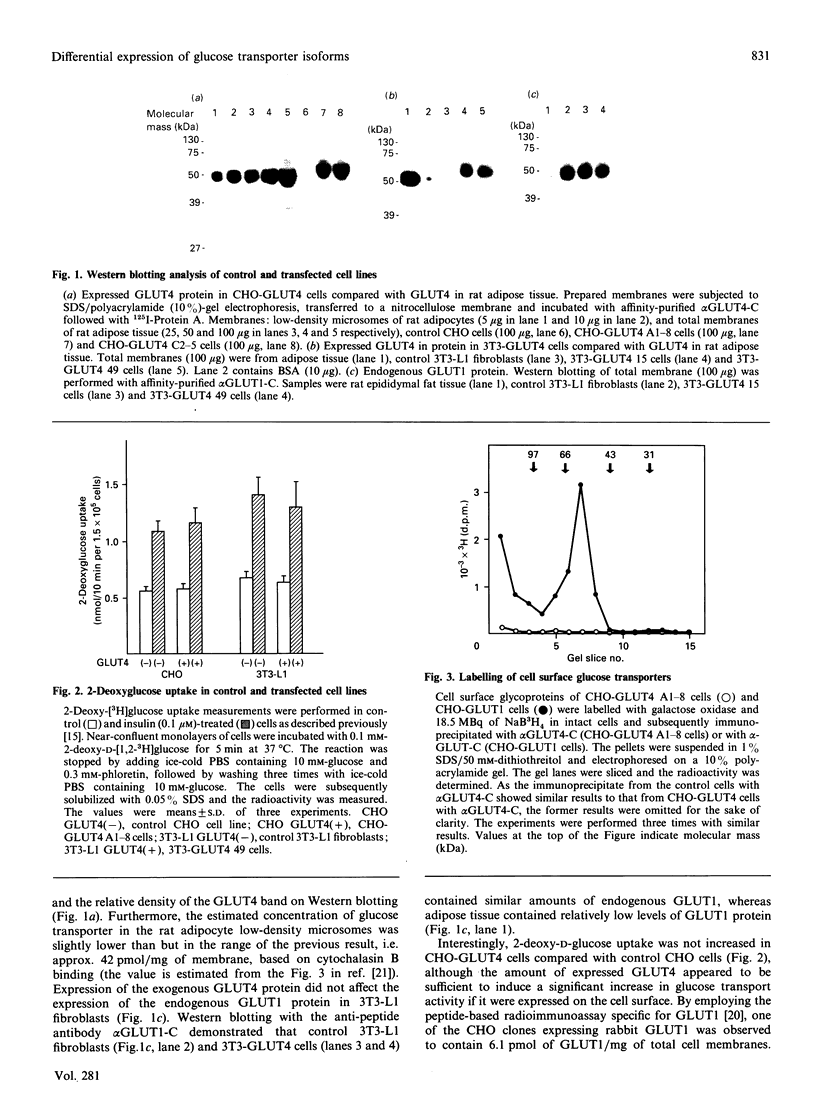
Abstract
Rat GLUT4 (adipocyte/muscle-type glucose transporter) was expressed in two fibroblastic cell lines, Chinese hamster ovary (CHO) cells and 3T3-L1 fibroblasts, under the control of the methallothionein I promoter. Although immunoblotting with a GLUT4-specific anti-peptide antibody demonstrated that the amount of GLUT4 expressed was comparable with that in 3T3-L1 adipocytes and rat adipose tissues, no increase in 2-deoxy-D-glucose uptake was observed in the basal state in fibroblasts. Immunocytochemical studies showed that the expressed GLUT4 appeared to be localized in a specific region in the cytoplasm. These results were in marked contrast to those obtained in CHO cells expressing GLUT1 (HepG2/erythrocyte-type glucose transporter) using the same expression vector. In this case the expressed GLUT1 protein appeared to reside mainly on the plasma membranes, and a significant increase in glucose uptake was observed. Although insulin increased glucose uptake in CHO cells and 3T3-L1 fibroblasts as well as in the cells expressing rat GLUT4, an increment due to insulin above basal values was small, at most 2-fold, and no significant differences were observed in insulin-stimulated glucose uptake between transfected and parental cells. In addition, no apparent differences in the subcellular distribution of expressed GLUT4 were observed between the insulin-stimulated and the basal state. These results indicate that in fibroblastic cell lines GLUT1 and GLUT4 proteins are sorted in a different fashion, and the expression of GLUT4 protein per se is not enough to produce a large insulin-induced increase in glucose transport activity such as that observed in rat adipocytes and 3T3-L1 adipocytes. Thus unidentified aspects of the cellular environment which are present in the adipocytes but not in fibroblastic cell lines may be required for a large insulin-induced increase in glucose transport activity to be observed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano T., Shibasaki Y., Ohno S., Taira H., Lin J. L., Kasuga M., Kanazawa Y., Akanuma Y., Takaku F., Oka Y. Rabbit brain glucose transporter responds to insulin when expressed in insulin-sensitive Chinese hamster ovary cells. J Biol Chem. 1989 Feb 25;264(6):3416–3420. [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum M. J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989 Apr 21;57(2):305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Calderhead D. M., Lienhard G. E. Labeling of glucose transporters at the cell surface in 3T3-L1 adipocytes. Evidence for both translocation and a second mechanism in the insulin stimulation of transport. J Biol Chem. 1988 Sep 5;263(25):12171–12174. [PubMed] [Google Scholar]
- Charron M. J., Brosius F. C., 3rd, Alper S. L., Lodish H. F. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2535–2539. doi: 10.1073/pnas.86.8.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto H., Kayano T., Buse J. B., Edwards Y., Pilch P. F., Bell G. I., Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989 May 15;264(14):7776–7779. [PubMed] [Google Scholar]
- Fukumoto H., Seino S., Imura H., Seino Y., Eddy R. L., Fukushima Y., Byers M. G., Shows T. B., Bell G. I. Sequence, tissue distribution, and chromosomal localization of mRNA encoding a human glucose transporter-like protein. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5434–5438. doi: 10.1073/pnas.85.15.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia de Herreros A., Birnbaum M. J. The acquisition of increased insulin-responsive hexose transport in 3T3-L1 adipocytes correlates with expression of a novel transporter gene. J Biol Chem. 1989 Nov 25;264(33):19994–19999. [PubMed] [Google Scholar]
