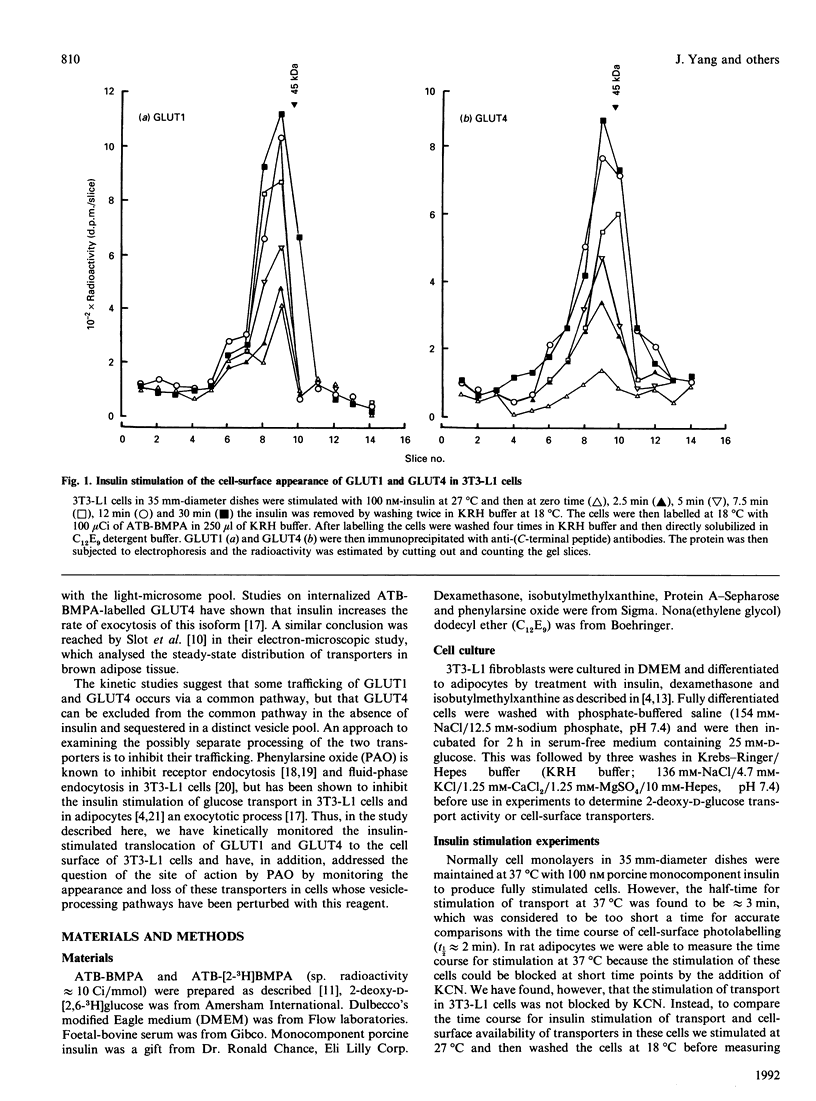
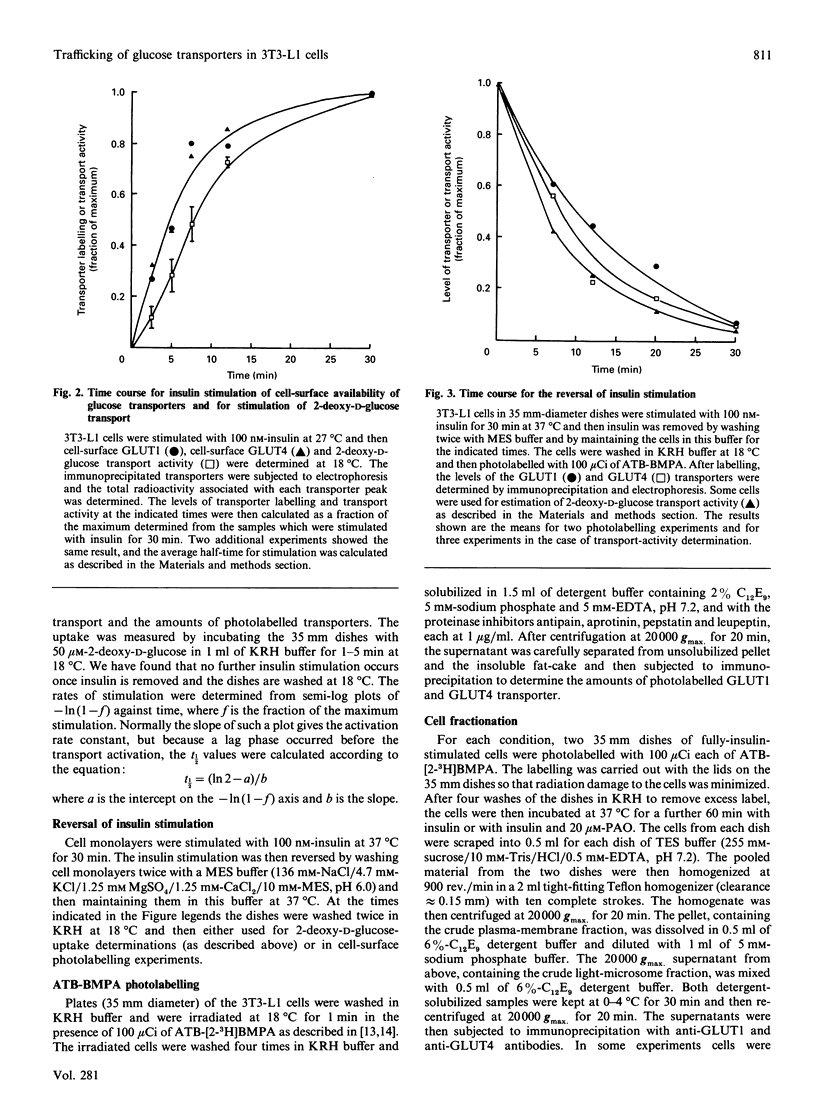
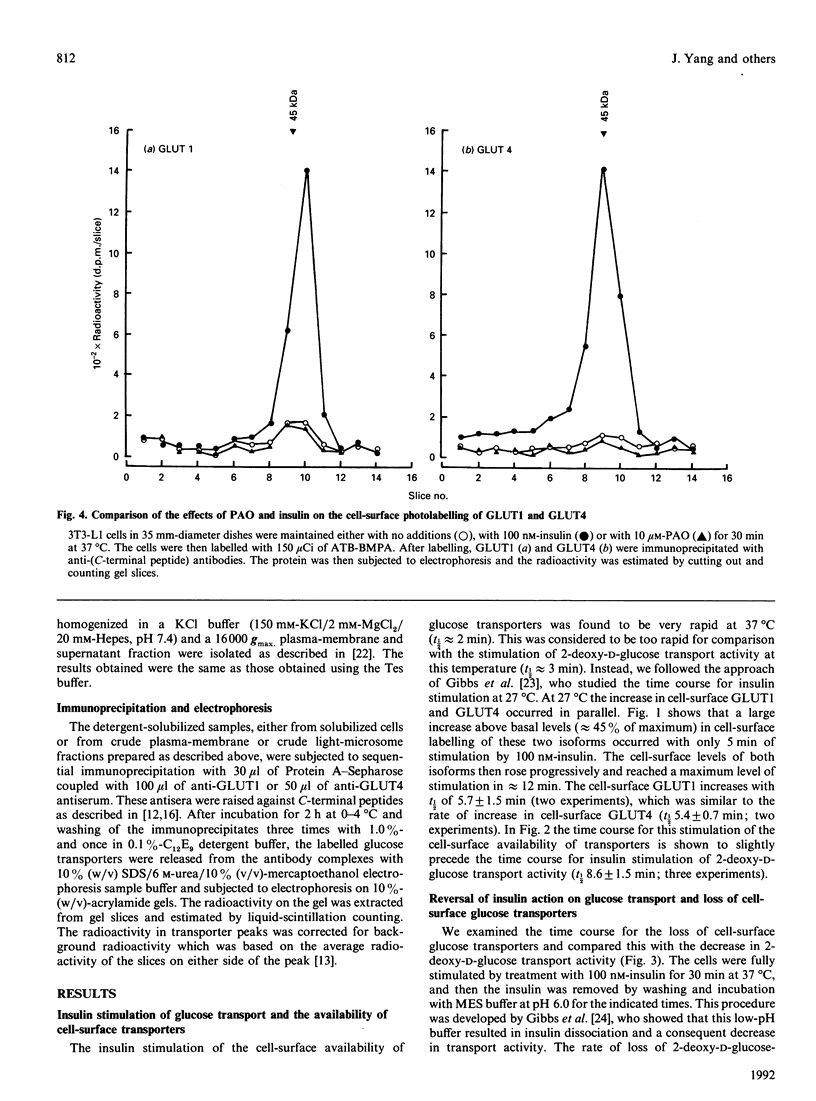
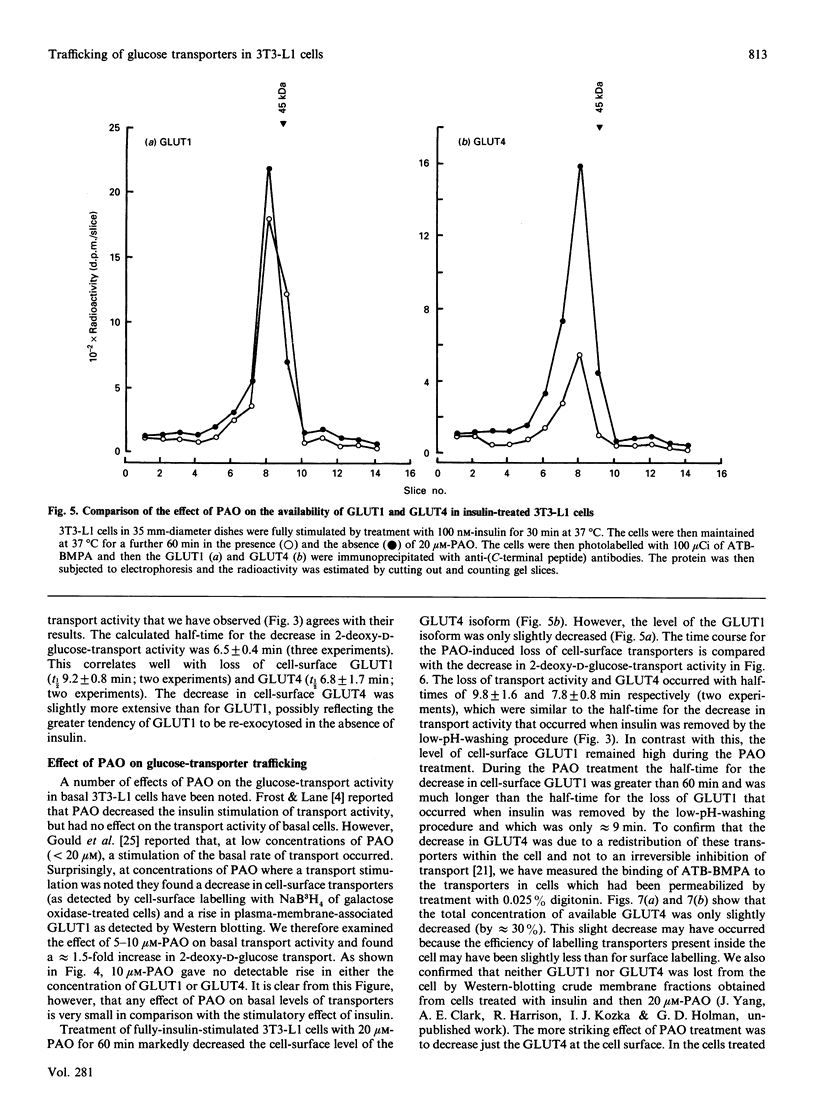
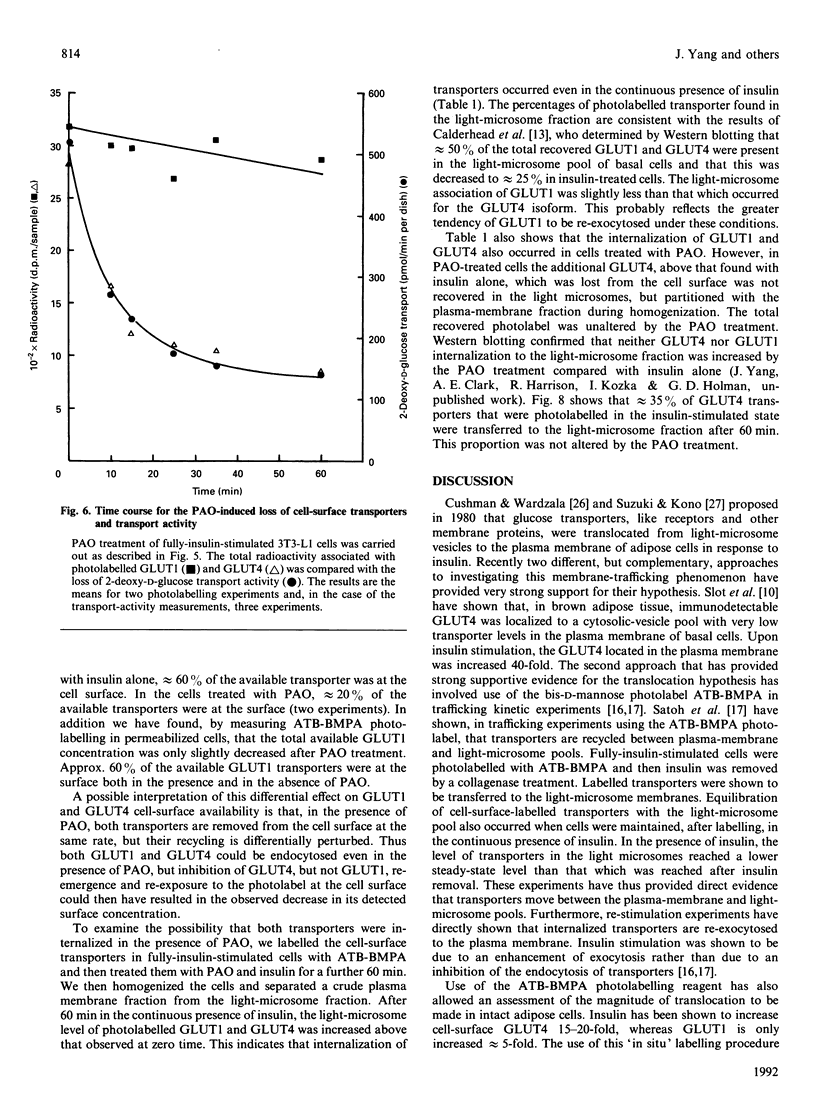
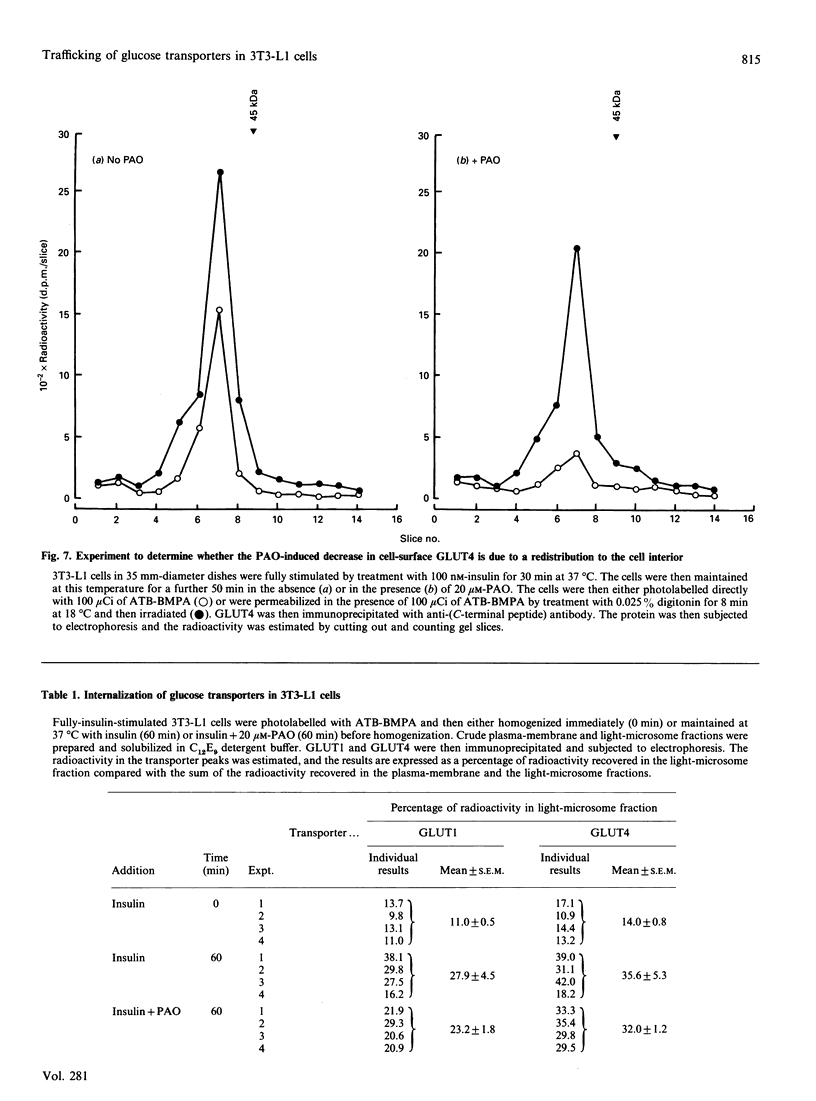
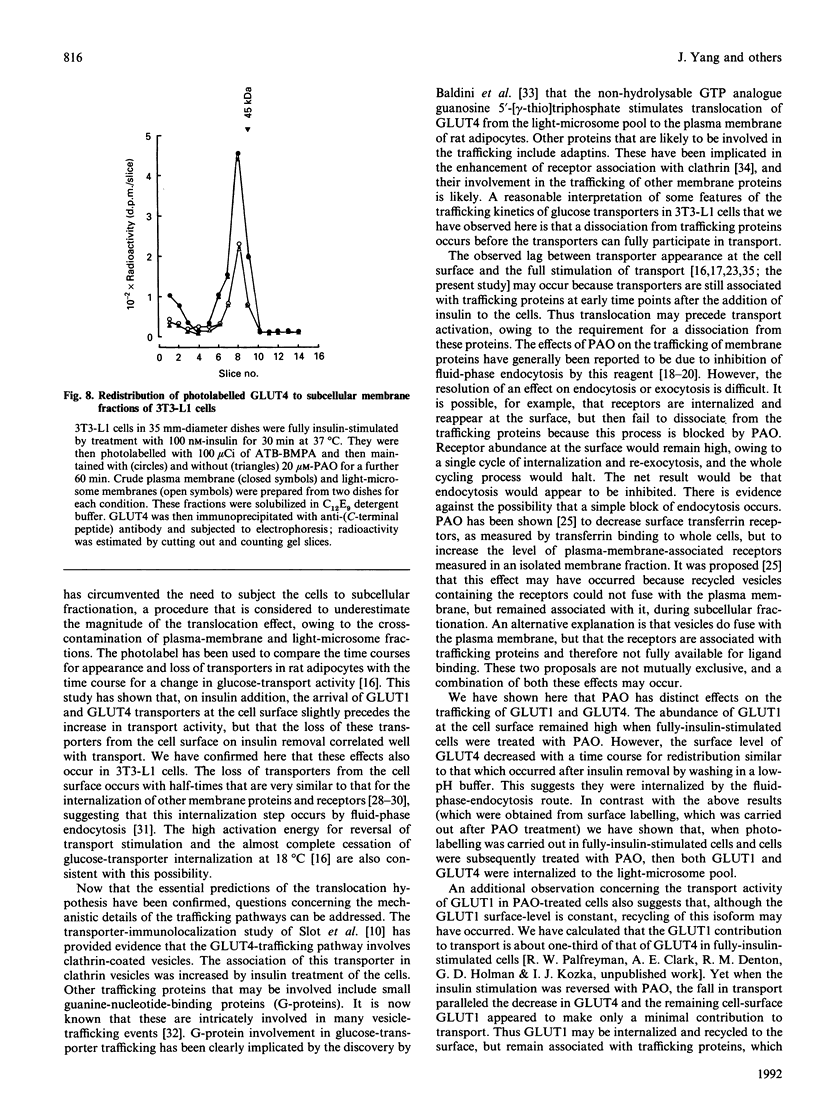
We have compared the rates of insulin stimulation of cell-surface availability of glucose-transporter isoforms (GLUT1 and GLUT4) and the stimulation of 2-deoxy-D-glucose transport in 3T3-L1 cells. The levels of cell-surface transporters have been assessed by using the bismannose compound 2-N-[4-(1-azi-2,2,2-trifluoroethyl)benzoyl]-1,3-bis(D-mannos -4-yloxy) propyl-2-amine (ATB-BMPA). At 27 degrees C the half-times for the appearance of GLUT1 and GLUT4 at the cell surface were 5.7 and 5.4 min respectively and were slightly shorter than that for the observed stimulation of transport activity (t 1/2 8.6 min). This lag may be due to a slow dissociation of surface transporters from trafficking proteins responsible for translocation. When fully-insulin-stimulated cells were subjected to a low-pH washing procedure to remove insulin at 37 degrees C, the cell-surface levels of GLUT1 and GLUT4 decreased, with half-times of 9.2 and 6.8 min respectively. These times correlated well with decrease in 2-deoxy-D-glucose transport activity that occurred during this washing procedure (t1/2 6.5 min). When fully-insulin-stimulated cells were treated with phenylarsine oxide (PAO), a similar decrease in transport activity occurred (t1/2 9.8 min). However, surface labelling showed that this corresponded with a decrease in GLUT4 only (t1/2 7.8 min). The cell-surface level of GLUT1 remained high throughout the PAO treatment. Light-microsome membranes were isolated from cells which had been cell-surface-labelled with ATB-BMPA. Internalization of both transporter isoforms to this pool occurred when cells were maintained in the presence of insulin for 60 min. In contrast with the surface-labelling results, we have shown that the transfer to the light-microsome pool of both transporters occurred in cells treated with insulin and PAO. These results suggest that both transporters are recycled by fluid-phase endocytosis and exocytosis. PAO may inhibit this recycling at a stage which involves the re-emergence of internalized transporters at the plasma membrane. The GLUT1 transporters that are recycled to the surface in insulin- and PAO-treated cells appear to have low transport activity. This may be because of a failure to dissociate fully from trafficking proteins at the cell surface. GLUT4 transporters appear to have a greater tendency to remain internalized if the normal mechanisms that commit transporters to the cell surface, such as dissociation from trafficking proteins, are uncoupled.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldini G., Hohman R., Charron M. J., Lodish H. F. Insulin and nonhydrolyzable GTP analogs induce translocation of GLUT 4 to the plasma membrane in alpha-toxin-permeabilized rat adipose cells. J Biol Chem. 1991 Mar 5;266(7):4037–4040. [PubMed] [Google Scholar]
- Birnbaum M. J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989 Apr 21;57(2):305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Brown S. J., Gould G. W., Davies A., Baldwin S. A., Lienhard G. E., Gibbs E. M. Characterization of vesicles containing insulin-responsive intracellular glucose transporters isolated from 3T3-L1 adipocytes by an improved procedure. Biochim Biophys Acta. 1988 Oct 7;971(3):339–350. doi: 10.1016/0167-4889(88)90150-4. [DOI] [PubMed] [Google Scholar]
- Calderhead D. M., Kitagawa K., Tanner L. I., Holman G. D., Lienhard G. E. Insulin regulation of the two glucose transporters in 3T3-L1 adipocytes. J Biol Chem. 1990 Aug 15;265(23):13801–13808. [PubMed] [Google Scholar]