- Hamer D. H., Walling M. Regulation in vivo of a cloned mammalian gene: cadmium induces the transcription of a mouse metallothionein gene in SV40 vectors. J Mol Appl Genet. 1982;1(4):273–288. [PubMed] [Google Scholar]
- Holman G. D. Side-specific photolabelling of the hexose transporter. Biochem Soc Trans. 1989 Jun;17(3):438–440. doi: 10.1042/bst0170438. [DOI] [PubMed] [Google Scholar]
- James D. E., Brown R., Navarro J., Pilch P. F. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988 May 12;333(6169):183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Joost H. G., Weber T. M., Cushman S. W., Simpson I. A. Activity and phosphorylation state of glucose transporters in plasma membranes from insulin-, isoproterenol-, and phorbol ester-treated rat adipose cells. J Biol Chem. 1987 Aug 15;262(23):11261–11267. [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., McLenithan J. C., Braiterman L. T., Cornelius P., Pekala P. H., Lane M. D. Sequence, tissue distribution, and differential expression of mRNA for a putative insulin-responsive glucose transporter in mouse 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1989 May;86(9):3150–3154. doi: 10.1073/pnas.86.9.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayano T., Burant C. F., Fukumoto H., Gould G. W., Fan Y. S., Eddy R. L., Byers M. G., Shows T. B., Seino S., Bell G. I. Human facilitative glucose transporters. Isolation, functional characterization, and gene localization of cDNAs encoding an isoform (GLUT5) expressed in small intestine, kidney, muscle, and adipose tissue and an unusual glucose transporter pseudogene-like sequence (GLUT6). J Biol Chem. 1990 Aug 5;265(22):13276–13282. [PubMed] [Google Scholar]
- Lin J. L., Asano T., Shibasaki Y., Tsukuda K., Katagiri H., Ishihara H., Takaku F., Oka Y. Altered expression of glucose transporter isoforms with aging in rats--selective decrease in GluT4 in the fat tissue and skeletal muscle. Diabetologia. 1991 Jul;34(7):477–482. doi: 10.1007/BF00403283. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Oka Y., Asano T., Shibasaki Y., Kasuga M., Kanazawa Y., Takaku F. Studies with antipeptide antibody suggest the presence of at least two types of glucose transporter in rat brain and adipocyte. J Biol Chem. 1988 Sep 15;263(26):13432–13439. [PubMed] [Google Scholar]
- Oka Y., Asano T., Shibasaki Y., Lin J. L., Tsukuda K., Katagiri H., Akanuma Y., Takaku F. C-terminal truncated glucose transporter is locked into an inward-facing form without transport activity. Nature. 1990 Jun 7;345(6275):550–553. doi: 10.1038/345550a0. [DOI] [PubMed] [Google Scholar]
- Pilch P. F. Glucose transporters: what's in a name? Endocrinology. 1990 Jan;126(1):3–5. doi: 10.1210/endo-126-1-3. [DOI] [PubMed] [Google Scholar]
- Piper R. C., Hess L. J., James D. E. Differential sorting of two glucose transporters expressed in insulin-sensitive cells. Am J Physiol. 1991 Mar;260(3 Pt 1):C570–C580. doi: 10.1152/ajpcell.1991.260.3.C570. [DOI] [PubMed] [Google Scholar]
- Student A. K., Hsu R. Y., Lane M. D. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem. 1980 May 25;255(10):4745–4750. [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Tsukuda K., Asano T., Lin J. L., Katagiri H., Ishihara H., Takaku F., Oka Y. Peptide-based radioimmunoassay specific for GLUT1 glucose transporter. Diabetes. 1991 Mar;40(3):315–318. doi: 10.2337/diab.40.3.315. [DOI] [PubMed] [Google Scholar]
- Zorzano A., Wilkinson W., Kotliar N., Thoidis G., Wadzinkski B. E., Ruoho A. E., Pilch P. F. Insulin-regulated glucose uptake in rat adipocytes is mediated by two transporter isoforms present in at least two vesicle populations. J Biol Chem. 1989 Jul 25;264(21):12358–12363. [PubMed] [Google Scholar]