- Charron M. J., Brosius F. C., 3rd, Alper S. L., Lodish H. F. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2535–2539. doi: 10.1073/pnas.86.8.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B. M., Czech M. P. Hexose transport stimulation and membrane redistribution of glucose transporter isoforms in response to cholera toxin, dibutyryl cyclic AMP, and insulin in 3T3-L1 adipocytes. J Biol Chem. 1990 Jul 25;265(21):12434–12443. [PubMed] [Google Scholar]
- Clark A. E., Holman G. D. Exofacial photolabelling of the human erythrocyte glucose transporter with an azitrifluoroethylbenzoyl-substituted bismannose. Biochem J. 1990 Aug 1;269(3):615–622. doi: 10.1042/bj2690615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. E., Holman G. D., Kozka I. J. Determination of the rates of appearance and loss of glucose transporters at the cell surface of rat adipose cells. Biochem J. 1991 Aug 15;278(Pt 1):235–241. doi: 10.1042/bj2780235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Douen A. G., Jones M. N. The action of phenylarsine oxide on the stereospecific uptake of D-glucose in basal and insulin-stimulated rat adipocytes. Biochim Biophys Acta. 1988 Jan 18;968(1):109–118. doi: 10.1016/0167-4889(88)90050-x. [DOI] [PubMed] [Google Scholar]
- Frost S. C., Lane M. D. Evidence for the involvement of vicinal sulfhydryl groups in insulin-activated hexose transport by 3T3-L1 adipocytes. J Biol Chem. 1985 Mar 10;260(5):2646–2652. [PubMed] [Google Scholar]
- Frost S. C., Lane M. D., Gibbs E. M. Effect of phenylarsine oxide on fluid phase endocytosis: further evidence for activation of the glucose transporter. J Cell Physiol. 1989 Dec;141(3):467–474. doi: 10.1002/jcp.1041410304. [DOI] [PubMed] [Google Scholar]
- Fukumoto H., Kayano T., Buse J. B., Edwards Y., Pilch P. F., Bell G. I., Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989 May 15;264(14):7776–7779. [PubMed] [Google Scholar]
- Gibbs E. M., Allard W. J., Lienhard G. E. The glucose transporter in 3T3-L1 adipocytes is phosphorylated in response to phorbol ester but not in response to insulin. J Biol Chem. 1986 Dec 15;261(35):16597–16603. [PubMed] [Google Scholar]
- Gibbs E. M., Lienhard G. E., Appleman J. R., Lane M. D., Frost S. C. Insulin stimulates fluid-phase endocytosis and exocytosis in 3T3-L1 adipocytes. J Biol Chem. 1986 Mar 25;261(9):3944–3951. [PubMed] [Google Scholar]
- Gibbs E. M., Lienhard G. E., Gould G. W. Insulin-induced translocation of glucose transporters to the plasma membrane precedes full stimulation of hexose transport. Biochemistry. 1988 Sep 6;27(18):6681–6685. doi: 10.1021/bi00418a006. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Lienhard G. E., Tanner L. I., Gibbs E. M. Phenylarsine oxide stimulates hexose transport in 3T3-L1 adipocytes by a mechanism other than an increase in surface transporters. Arch Biochem Biophys. 1989 Jan;268(1):264–275. doi: 10.1016/0003-9861(89)90588-2. [DOI] [PubMed] [Google Scholar]
- Harrison S. A., Buxton J. M., Clancy B. M., Czech M. P. Insulin regulation of hexose transport in mouse 3T3-L1 cells expressing the human HepG2 glucose transporter. J Biol Chem. 1990 Nov 25;265(33):20106–20116. [PubMed] [Google Scholar]
- Holman G. D., Kozka I. J., Clark A. E., Flower C. J., Saltis J., Habberfield A. D., Simpson I. A., Cushman S. W. Cell surface labeling of glucose transporter isoform GLUT4 by bis-mannose photolabel. Correlation with stimulation of glucose transport in rat adipose cells by insulin and phorbol ester. J Biol Chem. 1990 Oct 25;265(30):18172–18179. [PubMed] [Google Scholar]
- James D. E., Brown R., Navarro J., Pilch P. F. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988 May 12;333(6169):183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Joost H. G., Weber T. M., Cushman S. W., Simpson I. A. Activity and phosphorylation state of glucose transporters in plasma membranes from insulin-, isoproterenol-, and phorbol ester-treated rat adipose cells. J Biol Chem. 1987 Aug 15;262(23):11261–11267. [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., McLenithan J. C., Braiterman L. T., Cornelius P., Pekala P. H., Lane M. D. Sequence, tissue distribution, and differential expression of mRNA for a putative insulin-responsive glucose transporter in mouse 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1989 May;86(9):3150–3154. doi: 10.1073/pnas.86.9.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnieli E., Zarnowski M. J., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981 May 25;256(10):4772–4777. [PubMed] [Google Scholar]
- Knutson V. P., Ronnett G. V., Lane M. D. Rapid, reversible internalization of cell surface insulin receptors. Correlation with insulin-induced down-regulation. J Biol Chem. 1983 Oct 25;258(20):12139–12142. [PubMed] [Google Scholar]
- Robinson M. S. 100-kD coated vesicle proteins: molecular heterogeneity and intracellular distribution studied with monoclonal antibodies. J Cell Biol. 1987 Apr;104(4):887–895. doi: 10.1083/jcb.104.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson I. A., Cushman S. W. Hormonal regulation of mammalian glucose transport. Annu Rev Biochem. 1986;55:1059–1089. doi: 10.1146/annurev.bi.55.070186.005211. [DOI] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J., Gigengack S., Lienhard G. E., James D. E. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991 Apr;113(1):123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W., Geuze H. J., Strous G. J. Sorting of endocytosed transferrin and asialoglycoprotein occurs immediately after internalization in HepG2 cells. J Cell Biol. 1987 May;104(5):1261–1268. doi: 10.1083/jcb.104.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L. P., Holman G. D. Symmetrical kinetic parameters for 3-O-methyl-D-glucose transport in adipocytes in the presence and in the absence of insulin. Biochim Biophys Acta. 1981 Apr 6;642(2):325–335. doi: 10.1016/0005-2736(81)90449-1. [DOI] [PubMed] [Google Scholar]
- Vinten J., Gliemann J., Osterlind K. Exchange of 3-O-methylglucose in isolated fat cells. Concentration dependence and effect of insulin. J Biol Chem. 1976 Feb 10;251(3):794–800. [PubMed] [Google Scholar]
- Wiley H. S., Cunningham D. D. The endocytotic rate constant. A cellular parameter for quantitating receptor-mediated endocytosis. J Biol Chem. 1982 Apr 25;257(8):4222–4229. [PubMed] [Google Scholar]


